Abstract
Introduction
Gastric tube necrosis following oesophagectomy is thought to have an increased association with a minimally invasive technique. Some suggest gastric ischaemic preconditioning may reduce ischaemic complications. We discuss our series of 155 consecutive minimally invasive oesophagectomies (MIOs), including a number of cases of gastric tube ischaemia, of which 4 (2.6%) developed conduit necrosis.
Methods
Data were collected prospectively of MIOs carried out by a single surgeon between 2005 and 2011. Cases of gastric tube necrosis were identified.
Results
Overall, 155 patients were identified. The inpatient mortality rate was 2.6%. Gastric tube necrosis occurred in four patients (2.6%). An ultrasonic dissector injury to the gastroepiploic arcade had occurred in two cases. In another case, the gastric tube was strangulated in the hiatus. In the remaining case, no clear mechanical cause was identified. All 4 cases occurred within the first 73 cases. The gastric tube necrosis rate of the first 50 cases versus cases 51–155 was 4% and 2% respectively (p=0.5948). The anastomotic leak rate in these two cohorts was 18% and 7% respectively (p=0.0457). There was a significant reduction in overall gastric tube complications from 22% to 10% following the learning curve of the initial 50 cases (p=0.0447).
Conclusions
In our series, gastric tube necrosis appears to be a learning curve issue. Prophylactic measures such as ischaemic preconditioning become less relevant as the operating surgeon’s experience increases. Instead, meticulous attention to preserving the gastroepiploic arcade, avoidance of tension in the tube and careful positioning of the gastric conduit through an adequately sized hiatus are key factors.
Keywords: Minimally invasive, Oesophagectomy, Ischaemia, Necrosis
Minimally invasive oesophagectomies (MIOs) have become increasingly popular, with 659 being carried out in England and Wales between 2007 and 2009, accounting for 30% of all oesophagectomies performed during this period. 1 The transition from an open procedure to an MIO has raised some concerns. In cancer patients, the initial concerns about lymph gland dissection and oncological parity with open surgery have been shown to be largely misplaced. 2 Another concern is the perceived increased incidence of gastric conduit ischaemia, necrosis and anastomotic leakage, which may result in increased morbidity and mortality (Fig 1). 1
Figure 1. Gastroscopic images with varying degrees of ischaemia of the tip of the gastric tube: a wedge shaped segment of complete necrosis around the proximal staple line of the gastric tube (arrow) (A), circumferential partial ischaemia of the proximal gastric tube with islands of necrosis surrounded by viable mucosa (B) and circumferential complete necrosis of the proximal gastric tube (C).
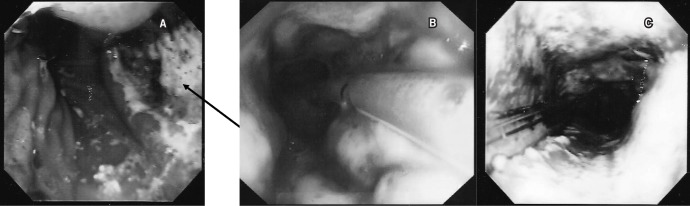
The published rates of early gastric tube ischaemic complications after MIO vary from 3.3% to 10.5%. 1,3–5 This is in contrast to open oesophagectomies where the reported rates are 0.5–7.4%. 1,6 The consensus group for the Association of Upper Gastrointestinal Surgeons (AUGIS) stated that the learning curve for a surgeon developing his or her MIO technique is substantial, and thought to be between 20 and 50 cases. 7 We have previously shown a learning curve in relation to the lymph gland yield when introducing MIO for cancer. 6 We therefore hypothesised that the increased incidence of gastric tube ischaemic complications associated with MIO is also a learning curve issue.
The aims of this study were to identify all those patients who developed gastric tube necrosis and/or leakage in our large series of MIOs, and to discuss the presentation, aetiology, management and outcome for each of these cases. The literature regarding gastric tube necrosis is reviewed and discussed alongside our own findings to determine a strategy to avoid these complications.
Methods
The case notes of all consecutive cases of MIO carried out in our unit between 2005 and 2011 were reviewed. Patient data were identified prospectively and collated on an Access® database (Microsoft, Redmond WA, US). Inclusion criteria were all patients who had an oesophagogastrectomy with a fully laparoscopic abdominal phase and either video assisted thoracoscopic surgery (VATS) or a mini-thoracotomy. Patients whose laparoscopy was converted to a laparotomy were included.
