Abstract
Introduction
Oral squamous cell carcinoma (SCC) can present with a wide range of clinical appearances. Consequently, an oral SCC, particularly in its early stage, may not be considered suspicious by a clinician, thereby delaying diagnosis. Delayed diagnosis of an oral SCC could result in more advanced disease at the time of treatment, leading to more extensive and costly treatment, greater morbidity and poorer survival. The aim of this study was to identify cases of oral SCC treated at Southern Heath (Melbourne, Australia) with a history of prediagnosis dental treatment, and to determine the delay between dental treatment and appropriate surgical assessment of the oral SCC.
Methods
Patients were identified from the head and neck tumour database at Southern Health who met the inclusion criteria and relevant data were recorded.
Results
Twelve patients met the inclusion criteria and 83% of cases involved the mandible. Dental extraction was the most common prediagnosis treatment performed (75%). The average delay from dental treatment to surgical assessment was just over eight weeks and all patients were found to have stage IV disease. Most patients had received extensive surgical resections (83%), neck dissections (75%) and adjunctive therapy (83%).
Conclusions
Oral SCC can sometimes be difficult to diagnose, which can result in more extensive treatment and greater morbidity. Health professionals and patients need to be aware that non-healing oral lesions, even after dental treatment such as a dental extraction, need to be considered as suspicious and an appropriate surgical referral should be made.
Keywords: Oral cancer, Carcinoma, Dental treatment, Extraction, Delay
Oral squamous cell carcinoma (SCC) accounts for 1–3% of all cancers and cancer-related deaths. 1–3 Approximately 2,000 new cases of oral SCC are diagnosed every year in Australia. 1,2 It presents with a wide range of clinical signs and symptoms, including pain, swelling, ulceration, mobile teeth and bleeding. These also occur in common non-neoplastic conditions of the oral cavity such as periodontal disease and dental abscesses. Recognising a lesion that is suspicious for being an oral SCC can therefore be confounding as they have characteristics that mimic these other common inflammatory and infective conditions. The presence of an oral SCC involving the dentition, particularly in its early stages, may consequently be overlooked by a clinician as a possible diagnosis.
If healing following dental treatment does not progress as expected, suspicion for other lesions such as an oral SCC should be considered. Delayed diagnosis of an oral SCC can potentially result in more advanced disease at the time of treatment, leading to more extensive and costly treatment, greater morbidity and poorer survival than may occur with an earlier diagnosis. In addition, this group of patients and their families may feel frustration and loss of confidence in the healthcare system.
The Southern Health head and neck cancer multidisciplinary team (MDT) (Melbourne, Australia) manages over 200 new head and neck malignancies annually. Some oral SCC patients managed by this team have a history of dental treatment prior to the diagnosis of their cancer. With this in mind, the aim of our study was to determine the time delay between dental treatment and subsequent referral, diagnosis and surgical assessment of oral SCC in patients treated by the Southern Health head and neck cancer MDT, and to identify any common characteristics and risk factors in this group. We wish to reinforce the doctrine of always being vigilant for the presence of an oral SCC, particularly when lesions of the oral cavity do not respond to treatment as would usually be expected by the clinician.
Methods
The head and neck oncology research committee at Southern Health granted approval to conduct this retrospective study. A senior oral and maxillofacial surgery trainee (TS) comprehensively reviewed the medical records of 179 cases of oral SCC from the Southern Health head and neck oncology database.
Two inclusion criteria were applied to patients examined for this study. First, a patient was included when there was a diagnosis of oral SCC if the patient had documented treatment with a general dental practitioner, dental specialist or other oral health professional prior to diagnosis. Treatment could include any form of invasive or non-invasive management related to the undiagnosed SCC, including dental extractions, periodontal treatment and the prescription of medications (eg antibiotics). Second, sufficient details regarding pretreatment symptoms or signs present prior to the diagnosis of oral SCC needed to be documented in the patient’s medical records. Appropriate surgical assessment was defined as the first consultation at the oral and maxillofacial surgery clinic, otolaryngology clinic or head and neck cancer clinic.
