Abstract
Perthes disease is an idiopathic avascular necrosis of a juvenile hip. Although 2010 marked a century since it was first described, the aetiology remains unknown. It is suggested that adverse socioeconomic circumstances may be a key precipitant. This work describes recent studies that explore the disease epidemiology.
Descriptive studies include a case register from Merseyside, hospital discharge data from Scotland, analysis of the world’s largest community disease register (General Practice Research Database [GPRD]) and a systematic review of incidence. Analytical studies include a nested case-controlled study in the GPRD and a hospital case-controlled study. The studies demonstrated a striking north–south divide in the UK incidence of Perthes disease, similar to that seen in many adult diseases. There was a sustained fall in disease frequency in all studies, with a narrowing of the north–south divide. There was a strong association with area deprivation, independent of living in an urban environment. Internationally, equatorial regions were unaffected by disease and northern Europe had the highest incidence, which was primarily a function of race although latitude was an independent predictor. Individual characteristics associated with the disease were congenital anomalies of the genitourinary tract and a structural abnormality of arterial calibre.
Despite a falling incidence, Perthes disease remains an important cause of child morbidity and exemplifies socioeconomic inequalities. A deprivation-related exposure, acting early in development, appears critical. The aetiological factor in Perthes disease remains elusive but it is likely that unravelling this enigma may unlock additional secrets pertaining to the developmental origins of this and other diseases.
Keywords: Epidemiology, Aetiology, Legg–Calvé–Perthes disease, Osteonecrosis, Osteoarthritis
In 1910 three independent observers, Arthur Legg (Boston, US), Jacques Calvé (Berck, France) and Georg Perthes (Tübigen, Germany), 1–3 described a debilitating condition of the developing hip. Georg Perthes later wrote the classic monograph of this disease, describing a self-limiting, non-inflammatory condition, affecting the capital femoral epiphysis with stages of degeneration and regeneration, leading to a restoration of the bone nucleus. 4 The disease is known by many names (coxa plana, Legg–Calvé–Perthes, Legg–Perthes, Legg–Calvé) although it is commonly known simply as Perthes disease.
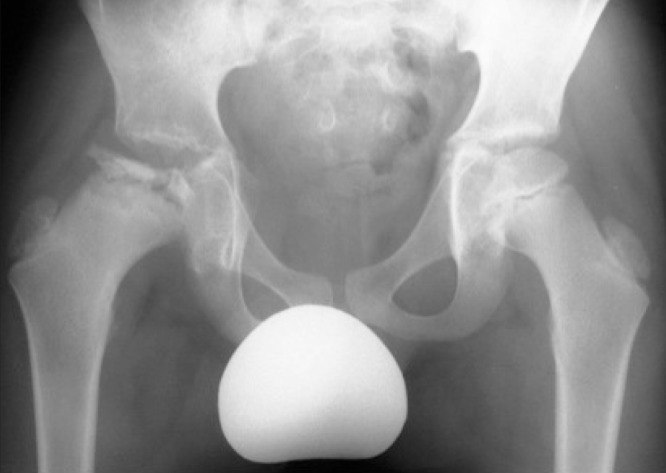
Perthes disease results in osteonecrosis of the femoral head (Fig 1), leading to hip pain and restricted mobility throughout childhood. The femoral head frequently deforms during the disease process, resulting in the development of an aspherical hip with ensuing osteoarthritis in adulthood. 5
Figure 1. Anteroposterior pelvic radiography: The right hip demonstrates typical features of Perthes disease (ie flattening and fragmentation of the proximal femoral epiphysis, with metaphyseal cysts and lateral extrusion of the hip). The left hip is normal.
Despite a century of research, Perthes disease is still poorly understood. There remains no consensus as to who gets the disease, the mechanism of disease or how to treat the disease. In particular, until recently there has been a paucity of robust epidemiological studies, which has extended the formulation of a number of poorly substantiated hypotheses pertaining to the disease aetiology. This paper considers recent epidemiological studies undertaken by the author and uses these to synthesise ideas in the quest to discover the aetiology of this puzzling disease.