Details collected included patient demographics, preoperative chemoradiotherapy details, intraoperative details, postoperative complications and final histology. To assess for a learning curve effect on gastric tube complications, we arbitrarily compared the outcome of the first 50 cases with the subsequent 105.
Operative technique
All operations were carried out by or under the close supervision of a single consultant surgeon who had personal experience of over 100 open oesophagectomies as well as extensive experience in advanced laparoscopic surgery. Patients were intubated using a double lumen endotracheal tube. All patients received a thoracic epidural prior to commencement of surgery. The patient was positioned in a Lloyd-Davies position with the operating surgeon standing between the patient’s legs. Usually, five ports were introduced. An ultrasonic dissector was used to mobilise the stomach along the greater curve while taking care to preserve the gastroepiploic arcade. All patients underwent pyloroplasty. Lymph node harvesting was performed through the use of the Harmonic® scalpel (Ethicon Endo-Surgery, Cincinnati, OH, US). Ligaclips were used to secure the left gastric artery.
Dissection into the chest was carried out for at least 10cm in order to facilitate the thoracic phase of the operation, according to the principles of total adventitial resection of the cardia (TARC), as described previously. 8 If the hiatal orifice after TARC was deemed too wide, the anterior tendinous part of the hiatus was repaired with size 0 Ethibond® sutures (Ethicon, Somerville, NJ, US). Overtightening of the hiatus is avoided by ‘sizing’ the orifice with laparoscopic forceps.
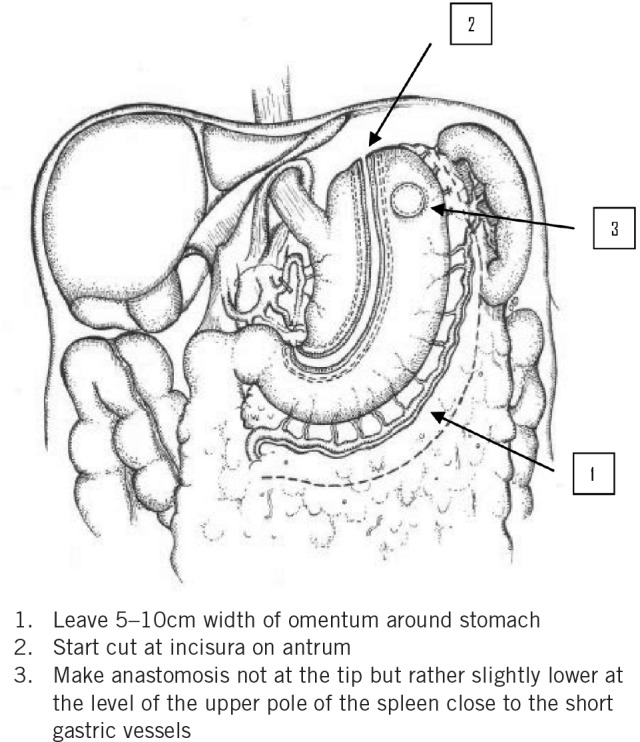
For oncological reasons, the lesser curve of the stomach and lymph nodes were removed by creating a greater curve tube. The gastric tube was constructed intracorporeally starting from below the incisura approximately 5cm proximal to the pylorus through the use of an Echelon™ 60mm Endopath® (Ethicon Endo-Surgery) stapling gun. The width of the gastric tube was approximately 5cm. The tube was not fully divided at the fundus to enable later pull-up into the thorax. Left and right transhiatal chest drains were introduced under direct vision through the abdominal port sites. 9
For the thoracic phase, the patient was in the left lateral position for a posterolateral thoracotomy incision. In the case of the VATS procedures, two or three 12mm thoracoscopic ports were inserted, allowing the thoracotomy incision length to be reduced to 10–15cm with sparing of the latissimus dorsi and serratus anterior muscles. The azygos vein was divided before harvesting of the paraoesophageal and subcarinal nodes. The oesophagus was divided in the upper mediastinum and the intrathoracic end-to-side oesophagogastric anastomosis to the anterior wall of the gastric tube (Fig 2) was fashioned either through hand sewing or the use of a 25mm CEEA™ gun (Covidien, Dublin, Ireland). 10 A triple lumen nasojejunal tube (Freka®; Fresenius Kabi, Bad Homburg, Germany) was placed under flexible gastroscopic vision to allow gastric decompression and jejunal feeding in the immediate postoperative phase.
