Abstract
Background
Most ocular changes in pregnancy are harmless. For example, 14% of pregnant women need a new eyeglass prescription. Some changes, however, are serious, such as retinal effects of hypertension, which can be a sign of pre-eclampsia. Ocular changes may give rise to uncertainty about the administration of ophthalmological drugs or the optimal method of childbirth.
Method
This review is based on pertinent literature retrieved by a selective search in Medline and on guidelines from Germany and abroad. Recommendations about drugs were taken from the Embryotox and Reprotox databases, the German Red List, and the United States Food and Drug Administration (FDA).
Results
40% to 100% of pregnant women with high blood pressure have retinal changes whose severity is correlated with the severity of pre-eclampsia or eclampsia. Diabetic women should undergo ocular examination before and during pregnancy. Pre-existing retinal changes worsen during pregnancy in 55% of cases. Most ocular diseases can be treated with the usual drugs in pregnant women and nursing mothers, although the evidence for drug safety is derived from case series and the treatment is usually provided off label. Ocular conditions that are present before pregnancy are irrelevant to the choice of a method of childbirth.
Conclusion
Pregnant women and nursing mothers can undergo most types of ophthalmological examination and treatment. Recommendations about drug treatment should be checked against current information that can be found on the embryotox.de and reprotox.de websites.
Visual impairment and other ocular changes are rare in pregnancy. They arise in at most 15% of pregnant women and are usually harmless, but are nonetheless a cause of concern among the women who have them and their non-ophthalmologist treating physicians. Women with pre-existing ocular conditions often wonder, even before they become pregnant, how pregnancy might affect their condition and its treatment, and how the treatment of the ocular condition might affect the unborn child.
The few available epidemiologic studies of ocular changes in pregnancy have mainly concerned retinopathies (1) and refractive changes (2). Nearly all pregnant women have reactive changes of the retinal vessels (e1); clearly visible changes arise only in the setting of hypertension, pre-eclampsia, or eclampsia. One in six pregnant women experiences a change in the tear film or eyeglass prescription—the most common reasons for referral for an outpatient ophthalmological examination. Other questions often asked by pregnant women and their physicians concern the diagnostic and therapeutic use of ocular drugs, the significance of pre-existing ocular conditions in pregnancy, and the relevance of such conditions to the choice of childbirth method. We address these issues in the light of the literature and our personal experience.
Frequency.
Nearly all pregnant women have reactive changes of the retinal vessels, but clearly visible changes arise only in the setting of hypertension, pre-eclampsia, or eclampsia.
Methods
We searched the Medline database selectively for pertinent publications up to May 2014 with the keywords “eye,” “uveitis,” “diabetic retinopathy,” and “pregnancy” and supplemented the findings with articles from the reference lists of earlier reviews. We also considered Guideline No. 20 (2011) on diabetic retinopathy of the Association of German Ophthalmologists (Berufsverband der Augenärzte Deutschlands), the American Diabetic Association (ADA) guideline of 2013 (3), and NICE Guideline No. 63 of the UK National Institute for Health (2008); these are the only extant guidelines on the subject. We base our therapeutic recommendations for specific drugs on the current recommendations of Embryotox, Reprotox, the German Red List, and the FDA. In case of discrepancies among these sources, we recommend the drug with the greatest potential to preserve maternal visual acuity while carrying the lowest risk for the unborn child.
Increased skin pigmentation.
90% of pregnant women have increased skin pigmentation (4), and 5% to 70% have chloasma, i.e., reversibly increased skin pigmentation in the face, on the dorsum of the nose, and on the eyelids.
Learning objectives
After reading this article, readers should be able to:
distinguish harmless pregnancy-related changes in the eye from pathological ones,
know that ocular drugs are, in general, compatible with pregnancy and breastfeeding,
be aware of the Internet addresses where specific information can be sought for the proper counseling of pregnant women and nursing mothers,
assess the relevance, if any, of ocular disease to the choice of childbirth method.
Physiological and harmless ocular changes
All structures in and around the eye can undergo change during and after pregnancy. The most important changes are listed in Table 1. The frequency of each type of change is indicated in the table and in the text whenever such information is available.
Table 1. Ocular changes during pregnancy (modified from [36]). Data on the frequency of such changes are given when available.
| Structure | Physiological/innocuous | Pathological |
|---|---|---|
| General | Lowering of intraocular pressure | |
| Lid | Chloasma (5%—703) (4, e2, e3) | |
| Conjunctiva | Hyposphagma (10%) (e4) | Vasospasm in pre-eclampsia |
| Cornea | ||
| Lens | Increased thickness, refractive change (14%) (2) | |
| Retina | ||
| Optic nerve /optic pathway | Enlargement of pituitary gland |
|
| Orbit |
|
90% of pregnant women have increased skin pigmentation (4), and 5% to 70% (4, e2, e3) have chloasma (the “mask of pregnancy”), i.e., reversibly increased skin pigmentation in the face, on the dorsum of the nose, and on the eyelids. The probability of chloasma depends on
sunlight exposure,
genetic predisposition,
and skin type.
Intraocular pressure.
Intraocular pressure decreases mildly during pregnancy, by 2–3 mmHg, under the influence of hormones (mainly progesterone). The mechanism is by way of lower episcleral venous pressure and an ensuing increased efflux of aqueous humor.
A connection to thyroid disease is also suspected.
Similar changes can be seen in women taking oral contraceptive drugs. 3% of pregnant women have reversible pigmentation of the posterior surface of the cornea (Krukenberg's spindle) (5) as a non-pathological finding.