The foundations of epidemiology
The cornerstone of epidemiology is the ability to describe a disease in terms of geography, time and person: Is an individual more likely to get the disease if he or she lives in a certain location? Has the risk of disease to an individual changed over time? Do some individuals possess features that heighten their risk of disease? The answers to each of these questions may give important clues to the aetiology of disease and open avenues for further exploration.
Geography
International incidence
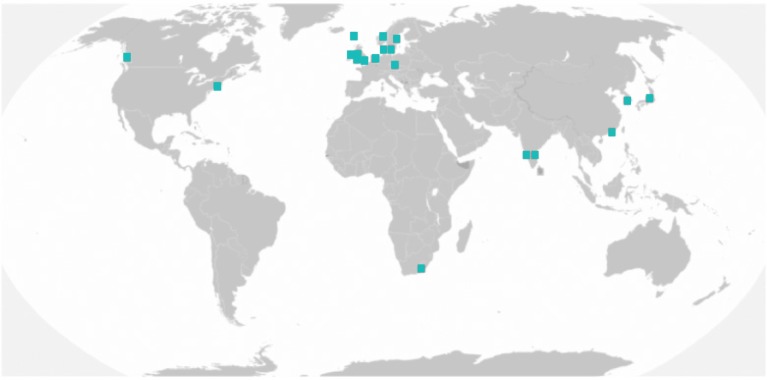
There have been a number of studies that have considered the distribution of Perthes disease in different countries. However, the heterogeneity in study methodologies and widespread confusion due to differing population denominators have limited their utility as it is difficult to compare rates between regions. A systematic review of incidence studies of Perthes disease has overcome this problem. 6 This review included 21 robust studies of incidence and made adjustments to standardise the population denominators, with a marked international variation in the annual incidence ranging from 0.2 and 19.9 cases per 100,000 children aged 0–14 years.
Internationally, race had the most marked influence on Perthes disease incidence, with East Asian individuals least affected, white individuals most affected (8.8 times the rate of East Asian individuals) and south Asian individuals in between (2.9 times the rate of East Asian individuals). There were no robust studies of incidence among individuals of predominantly black ancestry, which is likely to be a reflection of the scarcity of disease in this group (supported by a paucity of black people featuring among larger published series of disease). 7,8
Most of the studies of Perthes disease incidence arose in northern Europe (Fig 2), prompting the suggestion that latitude may be associated with the disease. After adjustment for race and exclusion of the single study conducted in the southern hemisphere, every 10 degrees further north from the equator resulted in an increase in the incidence of disease by almost 50%. This is an association shared with other diseases such as multiple sclerosis and osteoporosis. 9,10
Figure 2. Map demonstrating the incidence studies included in a systematic review (showing a northern European research focus) 6 .
National incidence
In 1978 a marked variation in incidence was observed between three different health board regions of the UK. 11 It was demonstrated that Wessex in the south of England had half the incidence of Merseyside in the north, with Trent in the Midlands having an intermediate value (11.1 vs 7.6 vs 5.5 cases per 100,000 children aged 0–14 years per annum). However, the true disease distribution across the whole UK was unknown.
