Abstract
Introduction
Pelvic acetabular injuries are associated with significant blood loss. This is compounded by multiple surgical interventions including definitive fracture fixation, which put patients at further risk of postoperative transfusion. We use intraoperative cell salvage routinely as a blood conservation strategy to address this issue. This is a prospective evaluation of the clinical efficacy and cost effectiveness of using intraoperative cell salvage in patients with pelvic acetabular injuries.
Methods
Data were collected prospectively for all the patients who underwent pelvic acetabular fracture fixation at our institution. A total of 30 patients (25 men, 5 women) with a mean age of 41 years (range: 31–79 years) were assessed over a period of 10 months.
Results
The mean preoperative and postoperative haemoglobin levels were 11.8g/dl and 9.9g/dl respectively. The mean intraoperative blood loss was 1,232.5ml (range: 150–2,693ml). The mean amount of blood salvaged and retransfused through a cell saver was 388ml. Of the 30 patients, 14 (47%) required transfusion after surgery and 26 units of blood were transfused. In terms of cost effectiveness, a total of £2,572 in 30 patients or £86 per patient were saved.
Conclusions
We found intraoperative cell salvage to be clinically efficacious and cost effective in patients with pelvic acetabular injuries.
Keywords: Pelvis, Acetabular, Cell salvage, Cell saver, Transfusion
Pelvic fractures account for 3–6% of all fractures in adults and about 20% are associated with multiple trauma. 1 The majority of these patients require several surgical interventions along with pelvic fracture fixation. 2 As a result, surgery in these patients is associated with significant perioperative blood loss and significant risk of postoperative transfusion. 3
The British Committee for Standards in Haematology has recommended several blood conservation strategies to reduce intraoperative blood loss and subsequent requirement of postoperative transfusion in patients undergoing major surgery. 4 These include normovolaemic haemodilution and autologous transfusion. 5,6 Use of intraoperative cell salvage is well documented in major cardiothoracic, spinal, vascular and elective orthopaedic surgery. 7 However, its use and efficacy in pelvic acetabular trauma surgery has not yet been fully established.
We have introduced intraoperative cell salvage in pelvic surgery as part of our standard blood conservation strategy. This strategy takes into account several factors including the ability to use patients’ own blood, immediate availability, relative safety and ease of application. The aim of this study was to assess the clinical efficacy in terms of amount of blood salvaged intraoperatively and reduction of postoperative transfusion with use of cell salvage in patients undergoing major pelvic trauma surgery. The cost effectiveness of using a cell saver in this patient group was also assessed.
Methods
This was an observational study carried out over a period of ten months. As a departmental policy, intraoperative cell salvage was used in all the patients admitted to the unit in whom significant intraoperative blood loss was anticipated. These were patients with type C (Tile’s classification) pelvic ring injuries, 8 patients with acetabular fractures or fracture dislocations and patients with both pelvic ring injuries and acetabular fractures requiring surgical fixation. Patients with contaminated/open pelvic acetabular fractures were excluded owing to the risk of transmission of infection, as were those for whom the anticipated intraoperative blood loss was not significant (such as patients with open book/ type B pelvic ring injuries) and those in whom external fixation was used as a definitive method of fixation. 8
Being a regional pelvic trauma unit, the majority of these patients were transferred from the base hospitals where they were initially admitted and treated. This also comprises any interim surgical/orthopaedic procedures including treatment of other injuries. Once stabilised, these patients were transferred to our unit for definitive pelvic fracture fixation.
All the type B fractures were fixed with open anterior double plating using a Pfannenstiel incision with the patient in the supine position. In patients with type C fractures, initially, anterior fixation was carried out using the above method. This was followed by turning the patient prone for posterior sacral fixation. Some cases were not suitable for anterior plating and in these patients posterior sacral plating was initially carried out, followed by anterior fixation using external fixators. These were left in situ for 10–12 weeks and were removed as a day-case procedure. Acetabular fractures were either fixed with a traditional Kocher– Langenbeck approach with the patient in the lateral position or using an ilioinguinal approach with the patient in the supine position, depending on the fracture type.
A ‘transfusion nurse’, who was also in charge of managing the cell salvage service in the trust, collected all the data in these patients prospectively including the number of units transfused postoperatively. This was also confirmed by the electronic database from the trust’s blood bank.
Haemoglobin levels were routinely checked preoperatively, on postoperative days 1 and 3 and following the transfusion. Intraoperative blood loss was calculated by adding the amount of blood salvaged and weighing the swabs used during the surgery. This was further verified by the theatre staff and anaesthetic records. No data were available with regard to any blood transfusion the patients might have had in the transferring base hospital. All patients were followed up until the time of discharge.