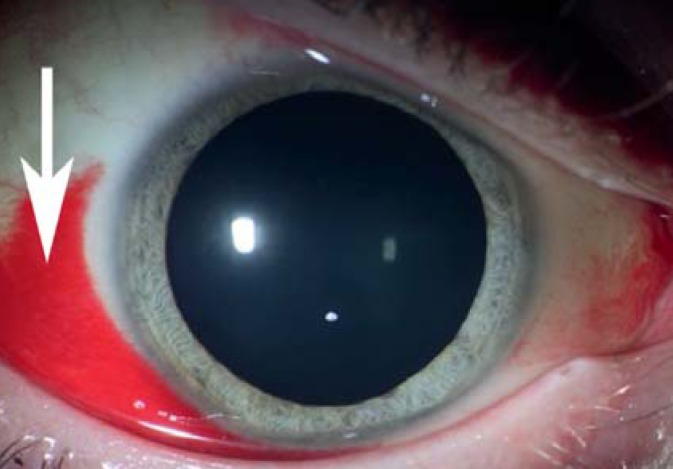
Conjunctival hemorrhage (hyposphagma) is a harmless finding that is seen in about 10% of women during and after delivery (e4); it calls for no measures other than a check of blood pressure (Figure 1). Patients are made more uncomfortable by symptoms resulting from the reduced production of tear fluid. Lessened tear fluid, together with altered corneal curvature, increased corneal thickness, and influx of water into the lens, causes refractive changes toward the myopic range in about 14% of all pregnant women, as well as contact lens intolerance (2). For this reason, refractive surgery should not be performed during pregnancy or in the first year after delivery.
Figure 1.
An innocuous conjunctival hemorrhage (hyposphagma, arrow) that was spontaneously resorbed within 2–3 weeks. Such hemorrhages arise in about 11% of pregnancies, usually after delivery.
Intraocular pressure decreases mildly during pregnancy, by 2–3 mmHg, under the influence of hormones (mainly progesterone). The mechanism is by way of lower episcleral venous pressure and an ensuing increased efflux of aqueous humor (5). No physiological, funduscopically visible changes of the retina arise in the course of a normal pregnancy.
Pathological ocular changes
A few cases of ptosis due to hormonally induced weakening of the attachment of the levator palpebrae have been described in the literature (6). This benign change must be distinguished from an oculomotor nerve palsy (in which ptosis is accompanied by extraocular muscle weakness leading to diplopia, and by enlargement of the pupil), which requires a further neurological work-up. Reversible Horner's syndrome is reported to arise after epidural anesthesia in 0.4% to 2.5% of cases (e5). The probability of development of a transient facial nerve palsy during pregnancy is 38–45 per 100 000 births, or three times higher than at other times (7).
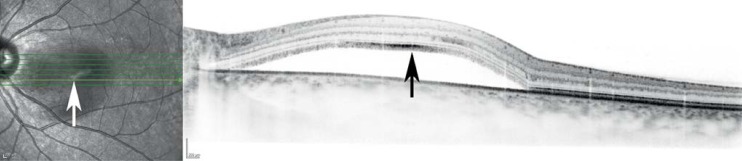
Central serous chorioretinopathy is an idiopathic condition involving central retinal detachment (Figure 2). It arises in 0.008% (4 of 17 000) women in the course of an otherwise unremarkable pregnancy (8). The high cortisol concentration during pregnancy is thought to be the main precipitant of this condition. It usually arises in the third trimester and regresses spontaneously a few weeks to months after delivery (e6, e7).
Figure 2.
A typical image by optical coherence tomography (OCT) of central serous chorioretinitis, showing fluid deposition under the retinal layers (arrow).
Refractive surgery.
Refractive surgery should not be performed during pregnancy or in the first year after delivery.
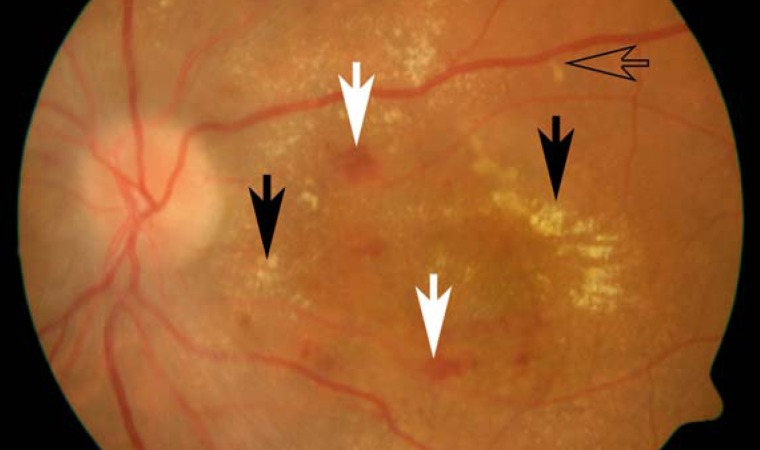
Pregnancy-related hypertension arises in 5% to 11% of pregnant women (9). 40% to 100% of these patients show signs of hypertensive retinopathy (10, e1), ranging from mild focal retinal vascular spasm to cotton-wool spots and hemorrhages to papilledema. These changes, when detected, should prompt further diagnostic and therapeutic steps, even though only 25% to 50% of the affected women are symptomatic (blurred vision, photopsia, visual field defects) (1). The retinal changes reflect the severity of hypertension: the most severe changes are seen in (pre-)eclampsia, ranging to reversible serous retinal detachment (Figure 3) (1 in 18 524 pregnancies) (11, e8). When severe retinovascular changes are seen, delivery is recommended as soon as the pregnancy is sufficiently advanced, in order to achieve the optimal benefit to the mother's vision without endangering the unborn child. More severe vascular changes in eclampsia, leading to anterior ischemic optic neuropathy (AION) or cortical blindness, are rare (summary, Refs. [1, 6, 12]). Fortunately, cortical blindness and other visual changes resulting from eclampsia generally resolve within hours or days, although subjective visual impairment persists in rare cases (13).
Figure 3.
Hypertensive retinopathy with hemorrhages (solid white arrows), cotton-wool spots (open arrow), and exudates (solid black arrows) in eclampsia.
Ophthalmic drugs
Early pregnancy is the sensitive phase in which organs are formed in the developing embryo. Half of all pregnant women take medication in early pregnancy before they know that they are pregnant.
Pregnancy-induced hypertension.
