Abstract
Localization of RNA replication to intracellular membranes is a universal feature of positive-strand RNA viruses. The betanodavirus greasy grouper (Epinephelus tauvina) nervous necrosis virus (GGNNV) is a positive-RNA virus with one of the smallest genomes among RNA viruses replicating in fish cells. To understand the localization of GGNNV replication complexes, we generated polyclonal antisera against protein A, the GGNNV RNA-dependent RNA polymerase. Protein A was detected at 5 h postinfection in infected sea bass cells. Biochemical fractionation experiments revealed that GGNNV protein A sedimented with intracellular membranes upon treatment with an alkaline pH and a high salt concentration, indicating that GGNNV protein A is tightly associated with intracellular membranes in infected cells. Confocal immunofluorescence microscopy and bromo-UTP incorporation studies identified mitochondria as the intracellular site of protein A localization and viral RNA synthesis. In addition, protein A fused with green fluorescent protein (GFP) was detected in the mitochondria in transfected cells and was demonstrated to be tightly associated with intracellular membranes by biochemical fractionation analysis and membrane flotation assays, indicating that protein A alone was sufficient for mitochondrial localization in the absence of RNA replication, nonstructural protein B, or capsid proteins. Three sequence analysis programs showed two regions of hydrophobic amino acid residues, amino acids 153 to 173 and 229 to 249, to be transmembrane domains (TMD) that might contain a membrane association domain. Membrane fraction analysis showed that the major domain is N-terminal amino acids 215 to 255, containing the predicted TMD from amino acids 229 to 249. Using GFP as the reporter by systematically introducing deletions of these two regions in the constructs, we further confirmed that the N-terminal amino acids 215 to 255 of protein A function as a mitochondrial targeting signal.
Cellular membranes are complex structures composed of lipids and proteins that compartmentalize the intracellular space and serve as the physical matrix upon which numerous biosynthetic events are performed. The RNA replication complexes of all eukaryotic positive-stranded RNA viruses studied to date are associated with intracellular membranes, but different RNA viruses seem to target or recruit distinct membranes for the assembly of their replication complexes.
Proper intracellular localization of proteins is critical for their integration into the biochemical and metabolic processes in which they participate. Mitochondria are essential organelles that carry out oxidative phosphorylation and many other important functions, such as synthesis of lipids, heme, and amino acids. Most of the known outer membrane proteins contain one or several hydrophobic transmembrane domains (TMD) which span the membrane several times. The TMD, in conjunction with the N-terminal or C-terminal flanking regions, functions as the mitochondrial targeting and membrane insertion signal (22). Targeting and translocation of most nucleus-encoded mitochondrial proteins depend on N-terminal extensions referred to as mitochondrial targeting sequences or presequences (26, 27, 32, 34). A presequence typically consists of 15 to 40 amino acid residues and is enriched in positively charged and hydroxylated (mostly serine) residues. The ability of most presequence peptides to form amphipathic α-helices is thought to be important for their recognition by the translocation machineries in the mitochondrial outer and inner membranes (31, 45).
Greasy grouper (Epinephelus tauvina) nervous necrosis virus (GGNNV), a member of the betanodaviruses that usually infects a wide variety of larval and juvenile marine fish, belongs to Nodaviridae family. GGNNVs are small, nonenveloped, spherical viruses with bipartite positive-sense RNA genomes which are capped but not polyadenylated (1, 2). The genome of GGNNV has two encapsidated genomic RNA segments (RNA1 and RNA2), both of which are required for infectivity. RNA1 (3.1 kb) encodes protein A, a 110-kDa protein which is the viral component of the viral RNA-dependent RNA polymerase, and RNA2 (1.4 kb) encodes the 37-kDa protein α, a proteolytic precursor of viral capsid proteins which is required for the production of whole virions. During replication, GGNNV produces a subgenomic RNA3 that is colinear with the 3′ end of RNA1 and encodes one or two small proteins (B1 and B2) of unknown functions (39). In our previous work, GGNNV was demonstrated to be able to induce caspase-dependent apoptosis in GGNNV-infected sea bass (SB) cells and protein α was shown to be a possible apoptotic inducer in transfected cells (14). A nucleolus localization signal was determined to be present in protein α (13). However, the mechanism and site of assembly of the replication complexes of GGNNV are less studied and unclear.
The present study identifies and characterizes the GGNNV replication complex by using antibodies against protein A, the GGNNV RNA-dependent RNA polymerase. We used confocal microscopy to localize protein A in GGNNV-infected SB cells. We demonstrated that the replication complex was overlaid with the image of the mitochondrial tracker. Dual labeling of GGNNV-infected cells with 5′-bromouridine (BrU) 5′-triphosphate (BrUTP) and anti-protein A sera showed that the protein A was colocalized with viral RNA, suggesting that the GGNNV RNA replication complex was associated with mitochondria. Biochemical fractionation experiments revealed that GGNNV protein A sedimented with intracellular membranes, indicating that GGNNV protein A is tightly associated with intracellular membranes in infected cells. In addition, we expressed GGNNV protein A in the absence of other viral proteins in mammalian cells and observed its localization by confocal microscopy. The results showed that the distribution of protein A is overlaid with mitochondrial tracker, consistent with its location in GGNNV-infected SB cells. Biochemical fractionation analysis of GGNNV protein A expressed in mammalian cells further confirmed that GGNNV protein A is tightly membrane associated. Furthermore, using several sequence analysis programs, two regions of hydrophobic amino acid residues, amino acids 153 to 173 and 229 to 249, were predicted to be TMD and might contain a membrane association domain. Membrane fraction analysis showed that N-terminal amino acids 215 to 255 containing the predicted TMD from amino acids 229 to 249 was the major domain, while N-terminal amino acids 145 to 185 containing the predicted TMD from amino acids 153 to 173 showed a very weak signal. We further confirmed, using confocal studies, that N-terminal amino acids 215 to 255 of protein A function as a mitochondrial targeting signal.
MATERIALS AND METHODS
Cells and viruses.
The betanodavirus (GGNNV) and the SB cell line (4, 5) were obtained from the Agri-Food and Veterinary Authority of Singapore. The SB cells were grown in modified Eagle's medium (GIBCO) supplemented with 10% fetal bovine serum (GIBCO), 0.34% NaCl, 0.12% HEPES, and 2 mM glutamine at 23°C. GGNNV was originally isolated in 1992 from brain, head, kidney, and liver of greasy grouper E. tauvina in Singapore. The SB cells were infected with the betanodavirus GGNNV for propagation, and inoculated cultures were harvested when 90% of cells in the monolayer showed a specific cytopathic effect.
Cos-7 cells (a monkey kidney cell line) were maintained in Dulbecco modified Eagle's medium (GIBCO.) supplemented with 10% fetal bovine serum and antibiotics (GIBCO) and grown at 37°C with 5% CO2.
Transient expression of protein A in vitro.