The current postoperative hospital transfusion policy is to transfuse in patients with two of the following three parameters: (i) haemodynamic compromise, (ii) symptomatic anaemia secondary to blood loss or (iii) patients with a postoperative haemoglobin value of less than 8.0g/dl. Based on the intraoperative blood loss, the expected transfusion requirement was calculated for every patient. This was compared against the actual number of units of blood transfused to give an estimate of units of blood saved from transfusion. The direct cost of transfusing one unit of blood was compared against the direct cost of running the cell saver per patient for cost analysis purposes. SPSS® version 17 (SPSS, Chicago, IL, US) was used for all statistical analysis.
Results
A total of 49 patients (39 men and 10 women) were admitted to the pelvic trauma unit during the study period. Of these, 30 patients (25 men and 5 women) with a mean age of 41 years (range: 31–79 years) required intraoperative cell salvage. Of the 19 patients excluded, 13 patients had anterior pubic symphyseal double plating through a single anterior approach, 2 patients required external fixation only, 3 patients had posterior sacral plating and 1 patient was treated non-operatively.
Table 1 illustrates the frequency and distribution of the fracture type in the cell salvage group while Table 2 shows the mean preoperative and day 1 postoperative haemoglobin levels, the amount of blood salvaged per patient intraoperatively and the expected intraoperative blood loss. All the blood salvaged intraoperatively was reinfused in the immediate postoperative period.
Table 1.
Frequency and distribution of fracture type
| Fracture type | Frequency |
|---|---|
| Pelvic ring injury (type C) | 9 (30%) |
| Acetabular fracture | 21 (70%) |
| Both columns fractures | 5 (17%) |
| Fracture dislocation | 16 (53%) |
Table 2.
Mean haemoglobin levels, amount of blood salvaged per patient and expected intraoperative blood loss
| Mean | Standard deviation | Range | |
|---|---|---|---|
| Preoperative haemoglobin | 11.8g/dl | 2.0g/dl | 8.1–15.0g/dl |
| Postoperative haemoglobin | 9.9g/dl | 1.3g/dl | 7–13g/dl |
| Intraoperative blood loss | 1,232.5ml | 815.5ml | 150–2,693ml |
| Blood salvaged intraoperatively | 388ml | 270ml | 101–1,231ml |
Postoperative transfusion
Fourteen patients (47%) required postoperative transfusion and a total of twenty-six units of blood were transfused postoperatively. None of the patients required any intraoperative transfusion. Figure 1 illustrates the frequency of the units of allogeneic blood transfused postoperatively.
Figure 1. Units of allogenic blood transfused postoperatively.
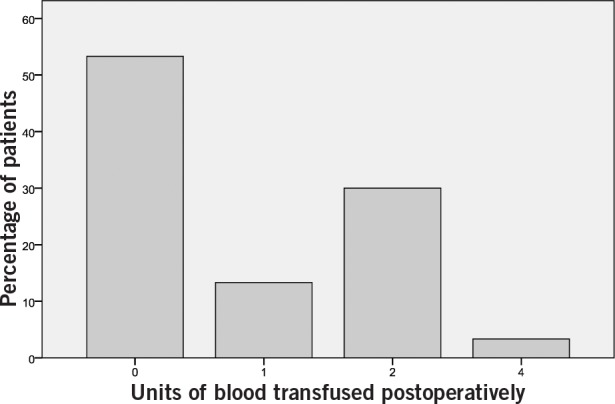
As noted in Table 2, the mean intraoperative blood loss was 1,232.5ml, which would lead to a drop in haemoglobin value of 5g/dl. The expected drop in postoperative haemoglobin as compared with the preoperative level would therefore have been 6.8g/dl (11.8g/dl - 5g/dl = 6.8g/dl). Based on the current hospital transfusion policies, this could mean transfusing 2 units (1 unit = 250ml) of allogeneic blood per patient or 60 units in total. However, as noted above, only 26 units of blood were transfused.
There were no complications or side effects secondary to the use of the cell saver.
Statistical analysis
A bivariate parametric correlation analysis (using the Pearson correlation coefficient) between the amount of blood salvaged intraoperatively and the amount of blood transfused postoperatively revealed a significant inverse correlation of 0.42 (p=0.02) with a moderate effect size.