Figure 2. Illustration showing formation of the gastric tube.
Perioperative care
The general pre and postoperative care was instigated through the use of patient management protocols. Postoperatively, all patients were nursed in an overnight intensive recovery unit and extubated when physiologically stable. A systolic blood pressure of around 120mmHg was maintained through the combination of crystalloid infusions and low dose noradrenaline infusion. Blood transfusion was considered in all patients with a postoperative haemoglobin level of <8g/dl.
On the first postoperative day, patients were transferred to the surgical high dependency unit or a normal surgical ward depending on their clinical and physiological parameters. Patients were allowed sips of water and then built up oral fluids slowly over the first week. Soft food was commenced after a week and the nasojejunal tube removed. For the first 50 cases, routine water soluble contrast swallows were performed on the fifth postoperative day to examine for anastomotic leaks but this practice was thereafter abandoned in favour of selective investigation. Close observation was performed to monitor for early signs of a leak (eg persistent tachycardia, pyrexia, pleural effusion or laboratory indicators of sepsis). These patients underwent contrast computed tomography or a water soluble swallow, followed by gastroscopic assessment under sedation to assess for gastric tube ischaemia and/or a demonstrable anastomotic leak. On discharge, patients were followed up closely at regular intervals.
Results
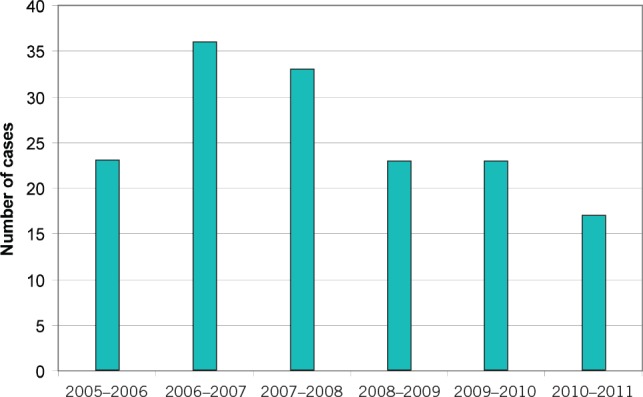
A total of 158 records were obtained over a 6-year period from May 2005 to April 2011. Two cases were excluded as these were extended total gastrectomies. A further case was excluded as it began as an open procedure. There were therefore 155 cases of laparoscopically assisted Ivor–Lewis oesophagectomies (Fig 3). Of these, 149 operations were carried out as treatment for a primary oesophageal malignancy, the others for resection of benign disease.
Figure 3. Number of oesophagectomies carried out per year.
Of the 155 patients, 115 were male (Table 1). The median patient age at the time of surgery was 63 years. The mean operating time per case was 280 minutes and the median inpatient stay was 14 days (Table 2).
Table 1.
Patient characteristics and pathology
| Median age (range) | 63 (41–82) |
| <55 years | 31 (20%) |
| <45 years | 7 (5%) |
| Male-to-female ratio | 2.88 |
| ASA grade: | |
| 1 | 5 |
| 2 | 95 |
| 3 | 34 |
| Not recorded | 21 |
| Histopathology: | |
| Adenocarcinoma | 112 (72%) |
| Squamous cell carcinoma | 23 |
| Adenosquamous carcinoma | 4 |
| Leiomyoma | 3 |
| Gastrointestinal stromal tumour | 1 |
| High grade dysplasia | 1 |
| Other | 1 |
| Not recorded | 10 |
| Neoadjuvant chemotherapy | 112 (72%) |
| Unknown | 10 (6%) |
| Median length of tumour (range) | 4.4cm (1–15cm) |
| pTNM classification: | |
| T0 (complete response) | 8 |
| T1 | 21 |
| T2 | 44 |
| T3 | 63 |
| T4 | 3 |
| Benign | 6 |
| Data incomplete | 10 |
ASA = American Society of Anesthesiologists
Table 2.