Medical records were either in electronic or hard copy format, and all relevant consultation and follow-up clinical notes, biopsy results and management details were reviewed. Demographics, signs and symptoms, delay in diagnosis, TNM (tumour, lymph nodes, metastasis) staging, method of treatment, follow-up and outcome were recorded in a secure database (Table 1). The TNM staging system for oral cancer was used in this study as it is recognised internationally and has been validated recently. 4,5 Staging was pathological where available, otherwise staging was determined based on clinical and radiological findings.
Table 1.
Summary of results
| Case | Sex | Age (yrs) | Location | Subsite | Dental Tx | Delay (wks) | Staging | Primary Tx | Adjuncts |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 61 | Mandible | Posterior | Extraction | 3 | T4N2M0 | Surgical | ChemoRT |
| 2 | F | 72 | Mandible | Posterior | Periodontal Tx and extraction | 24 | T4N2M0 | Surgical | ChemoRT |
| 3 | F | 73 | Mandible | Posterior | Extraction | 4 | T4N1M0 | Surgical | RT |
| 4 | M | 47 | Mandible | Anterior | Extraction | 5 | T4N2M0 | ChemoRT | |
| 5 | M | 71 | Mandible | Posterior | Extraction | 3 | T4N2M0 | Surgical | RT |
| 6 | M | 50 | Mandible | Posterior | Extraction | 16 | N1M0 | Surgical | RT |
| 7 | M | 82 | Mandible | Posterior | Extraction | 3 | T4N0M0 | Surgical | RT |
| 8 | M | 48 | Mandible | Anterior | Antibiotics | 8 | T4N2M0 | Surgical | RT |
| 9 | F | 90 | Mandible | Anterior | Antibiotics | 3 | T4N2M0 | Palliative | |
| 10 | F | 60 | Maxilla | Posterior | Periodontal Tx | 10 | T4N2M0 | Surgical | ChemoRT |
| 11 | F | 32 | Maxilla | Posterior | Extraction | 6 | T4N0M0 | Surgical | RT |
| 12 | F | 86 | Mandible | Posterior | Extraction | 14 | T4N2M0 | Surgical | RT |
M = male; F = female; Tx = treatment; ChemoRT = chemotherapy and radiotherapy; RT = radiotherapy
Results
Of the 179 cases of oral SCC cases reviewed, 12 cases met the inclusion criteria. Ten of the cases were from the period 2009–2012, which likely reflects the recent changes to our database. From 2009 there was an upgrade to the database software that enabled more thorough and efficient recording of data, capturing of a larger cohort of patients from different departments across the Southern Health network.
The age range at diagnosis was 32–90 years, with the average being 64 years. There were equal numbers of men and women. Six patients gave a history of smoking (either current or ex-smokers). Alcohol consumption was not recorded in any of the patients’ records so this important factor could not be assessed.
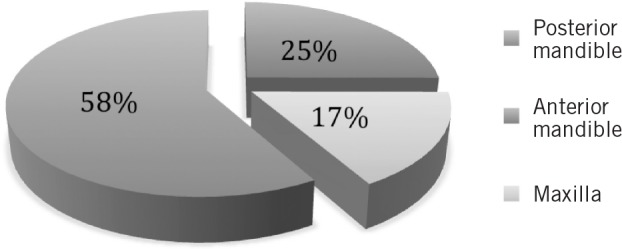
Ten cases (83%) involved the mandible and there were two cases (17%) in the maxilla. The posterior mandible was the most common subsite affected (58%) (Fig 1).
Figure 1. Location of squamous cell carcinomas.
Pain (25%) and loose teeth (25%) were the most common documented presenting symptoms prior to receiving dental treatment. Other signs and symptoms included ulceration, ill fitting dentures, swelling and ‘gingivitis’. Although these signs and symptoms may have reflected true dental pathology, they equally may be manifestations of the cancer process. Interestingly, classic ‘dental’ symptoms of sensitivity to cold or hot stimuli, or the tenderness to percussion of a tooth was not recorded in the histories of any of the 12 cases. However, conclusions cannot be drawn from this as these symptoms may have been experienced by the patient but not documented.