Pregnancy-induced hypertension can cause hypertensive retinopathy.
There is uncertainty about the use of medications during pregnancy and breastfeeding. It is stated in the manufacturer-supplied package inserts of most drugs that the drug is either contraindicated in these situations or should be used only under strictly defined circumstances. The German Red List contains a classification analogous to that of the United States Food and Drug Administration (FDA).
Clinical trials are hardly ever performed on pregnant women, for ethical reasons; thus, information about drug safety during pregnancy and breastfeeding is often derived mainly from animal experimentation, the results of which cannot necessarily be extrapolated to human beings. Often, reliable knowledge about safety is not obtainable until years after a drug has been introduced.
Whenever drug therapy for an ocular condition is begun in a woman of childbearing age, her desires and intentions about future motherhood should be discussed. In some cases, a suitable method of contraception may need to be given simultaneously, or else the ophthalmological treatment may need to be modified to permit pregnancy. The systemic resorption of eye drops can be lessened by occlusion of the lacrimal ducts, or local measures may be used temporarily instead of systemic drug treatment.
Counseling centers for reproductive toxicology and pharmacovigilance provide information about drug safety during pregnancy and breastfeeding. In Germany, the main institutions of this type are Reprotox in Ravensburg (www.reprotox.de) and Embryotox in Berlin (www.embryotox.de), where physicians and patients can obtain, at no cost, advice on these matters that is not derived from the pharmaceutical industry.
Use of medications.
Ophthalmic drugs can be given during pregnancy for diagnostic or therapeutic purposes.
The recommendations on treatment in the remainder of this article are based on those of the Red List and the FDA, on information supplied by the pharmacovigilance centers, and on the pertinent scientific literature. The data in Table 2 represent current knowledge as of the date of writing; they will need ongoing revision as new information about drug safety during pregnancy and breastfeeding comes to light. All of the recommendations are based on grade III or grade IV evidence.
Table 2. Recommendations on the use of ophthalmic drugs during pregnancy (as of December 2013)*.
| a) Local drugs (revised from [18], evidence levels III–IV) | |||
| Safe | Little concern about safety | Use not recommended;considered justified only when there is no alternative | Avoid use |
|
|
|
|
| b) Systemic drugs (revised from [18], evidence levels III–IV) | |||
| Safe | Little concern about safety | Use not recommended;considered justified only when there is no alternative | Avoid use |
|
|
|
|
*These recommendations may change, please check for updates! Suitable places to seek such information are www.embryotox.de and www.reprotox.de. Evidence for safety during pregnancy is based on animal experiments, case series, and databases rather than on clinical trials, and is therefore only of level III to IV.
Most drugs have not been specifically approved for use during pregnancy and breastfeeding, and their use at these times is off label. In consequence, written documentation of informed consent is advisable.
Ophthalmological evaluation
Dilation of the pupils is essential for adequate visualization of the fundi, e.g., if retinal detachment is suspected. The available substances for this purpose (tropicamide and phenylephrine HCL) are, however, contraindicated according to the German Red List, where they appear under Group 5. This designation means that adequate information about the substance is not available to date and thus implies that there is no evidence that these substances are harmful during pregnancy. The pharmacovigilance centers have not documented any higher rate of congenital malformations after the administration of these drugs for local diagnostic purposes, nor does a single administration generate a serum concentration of the drug that would be high enough to affect the fetus. The FDA assigns these two substances to Category C, in which the potential benefit is said to justify use, despite the potential risk. The authors' view is that both substances are safe for diagnostic use in pregnancy. For longer-lasting, therapeutic pupillary dilation, scopolamine and atropine are used locally (14).
Diabetic retinopathy.
The risk that diabetic retinopathy will worsen during pregnancy is a function of the pre-existing retinal findings.
Applanation tonometry with a combination of local anesthetic and fluorescein can be performed in pregnancy without restriction; corneal defect staining with fluorescein is likewise unproblematic (15). Alternative methods of measuring intraocular pressure are also available (e.g., Tonopen, iCare).
The systemic administration of fluorescein for angiography is not associated with fetal damage (16, e9, e10). A clinically relevant concentration of fluorescein can be detected in milk during breastfeeding; thus, breastfeeding should be temporarily interrupted in case the mother has received systemic fluorescein and the child is being treated with phototherapy, because a few cases of fluorescein-associated phototoxicity have been reported (15, 17, e11).
Treatment
The systemic drugs of first choice for bacterial infections of the eye are beta-lactam antibiotics and, in case of intolerance, macrolides. Erythromycin, fluoroquinolones, and aminoglycosides are recommended for local use (18). Conjunctivitis is nearly always of viral origin and does not require antibiotic treatment.
Herpes virus infections can be treated with virostatic agents either locally or systemically in all phases of pregnancy (19).
The treatment of allergic reactions
Allergic conjunctivitis can be treated locally with mast-cell stabilizers, antihistamines, and steroids in all phases of pregnancy and breastfeeding (e12, e13).
The treatment of glaucoma
In women of childbearing age, glaucoma is rare and the question of its appropriate treatment seldom arises. There is no consensus on this matter: the FDA lists only sympathomimetic drugs as Category B agents, while the pharmacovigilance centers recommend beta-blockers and the Red List designates beta-blockers, sympathomimetic drugs, prostaglandin analogues, and carbonic anhydrase inhibitors as Group 5, 6, or 7 agents, meaning that they are contraindicated or should be used only for highly restricted indications. These inconsistent recommendations reflect the inadequate scientific evidence available to date.
The drugs of first choice are beta-blockers, specifically timolol, in view of their longstanding use. If timolol is used locally twice daily, systemic side effects such as fetal bradycardia are unlikely (20, e14).
Intraocular pressure measurement.
Applanation tonometry with a combination of local anesthetic and fluorescein can be performed in pregnancy without restriction; corneal defect staining with fluorescein is likewise unproblematic.