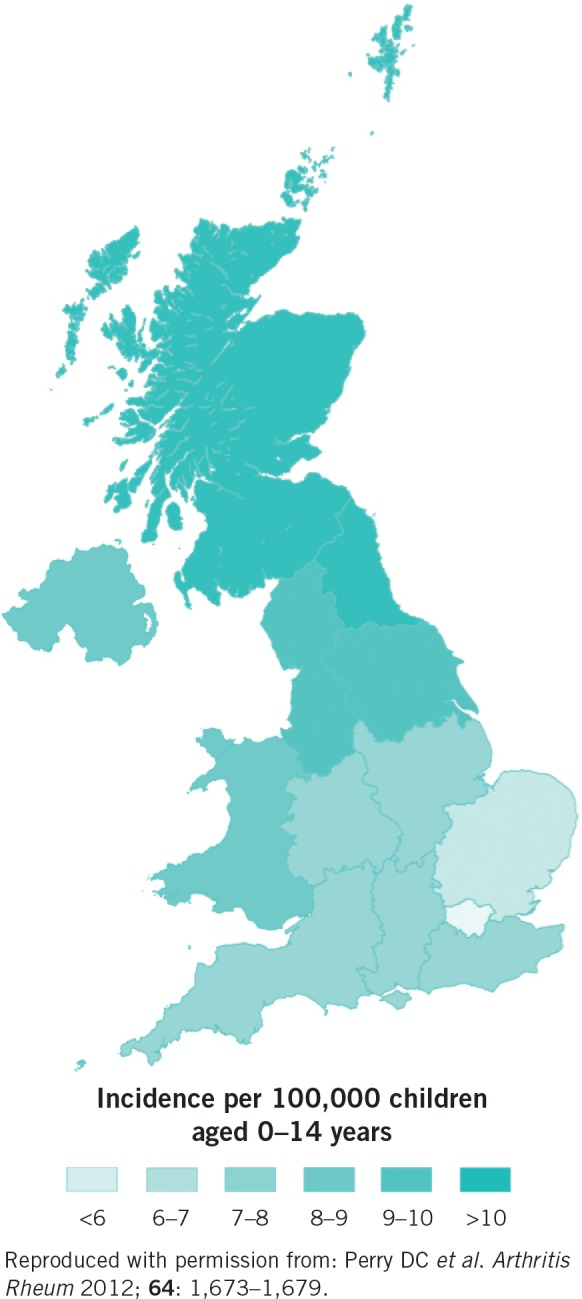
A investigation published in 2012 mapped Perthes disease across the UK, at a health board level. 12 This investigation used, for the first time, a community database to investigate the incidence of Perthes disease: the General Practice Research Database (GPRD). GPRD is the world’s largest primary database, extensively validated and collected as part of ‘routine patient care’. It encompasses over 68 million person years of data and enumerates approximately 8% of the UK population. This community-based study has advantages over hospital-based incidence studies, including a clear denominator population (owing to the general practitioner having a known number of patients) and a reliable numerator (owing to the practitioner being the ‘gatekeeper’ to secondary care services).
The GPRD study identified a striking pattern of Perthes disease incidence across the UK (Fig 3). 12 London and the south east of England were least affected by Perthes disease, with a gradual increase in incidence in more northerly regions such that the incidence of Perthes disease in Scotland was more than double that in London. This geographic pattern is striking and is a common pattern seen when mapping diseases in the UK. For example, it is seen in ischaemic heart disease, 13 hypertension 13 and all-cause mortality. 14
Figure 3. Geographic illustration of the incidence of Perthes disease (by English strategic health authority, Wales, Scotland and Northern Ireland).
Local incidence
Even at a local level, the incidence of Perthes disease varies considerably by area. Investigations of the regional incidence in Merseyside and Yorkshire in the 1970s and 1980s demonstrated marked variation in these counties. 15–17 Analysis of the Merseyside Perthes register from 2011 continues to show marked variation in regional incidence across Merseyside. 8 This analysis used computerised allocation of postcodes to ward regions (primary unit of administrative electoral geography), a tightly defined case definition and multiple case finding methods to ensure that the observed differences were real. It was identified that incidence rates varied by around five times between wards after excluding outliers.