The coding sequence of protein A was amplified by PCR and inserted into pEGFP-C1 vector (Clontech) under the control of the human cytomegalovirus promoter to obtain pEGFP-RNA1 and was transiently expressed in Cos-7 cells as described previously (13).
Antibodies and fluorescent reagents.
Polyclonal anti-protein A sera were produced by immunizing guinea pigs with recombinant protein A expressed in Escherichia coli. The sera were obtained after the animals were immunized two times. The antibody was confirmed by Western blot analysis with GGNNV-infected SB cells, and a specific 110-kDa protein could be detected (14). Polyclonal anti-green fluorescent protein (anti-GFP) sera were purchased from Molecular Probes, and monoclonal mouse antibromodeoxyuridine was from Sigma. MitoTracker Red CMXRos was from Molecular Probes.
IFA and confocal microscopy.
GGNNV-infected SB cells were cultured on four-well chamber slides (IWAKI). At 8 and 14 h postinfection (p.i.), cells were rinsed once with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 25 min at room temperature, and then permeabilized for 5 min in 0.2% Triton X-100 in PBS at room temperature for immunofluorescence assay (IFA). IFA was performed by incubating cells with primary antibodies for 1 h at room temperature, washing with PBS with 0.05% Tween 20 (PBST), incubating with secondary antibodies conjugated with tetramethyl rhodamine isocyanate or fluorescein isothiocyanate for 40 min at room temperature, and washing three times with PBST. For immunofluorescence assays with MitoTracker Red CMXRos, live cells were labeled with the mitochondrion-specific dye in accordance with the manufacturer's instructions. Images were viewed and collected with a Zeiss LSM510 confocal microscope.
Northern blot analysis.
Total RNA was isolated with an RNeasy kit (Qiagen). RNA samples were prepared in 60% formamide sample buffer, heated at 65°C for 10 min, separated on formaldehyde-1.2% agarose gels, and transferred to Nytran nylon membranes via horizontal capillary blotting in a solution containing 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membranes were UV cross-linked, prehybridized with a solution containing digoxigenin (DIG) Easy Hyb (Roche), and hybridized at 60°C with a strand-specific, DIG-labeled riboprobe that corresponded to nucleotides 2753 to 2980 from GGNNV RNA1. DIG signals were detected with a monoclonal antibody (MAb) against DIG and visualized by enhanced chemiluminescence (Pierce).
Viral RNA labeling and localization.
Newly synthesized GGNNV RNA was detected by BrU incorporation. GGNNV-infected or mock-infected SB cells were treated with 20 μg of actinomycin D (Sigma) at 14 h p.i. for 4 h to shut off host cell mRNA transcription. BrUTP (Sigma) (1 mM) was introduced into cells by using 7.5 μl of Superfect (Qiagen), and the cells were incubated for another 1 h before fixation and permeabilization for immunofluorescent staining as described above.
Immunoblot analysis.
Protein samples were separated by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE) and transferred to Hybond nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% skim milk in PBST for 1 h at room temperature, washed with PBST once, incubated with polyclonal anti-protein A sera or polyclonal anti-GFP sera at room temperature for 1 h, and then washed with PBST three times and incubated with secondary antibody conjugated with horseradish peroxidase at room temperature for 40 min. After rinsing three times, the specific proteins were visualized by enhanced chemiluminescence (Pierce) or with Superfect (Pierce).
Cell fractionation and membrane association assays.
Intracellular membranes were isolated from GGNNV-infected SB cells and Cos-7 cells transfected with pEGFP/RNA1. Briefly, the cell monolayers were washed twice with cold PBS, scraped in 1 ml of PBS, and pelleted by centrifugation at 500 × g for 5 min. The cell pellet was resuspended in hypotonic buffer (1 mM Tris-HCl [pH 7.4], 0.1 mM EDTA, 15 mM NaCl) containing 0.4 mM phenylmethylsulfonyl fluoride and a mammalian protease inhibitor cocktail (Sigma) and rocked at 4°C for 15 min. Cell debris and nuclei were removed by centrifugation at 1,500 × g for 10 min at 4°C. The membranes (postnuclear fraction) were pelleted by ultracentrifugation through a 6% sucrose cushion in a Beckman TLA 55 rotor (150,000 × g for 30 min at 4°C). For membrane association assays, the membrane fraction was treated with either 1% Triton X-100, 100 mM Na2CO3 (pH 11), or 1 M KCl (high salt) for 30 min on ice and then ultracentrifuged to separate the soluble contents and pellets. Pellets were separated by SDS-PAGE and immunoblotted as described above.
Membrane flotation assays.
Cells were recovered by scraping and centrifugation and resuspended in hypotonic buffer (1 mM Tris-HCl [pH 7.4], 0.1 mM EDTA, 15 mM NaCl) containing 0.4 mM phenylmethylsulfonyl fluoride and a mammalian protease inhibitor cocktail (Sigma) and rocked at 4°C for 15 min. Unbroken cells, nuclei, and large debris were removed by centrifugation at 500 × g for 5 min to obtain the initial total lysate (23, 24). Nycodenz was added to total lysates to a final concentration of 37.5% (wt/vol), and samples were loaded under a 5 to 25% discontinuous Nycondenz gradient prepared in hypotonic buffer. Samples were centrifuged at 100,000 × g for 20 h at 4°C, and equal-volume gradient fractions were recovered manually, separated by SDS-PAGE, and immunoblotted as described above.
Sequence analysis and TMD and localization prediction.
We performed sequence analysis with the following programs: DAS (7) (www.sbc.su.se/∼miklos/DAS/), Toppred2 (46) (www.sbc.su.se/∼erikw/topred2), Tmpred (www.ch.embnet.org/software/TMPRED/), HMMTOP (41) (www.enzim.hu/hmmtop/), TMHMM version 2 (19) (www.cbs.dtu.dk/services/TMHMM-2.0), MitoProt (6) (http://www.mips.biochem.mpg.de/cgi-bin/proj/medgen/mitofilter), and TargetP version 1.0 (9).
RESULTS
Temporal pattern of GGNNV protein A expression and localization of GGNNV protein A in infected SB cells.