No data are available on the use of sympathomimetic ocular drugs (brimonidine, clonidine, dipivefrin) in pregnancy. Local use in the eye can have systemic side effects (headache, drowsiness, circulatory symptoms); thus, these drugs should be used during pregnancy only if no alternative is available.
The topical use of carbonic anhydrase inhibitors is associated with a very low systemic concentration of the active substance; thus, no negative effects of their use during pregnancy are to be expected, and, indeed, none have been reported to date. Experiments in rodents have shown that the systemic use of acetazolamide during gestation can cause malformations of the extremities, but nothing comparable has been observed in the few documented human pregnancies under such treatment. The use of acetazolamide is considered justified if no alternative is available (21). The drug appears in mother's milk in a low concentration that would not be expected to have any effect on the child. In case of doubt, measurement of the child's serum electrolyte concentrations is recommended.
Prostaglandins should be used with caution in pregnancy, as they increase uterine smooth muscle tone and may impair fetal perfusion. When given locally, their systemic concentration is low and they are rapidly catabolized. There have been no reports of prostaglandin-induced fetal or embryonal toxicity in the few pregnancies observed to date (about 50, according to Embryotox).
Sympathomimetic drugs.
Local use in the eye can have systemic side effects (headache, drowsiness, circulatory symptoms); thus, these drugs should be used during pregnancy only if no alternative is available.
The effect of pilocarpine on human pregnancy has been little studied. Very high doses cause skeletal malformations and stillbirths in rodents. The low medical benefit of pilocarpine does not justify its use in pregnancy.
As an alternative to drug treatment, the intraocular pressure can be temporarily lowered during pregnancy with laser trabeculoplasty. A filtering operation can be performed before a pregnancy is planned.
The treatment of pain
Local anesthetics such as tetracaine eyedrops can be used safely for superficial anesthesia. Paracetamol (acetaminophen) and ibuprofen can be given systemically to treat pain of mild to moderate severity. Ibuprofen and other non-steroidal anti-inflammatory drugs should not be given after the 28th week of gestation, as they can cause premature closure of the ductus arteriosus. Paracetamol (up to 2 g/day) and ibuprofen (up to 1.6 g/day) can be given during breastfeeding.
Prostaglandins.
Prostaglandins should be used with caution in pregnancy, as they increase uterine smooth muscle tone and may impair fetal perfusion. When given locally, their systemic concentration is low and they are rapidly catabolized.
The treatment of inflammatory conditions
If medically necessary, anti-inflammatory treatment for uveitis or status post corneal transplantation can be provided relatively safely during pregnancy and breastfeeding with glucocorticoids, cyclosporine A (22, e15), azathioprine (23, 24, e15), interferons (25– 28, e16), and TNF-alpha antagonists (23, 29, e17). On the other hand, it is recommended that mycophenolate mofetil / mycophenolic acid, methotrexate, leflunomide, and cyclophosphamide should not be given, as these substances have been documented to be embryotoxic and fetotoxic (23, 30, 31, e18).
The treatment of pain.
Local anesthetics such as tetracaine eyedrops can be used safely for superficial anesthesia.
Prednisone and prednisolone cross the placenta only to a small extent (10% to 20%), while dexamethasone does so fully (100%). Thus, prednisone and prednisolone are the drugs of choice for local or systemic treatment of the mother. There have been inconsistent observations regarding an association of orofacial cleft malformations with the use of these drugs in early pregnancy (e19, 32); thus, they should be given in doses less than 15 mg/day, if possible, in the 9th to 12th week of gestation.
The treatment of neovascularization
The injection of VEGF inhibitors into the vitreous humor is now well established as the main mode of treatment of vascular diseases of the retina. Only individual case reports of the provision of such treatment during pregnancy are available; uncomplicated pregnancies have been reported, but miscarriages have been reported as well (e20– e22). The decision whether to treat with VEGF inhibitors should be made individually by a well-informed patient and her physician during a carefully monitored pregnancy, because theoretical considerations of the mechanism of action of these drugs tend to imply that they should not be used. The complete antibody bevacizumab is to be preferred over the Fab fragment ranibizumab for this purpose, as it does not cross the placenta.
Selected ocular diseases
Pregnancy has a substantial effect on inflammatory conditions of the interior of the eye (uveitis) and on the course of diabetic retinopathy. These ocular conditions can arise during childbearing age and therefore merit individual discussion here.
Diabetic retinopathy
Some 2%–5% of pregnant women already had diabetes before conception (NICE guideline). Pregnancy is an independent risk factor for the worsening of diabetic retinopathy, mainly in type 1 diabetics (1). In contrast, purely gestational diabetes does not confer any risk of developing diabetic retinopathy (33).
Further risk factors include
the duration of diabetes,
the need for regular blood-sugar measurement before conception,
high blood pressure,
and the degree of pre-existing retinal changes (this is the most important risk factor).
Uveitis & status post corneal transplantation.
If necessary, in these cases anti-inflammatory treatment can be given with only little concern about safety during pregnancy and breastfeeding with glucocorticoids, cyclosporine A, azathioprine, interferons, and TNF-alpha antagonists.
It was shown in the Diabetes in Early Pregnancy Study, and confirmed in a large-scale review, that pregnant women with diabetes who have little or no proliferative change in the fundi at the onset of pregnancy or before it are unlikely to suffer a worsening of diabetic neuropathy during pregnancy: mild worsening was seen in only 10% to 20% of such patients. Women with moderately severe non-proliferative retinopathy before pregnancy suffered a worsening of retinopathy during pregnancy in 55% of cases, with vascular proliferation in one-third (34, 35).
These facts provide the basis for recommendations concerning ocular examination in pregnancy for diabetic patients. Funduscopy should be performed before pregnancy and once per trimester (NICE guideline 63, BVA guideline No. 20). For high-risk patients with moderate to severe non-proliferative diabetic retinopathy, more frequent follow-up examinations are recommended, in particular in the second and third trimesters (once every four weeks) and one year after delivery (evidence level B, ADA recommendation [3]). Even before the patient becomes pregnant, an interdisciplinary collaboration should be arranged, involving her gynecologist, internist, and ophthalmologist. Decisions about laser treatment or vitrectomy for retinopathic changes should be made by the ophthalmologist soon after diabetic retinopathy is diagnosed on the basis of the ocular changes alone, independently of the pregnancy (1). An exception to this rule is diabetic macular edema arising during pregnancy, which may regress after delivery (36).