Explaining geographic variation
Deprivation
The 1980s Merseyside incidence studies observed a very high incidence of Perthes disease in inner-city Liverpool, prompting investigators to examine the relationship between deprivation and disease. 15,17 This revealed a steep social class gradient associated with Perthes disease, with incidence rates rising from 4 per 100,000 per annum in Social Class 1 (highest social standing) to 31.7 per 100,000 per annum in Social Class 5 (lowest social standing). 15 However, social class and deprivation trends have remained a source of controversy. 18
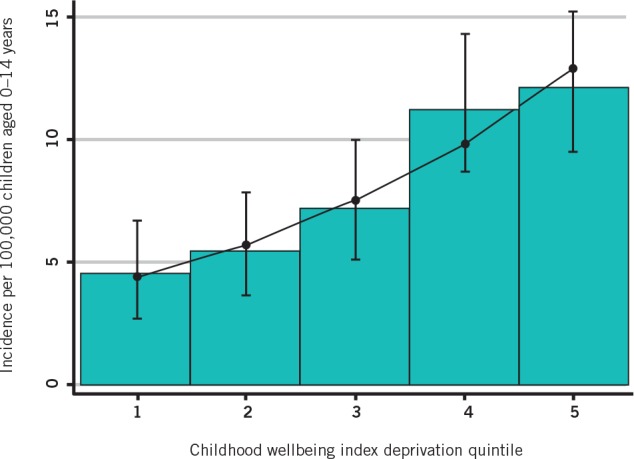
The recent Merseyside and GPRD studies have re-examined deprivation at a local and national level. In Merseyside, deprivation was defined using area deprivation scores at a ward level and at the lower layer super output area level, which is the smallest administrative subunit of UK geography. Both revealed a very strong correlation between Perthes disease incidence and deprivation. The strongest correlation was seen using a child-specific measure of deprivation (the child wellbeing index), 19 which is derived from the extensively validated English indices of multiple deprivation. Perthes disease was three times more frequent in the most deprived quintile compared with the most affluent quintile, with a step-wise progression (Fig 4).
Figure 4. Relationship between area deprivation and Perthes disease incidence (adapted from data in Perry et al). 8 (Childhood wellbeing index: 1= least deprived, 5 = most deprived. Bars indicate measured values. Error bars indicate 95% confidence interval. Poisson regression line is superimposed.).
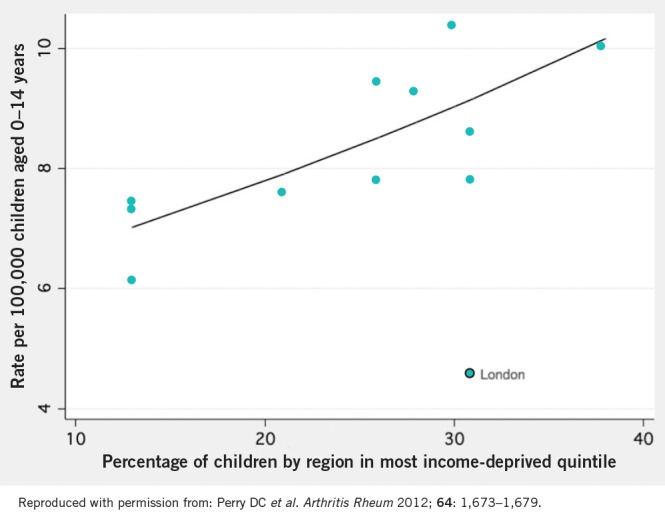
At the national level, deprivation inequalities were assessed using the percentage of children, by region, in the most deprived quintile of UK deprivation (Fig 5). 12 Using deprivation markers adapted for strategic health authorities, it was shown that after excluding London, Perthes disease incidence appeared intimately related to regional deprivation. London may have differed owing to its multi-ethnicity and London similarly acts as an outlier in numerous deprivation-related diseases such as cardiovascular disease. 20 Its exclusion therefore appears plausible.
Figure 5. Relationship between Perthes disease incidence and income deprivation. (Smooth line = Poisson regression line after excluding London. London is illustrated as an outlier.).