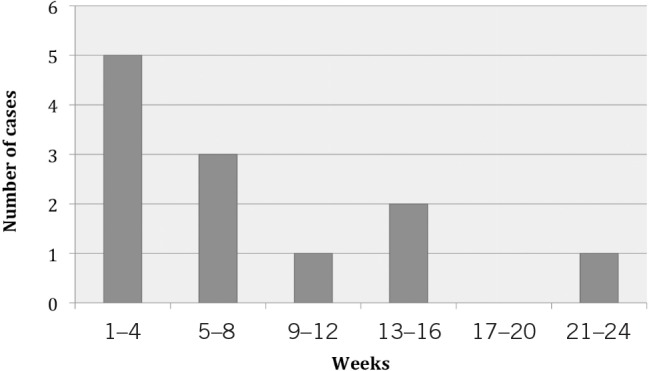
Nine patients (75%) received dental extractions after presenting to a dental practitioner before a diagnosis of oral SCC was made. Three cases underwent more than one dental extraction. Two patients were treated on multiple courses of antibiotics and two patients received periodontal treatment for a lengthy period of time (10–24 weeks). There was an overall average delay of 8.25 weeks between first dental treatment and a surgical consultation, ranging from 3 to 24 weeks (Fig 2).
Figure 2. Delay in diagnosis.
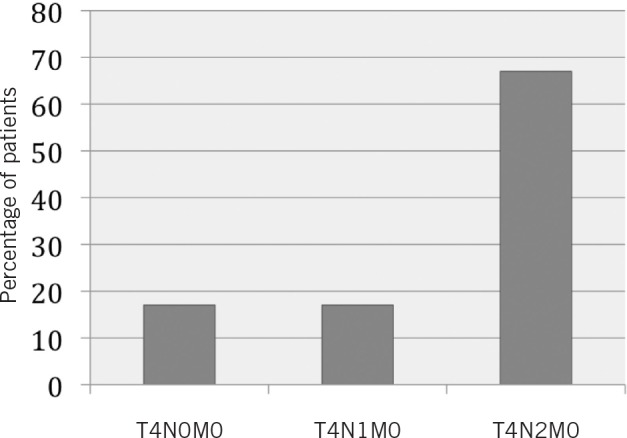
All 12 cases were stage IV due to T4 disease (tumour invades adjacent or deep structures) 4,5 and 84% had cervical metastases (Fig 3). This staging was derived from the final histopathology in surgical cases (n=10) and clinicoradiological evaluation in non-surgical cases (n=2). Treatment plans for each patient were determined at the head and neck MDT. The treatment received by ten of the patients was surgical resection, tracheostomy and neck dissections as the primary treatment modality. Surgical resection included mandibulectomy (eight cases) or maxillectomy (two cases). One patient declined surgery and was treated with primary chemoradiotherapy while another received palliative management owing to very extensive disease judged by the MDT as non-treatable with surgery.
Figure 4. Clinical photograph of a proliferative growth of the right posterior mandibular gingiva where recent dental extractions had been performed. Biopsy confirmed oral squamous cell carcinoma.
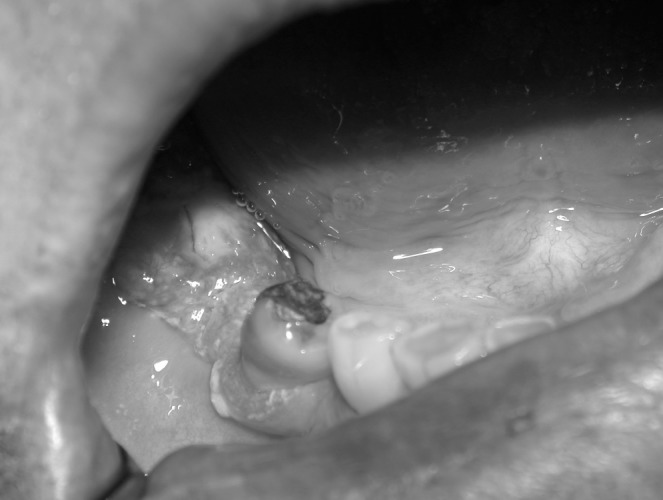
Figure 5. Orthopantomography demonstrating a large, ill-defined, radiolucency in the right posterior mandible, consistent with malignancy.