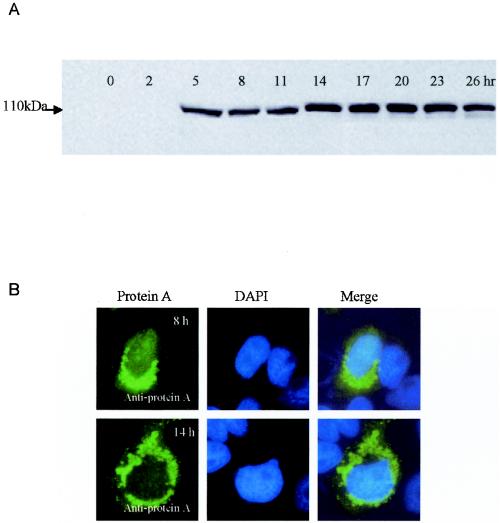
To identify the time point with maximal protein A expression early after infection, we examined GGNNV protein A levels by immunoblot analysis at 3-h intervals up to 26 h p.i. Protein A was first detectable at 5 h p.i., increased to a maximal level by 14 h p.i., remained stable to 23 h p.i., and declined slightly thereafter (Fig. 1A). We also examined the localization of GGNNV protein A in infected SB cells. GGNNV-infected SB cells were fixed at 5, 8, and 14 h p.i. and analyzed by IFA. A polyclonal guinea pig anti-protein A serum was used as the primary antibody, followed by fluorescein isothiocyanate-conjugated secondary antibody, and the cells were observed by confocal immunofluorescence microscopy. Although protein A was first detected by immunoblotting at 5 h p.i., it was detected by IFA at 8 h p.i. and became more prominent at 14 h p.i. (Fig. 1B). In GGNNV-infected SB cells, protein A showed a perinuclear, punctate staining pattern, suggesting that it may be localized to intracellular membrane compartments. DAPI (4′,6′-diamidino-2-phenylindole) staining was used to show the nuclei, and the merged images clearly showed the cytoplasmic localization of GGNNV protein A in infected SB cells (Fig. 1B). From the results described above, we choose 14 h p.i. as the time point at which to evaluate the RNA replication in all subsequent experiments, unless otherwise indicated.
FIG. 1.
(A) Time course study of protein A expression in GGNNV-infected SB cells. SB cells were infected with GGNNV, and protein A was detected by immunoblotting with anti-protein A sera at 3-h intervals up to 26 h. (B) Localization of protein A in GGNNV-infected SB cells. SB cells infected with GGNNV were fixed at 8 h (top row) and 14 h (bottom row) p.i., and IFA was carried out to detect the expression and localization of protein A with anti-protein A sera. DAPI staining was used to show the nucleus (blue). The images were viewed with a confocal microscope. Magnification, ×630.
Membrane association of GGNNV protein A in infected SB cells.
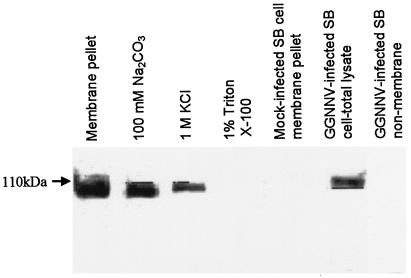
The subcellular localization profiles of protein A in infected SB cells strongly suggested that it is associated with intracellular membranes. To confirm the membrane association of GGNNV protein A, the membrane fraction of GGNNV-infected SB cells was extracted after removal of the nuclei and cell debris by centrifugation at low speed, and protein A was detected in the membrane fraction pellet with anti-protein A sera (Fig. 2). Biochemical studies were then carried out to ascertain whether the protein A is an integral membrane protein or a peripherally associated membrane protein. The membrane fractions were treated with different buffers designed to remove peripheral proteins, which are loosely associated with intracellular membranes. Most peripheral membrane proteins are dissociated from membranes by high pH, high ionic strength, or chaotropic agents. Only proteins that are tightly associated with the membrane, such as integral membrane proteins, will remain in the membrane pellets, which require the presence of a detergent for solubilization. After treatment with Na2CO3 (pH 11) and 1 M KCl, the protein A was detected in the pellets. However, treatment of the same membrane pellets with 1% Triton X-100 led to a decrease in the amount of protein A in membrane pellets (Fig. 2A), suggesting that protein A was partially soluble in the supernatant. We concluded from these results that GGNNV protein A was membrane associated through a mechanism that imparted significant stability to the protein-membrane interaction.
FIG. 2.
Membrane association of protein A in GGNNV-infected SB cells. The membrane fraction of SB cells infected with GGNNV at 14 h p.i. was extracted to detect membrane association of protein A with anti-protein A sera by Western blot analysis.
Subcellular localization of GGNNV protein A in infected SB cells.
Previous studies have suggested an association of one or more steps in alphanodavirus replication with mitochondria (3, 11, 23). Here, we predicted the subcellular localization of GGNNV protein A by using different analysis programs. Our analysis of GGNNV protein A by using TargetP analysis indicated that protein A has a 76.1% probability of containing a mitochondrial targeting peptide, and the reliability of mitochondrial localization is between 0.6 and 0.8. Sequence analysis of the GGNNV protein A by using the MitoProt II program suggested that protein A has a >85.43% probability of export to the mitochondria.
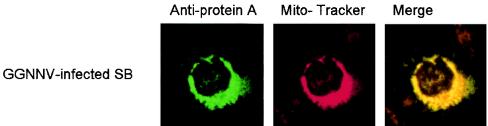
To determine the relationship between betanodavirus GGNNV replication and mitochondria, we used confocal immunofluorescence microscopy to compare the intracellular localization of GGNNV protein A to that of mitochondria in infected SB cells. MitoTracker Red CMXRos was used to stain the mitochondria of SB cells (Fig. 3). IFA was then carried out to show the subcellular localization of protein A (Fig. 3). Figure 3 shows the areas where labeled protein A was completely overlaid with MitoTracker.
FIG. 3.
Subcellular localization of GGNNV protein A in infected SB cells. SB cells infected with GGNNV were fixed at 14 h p.i. IFA was performed with anti-protein A sera to show the expression of protein A (green) in SB cells. MitoTracker was used to show mitochondria. The merged image represents digital superimposition of green and red signals, where areas of fluorescence colocalization are yellow-orange. Magnification, ×630.
Colocalization of GGNNV protein A with nascent viral RNA in infected SB cells.
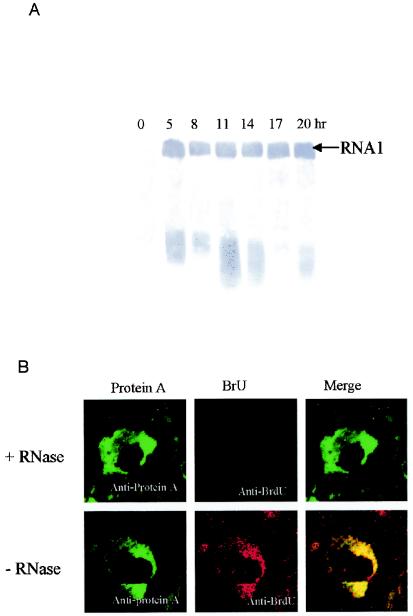
We used Northern blot analysis to check GGNNV RNA levels at 3-h intervals from 5 h p.i. up to 20 h p.i. (Fig. 4A). Amplification of positive-stranded RNA1 initiated at around 5 h p.i., and it was detectable at all time points, consistent with its presence in whole virions. Therefore, we choose 14 h p.i. to analyze the site of RNA synthesis in all subsequent experiments.
FIG. 4.