Pregnancy is not known to have any negative effect on the long-term course of diabetic retinopathy (37).
Uveitis
The mean age at the time of diagnosis of uveitis is 39 years (e23); thus, some patients with uveitis are women of childbearing age. Although this condition is rare in the general population, with a 12-month prevalence of 115 per 100 000 persons (e24), it is the second most common reason for an ophthalmologist to be asked about potential problems during pregnancy or breastfeeding, in the authors' personal experience. Theoretically, autoimmune conditions would be expected to improve during pregnancy because of the elevated circulating cortisol level and other pregnancy-associated immunosuppressive mechanisms (for a review, see [(34]).
Nonetheless, studies have shown that the rate of new episodes of uveitis is unchanged or elevated in the first trimester; it decreases in the second and third trimesters, then rises again after delivery (38, e25, e26). In principle, uveitis in a woman who desires to become pregnant or is already pregnant can be treated either locally with eyedrops or injections (either parabulbar or intraocular), or else with systemic immunosuppression. The proper choice of drug is of paramount importance.
Diabetic retinopathy.
Funduscopy should be performed before pregnancy and once per trimester. For high-risk patients, more frequent follow-up examinations are recommended, in the second and third trimesters (once every four weeks) and one year after delivery.
Infectious varieties of uveitis, such as chorioretinitis due to toxoplasmosis, which is the most common type of posterior uveitis, accounting for 7% of cases (e23), may be reactivated during pregnancy, but case series have not shown more common reactivation or a more severe course during pregnancy (e27– e29). It follows that there is no particular need for ophthalmologic follow-up examination during pregnancy. In case toxoplasmosis chorioretinitis is reactivated during pregnancy and central vision is threatened, the patient can be treated with spiramycin in the first trimester, and with clindamycin or sulfadiazine and pyrimethamine in combination with oral steroids (0.5 to 1.0 mg/kg BW) from the second trimester onward. Alternatively, clindamycin and steroids can be given by intravitreous injection (e30, e31). Reactivation of toxoplasmosis during pregnancy does not confer any risk of transmission of the disease to the fetus, which is protected by maternal immunity.
Method of delivery
Nowadays nearly one child in three is delivered by caesarean section. In a retrospective analysis of 4895 caesarean sections from 2000 to 2008, 100 (2.04%) were performed because of an ocular condition (39), most commonly myopia (57%) or glaucoma (5%).
Uveitis.
The rate of new episodes of uveitis rises in the first trimester, falls in the second and third trimesters, then rises again after delivery.
Recommendations regarding delivery methods in women with pre-existing ocular disease are not evidence-based (40). The question whether a caesarean section should be performed for ophthalmological reasons is most commonly asked, in the authors' experience, in cases of glaucoma, myopia, peripheral retinal degeneration, and status post retinal detachment. The arguments made for a caesarean section in women with glaucoma involve the elevated intraocular pressure and the potential diminution of ocular perfusion by the Valsalva maneuvers that occur during normal vaginal delivery. Nonetheless, a study of this question showed no reduction of ocular perfusion during delivery (e32), and the rise of intraocular pressure during Valsalva maneuvers was only modest, with a mean of 4 mmHg and a maximum of 12 mmHg (e33). These brief pressure fluctuations would be unproblematic even for already damaged optic nerves. For women with high myopia, retinal degeneration, or status post retinal detachment, studies have revealed no evidence of an association between vaginal delivery and new retinal detachment or retinal degeneration, regardless of the current refractive error or prior vitreoretinal interventions (e34, e35). In summary, it can be stated that the fear of worsening because of vaginal delivery has no known justification for any type of ocular condition. Pre-existing eye disease alone is not an indication for caesarean section.
No indication for caesarean section.
Pre-existing eye disease alone is not an indication for caesarean section.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant's CME certificate.
This CME unit can be accessed until 9 November 2014, and earlier CME units until the dates indicated:
“The Diagnosis and Graded Treatment of Atopic Dermatitis” (Issue 29–30/2014) until 12 October 2014,
“Assessing Preoperative Risk” (issue 25/2014) until 14 September 2014.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following ocular changes during pregnancy should prompt further differential diagnostic evaluation and treatment?
chloasma
reduced corneal sensitivity
hyposphagma
increased thickness of the lens
cotton-wool spots
Question 2
How common is central serous chorioretinopathy during an otherwise uncomplicated pregnancy?
0.0008%
0.008%
0.08%
0.8%
8%
Question 3
Which of the following systemic drugs is currently considered safe for ocular use during pregnancy?
acyclovir
methotrexate
cyclophoshamide
leflunomide
mycophenolate mofetil
Question 4
How often should a woman with type 1 diabetes and severe, nonproliferative diabetic retinopathy undergo ophthalmological examination in the last two trimesters of pregnancy?
daily
once a week
every four weeks
every two months
every three months
Question 5
How many patients have conjunctival hemorrhages during pregnancy and after delivery?