Cost analysis
The initial cost to purchase the cell saver was £12,000 and it was used routinely for elective or emergency vascular surgery such as repair of ruptured abdominal aortic aneurism along with major revision arthroplasty surgery prior to its use in pelvic trauma surgery. As per the manufacturer, the machine could repay its installation cost after 7,000 cycles. Staff training was provided for free by the manufacturer. At the time of commencement of this study, the cell saver had generated savings covering its installation cost.
The direct cost of one unit of allogeneic blood as determined by the hospital’s finances department is £133 while the average cost of running one cycle of cell salvage per procedure is £65. As 34 units of blood were saved from being transfused using intraoperative cell salvage, this roughly equates to a total of £2,572 or £86 per patient. It should be noted that we did not include the indirect costs incurred (eg salary of transfusion nurse, cost of regular maintenance of the cell saver, cost for cross-matching).
Discussion
Allogeneic blood transfusion is potentially associated with significant hazards such as transfusion reactions, 9–11 transmission of blood borne infections 12–15 and delayed wound healing. Although the majority of these complications are historical, some can be life threatening. 16–18 These risks, together with the cost for collection, storage and processing of donor blood, have led to alternative methods of blood conservation.
Cell salvage and autotransfusion has been described for more than 40 years as a way to reduce intraoperative blood loss. 19 It has been used mainly in open heart surgery, vascular surgery, transplantation surgery, elective orthopaedic surgery, ruptured ectopic pregnancy and some neurosurgical procedures. 6,7 The current literature regarding the role of cell salvage in orthopaedics is limited to adult limb reconstruction including major revision arthroplasty and spinal surgery.7,11,20–22,33 Several authors have reported favourable outcomes using an intraoperative cell saver.
In a systematic review from 2010, the authors justified the use of a cell saver in elective orthopaedic surgery based on the finding of a 55% reduction in relative risk of postoperative transfusion using cell salvage. 7 Zarin et al reported a net decrease in perioperative blood loss in revision surgery using cell salvage. 24 Furthermore, Bridgens et al reported a significant reduction in postoperative transfusion with the use of cell salvage. 20 Savvidou et al not only found the cell saver to be clinically effective in reducing blood loss but also cost effective when used for posterior lumbar fusion. 25
Contrary to the above mentioned studies, Gause et al did not find such favourable results and reported a significantly increased blood loss and an increased number of postoperative blood transfusions when cell salvage was used in lumbar spinal fusions. 21 Scannell et al reported similar results, along with significantly increased blood-related charges when a cell saver was used in the treatment of acetabular fractures. 3 Although the cause of this paradoxical increase was unclear, in both case series, the authors suggested it was due to the increased likelihood of using a cell saver in complex cases where increased blood loss was anticipated.
In our study, a statistically significant inverse relationship was noted between the amount of intraoperative cell salvage and postoperative blood transfusion (Pearson’s correlation coefficient 0.42, p=0.02). Similarly, considering the nature of the injury and the complexity of the surgical procedure undertaken after using the intraoperative cell saver, an average of only two units of blood (500ml) were transfused postoperatively, proving it to be clinically efficacious. Furthermore, this study shows that the use of cell salvage is also cost effective, with average savings of £87 per patient.
Our study was limited by the lack of a control group and so a direct comparison was not possible. In addition, a relatively small sample size precluded analysing the effect of different patient-related preoperative variables on the postoperative outcome of blood transfusion. To account for this, a long-term study with a larger sample size is planned. Lastly, despite an anticipated significant loss in postoperative haemoglobin values, not all the patients would have required postoperative transfusion.
Conclusions
Patients with pelvic acetabular injuries are at higher risk of anaemia secondary to blood loss owing to the nature of the injury, the vascularity of the pelvis and the increased number of surgical interventions. This is compounded by lengthy surgical procedures and the extended exposures required for definitive pelvic fracture fixation. Implementation of alternatives for intraoperative blood conservation is therefore imperative in these patients. We found intraoperative cell salvage to be a clinically efficacious and cost effective means to achieve this.