(A) Temporal pattern of GGNNV RNA1 replication in infected SB cells. Total RNA of SB cells infected with GGNNV was extracted at 3-h intervals from 5 h p.i. up to 20 h p.i. Total RNA was separated on denatured formaldehyde-agarose gels, transferred to a nylon membrane, and blotted with DIG-labeled probe that detected positive-strand RNA1. (B) Colocalization of GGNNV protein A with nascent viral RNA synthesis in infected SB cells. GGNNV-infected SB cells were BrUTP labeled at 14 h p.i. for 1 h and dual labeled with anti-protein A sera (red) and anti-BrdU MAb (green). Cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and immunostained. As a specificity control, BrUTP-labeled cells were treated with RNase after fixation and permeabilization but before immunostaining (top). The merged images represent digital superimposition of green and red signals, where areas of fluorescence colocalization are yellow-orange. Magnification, ×630.
To determine whether the localization of the protein A indeed corresponds to the viral RNA synthesis in infected cells, a metabolic in vivo labeling of de novo-synthesized GGNNV RNA was performed by using BrUTP. It has been shown that this UTP analog can be incorporated into nascent RNA transcripts by both cellular (48) and viral DNA-dependent (28) RNA polymerases, including the viral RNA-dependent RNA polymerases (29). Antibodies originally raised to detect BrdU-labeled DNA are also able to recognize BrUTP-labeled RNA transcripts (43). This makes it is possible to visualize de novo-synthesized RNA by using indirect immunofluorescence techniques. Before and during the labeling, the cells were treated with actinomycin D to shut off the host cell mRNA synthesis. GGNNV-infected SB cells were BrUTP labeled at 14 h p.i. for 1 h and dual labeled with anti-protein A sera and anti-BrdU MAb (Fig. 4B). This showed a perinuclear vesicular staining pattern. A clear overlap between the two labeling patterns was observed (Fig. 4B). BrU activity was eliminated by RNase treatment after transfection, indicating that the BrU signal was due to ribonucleotide incorporation into newly synthesized viral RNA and not to localized pools of unincorporated BrUTP. This in situ RNA labeling confirmed that the membrane-associated complex in which GGNNV protein A accumulated was also the site of viral RNA synthesis in infected SB cells.
Membrane association of GGNNV protein A in transfected cells.
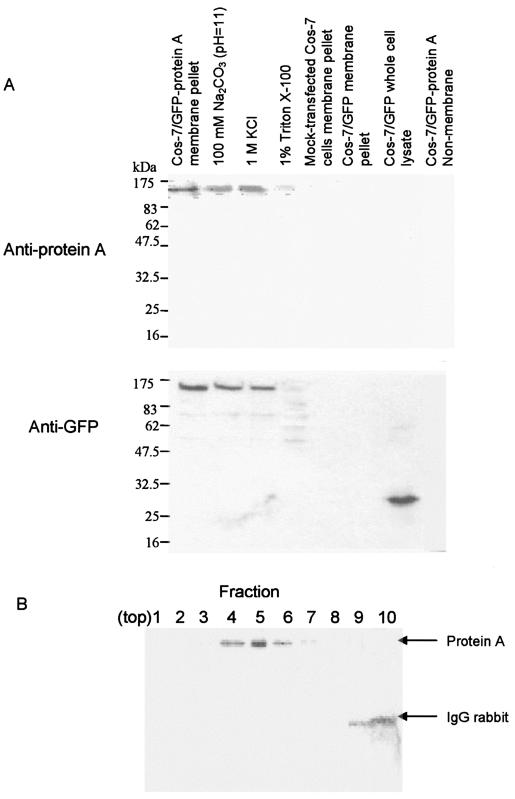
To further investigate the independent localization properties of the GGNNV protein A, without the present of other viral proteins, we inserted the open reading frame of RNA1 cDNA into the pEGFP-C1 vector and transiently expressed it in Cos-7 cells. At 36 h posttransfection, Cos-7 cells transfected with pEGFP/RNA1 were lysed in hypotonic buffer and the membrane fraction was extracted. The enhanced GFP (EGFP)-protein A was found exclusively in the membrane fraction pellet by immunoblotting with anti-protein A sera or anti-GFP sera (Fig. 5A) and anti-B2 sera (data not shown). The membrane fractions were further extracted with buffers that discriminate between peripheral membrane proteins (which are attached to membranes by ionic interactions with membrane integral proteins or phospholipid head groups) and integral membrane proteins (which are embedded in the phospholipid bilayer). The soluble contents were separated from the pellets by ultracentrifugation to separate the soluble contents from the pellets and the pellets were analyzed by immunoblot with anti-protein A sera or anti-GFP sera (Fig. 5A). Cos-7 cells transfected with the pEGFP-C1 vector were used as a negative control. As expected, EGFP-protein A was detected in the membrane fraction pellet by anti-protein A sera after treatment with 100 mM Na2CO3 (pH 11) or 1 M KCl (high salt) (Fig. 5A), showing a 140-kDa band. However, no specific bands were detected in the membrane fraction pellet extracted from Cos-7 cells, the membrane fraction pellet, or whole lysates extracted from pEGFP-C1-transfected Cos-7 cells (Fig. 5A). Similarly, anti-GFP sera could detect 140-kDa bands in the membrane fraction pellet of pEGFP/RNA1-transfected Cos-7cells which was treated with 100 mM Na2CO3 (pH 11) or 1 M KCl (high salt) (Fig. 5A) but not in the membrane fraction pellet extracted from Cos-7 cells or pEGFP-C1-transfected Cos-7 cells (Fig. 5A). We found that treatment of the same membrane pellets with 1% Triton X-100 led to the disappearance of EGFP-protein A in membrane fraction pellet (Fig. 5A). In addition, anti-GFP sera could detect expression of EGFP in whole lysates of pEGFP-C1-transfected Cos-7 cells but not in the membrane fraction pellets (Fig. 5A). These results indicate that there is a tight membrane association of EGFP-protein A in transfected cells that is due to protein A, not EGFP. We also found that no B2 protein expression occurs in Cos-7 cells transfected with pEGFP-RNA1 (data not shown).
FIG. 5.
Membrane association of GGNNV protein A in transfected Cos-7 cells. (A) The membrane fraction of Cos-7 cells transfected with pEGFP-RNA1 or pEGFP-C1 was extracted at 36 h posttransfection to analyze the membrane association of protein A in transfected cells by using anti-protein A sera (upper panel) and anti-GFP sera (lower panel) by Western blot analysis. (B) Equilibrium density Nycondenz gradient fractionation of lysates from pEGFP-RNA1-transfected Cos-7 cells. Equal-volume fractions were recovered from the top of the gradient, and samples were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with rabbit anti-GFP. The position of protein A is indicated on the right. The 50-kDa rabbit immunoglobulin G (IgG) heavy chain was detected by the secondary reagent, and its position is also indicated on the right.