1%
5%
10%
15%
20%
Question 6
What can be done to lower intraocular pressure temporarily during pregnancy without using drugs?
phototherapeutic keratectomy
phakoemulsification
vasoconstrictive therapy with pulsed infrared lasers
ionizing radiotherapy of the eye
laser trabeculoplasty
Question 7
What is the drug of first choice for glaucoma that needs to be treated during pregnancy?
timolol, because of longstanding experience and a favorable risk-benefit profile
brimonidine, because the FDA lists it as a category B drug
prostaglandin analogues, because their systemic absorption is negligible if they are given in eyedrops
pilocarpine, because it is the oldest anti-glaucoma drug still in use
carbonic anhydrase inhibitors, because the blood level of the active substance is so low that no negative effects are to be expected
Question 8
Which of the following ocular changes is an innocuous finding associated with pregnancy?
elevation of the intraocular pressure by ca. 2 mmHg
inflammatory cells in the anterior chamber
cotton-wool spots
diabetic retinopathy
corneal pigmentation (Krukenberg's spindles)
Question 9
Which immunosuppressive drug can be given relatively safely, if indicated, during pregnancy without increasing the risk of congenital malformations?
mycophenolate mofetil
cyclophosphamide
azathioprine
leflunomide
methotrexate
Question 10
Which drug can be given to treat reactivated chorioretinitis due to toxoplasmosis in the first trimester of pregnancy?
methotrexate
pyrimethamine
sulfadiazine
clindamycin
spiramycin
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Mackensen has served as a paid consultant for Merck Serono and has received payment for continuing medical education events from Heidelberg Engineering.
Dr. Paulus has received payment for continuing medical education seminars from Pfizer Pharma GmbH.
PD Dr. Ness has served as a paid consultant for Abbvie. He has received reimbursement of meeting participation fees and travel and accommodation expenses from Bayer Health Care, as well as payment for continuing medical education events form Abbvie and Novartis and for the performance of clinical trials on behalf of Novartis, Santen, Abbvie, Sanofi, Allergan, and pSivida corp.
Dr. Max states that she has no conflict of interest.
References
- 1.Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013;58:127–142. doi: 10.1016/j.survophthal.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S RW, Sharma T, Downey G. Refractive issues in pregnancy. Aust N Z J Obstet Gynaecol. 2006;46:186–188. doi: 10.1111/j.1479-828X.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes A. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37:14–80. [Google Scholar]
- 4.Tunzi M, Gray GR. Common skin conditions during pregnancy. American Family Physician. 2007;75:211–218. [PubMed] [Google Scholar]
- 5.Weinreb RN, Lu A, Key T. Maternal ocular adaptations during pregnancy. Obstetrical and Gynecological Survey. 1987;42:471–483. doi: 10.1097/00006254-198708000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Grant AD, Chung SM. The eye in pregnancy: ophthalmologic and neuro-ophthalmologic changes. Clin Obstet Gynecol. 2013;56:397–412. doi: 10.1097/GRF.0b013e31828f273c. [DOI] [PubMed] [Google Scholar]
- 7.Cohen Y, Lavie O, Granovsky-Grisaru S, Aboulafia Y, Diamant YZ. Bell palsy complicating pregnancy: a review. Obstet Gynecol Surv. 2000;55:184–188. doi: 10.1097/00006254-200003000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Said-Ahmed K, Moustafa G, Fawzy M. Incidence and natural course of symptomatic central serous chorioretinopathy in pregnant women in a maternity hospital in Kuwait. Middle East Afr J Ophthalmol. 2012;19:273–276. doi: 10.4103/0974-9233.97920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang A, Struben H. Präeklampsie-Screening im 1 und 2. Trimenon. Therapeutische Umschau. 2008;65:663–666. doi: 10.1024/0040-5930.65.11.663. [DOI] [PubMed] [Google Scholar]
- 10.Reddy SC NS George SRa, Who TS. Fundus changes in pregnancy induced hypertension. Int J Ophthalmol. 2012;5:694–697. [Google Scholar]
- 11.Bosco JAS. Spontaneous nontraumatic retinal detachment in pregnancy. American Journal of Obstetrics & Gynecology. 1961;82 [Google Scholar]
- 12.Achanna S, Monga D, Sivagnanam Transient blindness in pregnancy induced hypertension. Asia-Oceania Journal of Obstetrics and Gynaecology/AOFOG. 1994;20:49–52. doi: 10.1111/j.1447-0756.1994.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Wiegman MJ, de Groot JC, Jansonius NM, et al. Long-term visual functioning after eclampsia. Obstetrics and Gynocology. 2012;119:959–966. doi: 10.1097/AOG.0b013e31824da5a8. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen O, Slone D, Shapiro S. Birth defects and drugs in pregnancy. Littleton, MA: Publishing Sciences Group. 1977 [Google Scholar]
- 15.American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137–150. [PubMed] [Google Scholar]
- 16.McEnerney JK, Wong WP, Peyman GA. Evaluation of the teratogenicity of fluorescein sodium. Am J Ophthalmol. 1977;84:847–850. doi: 10.1016/0002-9394(77)90508-6. [DOI] [PubMed] [Google Scholar]
- 17.Kearns GL, Williams BJ, Timmons OD. Fluorescein phototoxicity in a premature infant. The Journal of Pediatrics. 1985;107:796–798. doi: 10.1016/s0022-3476(85)80421-2. [DOI] [PubMed] [Google Scholar]
- 18.Ness T, Paulus W. Ophthalmika während Schwangerschaft und Stillzeit. Ophthalmology. 2011;109:221–228. doi: 10.1007/s00347-011-2459-x. [DOI] [PubMed] [Google Scholar]
- 19.Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304:859–866. doi: 10.1001/jama.2010.1206. [DOI] [PubMed] [Google Scholar]