On an international scale, it was difficult to examine the effects of deprivation, particularly owing to the wide time period of the studies used. The high disease incidence in wealthy northern Europe suggests that more than deprivation is a factor on an international scale. Race is clearly important, which may indicate a genetic predisposition to disease or differential exposure to an environmental determinant by race. Studies of Perthes disease among migrant populations living in western cultures would help to determine this relationship. The latitude effect is also difficult to resolve although other diseases show a similar pattern of incidence. Multiple sclerosis is the disease most widely known to be associated with northerly latitudes. 9 Aetiological determinants that have been suggested to explain this phenomenon include vitamin D deficiency (through inadequate sunlight exposure) or exposure to infectious agents. Further consideration therefore needs to be given to this observation in Perthes disease.
The urban environment
Since early observation of a high disease frequency in innercity Liverpool, the notion has arisen that Perthes disease is a condition of urban environment and particularly deprived individuals in urban environments. Understanding the relationship between Perthes disease, socioeconomic deprivation and the urban environment is important as it may offer insights into whether disease determinants relate to geographical exposures such as air or water pollution.
Perthes disease discharge data from Scotland were used in 2012 to investigate this relationship. 21 Scotland is a particularly good setting for this investigation as it is largely rural despite having a number of major cities and some of the UK’s most deprived areas. This study observed more Perthes disease in urban environments. However, this seemed to reflect a greater propensity to deprivation in these settings. When the environment was considered together with deprivation (using the Scottish urban–rural classification and the Scottish indices of multiple deprivation), then deprivation appeared the crucial determinant, with little or no additional effect from the urban environment. This study is supported by others that have indicated high rates of Perthes disease in deprived rural communities. 22,23
Time
Up to 2011 only one study had offered any insight to the temporal trends in Perthes disease incidence. This study used the Merseyside Perthes disease register to compare incidence rates between the 1980s and 1990s, and demonstrated a marked reduction over this time period. 17 The 2011 analysis of the Merseyside Perthes disease register has examined temporal trends over a 34-year period. 8 In Liverpool, Perthes disease rates had almost halved over this time period, from 14.2 to 7.7 cases per 100,000 children aged 0–14 years. Nearby Knowsley showed a similar decline in incidence while the more affluent neighbouring area of Sefton demonstrated no such decline. The most marked decline in incidence therefore appeared in the socioeconomically deprived regions of Liverpool and Knowsley, yet there was no significant fall in the more affluent neighbouring region of Sefton.
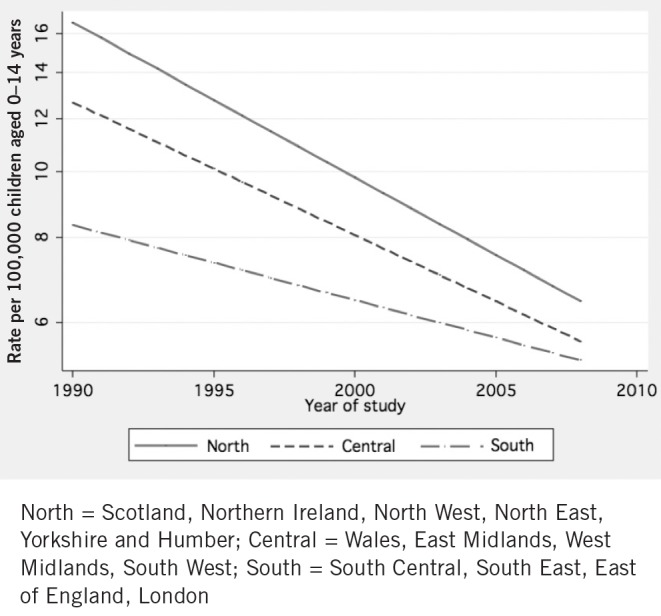
At a national level, similar trends were identified using the GPRD. Over the 19-year study period, there was a year-on-year decline in incidence of 4.2% (2.7–5.5%), with the national Perthes disease incidence rate falling from 12.2 in 1990 to 5.7 in 2008 (cases per 100,000 children aged 0–14 years). The rate of decline was greatest in areas in the north of the UK, negligible in the south and intermediate in central areas. Therefore, the most marked decline was apparent in the most deprived areas and the most affluent areas had a decline of borderline significance (Fig 6).