The sedimentation behavior of GGNNV protein A in differential centrifugation assays might have represented self-aggregation rather than membrane association, so we used equilibrium density gradient centrifugation to examine the flotation behavior of protein A from Cos-7 cell lysates. Total lysates were adjusted to 37.5% Nycondenz, loaded under a 5 to 25% Nycondenz gradient, and centrifuged to equilibrium. We analyzed equal-volume gradient fractions by immunoblot assay for protein A and rabbit immunoglobulin, which we added to cell lysates prior to centrifugation in order to easily monitor the soluble protein distribution on a single blot. Consistent with membrane association, protein A floated up into and was recovered in lower-density gradient fractions (Fig. 5B). These results support the conclusion from differential centrifugation that GGNNV protein A is tightly membrane associated in transfected Cos-7 cells.
Subcellular localization of GGNNV protein A in transfected cells.
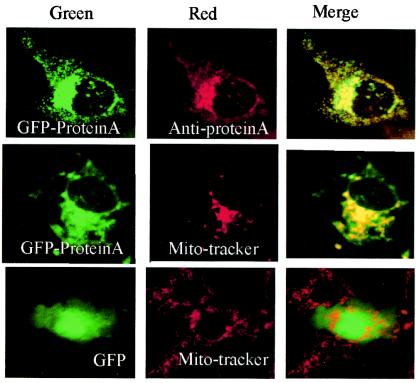
In the past few years, GFP from jellyfish has been a popular tool in many studies for localization of a specific protein distribution inside cells when the GFP is fused with that particular protein (25, 37, 49). We constructed EGFP-protein A fusion proteins with the N terminus of the RNA-dependent RNA polymerase protein fused to the C terminus of EGFP. Cos-7 cells overexpressing EGFP-protein A showed a perinuclear, punctate staining pattern, which completely overlapped anti-protein A serum staining (Fig. 6, upper row). MitoTracker Red CMXRos was used to determine whether EGFP-protein A was located to mitochondria. The green pattern of EGFP-protein A was colocalized with the MitoTracker Red dye signal, showing yellow in the merged pattern color (Fig. 6, middle row). These results demonstrated that EGFP-protein A localized to mitochondria in the absence of other viral proteins. Cos-7 cells overexpressing EGFP showed a diffuse staining and did not overlap with the pattern of MitoTracker (Fig. 6, lower row), indicating that protein A is responsible for the mitochondrial localization of the EGFP-protein A fusion protein.
FIG. 6.
Subcellular localization of GGNNV protein A in transfected Cos-7 cells. Cos-7 cells were transfected with pEGFP-RNA1 plasmid and labeled with anti-protein A sera (upper panel) and MitoTracker (middle panel) at 24 h posttransfection to show its subcellular localization. The merged images represent digital superimposition of green and red signals, where areas of fluorescence colocalization are yellow-orange. GFP (lower panel) was used as a control. Magnification, ×630.
Membrane association of protein A deletion mutants.
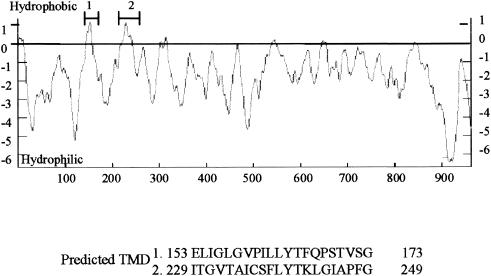
To identify regions within protein A that mediated membrane association, we analyzed the protein A amino acid sequence by using several programs to identify potential hydrophobic regions and TMD. Three sequence analysis programs (DAS, Topred2, and TMpred) predicted with high probability that two internal regions of protein A, residues 153 to 173 and 229 to 249, had sufficient hydrophobicity. These two regions might contain a membrane association domain (Fig. 7).
FIG. 7.
Potential hydrophobic regions and TMD of protein A. The underlined amino acids represent the predicted TMD identified by four different structural prediction programs (see text), and the primary sequences of predicted TMD (amino acids 153 to 173 and 229 to 249) are shown.
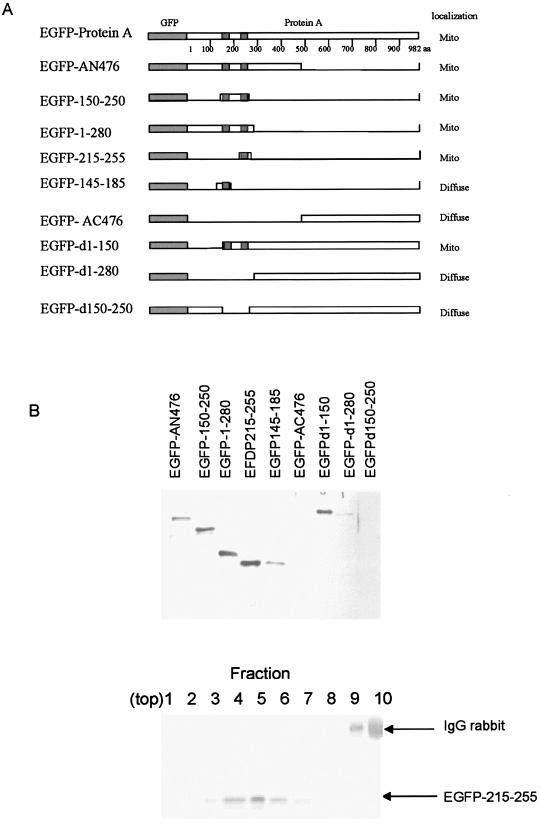
According to the predicted TMD, we constructed a series of EGFP-protein A fusion proteins carrying deletions to identify regions within protein A that mediated membrane association (Fig. 8A). Polyclonal GFP sera were used to determine the sensitivity for immunoblotting. At 36 h posttransfection, membrane fraction pellets extracted from Cos-7 cells transfected with the recombinant plasmids of these constructs (Fig. 8A) were analyzed by immunoblotting (Fig. 8B, top). EGFP-AN476 fusion protein was detected in the membrane fraction pellets; however, EGFP-AC476 could not be found in the membrane fraction pellets, suggesting that the N terminus of protein A was likely to be involved in the overall association of protein A with membranes. Further deletions of N-terminal amino acids from protein A, such as deleting N-terminal amino acids 1 to 280 (EGFP-d1-280) or 150 to 250 (EGFP-d150-250), showed that the EGFP fusion proteins could not be detected in the membrane fraction pellets. However, EGFP fusion protein was detected in the membrane fraction pellets when N-terminal amino acids 1 to 150 were deleted (EGFP-d1-150). From these results, we concluded that protein A N-terminal amino acids 150 to 250 might be responsible for membrane association of protein A and was sufficient to target a heterologous protein EGFP to membranes.
FIG. 8.