Perioperative outcome
| Operating time | |
| Mean (SD) | 280 mins (49 mins) |
| Median (range) | 270 mins (181–480 mins) |
| Blood transfusions | |
| Patients transfused in first 72 hours | 35 (23%) |
| Patients transfused beyond 72 hours | 24 (16%) |
| Patients not transfused | 110 (71%) |
| Mean units transfused per patient (range) | 1.59 (0–24) |
| Postoperative controlled ventilation | |
| Mean (SD) | 11.6 hrs (21.6 hrs) |
| Median (range) | 11 hrs (2–240 hrs) |
| Overnight intensive recovery | |
| Mean (SD) | 27.3 hrs (15.9 hrs) |
| Median (range) | 22 hrs (5–94 hrs) |
| Step-down facility from overnight intensive recovery | 19 (12%) |
| Ward High dependency unit | 90 (58%) |
| Intensive care unit | 22 (14%) |
| Unknown | 25 (16%) |
| Mean primary stay in critical care | |
| Mean (SD) | 8.9 days (15.4 days) |
| Median (range) | 4 days (1–90 days) |
| Patients readmitted to critical care | 14 (9%) |
| Total length of hospital stay | |
| Median (range) | 14 days (6–210 days) |
SD = standard deviation
All 155 cases had a full laparoscopy with no planned laparotomy incision. Two cases were converted to an open procedure, both due to significant intraoperative bleeding. In terms of the thoracic component, 128 cases were performed through VATS whereas 10 had a standard thoracotomy incision. In 17 cases, the thoracic approach was unrecorded. The mean intraoperative lymph node harvest was 20 (standard deviation: 7) in those cases with a primary oesophageal malignancy (n=149). Resection margins were deemed positive when the tumour was present within <1mm from the cut edge. The resection margin was positive in 46/149 cases and longitudinal margins were involved in 4/149 cases.
Overall, 159 complications were recorded in 89 patients (57%), 100 (63%) of which were considered to be minor and 59 (37%) major (Table 3). Respiratory complications accounted for 47% of the total complications encountered. The majority of pneumothoraces and pleural effusions coincided with the occurrence of other complications. Eleven patients (7%) required reoperation (Table 4); one patient returned to theatre twice (once for resection of a necrotic tube and a second time for bleeding at the cervical oesophagostomy site). No complications occurred relating to either intraoperative or postoperative flexible gastroscopy performed by the surgical team.
Table 3.
Postoperative complications
| Major complications (n=59) | Minor complications (n=100) | ||
|---|---|---|---|
| Haemorrhage | 5 | Wound infection | 7 |
| Gastric tube necrosis | 4 | Atrial fibrillation | 13 |
| Septicaemia | 3 | Unary tract infection | 1 |
| Clinical anastomotic leak | 8 | Jejunostomy site problems | 3 |
| Myocardial infarction | 2 | Gastric tube ischaemia | 1 |
| Delayed gastric emptying | 1 | Radiological anastomotic leak 8 | |
| Chyle leak | 5 | ||
| Pneumonia | 27 | Stricture | 2 |
| Respiratory failure | 4 | Pneumothorax | 7 |
| Pleural effusion | 33 | ||
| Pulmonary oedema | 3 | ||
| Other | 22 | ||
Table 4.
Patients requiring reoperation
| Complication requiring reoperation | Number of patients |
|---|---|
| Gastric tube necrosis | 4 |
| Hiatus hernia with intrathoracic bowel | 1 |
| Haemorrhage | 2 |
| Anastomotic leak | 3 |
| Chyle leak | 1 |
Four patients died during their inpatient stay. The overall inpatient mortality rate was 2.6%. One patient died on day 10 after a massive intraoperative haemorrhage following iatrogenic damage to the aorta. A further patient died on day 12 following a myocardial infarction and anastomotic leak. Another died on day 70 of multiple organ failure following major gastrointestinal bleeding. The fourth patient died on day 100 following gastric tube necrosis and multiple episodes of chest sepsis.
Gastric tube complications
There were 16 anastomotic leaks in this series. Eight were classified as radiological only with no clinical sequelae. Of the eight clinical anastomotic leaks, one was associated with a necrotic gastric tube requiring reoperation and another three also required reoperation.
Four patients (2.6%) developed gastric tube necrosis (Table 5). All cases occurred in the first 73 patients, namely cases 8, 44, 59 and 73. Three cases presented on days 5–7 with a pleural effusion and sepsis. The diagnosis was made at endoscopy, where necrotic mucosa was seen but the gastric tube and anastomosis were intact. The fourth patient presented with an anastomotic leak on the fourth postoperative day. All four patients had a resection of the gastric tube with formation of a cervical oesophagostomy and placement of a feeding jejunostomy.