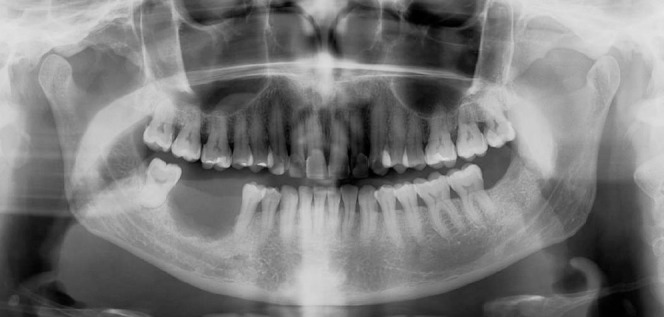
Figure 6. Orthopantomography of the same patient from Figure 5. A segmental mandibulectomy had been conducted with a fibula free flap and titanium plate reconstruction.
Figure 3. TNM staging.
Outcomes were difficult to assess owing to a relatively short follow-up period. Nine patients were alive and free of disease after a follow-up of three years or less while two cases showed no signs of recurrence five years after treatment. One patient died of disease seven months after commencement of palliative treatment.
Discussion
This study found a mean of 8.25 weeks (range: 3–24 weeks) between the patient first receiving dental treatment and subsequent surgical assessment for an oral SCC. The largest delay in diagnosis in our cohort of patients was 24 weeks. This is consistent with other studies on this subject, which have shown an average delay of 7–11 weeks. 6–8 Other studies reported a delay of several years before appropriate surgical assessment was conducted. 9–11 A review of the literature reveals few articles examining this topic. Existing studies are mainly retrospective and from the 1990s. 6–11 Certainly, there is a need for larger prospective studies on this cohort of patients to determine accurately whether this delay in diagnosis has a significant affect on a patient’s long-term outcome.
The reasons for a delay in diagnosis of an oral SCC can be multifactorial including:
-
>
Clinical presentation of an oral SCC, particularly in the early stage, can mimic common conditions of the oral cavity.
-
>
These patients can present with an array of non-specific signs and symptoms to health professionals, which can make clinical diagnosis difficult. 6–13
-
>
Patients themselves can have trouble attributing their symptoms to a clinical problem, which can result in 3–4.5 months’ delay before seeking professional treatment. 6,8
-
>
Referral by a dentist to oral specialists (eg oral medicine specialists, periodontists, dental hygienists) to manage an oral condition that is initially not recognised as a malignancy can subsequently add further to the delay in diagnosis owing to the added time required waiting to be seen by the oral specialist.
A range of factors should increase a clinician’s suspicion that a neoplastic process may be masquerading as a common non-neoplastic condition of the oral cavity. These factors include:
-
>
The patient is over 60 years of age. A number of studies, including the present one, have shown that patients over the age of 60 have an increased incidence of oral SCC compared with younger patients.6,8,12
-
>
Localised gingival and mucosal lesions, loose teeth or other signs of periodontitis in an otherwise healthy mouth should be regarded as suspicious.
-
>
Response to therapy that does not follow the normal pathway should be investigated further, especially in those patients with a history of known risk factors, in particular tobacco smoking and alcohol consumption.
-
>
This study shows the mandible (in particular, the posterior mandible) to be a potential high-risk area for the development of oral SCC in the setting of non-specific signs and symptoms such as pain and mobile teeth.
In our study, all patients had stage IV disease, with 7 of the 9 (78%) postextraction cases having cervical metastatic disease. Other studies have shown a similar increased incidence of cervical metastases in postextraction cases compared with those with similar lesions who had not received dental extractions prior to diagnosis of oral SCC. 7,13 As suggested in the literature, this may reflect more aggressive tumour behaviour after dental extraction or surgical manipulation.7,9,12
Study limitations
There are several limiting factors in our study. It is a retrospective study and therefore entails bias from a number of areas including the accuracy of medical records. However, cases were only included in the study where there was documentation (rather than speculation) on the pretreatment signs and symptoms, and the type of dental treatment the patient received.