Figure 6. Annual incidence of Perthes disease in the General Practice Research Database with Poisson regression lines by region. (Y-axis is logarithmic.).
Data concerning Perthes disease discharges in Scotland 21 and unpublished data from the Perthes disease register from Northern Ireland (presented at the British Orthopaedic Association congress in 2012) have similarly confirmed a decline in Perthes disease incidence, adding support to the validity and generalisability of these findings.
Explaining longitudinal trends
It is difficult to attribute directly the change in incidence with time to changes in deprivation over this period. This is both for theoretical and practical reasons. ‘Deprivation’ is an arbitrary term used to differentiate access to material goods and services between individuals, and is relative to the setting and time (ie access to goods and services of a deprived individual in 1980 is likely to be quite different to that in 2010). Practically, the means by which deprivation is measured also changes often, in part to reflect changes in society (ie access to technology and transport) and in part to reflect government policy. Proxy measures of deprivation (such as health measures) are a useful way to consider the temporal effects.
The decline in the incidence of Perthes disease reflects many general markers of health over this period. Among children, there was a decline in the UK infant mortality rate between 1990 and 2009 from 8.0 to 4.6 per 1,000 live births 24 along with a decline in sudden infant death syndrome. 25 Some of these effects have been attributed partly to a decline in the smoking prevalence in the UK, which fell from 30% in 1990 to 22% in 2007. 26
Person
The most widely recognised individual characteristics to heighten the risk of Perthes disease are the age and sex of an individual. Perthes disease affects boys around four times more commonly than girls. 8,12 The age of onset of disease demonstrates a marked positive skew, meaning that most cases present between 4 and 7 years old, with a few cases still appearing late in adolescence. 12
Another well-described individual characteristic is a growth abnormality. 27 Children with Perthes disease are short and, anecdotally, often the smallest child in their class. Their head size is known to be normal, yet body proportions reduce disproportionately in size as one progresses distally along limb units, a pattern of growth described as rostral sparing. Disproportionate arterial size has also been demonstrated, which may have a role in the disease aetiology. 28
Congenital disease associations have also been observed in Perthes disease, which suggest that a prenatal event/factor may be important in the disease aetiology. The lack of control populations and ‘specialist centre’ case ascertainment has complicated the validity of such studies, and has introduced the potential for bias. A large nested case-controlled study in the GPRD has overcome some of the previous methodological concerns. 29 This study was able to confirm an association between Perthes disease and congenital genitourinary disease (hypospadias, inguinal hernia and undescended testes), an association first proposed by Catterall et al. 30 This may indicate that the aetiological factor responsible for these diseases, believed to be fetal androgen metabolism, may be influential in the onset of Perthes disease. This may go some way to explaining the male disease preponderance.
Discussion
The studies have confirmed a close relationship between Perthes disease and deprivation. This relationship is present at both a local and national level, and is reproducible using a number of different tools measuring ‘socioeconomic deprivation’. The strength of this relationship is such that there are few other diseases in childhood that are so closely correlated with deprivation. However, the underlying ‘deprivation-related determinant’ remains unknown. Certainly, Perthes disease appears primarily a disease of environmental origin (ie a disease caused by an environmental trigger rather than an inherited genetic mutation).
The predilection for Perthes disease appears alike in similarly deprived urban and non-urban areas, suggesting that the determinant is unlikely to be attributable to the urban environment but is intimately intertwined with deprivation. It has also been established that the incidence of Perthes disease has declined in recent years in the populations studied. This is likely to reflect a reduction of a deprivation associated risk factor in the population (eg diet, smoking, exercise, injury, alcohol, drugs or infection) and mirrors trends in infant mortality.