Table 5.
Patients with gastric tube necrosis
| Case number | Age / sex | Cause | Presentation | Diagnosis | Outcome | Other relevant information |
|---|---|---|---|---|---|---|
| 7 | 73M | Nil noted | Sepsis, pleural effusion | Endoscopy (day 5) | Discharge day 47; deceased day 124 | Heavy ex-smoker (>50 pack years), stopped 6 months prior to surgery |
| 44 | 75M | Tight hiatus | Clinical leak | Thoracotomy (day 4) | Declined reconstruction; disease free | Cigar smoker (1/day) |
| 59 | 60F | Injury to gastroepiploic arcade | Pleural effusion | Endoscopy (day 7) | Colonic interposition graft; disease recurrence 3.5 years | Heavy ex-smoker (>50 pack years), stopped 5 months prior to surgery; developed acute onset AF postoperatively |
| 73 | 75M | Injury to gastroepiploic arcade | Sepsis | Endoscopy (day 5) | Endoscopy (day 5) | Pipe smoker; developed acute onset AF postoperatively |
M = male; F = female; AF = atrial fibrillation
Of these four patients, one died as an inpatient on day 100 following several septic episodes and another died at home of general exhaustion 2 months following discharge on the 124th postoperative day. Of the two survivors, one underwent reconstruction using a colonic interposition graft after six months. The final patient declined any reconstructive surgery and remains disease free more than five years after his operation.
The aetiology of the gastric tube necrosis was identified in two cases as injury to the gastroepiploic arcade at the time of the initial operation. Another patient was found to have the attached omentum impacted in the diaphragmatic hiatus, resulting in a cut-off point and necrosis of the intrathoracic conduit. In the remaining case, no identifiable cause for the necrosis was identified. All four patients had a positive smoking history, with two patients smoking up to the day of their resection and two who had stopped smoking shortly before their resections but had a pack-year history of >50 years.
The first 50 cases (so-called learning curve) were completed in 2 years. The gastric tube necrosis rate of the first 50 cases and that of cases 51–155 were 4% and 2% respectively (p=0.5948). The anastomotic leak rates in these two cohorts were 18% and 7% respectively (p=0.0457). The combined gastric tube necrosis and leak rate was significantly higher in the first 50 cases compared with the next 105 cases, at 22% and 10% respectively (p=0.0447).
Discussion
MIOs have become increasingly popular, with the first fully minimally invasive procedure being completed in 1999. 7 The initial concerns regarding oncological parity when compared with an open technique have now been largely discredited. In 2007 Gemmill and McCulloch reviewed 23 studies covering a total of 1,398 patients undergoing MIOs. 11 They found that the overall 30-day inpatient mortality rate was 2.3%, with a combined major and minor morbidity rate of 46.2%. In a more recent systematic review, Verhage et al combined the data from ten case-controlled studies and one systematic review, and found that minimally invasive oesophagectomy was associated with a decreased blood loss (312ml vs 577ml), a reduction in critical care stay (4.5 vs 7.6 days) and total inpatient stay (14.9 vs 19.6 days), and a reduced complication rate of 43.8% compared with 60.4% in the open group. 2 The mean lymph node retrieval was increased at 23.8 versus 20.2.