(A). Schematic representation of various constructs of GGNNV protein A deletion mutants and N-terminal amino acids fused with GFP and subcellular localization of their derivatives of protein A in transfected Cos-7 cells. (B) Top panel, membrane association assays of protein A deletion mutants and N-terminal amino acids in transfected Cos-7 cells. Membrane fractions of Cos-7 cells transfected with protein A deletion mutants fused with GFP or N-terminal amino acids fused with GFP were extracted at 36 h posttransfection, and samples were separated by SDS-PGE, transferred to a membrane, and immunoblotted with anti-GFP sera. Bottom panel, equilibrium density Nycondenz gradient fractionation of lysates from EGFP-215-255-transfected Cos-7 cells. Equal-volume fractions were recovered from the top of the gradient, and samples were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with rabbit anti-GFP. The position of protein A is indicated on the right. The 50-kDa rabbit immunoglobulin G (IgG) heavy chain was detected by the secondary reagent, and its position is also indicated on the right.
Various fusions of EGFP containing variable lengths of the protein A N-terminal segment were further constructed (Fig. 8A) and transiently expressed in Cos-7 cells. EGFP fusion proteins were detected in the membrane fraction pellets extracted from Cos-7 cells transfected with EGFP-1-280 and EGFP-150-250 at 36 h posttransfection (Fig. 8B, top). N-terminal amino acids 153 to 173 and 229 to 249 (the two predicted TMD) were fused with EGFP and transiently expressed in Cos-7 cells, but fusion proteins were not detected in the membrane fraction pellets (data not shown). Thus, longer fragments of EGFP fusion proteins were constructed, with N-terminal amino acids 215 to 255 (EGFP-215-255) and 145 to 185 (EGFP-145-185) transiently expressed in Cos-7 cells. Both fusion proteins were detected in the membrane fraction pellets. However, fusion protein EGFP-145-185 showed a very weak signal compared to EGFP-215-255. Taken together, these results identified the putative TMD of protein A in the regions consisting of N-terminal amino acids 145 to 185 and 215 to 255 of protein A.
To discriminate between sedimentation of these protein A deletion mutants due to membrane association or aggregation, we further confirmed that these protein A deletion mutants are membrane-associated proteins by using equilibrium density gradient centrifugation. All of the protein A deletion mutants which showed signals in immunoblotting floated up into and were recovered in lower-density gradient fractions (Fig. 8B, bottom [EGFP-215-255 was used as an example]), similar to the case for the wild-type EGFP-protein A (Fig. 5B).
Subcellular localization of protein A deletion mutants and characterization of the mitochondrial targeting sequence of protein A.
To characterize the mitochondrial targeting signal of protein A, different EGFP-protein A deletion mutants (Fig. 8A) were transiently expressed in Cos-7 cells to observe the subcellular localization by confocal microscopy with MitoTracker staining as the reference. Wild-type EGFP-protein A was colocalized with MitoTracker in the aggregated mitochondria at the perinuclear region (Fig. 6). The EGFP-AN476 fusion protein was colocalized with MitoTracker in the aggregated mitochondria at the perinuclear region (Fig. 8A) similar to the case for wild-type EGFP-protein A (Fig. 6). However, EGFP-AC476 showed a diffused pattern (Fig. 8A). The localization results for other constructs are summarized in Fig. 8A. EGFP-d1-280 or EGFP-d150-250 showed a diffused distribution. In contrast, EGFP-d1-150 was still able to target the EGFP fusion protein to mitochondria, the same as wild-type EGFP-protein A. These results indicated that the N-terminal portion of protein A, including amino acids 150 to 250, contains sufficient information to target EGFP to mitochondria.
To further confirm that the N-terminal region of protein A can function as a signal for mitochondrial targeting, other EGFP fusion proteins containing various lengths of protein A were used and individually transfected to Cos-7 cells. There was significant colocalization of EGFP-1-280 or EGFP-150-250 fusion protein and MitoTracker, clearly showing that the fusion proteins were targeted to mitochondria by the N-terminal signal in protein A. The data described above indicated that N-terminal residues 150 to 250 of protein A, including the two transmembrane domains, have a mitochondrion-targeting function.
We also tested two other fusion proteins, EGFP-145-185 and EGFP-215-255. EGFP-145-185 localized to the cytoplasm and nucleus, showing a diffuse pattern similar to that of EGFP, while EGFP-215-255 colocalized exclusively with mitochondria. These results indicated that N-terminal amino acids 215 to 255 of protein A function as a mitochondrial targeting signal.
DISCUSSION
In this study, we investigated the intracellular localization, membrane association, and organellar targeting signals of GGNNV protein A in SB cells and Cos-7 cells. We drew five main conclusions. First, protein A, the GGNNV RNA-dependent RNA polymerase, was tightly membrane associated. Second, protein A was localized to the mitochondria and colocalized with sites of viral RNA synthesis in GGNNV-infected SB cells. Third, in Cos-7 cells, protein A showed recapitulated cell biology features of GGNNV replication in infected SB cells, such as mitochondrial localization and tight membrane association. Fourth, amino acid sequence analysis identified two predicted regions of moderate hydrophobicity at amino acid residues 153 to 173 and 229 to 249 in the N terminus of protein A which are TMD and might contain a membrane association domain. Fifth, N-terminal amino acids 215 to 255 of protein A function as a mitochondrial targeting signal. These observations will contribute to understanding of the mechanism of betanodavirus replication.
The genome replication and mRNA transcription of eukaryotic positive-strand RNA viruses depend on a unique process, RNA-dependent RNA synthesis, that occurs in the cytoplasm of the infected cells. A common property among these viruses appears to be intimate association of their RNA-synthesizing machinery with intracellular membranes. The reasons for the membrane association of viral RNA synthesis are poorly understood. It is generally assumed that the membranes play a structural and/or organizational role in the complex, possibly by offering a suitable microenvironment for viral RNA synthesis and/or facilitating the use of membrane-bound host enzymes. Different virus groups use different intracellular membrane sites or compartments. For example, trans-Golgi membranes and the intermediate compartment are involved in events of flavivirus replication (20), Brome mosaic virus RNA replication takes place in the endoplasmic reticulum (30), alfalfa mosaic virus replicates at the vacuolar membrane (42), the RNA replicases of Semliki Forest and Sindbis viruses (two closely related alphaviruses) are located on the cytoplasmic surfaces of endosomes and lysosomes (10), Flock house virus (FHV) replication complexes are located at outer mitochondrial membranes (23, 24), and rubella virus replication complexes are membrane-bound cytoplasmic vacuoles (21). Previous studies have shown that mitochondria are involved in RNA synthesis by alphanodaviruses, such as FHV (23), Nodamura virus (11), and Boolarra virus (3). The replication of Nodamura virus, a species of the family Nodaviridae, is associated with mitochondria in the infected cells (11). In this study, the subcellular localization of the GGNNV protein A and its colocalization with BrUTP-labeled viral RNA indicated that the site of GGNNV RNA replication might be associated with mitochondria, suggesting that the Nodaviridae family might use the same cellular membrane for RNA replication and indicating a role of mitochondria as supports and/or energy suppliers for viral RNA synthesis and translation.