A complexity in understanding the underlying determinant is that it is unclear if it acts on the child, the mother or as part of a more complex gene–environment interaction. It is apparent that children with Perthes disease have a propensity to congenital disease of the genitourinary tract, therefore suggesting at least a prenatally determined tendency to disease. Future studies may usefully examine the growth trajectories of children prenatally or use prenatally determined markers of health to further examine the likelihood of the determinant acting in utero.
Conclusions
A century has now passed since Perthes disease was first described but the aetiological determinant remains elusive. It appears that the origin of the disease is environmental, with a determinant that is intertwined with socioeconomic deprivation. The decline in incidence is encouraging. Nevertheless, the disease remains a debilitating condition that affects around 650 children each year in the UK (based on Office for National Statistics 2010 population projections and GPRD incidence rates). It remains unclear how deprivation may exert an effect to precipitate osteonecrosis in the juvenile hip or when the exposure may act (ie acting on the child, acting on the mother or acting through an intergenerational epigenetic effect). The current period of economic recession and financial austerity appears a scientifically important and socially significant period in which to further investigate Perthes disease, and unravel this enigma definitively.
Acknowledgements
Funding was provided by the John Monk Hip Research Fund and the Doris Hillier Award from the British Medical Association.
Daniel Perry has also been awarded the Robert Jones Gold Medal and Association Prize from the British Orthopaedic Association (BOA). This text is, in part, derived from the Robert Jones essay, with permission granted by the BOA. The Hunterian lecture was presented at the BOA Annual Congress held in Manchester, September 2012.
Thank you for the help and support of my research supervisors: Colin Bruce, Daniel Pope, Peter Dangerfield, Mary Jane Platt and Professor Andy Hall. Thank you also to all the staff and children from D1 clinic at Alder Hey Hospital, Liverpool.
References
- 1.Perthes G. Über Arthritis deformans juvenilis. Deutsche Zeitschrift für Chirurgie 1910; 107: 111–159. [Google Scholar]
- 2.Legg AT. An obscure affection of the hip-joint. Boston Medical and Surgical Journal 1910; 162: 202–204. [Google Scholar]
- 3.Calvé J. Sur une forme particulière de pseudo-coxalgie greffée sur des déformations caractéristiques de I’extrémité supérieure du fémur. Rev Chir 1910; 30: 54–84. [Google Scholar]
- 4.Perthes G. Über osteochondritis deformans juvenilis. Archiv für klinische Chirurgie 1913; 101: 779–807. [Google Scholar]
- 5.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg–Calvé– Perthes disease. J Bone Joint Surg Am 1981; 63: 1,095–1,108. [PubMed] [Google Scholar]
- 6.Perry DC, Machin DM, Pope Det al Racial and geographic factors in the incidence of Legg–Calvé–Perthes’ disease: a systematic review. Am J Epidemiol 2012; 175: 159–166. [DOI] [PubMed] [Google Scholar]
- 7.Katz JF. Legg–Calve–Perthes disease: a statistical evaluation of 358 cases. Mt Sinai J Med 1973; 40: 20–47. [PubMed] [Google Scholar]
- 8.Perry DC, Bruce CE, Pope Det al Perthes’ disease: deprivation and decline. Arch Dis Child 2011; 96: 1,124–1,128. [DOI] [PubMed] [Google Scholar]
- 9.Hernán MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999; 53: 1,711–1,718. [DOI] [PubMed] [Google Scholar]