The incidence of ischaemia and necrosis of pedicled grafts has been reported extensively in the plastic surgery literature with the main patient risk factors being smoking, age, obesity and irradiation, and the main technical factors being arterial supply, venous drainage and tension in the graft. 12,13 Ischaemic failure of the gastric tube (also a pedicled graft) resulting in complications such as anastomotic leakage or frank necrosis is a well recognised problem with both open and laparoscopic oesophagectomy. Some authors have also linked the development of anastomotic strictures to gastric tube ischaemia. 14
Wormuth and Heitmiller found that the average rates of ischaemic complications for stomach, colon and jejunal conduits after oesophagectomy were 3.2%, 5.1% and 4.2% respectively. 15 They also stated that conduit ischaemia was influenced by operative technique, length of conduit and the site of the proximal anastomosis, with the neck anastomosis more likely to suffer ischaemia. In a case series of 47 patients, Scheepers et al reported the rate of ischaemic complications of the anastomosis following MIO as 23%. 16 In the third National Oesophago-Gastric Cancer Audit in the UK, the anastomotic leak rate after MIO was 10.6% compared with the open group’s 7.8%. 1 In a case series by Berrisford et al, the authors reported on 70 MIOs, with 9 patients (12.9%) suffering gastric conduit complications. 17
Several authors have tried to solve the problem of gastric tube ischaemia after laparoscopic oesophagectomy. In an experimental study in pigs, significant improvement in gastric tube blood flow was found following intravenous administration of unmodified prostaglandin E1 (PGE1) and lipo-PGE1 (p<0.01). 18 In a study of gastric ischaemic preconditioning, Beck et al showed in animal models that if the short gastric and left gastric vessels were ligated three weeks prior to oesophagectomy, formation of the gastric tube resulted in the gastric blood flow falling to only 60% of the baseline compared with 16% in those who were not preconditioned (p=0.07). 19 Hölscher et al described their study of 83 cases where laparoscopic preconditioning of the stomach was undertaken at an average of 4.3 days prior to the definitive procedure. 5 They showed a leak rate of 5%, a major morbidity rate of 13% and a 90-day mortality of 0%, and they identified no cases of tube necrosis. They felt that laparoscopic gastric preconditioning reduced patient morbidity and mortality.
Another suggestion has been the performance of extracorporeal gastric tube formation through a mini-laparotomy. 9 This may mean that the benefit of reduced perioperative pain with minimally invasive surgery is lost and it may also impact adversely on the respiratory complication rate.
Our retrospective study of MIO from 2005 to 2011 shows results comparable with other similar studies in that our mean operating time was 280 minutes, the median inpatient stay was 14 days, the inpatient mortality rate was 2.6% and the total patient complication rate was 57%. Of the 11 reoperations in the series, 7 were for anastomotic leaks or gastric tube necrosis. Not all anastomotic leaks are ischaemic in nature and ischaemia of the gastric tube does not invariably lead to a leak. In our series, three patients were reoperated on for clinical leaks but no ischaemia of the gastric tube was documented whereas of the four patients with documented tube ischaemia/necrosis, only one presented with a leak.
The subclinical conduit ischaemia rate is likely to be significantly higher but as we did not perform routine postoperative gastroscopies, it remains unknown. Because we also did not perform radiological investigation of the anastomosis routinely after the first 50 cases, we may have underestimated the total leak rate. However, this is not of clinical relevance.
The 4 cases of gastric tube necrosis in our MIO series occurred within the first 73 cases performed. The length of a learning curve varies significantly between different procedures. The learning curve also depends on whether the procedure is learnt in a mentoring or pioneering type model.
For example, the introduction of robot assisted urological surgery has sparked discussion regarding its steep learning curve, with Moreno Sierra et al stating that there was a consensus among 8 out of 13 urology teams that the learning curve lasted around 20–25 cases. 20 Kye et al evaluated the learning curve faced in laparoscopic right-sided colon cancer surgery, and concluded that the 18th case in a first-generation colorectal surgeon and the 8th case in a laparoscopically trained surgeon were the overall peak points in the learning curves. 21 AUGIS suggests a learning curve of around 20–50 cases for MIO, 7 and this was supported by our own data, which showed a significant reduction in the overall gastric tube complications after the first 50 cases.
Conclusions
Many patients undergoing oesophagectomy are elderly and have a smoking history, which increases the risk of graft ischaemic complications. Furthermore, gastric tube complications such as necrosis and leaks are increased during the learning curve of MIO and immediate pedicled gastric tube reconstruction. Meticulous attention to preserving the epiploic arterial blood supply, venous drainage, optimal sizing of the hiatus and avoiding tension in the tube are important factors in preventing ischaemia. Two stage gastric ischaemic preconditioning or open extracorporeal creation of the gastric tube may not be necessary. For those surgeons embarking on MIO, learning the operation in a mentoring model may reduce the length of the learning curve, thereby allowing them to achieve similar results to their open surgery in a shorter time frame.
Acknowledgements
The authors are grateful to Dr Cara Baker for the illustration of the stomach (Fig 2).
The material in this paper was presented as a poster at the International Surgical Congress of the Association of Surgeons of Great Britain and Ireland held in Bournemouth, May 2011.