Viral replicase proteins with integral membrane characteristics have been identified for hepatitis C virus (17, 36), poliovirus (40), equine arteritis virus (44), carnation Italian ringspot virus (33), tobacco etch virus (36), peanut clump virus (8), and mouse hepatitis virus (12). The membrane association of the GGNNV protein A was resistant to extraction buffers that release peripherally associated membrane proteins, indicating that one or more domains of the protein are embedded in the phospholipid bilayer rather than that the protein associates with membranes through electrostatic interactions with membrane proteins or the polar lipid head group. The membrane association of GGNNV protein A is consistent with previous studies showing that positive-strand RNA virus replication is associated with host intracellular membranes (29, 35). Through use of a sequence analysis program, N-terminal amino acids 153 to 173 and 229 to 249 of GGNNV protein A were predicted to be TMD. However, constructs of these two predicted TMD showed no signal in membrane fraction analysis. We then combined both TMD in the construct, and N-terminal amino acids 150 to 250 of protein A showed a strong signal in membrane fraction analysis, indicating that this region is membrane associated. In order to study the two predicted TMD individually, we then choose longer constructs, N-terminal amino acids 145 to 185 and N-terminal amino acids 215 to 255, covering the two predicted TMD, for further analysis, and both contributed to membrane association. We observed that the region from amino acid 215 to 255 of protein A showed stronger signals in the membrane fraction analysis than the region from amino acid 145 to 185. Confocal immunofluorescence studies further confirmed that N-terminal amino acids 215 to 255 of protein A function as a major mitochondrial targeting signal.
At the beginning of our research, experiments had been done with SB cells, but we found that the transfection rate of SB cells is very low, only around 1%. We then searched for another cell line that would be suitable for further studies. Thus, we used Cos-7 cells, which showed a transfection rate of more than 30%. Upon transient expression in transfected Cos-7 cells, the GGNNV protein A localized in the mitochondria and was tightly membrane associated, consistent with the mitochondrial localization in infected SB cells, suggesting that Cos-7 cells are suitable for use as a replacement for SB cells in transfection studies. Transfection of protein A with Cos-7 cells also showed that protein A alone was sufficient for mitochondrial localization in the absence of RNA replication, nonstructural protein B, or capsid proteins. Most mitochondrial proteins are nuclearly encoded and posttranslationally imported into mitochondria and therefore must contain signals for proper localization (16). Most known outer membrane proteins span the membrane either once or several times by use of an α-helical hydrophobic TMD within the molecule (38). There is an internal mitochondrial targeting sequence in carnation Italian ringspot virus 36-kDa protein (33). FHV protein A targets and anchors FHV RNA replication complexes to outer mitochondrial membranes, in part through an N-proximal mitochondrial localization signal and TMD (23, 24). Here, GGNNV protein A was demonstrated to be located to mitochondria in infected or transfected cells, which is consistent with the prediction of the program TargetP version 1.0. Localization of FHV protein A to the mitochondrial membrane requires a TMD with a hydrophobicity of 1.96 and the TMD sequence is immediately followed by a basic amino acid lysine (24). Similarly, Tom20 has a TMD with moderate hydrophobicity in the range of 1.97 to 2.15, followed within the next five amino acids by a net positive charge (18). Sequence analysis of the protein A gene shows two mitochondrial targeting sequences in N-terminal amino acids 1 to 250; however, transfections of Cos-7 cells with deletion fusion protein constructs showed that only N-terminal amino acids 215 to 255 of GGNNV protein A, containing a TMD (N-terminal amino acids 229 to 249), had a function to locate protein A to the mitochondria in transfected cells. This can be explained from the sequence analysis of the construct of N-terminal amino acids 215 to 255 (which contained the TMD from amino acids 229 to 249); this construct contained three amino acids with a net positive charge, while the construct of N-terminal amino acids 148 to 185 (which contained the TMD from amino acids 153 to 173) contained only one amino acid with a net positive charge. This result is consistent with reports by von Heijne (45, 47) and Hartl and Neupert (15) that many mitochondrial proteins contain hydrophobic α-helical TMD that are enriched in positively charged residues, lack negatively charged residues, and serve as sorting signals to direct proper localization within the organelle. N-terminal amino acids 153 to 173 might be sites for posttranslational modifications that increase membrane affinity or interact with membrane proteins. Identifying the mitochondrial localization of GGNNV protein A and characterizing the mitochondrial targeting sequence will be helpful in understanding the mechanism of viral replication and finding potential effective antiviral agents that inhibit fundamental steps in GGNNV replication.
Acknowledgments
This work is supported by Temasek Life Sciences Laboratory.
We gratefully acknowledge Siow Foong Chang from Agri-Food and Veterinary Authority, Singapore, for providing the SB cell line. We thank Volker Wachtler for technical assistance.
REFERENCES
- 1.Ball, L. A. 1994. Nodavirus, p. 919-925. In R. G. Webster and A. Granoff (ed.), Encyclopaedia of Virology. Academic Press Limited, London, United Kingdom.