- 10.Johnell O, Borgstrom F, Jonsson B, Kanis J. Latitude, socioeconomic prosperity, mobile phones and hip fracture risk. Osteoporos Int 2007; 18: 333–337. [DOI] [PubMed] [Google Scholar]
- 11.Barker DJ, Dixon E, Taylor JF. Perthes’ disease of the hip in three regions of England. J Bone Joint Surg Br 1978; 60: 478–480. [DOI] [PubMed] [Google Scholar]
- 12.Perry DC, Bruce CE, Pope Det al Legg–Calvé–Perthes disease in the UK: geographic and temporal trends in incidence reflecting differences in degree of deprivation in childhood. Arthritis Rheum 2012; 64: 1,673–1,679. [DOI] [PubMed] [Google Scholar]
- 13.Elford J, Phillips AN, Thomson AG, Shaper AG. Migration and geographic variations in ischaemic heart disease in Great Britain. Lancet 1989; 1: 343–346. [DOI] [PubMed] [Google Scholar]
- 14.Hacking JM, Muller S, Buchan IE. Trends in mortality from 1965 to 2008 across the English north-south divide: comparative observational study. BMJ 2011; 342: d508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AJ, Barker DJ, Dangerfield PH, Taylor JF. Perthes’ disease of the hip in Liverpool. BMJ 1983; 287: 1,757–1,759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall AJ, Barker DJ. Perthes’ disease in Yorkshire. J Bone Joint Surg Br 1989; 71: 229–233. [DOI] [PubMed] [Google Scholar]
- 17.Margetts BM, Perry CA, Taylor JF, Dangerfield PH. The incidence and distribution of Legg–Calvé–Perthes’ disease in Liverpool, 1982–95. Arch Dis Child 2001; 84: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Sibinski M, Sherlock DA. A profile of Perthes’ disease in Greater Glasgow: is there an association with deprivation? J Bone Joint Surg Br 2005; 87: 1,536–1,540. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw J, Noble M, Bloor Ket al A child well-being index at small area level in England. Child Indic Res 2009; 2: 201–219. [Google Scholar]
- 20.Walford H, Soljak M, Gordon F, Birger R. CHD Prevalence Modelling Briefing Document. London: APHO; 2008. [Google Scholar]
- 21.Perry DC, Bruce CE, Pope Det al Perthes’ disease of the hip: socioeconomic inequalities and the urban environment. Arch Dis Child 2012; 97: 1,053– 1,057. [DOI] [PubMed] [Google Scholar]
- 22.Pillai A, Atiya S, Costigan PS. The incidence of Perthes’ disease in Southwest Scotland. J Bone Joint Surg Br 2005; 87: 1,531–1,535. [DOI] [PubMed] [Google Scholar]
- 23.Kealey WD, Moore AJ, Cook S, Cosgrove AP. Deprivation, urbanisation and Perthes’ disease in Northern Ireland. J Bone Joint Surg Br 2000; 82: 167–171. [PubMed] [Google Scholar]
- 24.Indicators World Bank. http://data.worldbank.org/indicator/ (cited April2013).
- 25.Wood AM, Pasupathy D, Pell JPet al Trends in socioeconomic inequalities in risk of sudden infant death syndrome, other causes of infant mortality, and stillbirth in Scotland: population based study. BMJ 2012; 344: e1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson S, Lader D. Smoking and Drinking Among Adults, 2007. Newport: ONS; 2007. [Google Scholar]
- 27.Burwell RG, Dangerfield PH, Hall DJet al Perthes’ disease. J Bone Joint Surg Br 1978; 60: 461–477. [DOI] [PubMed] [Google Scholar]
- 28.Perry DC, Green DJ, Bruce CEet al Abnormalities of vascular structure and function in children with Perthes disease. Pediatrics 2012; 130: e126–e131. [DOI] [PubMed] [Google Scholar]
- 29.Perry DC, Bruce CE, Pope Det al Comorbidities in Perthes’ disease: a case control study using the General Practice Research database. J Bone Joint Surg Br 2012; 94: 1,684–1,689. [DOI] [PubMed] [Google Scholar]
- 30.Catterall A, Roberts GC, Wynne-Davies R. Association of Perthes’ disease with congenital anomalies of genitourinary tract and inguinal region. Lancet 1971; 1: 996–997. [DOI] [PubMed] [Google Scholar]