- 20.Wagenvoort AM, van Vugt JM, Sobotka M, van Geijn HP. Topical timolol therapy in pregnancy: is it safe for the fetus? Teratology. 1998;58:258–262. doi: 10.1002/(SICI)1096-9926(199812)58:6<258::AID-TERA7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Lee AG, Pless M, Falardeau J, Capozzoli T, Wall M, Kardon RH. The use of acetazolamide in idiopathic intracranial hypertension during pregnancy. Am J Ophthalmol. 2005;139:855–859. doi: 10.1016/j.ajo.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 22.Haugen G, Fauchald P, Sodal G, Halvorsen S, Oldereid N, Moe N. Pregnancy outcome in renal allograft recipients: influence of ciclosporin A. Eur J Obstet Gynecol Reprod Biol. 1991;39:25–29. doi: 10.1016/0028-2243(91)90137-a. [DOI] [PubMed] [Google Scholar]
- 23.Gisbert JP. Safety of immunomodulators and biologics for the treatment of inflammatory bowel disease during pregnancy and breast-feeding. Inflamm Bowel Dis. 2010;16:881–895. doi: 10.1002/ibd.21154. [DOI] [PubMed] [Google Scholar]
- 24.Chambers C, Koren G, Tutuncu ZN, Johnson D, Jones KL. Are new agents used to treat rheumatoid arthritis safe to take during pregnancy? Organization of Teratology Information Specialists (OTIS) study. Can Fam Physician. 2007;53:409–412. [PMC free article] [PubMed] [Google Scholar]
- 25.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology. 2010;75:1794–1802. doi: 10.1212/WNL.0b013e3181fd62bb. [DOI] [PubMed] [Google Scholar]
- 26.Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G. The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology. 2005;65:807–811. doi: 10.1212/01.wnl.0000180575.77021.c4. [DOI] [PubMed] [Google Scholar]
- 27.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011;17:423–430. doi: 10.1177/1352458510394610. [DOI] [PubMed] [Google Scholar]
- 28.Weber-Schoendorfer C, Schaefer C. Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler. 2009;15:1037–1042. doi: 10.1177/1352458509106543. [DOI] [PubMed] [Google Scholar]
- 29.Verstappen SM, King Y, Watson KD, Symmons DP, Hyrich KL. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823–826. doi: 10.1136/ard.2010.140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisoni CN, D'Cruz DP. The safety of mycophenolate mofetil in pregnancy. Expert Opin Drug Saf. 2008;7:219–222. doi: 10.1517/14740338.7.3.219. [DOI] [PubMed] [Google Scholar]
- 31.Martinez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding) Clin Exp Rheumatol. 2009;27:678–684. [PubMed] [Google Scholar]
- 32.Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796–804. doi: 10.1503/cmaj.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvat M, Maclean H, Goldberg L, Crock GW. Diabetic retinopathy in pregnancy: a 12-year prospective survey. British Journal of Ophthalmology. 1980;64:398–403. doi: 10.1136/bjo.64.6.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunness JS. The pregnant woman's eye. Survey of Ophthalmology. 1988;32:219–238. doi: 10.1016/0039-6257(88)90172-5. [DOI] [PubMed] [Google Scholar]
- 35.Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18:631–637. doi: 10.2337/diacare.18.5.631. [DOI] [PubMed] [Google Scholar]
- 36.Ness T, Paulus W. Auge und Schwangerschaft. Ophthalmologe. 2010;107:863–872. doi: 10.1007/s00347-010-2203-y. quiz 73. [DOI] [PubMed] [Google Scholar]
- 37.Verier-Mine O, Chaturvedi N, Webb D, Fuller JH. Is pregnancy a risk factor for microvascular complications? The EURODIAB Prospective Complications Study. Diabet Med. 2005;22:1503–1509. doi: 10.1111/j.1464-5491.2005.01682.x. [DOI] [PubMed] [Google Scholar]
- 38.Chiam NP, Hall AJ, Stawell RJ, Busija L, Lim LL. The course of uveitis in pregnancy and postpartum. Br J Ophthalmol. 2013;97:1284–1288. doi: 10.1136/bjophthalmol-2013-303358. [DOI] [PubMed] [Google Scholar]
- 39.Socha MW, Piotrowiak I, Jagielska I, et al. Retrospective analysis of ocular disorders and frequency of cesarean sections for ocular indications in 2000–2008-our own experience. Ginekol Pol. 2010;81:188–191. [PubMed] [Google Scholar]
- 40.Jünemann AG, Sterk N, Rejdak R. Einfluss des Geburtsmodus auf vorbestehende Augenerkrankungen. Ophthalmologe. 2012;109:229–234. doi: 10.1007/s00347-011-2460-4. [DOI] [PubMed] [Google Scholar]
- e1.Lupton SJ, Chiu CL, Hodgson LA, et al. Temporal changes in retinal microvascular caliber and blood pressure during pregnancy. Hypertension. 2013;61:880–885. doi: 10.1161/HYPERTENSIONAHA.111.00698. [DOI] [PubMed] [Google Scholar]
- e2.Esteve E, Saudeau L, Pierre F, Barruet K, Vaillant L, Lorette G. [Physiological cutaneous signs in normal pregnancy: a study of 60 pregnant women] Annales de dermatologie et de venereologie. 1994;121:227–231. [PubMed] [Google Scholar]
- e3.Moin A, Jabery Z, Fallah N. Prevalence and awareness of melasma during pregnancy. International Journal of Dermatology. 2006;45:285–288. doi: 10.1111/j.1365-4632.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- e4.Stolp W, Kamin W, Liedtke M, Borgmann H. [Eye diseases and control of labor Studies of changes in the eye in labor exemplified by subconjunctival hemorrhage (hyposphagmas)] Geburtshilfe Frauenheilkd. 1989;49:357–362. doi: 10.1055/s-2008-1026600. [DOI] [PubMed] [Google Scholar]
- e5.Biousse V, Guevara RA, Newman NJ. Transient Horner's syndrome after lumbar epidural anesthesia. Neurology. 1998;51:1473–1475. doi: 10.1212/wnl.51.5.1473. [DOI] [PubMed] [Google Scholar]
- e6.Gass JD. Central serous chorioretinopathy and white subretinal exudation during pregnancy. Arch Ophthalmol. 1991;109:677–681. doi: 10.1001/archopht.1991.01080050091036. [DOI] [PubMed] [Google Scholar]