- 2.Ball, L. A., D. A., Hendry, J. E., Johnson, R. R., Rueckert, and P. D. Scotti. 2000. Family Nodaviridae, p. 747-755. In M. H. V., van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 3.Bashiruddin, J. B., and J. L. Martin. 1987. Proteins induced by Boolarra virus in Drosophila melanogaster line 1 cells. Intervirology 28:122-124. [DOI] [PubMed] [Google Scholar]
- 4.Chong, S. Y., G. H. Ngoh, M. K. Ng, and K. T. Chu. 1987. Growth of lymphocystis virus in a sea bass, Lates calcarifer (Bloch) cell line. Singapore Vet. J. 11:78-85. [Google Scholar]
- 5.Chong, S. Y., G. H. Ngoh, and M. Chew-Lim. 1990. Study of three tissue culture viral isolates from marine food fish. Singapore J. Primary Industries 18:54-57. [Google Scholar]
- 6.Claros, M. G., and P. Vincens. 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241:779-786. [DOI] [PubMed] [Google Scholar]
- 7.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 8.Dunoyer, P., C. Ritzenthaler, O. Hemmer, P. Michler, and C. Fritsch. 2002. Intracellular localization of the peanut clump virus replication complex in tobacco BY-2 protoplasts containing green fluorescent protein-labeled endoplasmic reticulum or Golgi apparatus. J. Virol. 76:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emanuelsson, O., H. Nielsen, S. Brunak, and G. von Heijne. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300:1005-1016. [DOI] [PubMed] [Google Scholar]
- 10.Froshauer, S., J. Kartenbeck, and A. Helenius. 1988. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J. Cell Biol. 107:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garzon, S., H. Strykowski, and G. Charpentier. 1990. Implication of mitochondria in the replication of Nodamura virus in larvae of the Lepidoptera, Galleria mellonella (L.) and in suckling mice. Arch. Virol. 113:165-176. [DOI] [PubMed] [Google Scholar]
- 12.Gosert, R., A. Kanjanahaluethai, D. Egger, K. Bienz, and S. C. Baker. 2002. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 76:3697-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo, Y. X., K. Dallmann, and J. Kwang. 2003. Identification of nucleolus localization signal of betanodavirus GGNNV protein alpha. Virology 306:225-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, Y. X., T. Wei, K. Dallmann, and J. Kwang. 2003. Induction of caspase-dependent apoptosis by betanodaviruses GGNNV and demonstration of protein alpha as an apoptosis inducer. Virology 308:74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartl, F. U., and W. Neupert. 1990. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science 247:930-938. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann, J. M., and W. Neupert. 2000. Protein transport into mitochondria. Curr. Opin. Microbiol. 3:210-214. [DOI] [PubMed] [Google Scholar]
- 17.Hugle, T., F. Fehrmann, E. Bieck, M. Kohara, H. G. Krausslich, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. The hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology 284:70-81. [DOI] [PubMed] [Google Scholar]
- 18.Kanaji, S., J. Iwahashi, Y. Kida, M. Sakaguchi, and K. Mihara. 2000. Characterization of the signal that directs Tom20 to the mitochondrial outer membrane. J. Cell Biol. 151:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie, J. M., M. K. Jones, and E. G. Westaway. 1999. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J. Virol. 73:9555-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magliano, D., J. A. Marshall, D. S. Bowden, N. Vardaxis, J. Meanger, and J. Y. Lee. 1998. Rubella virus replication complexes are virus-modified lysosomes. Virology 240:57-63. [DOI] [PubMed] [Google Scholar]
- 22.Mihara, K. 2000. Targeting and insertion of nuclear-encoded preproteins into the mitochondrial outer membrane. Bioessays 22:364-371. [DOI] [PubMed] [Google Scholar]
- 23.Miller, D. J., M. D. Schwartz, and P. Ahlquist. 2001. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J. Virol. 75:11664-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, D. J., and P. Ahlquist. 2002. Flock house virus RNA polymerase is a transmembrane protein with amino-terminal sequences sufficient for mitochondrial localization and membrane insertion. J. Virol. 76:9856-9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misteli, T., and D. L. Spector. 1997. Applications of the green fluorescent protein in cell biology and biotechnology. Nat. Biotechnol. 15:961-964. [DOI] [PubMed] [Google Scholar]
- 26.Neupert, W. 1997. Protein import into mitochondria. Annu. Rev. Biochem. 66:863-917. [DOI] [PubMed] [Google Scholar]
- 27.Pfanner, N., E. A. Craig, and A. Honlinger. 1997. Mitochondrial preprotein translocase. Annu. Rev. Cell Dev. Biol. 13:25-51. [DOI] [PubMed] [Google Scholar]
- 28.Pombo, A., J. Ferreira, E. Bridge, and M. Carmo-Fonseca. 1994. Adenovirus replication and transcription sites are spatially separated in the nucleus of infected cells. EMBO J. 13:5075-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Restrepo-Hartwig, M. A., and P. Ahlquist. 1996. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J. Virol. 70:8908-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restrepo-Hartwig, M., and P. Ahlquist. 1999. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J. Virol. 73:10303-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roise, D., and G. Schatz. 1988. Mitochondrial presequences. J. Biol. Chem. 263:4509-4511. [PubMed] [Google Scholar]
- 32.Roise, D. 1997. Recognition and binding of mitochondrial presequences during the import of proteins into mitochondria. J. Bioenerg. Biomembr. 29:19-27. [DOI] [PubMed] [Google Scholar]
- 33.Rubino, L., and M. Russo. 1998. Membrane targeting sequences in tombusvirus infections. Virology 252:431-437. [DOI] [PubMed] [Google Scholar]
- 34.Schatz, G., and B. Dobberstein. 1996. Common principles of protein translocation across membranes. Science 271:1519-1526. [DOI] [PubMed] [Google Scholar]
- 35.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt-Mende, J., R. Tehranchi, A. M. Forsblom, B. Joseph, B. Christensson, B. Fadeel, B. Zhivotovsky, and E. Hellstrom-Lindberg. 2001. Granulocyte colony-stimulating factor inhibits Fas-triggered apoptosis in bone marrow cells isolated from patients with refractory anemia with ringed sideroblasts. Leukemia 15:742-751. [DOI] [PubMed] [Google Scholar]
- 37.Shih, K. N., and S. J. Lo. 2001. The HDV large-delta antigen fused with GFP remains functional and provides for studying its dynamic distribution. Virology 285:138-152. [DOI] [PubMed] [Google Scholar]
- 38.Shore, G. C., H. M. McBride, D. G. Millar, N. A. Steenaart, and M. Nguyen. 1995. Import and insertion of proteins into the mitochondrial outer membrane. Eur. J. Biochem. 227:9-18. [DOI] [PubMed] [Google Scholar]
- 39.Tan, C., B. Huang, S. F. Chang, G. H. Ngoh, B. Munday, S. C. Chen, and J. Kwang. 2001. Determination of the complete nucleotide sequences of RNA1 and RNA2 from greasy grouper (Epinephelus tauvina) nervous necrosis virus, Singapore strain. J. Gen. Virol. 82:647-653. [DOI] [PubMed] [Google Scholar]
- 40.Towner, J. S., T. V. Ho, and B. L. Semler. 1996. Determinants of membrane association for poliovirus protein 3AB. J. Biol. Chem. 271:26810-26818. [DOI] [PubMed] [Google Scholar]
- 41.Tusnady, G. E., and I. Simon. 1998. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol. 283:489-506. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Heijden, M. W., J. E. Carette, P. J. Reinhoud, A. Haegi, and J. F. Bol. 2001. Alfalfa mosaic virus replicase proteins P1 and P2 interact and colocalize at the vacuolar membrane. J. Virol. 75:1879-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderlaan. M., and C. B. Thomas. 1985. Characterization of monoclonal antibodies to bromodeoxyuridine. Cytometry 6:501-505. [DOI] [PubMed] [Google Scholar]
- 44.Van Der Meer, Y., H. van Tol, J. K. Locker, and E. J. Snijder. 1998. ORF1a-encoded replicase subunits are involved in the membrane association of the arterivirus replication complex. J. Virol. 72:6689-6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Heijne, G. 1986. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 5:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Heijne, G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 47.von Heijne, G. 1996. Principles of membrane protein assembly and structure. Prog. Biophys. Mol. Biol. 66:113-139. [DOI] [PubMed] [Google Scholar]
- 48.Wansink, D. G., W. Schul, I. van der Kraan, B. van Steensel, R. van Driel, and L. de Jong. 1993. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J. Cell Biol. 122:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White, J., and E. Stelzer. 1999. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 9:61-65. [DOI] [PubMed] [Google Scholar]