Furthermore, a change in the database recording method meant that cases from the period 2003–2008 might not have been identified accurately compared with when the new database was introduced in 2009. This is reflected in the larger number of cases recorded from 2009 onwards (10 cases).
Finally, outcome data have been difficult to ascertain due to the short follow-up period in many of our cases. Follow-up of this cohort of patients and comparison with a control group would be useful in the long term to determine whether this delay in diagnosis has any significant impact on patient outcomes.
Although survival data are important, one area that is often overlooked is the added psychological distress and frustrations often felt by patients and their family when an incorrect initial clinical diagnosis has been made by any health practitioner resulting in a delay in treatment. This can lead to a distrust of the healthcare service and can have litigious consequences. Likewise, the clinical pressure experienced by the health practitioners in assessing and managing their patients correctly can never be underestimated. By publishing our findings, we seek to reinforce to oral practitioners the importance of always being vigilant. In particular, an oral lesion that does not respond to dental treatment may in fact be an oral malignancy.
Conclusions
This study highlights the difficulty that oral health practitioners may face in the diagnosis of oral SCC. Dental treatment delayed the diagnosis and appropriate surgical assessment by a mean of 8.25 weeks in patients diagnosed with oral SCC in our cohort of patients. Failure of a lesion to show adequate signs of healing in a timely manner, particularly when risk factors for oral SCC are present, warrants prompt referral to an oral and maxillofacial surgeon for evaluation.
References
- 1.Sugerman PB, Savage NW. Oral cancer in Australia: 1983–1996. Aust Dent J 2002; 47: 45–56. [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of Health and Welfare, Australasian Association of Cancer Registries. Cancer in Australia: an Overview, 2008. Canberra: AIHW; 2008. [Google Scholar]
- 3.Cancer Research UK. UK Cancer Incidence (2010) and Mortality (2011) Summary – December 2012. London: CRUK; 2012. [Google Scholar]
- 4.Kreppel M, Eich HT, Kübler Aet al Prognostic value of the sixth edition of the UICC’s TNM classification and stage grouping for oral cancer. J Surg Oncol 2010; 102: 443–449. [DOI] [PubMed] [Google Scholar]
- 5.International Union Against Cancer. TNM Classification of Malignant Tumours. Geneva: UICC; 1968. [Google Scholar]
- 6.Obuekwe ON, Akpata O, Ojo MAet al Malignant tumours presenting after dental extraction: a case series. East Afr Med J 2005; 82: 256–259. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Shingaki S, Nomura T, Nakajima T. Oral carcinomas detected after extraction of teeth: a clinical and radiographic analysis of 32 cases with special reference to metastasis and survival. Int J Oral Maxillofac Surg 1998; 27: 290–294. [DOI] [PubMed] [Google Scholar]
- 8.Dimitroulis G, Reade P, Wiesenfeld D. Referral patterns of patients with oral squamous cell carcinoma, Australia. Eur J Cancer B Oral Oncol 1992; 28: 23–27. [DOI] [PubMed] [Google Scholar]
- 9.Papageorge MB, Lincoln RE. Nonhealing extraction sites: two case reports and a differential diagnosis. J Mass Dent Soc 1994; 43: 20–26. [PubMed] [Google Scholar]
- 10.Saxby PJ, Soutar DS. Intra-oral tumours presenting after dental extraction. Br Dent J 1989; 166: 337–338. [DOI] [PubMed] [Google Scholar]
- 11.Ventä I, Oikarinen VJ, Söderholm AL, Lindqvist C. Third molars confusing the diagnosis of carcinoma. Oral Surg Oral Med Oral Pathol 1993; 75: 551–555. [DOI] [PubMed] [Google Scholar]
- 12.Hong SX, Cha IH, Lee EW, Kim J. Mandibular invasion of the lower gingival carcinoma in the molar region: its clinical implications on the surgical management. Int J Oral Maxillofac Surg 2001; 30: 130–138. [DOI] [PubMed] [Google Scholar]
- 13.Shingaki S, Nomura T, Takada Met al Squamous cell carcinomas of the mandibular alveolus: analysis of prognostic factors. Oncology 2002; 62: 17–24. [DOI] [PubMed] [Google Scholar]