References
- 1.Demetriades D, Karaiskakis M, Toutouzas Ket al Pelvic fractures: epidemiology and predictors of associated abdominal injuries and outcomes. J Am Coll Surg 2002; 195: 1–10. [DOI] [PubMed] [Google Scholar]
- 2.Kregor PJ, Tempeman D. Associated injuries complicating the management of acetabular fractures: review and case studies. Orthop Clin Morth Am 2002; 33: 73–95. [DOI] [PubMed] [Google Scholar]
- 3.Scannell BP, Loeffler BJ, Bosse MJet al Efficacy of intraoperative red blood cell salvage and autotransfusion in the treatment of acetabular fractures. J Orthop Trauma 2009; 23: 340–345. [DOI] [PubMed] [Google Scholar]
- 4.Boulton FE, James V. Guidelines for policies on alternatives to allogeneic blood transfusion. 1. Predeposit autologous blood donation and transfusion. Transfus Med 2007; 17: 354–365. [DOI] [PubMed] [Google Scholar]
- 5.Lee D, Chapman C, Contreras Met al Guidelines for autologous transfusion. I. Pre-operative autologous donation. Transfus Med 1993; 3: 307–316. [Google Scholar]
- 6.Napier JA, Bruce M, Chapman Jet al Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. Br J Anaesth 1997; 78: 768–771. [DOI] [PubMed] [Google Scholar]
- 7.Carless PA, Henry DA, Moxey AJet al Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2010; 4: CD001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br 1988; 70: 1–12. [DOI] [PubMed] [Google Scholar]
- 9.Larsson LG, Welsh VJ, Ladd DJ. Acute intravascular hemolysis secondary to out-of-group platelet transfusion. Transfusion 2000; 40: 902–906. [DOI] [PubMed] [Google Scholar]
- 10.Ottenberg R, Kaliski DJ. Accidents in transfusion. JAMA 1913; 61: 2,138– 2,140. [Google Scholar]
- 11.Wiener AS, Maloney WC. Hemolytic transfusion reactions. IV. Differential diagnosis: ‘dangerous universal donor’ or intragroup incompatibility. Am J Clin Pathol 1943; 13: 74. [Google Scholar]
- 12.Feinstone SM, Kapikian AZ, Purcell RHet al Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 1975; 292: 767–770. [DOI] [PubMed] [Google Scholar]
- 13.Hill GE, Frawley WH, Griffith KEet al Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 2003; 54: 908–914. [DOI] [PubMed] [Google Scholar]
- 14.Peterman TA, Lui KJ, Lawrence DN, Allen JR. Estimating the risks of transfusion-associated acquired immune deficiency syndrome and human immunodeficiency virus infection. Transfusion 1987; 27: 371–374. [DOI] [PubMed] [Google Scholar]
- 15.Allen JR. Transmission of Human Immunodeficiency Virus (HIV) by Blood and Blood Components. In: Moore SB. Transfusion-Transmitted Viral Diseases. Arlington, VA: American Association of Blood Banks; 1987. pp37–51. [Google Scholar]
- 16.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood 2008; 112: 2,617–2,626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodnough LT. Risks of blood transfusion. Crit Care Med 2003; 31: S678– S686. [DOI] [PubMed] [Google Scholar]
- 18.Klein HG. The immunomodulatory effects of blood transfusion. Tumori 2001; 87: S17–S19. [PubMed] [Google Scholar]
- 19.Bjerre-Jepsen K, Kristensen P, Horn A, Rydahl K. Intraoperative autotransfusion. Acta Chir Scand 1982; 148: 557–561. [PubMed] [Google Scholar]
- 20.Bridgens JP, Evans CR, Dobson PM, Hamer AJ. Intraoperative blood-cell salvage in revision hip surgery: a case-matched study. J Bone Joint Surg Am 2007; 89: 270–275. [DOI] [PubMed] [Google Scholar]
- 21.Gause PR, Siska PA, Westrick ERet al Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine 2008; 33: 571–575. [DOI] [PubMed] [Google Scholar]
- 22.Guerra J, Cuckler J. Cost effectiveness of intraoperative autotransfusion in total hip arthroplasty surgery. Clin Orthop Relat Res 1995; 315: 212–222. [PubMed] [Google Scholar]
- 23.Reitman CA, Watters WC, Sassard WR. The cell saver in adult lumbar fusion surgery: a cost–benefit outcomes study. Spine 2004; 29; 1,580–1,583. [DOI] [PubMed] [Google Scholar]
- 24.Zarin J, Grosvenor D, Schurman D, Goodman S. Efficacy of intraoperative blood collection and reinfusion in revision total hip arthroplasty. J Bone Joint Surg Am 2003; 85: 2,147–2,151. [DOI] [PubMed] [Google Scholar]
- 25.Savvidou C, Chatziioannou SN, Pilichou A, Pneumaticos SG. Efficacy and cost-effectiveness of cell saving blood autotransfusion in adult lumbar fusion. Transfus Med 2009; 19: 202–206. [DOI] [PubMed] [Google Scholar]