References
- 1.National Oesophago-Gastric Cancer Audit 2010. Leeds: NHS Information Centre; 2010. [Google Scholar]
- 2.Verhage RJ, Hazebroek EJ, Boone J, Van Hillegersberg R. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009; 64: 135–146. [PubMed] [Google Scholar]
- 3.Hamouda AH, Forshaw MJ, Tsigritis Ket al Perioperative outcomes after transition from conventional to minimally invasive Ivor–Lewis esophagectomy in a specialized center. Surg Endosc 2010; 24: 865–869. [DOI] [PubMed] [Google Scholar]
- 4.Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 1999; 13: 293–297. [DOI] [PubMed] [Google Scholar]
- 5.Hölscher AH, Schneider PM, Gutschow C, Schröder W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 2007; 245: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003; 138: 303–330. [DOI] [PubMed] [Google Scholar]
- 7.Association of Upper Gastrointestinal Surgeons, Association of Laparoscopic Surgeons. A Consensus View and Recommendations on the Development and Practice of Minimally Invasive Oesophagectomy. London: AUGIS; 2008. [Google Scholar]
- 8.Botha AJ, Odendaal W, Patel Vet al Total adventitial resection of the cardia: ‘optimal local resection’ for tumours of the oesophagogastric junction. Ann R Coll Surg Engl 2011; 93: 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogalniceanu P, Crewdson K, Khan AZ, Botha AJ. Transhiatal chest drainage after oesophagectomy. Ann R Coll Surg Engl 2007; 89: 535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marangoni G, Villa F, Shamil E, Botha AJ. OrVil™-assisted anastomosis in laparoscopic upper gastrointestinal surgery: friend of the laparoscopic surgeon. Surg Endosc 2012; 26: 811–817. [DOI] [PubMed] [Google Scholar]
- 11.Gemmill EH, McCulloch P. Systematic review of minimally invasive resection for gastro-oesophageal cancer. Br J Surg 2007; 94: 1,461–1,467. [DOI] [PubMed] [Google Scholar]
- 12.Ducic I, Spear SL, Cuoco F, Hannan C. Safety and risk factors for breast reconstruction with pedicled transverse rectus abdominis musculocutaneous flaps: a 10-year analysis. Ann Plast Surg 2005; 55: 559–564. [DOI] [PubMed] [Google Scholar]
- 13.Gill PS, Hunt JP, Guerra ABet al A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg 2004; 113: 1,153–1,160. [DOI] [PubMed] [Google Scholar]
- 14.Bizekis C, Kent MS, Luketich JDet al Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2006; 82: 402–406. [DOI] [PubMed] [Google Scholar]
- 15.Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin 2006; 16: 11–22. [DOI] [PubMed] [Google Scholar]
- 16.Scheepers JJ, van der Peet DL, Veenhof AAet al Systematic approach of postoperative gastric conduit complications after esophageal resection. Dis Esophagus 2010; 23: 117–121. [DOI] [PubMed] [Google Scholar]
- 17.Berrisford RG, Wajed SA, Sanders D, Rucklidge MW. Short-term outcomes following total minimally invasive oesophagectomy. Br J Surg 2008; 95: 602–610. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda Y, Morita N. Long beneficial effects after lipo-prostaglandin E1 administration on the ischemic gastric tube in pigs. Dis Esophagus 2008; 21: 765–769. [DOI] [PubMed] [Google Scholar]
- 19.Beck SM, Malay MB, Gagné DJet al Experimental model of laparoscopic gastric ischemic preconditioning prior to transhiatal esophagectomy. Surg Endosc 2011; 25: 2,470–2,477. [DOI] [PubMed] [Google Scholar]
- 20.Moreno Sierra J, Fernández Pérez C, Ortiz Oshiro Eet al Key areas in the learning curve for robotic urological surgery: a Spanish multicentre survey. Urol Int 2011; 87: 64–69. [DOI] [PubMed] [Google Scholar]
- 21.Kye BH, Kim JG, Cho HMet al Learning curves in laparoscopic right-sided colon cancer surgery: a comparison of first-generation colorectal surgeon to advance laparoscopically trained surgeon. J Laparoendosc Adv Surg Tech A 2011; 21: 789–796. [DOI] [PubMed] [Google Scholar]