- e7.Sunness JS, Haller JA, Fine SL. Central serous chorioretinopathy and pregnancy. Arch Ophthalmol. 1993;111:360–364. doi: 10.1001/archopht.1993.01090030078043. [DOI] [PubMed] [Google Scholar]
- e8.Schiffman JS, Scherokman B, Tang RA, Dorotheo EU, Prieto P, Varon J. Evaluation and treatment of papilledema in pregnancy. Compr Ophthalmol Update. 2006;7:187–202. [PubMed] [Google Scholar]
- e9.Burnett CM, Goldenthal EI. The teratogenic potential in rats and rabbits of D & C Yellow No. 8. Food Chem Toxicol. 1986;24:819–823. doi: 10.1016/0278-6915(86)90071-2. [DOI] [PubMed] [Google Scholar]
- e10.Salem H, Loux JJ, Smith S, Nichols CW. Evaluation of the toxicologic and teratogenic potentials of sodium fluorescein in the rat. Toxicology. 1979;12:143–150. doi: 10.1016/0300-483x(79)90040-4. [DOI] [PubMed] [Google Scholar]
- e11.Danis RP, Wolverton S, Steffens T. Phototoxicity from systemic sodium fluorescein. Retina. 2000;20:370–373. doi: 10.1097/00006982-200007000-00008. [DOI] [PubMed] [Google Scholar]
- e12.Osur SL. The management of asthma and rhinitis during pregnancy. J Womens Health (Larchmt) 2005;14:263–276. doi: 10.1089/jwh.2005.14.263. [DOI] [PubMed] [Google Scholar]
- e13.The American College of Obstetricians and Gynecologists (ACOG) and The American College of Allergy, Asthma and Immunology (ACAAI) The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol. 2000;84:475–480. [PubMed] [Google Scholar]
- e14.Madadi P, Koren G, Freeman DJ, Oertel R, Campbell RJ, Trope GE. Timolol concentrations in breast milk of a woman treated for glaucoma: calculation of neonatal exposure. J Glaucoma. 2008;17:329–331. doi: 10.1097/IJG.0b013e31815c3a5b. [DOI] [PubMed] [Google Scholar]
- e15.Sivaraman P. Management of pregnancy in transplant recipients. Transplant Proc. 2004;36:1999–2000. doi: 10.1016/j.transproceed.2004.08.029. [DOI] [PubMed] [Google Scholar]
- e16.Pons JC, Lebon P, Frydman R, Delfraissy JF. Pharmacokinetics of interferon-alpha in pregnant women and fetoplacental passage. Fetal Diagn Ther. 1995;10:7–10. doi: 10.1159/000264183. [DOI] [PubMed] [Google Scholar]
- e17.Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71–76. doi: 10.1159/000262284. [DOI] [PubMed] [Google Scholar]
- e18.Ostensen M. [Antirheumatic therapy and reproduction. The influence on fertility, pregnancy and breast feeding] Z Rheumatol. 2006;65:217–220. doi: 10.1007/s00393-006-0052-5. 22-4. [DOI] [PubMed] [Google Scholar]
- e19.Carmichael SL, Shaw GM. Maternal corticosteroid use and risk of selected congenital anomalies. Am J Med Genet. 1999;86:242–244. doi: 10.1002/(sici)1096-8628(19990917)86:3<242::aid-ajmg9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- e20.Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88 doi: 10.1111/j.1755-3768.2009.01572.x. [DOI] [PubMed] [Google Scholar]
- e21.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30:1405–1411. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- e22.Wu Z, Huang J, Sadda S. Inadvertent use of bevacizumab to treat choroidal neovascularisation during pregnancy: a case report. Ann Acad Med Singapore. 2010;39:143–145. [PubMed] [Google Scholar]
- e23.Jakob E, Reuland MS, Mackensen F, et al. Uveitis subtypes in a German interdisciplinary uveitis center-analysis of 1916 patients. J Rheumatol. 2009;36:127–136. doi: 10.3899/jrheum.080102. [DOI] [PubMed] [Google Scholar]
- e24.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion. [DOI] [PubMed] [Google Scholar]
- e25.Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. American Journal of Ophthalmology. 2003;136:91–98. doi: 10.1016/s0002-9394(03)00110-7. [DOI] [PubMed] [Google Scholar]
- e26.Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS, Christen WG. Patterns of exacerbations of chronic non-infectious uveitis in pregnancy and puerperium. Ocular Immunology and Inflammation. 2006;14:99–104. doi: 10.1080/09273940500557027. [DOI] [PubMed] [Google Scholar]
- e27.Braakenburg AM, Rothova A. Clinical features of ocular toxoplasmosis during pregnancy. Retina. 2009;29:627–630. doi: 10.1097/IAE.0b013e31819a5ff0. [DOI] [PubMed] [Google Scholar]
- e28.Garweg JG, Scherrer J, Wallon M, Kodjikian L, Peyron F. Reactivation of ocular toxoplasmosis during pregnancy. BJOG. 2005;112:241–242. doi: 10.1111/j.1471-0528.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- e29.Reich M, Ruppenstein M, Becker MD, Mackensen F. Risk of Recurrence of Preexisting Ocular Toxoplasmosis during pregnancy. Ocul Immunol Inflamm. 2014:1–6. doi: 10.3109/09273948.2014.916306. [DOI] [PubMed] [Google Scholar]
- e30.Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118:134–141. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- e31.Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. Am J Ophthalmol. 2014;157:762–766. doi: 10.1016/j.ajo.2013.12.022. [DOI] [PubMed] [Google Scholar]
- e32.Lam AK, Lam CH. Effect of breath-holding on pulsatile ocular blood flow measurement in normal subjects. Optom Vis Sci. 2004;81:597–600. doi: 10.1097/01.opx.0000141795.95597.98. [DOI] [PubMed] [Google Scholar]
- e33.Atassi AR. [Intraocular pressure variations during delivery] Geburtshilfe Frauenheilkd. 1972;32:832–834. [PubMed] [Google Scholar]
- e34.Landau D, Seelenfreund MH, Tadmor O, Silverstone BZ, Diamant Y. The effect of normal childbirth on eyes with abnormalities predisposing to rhegmatogenous retinal detachment Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv für klinische und experimentelle. Ophthalmologie. 1995;233:598–600. doi: 10.1007/BF00404712. [DOI] [PubMed] [Google Scholar]
- e35.Neri A, Grausbord R, Kremer I, Ovadia J, Treister G. The management of labor in high myopic patients. Eur J Obstet Gynecol Reprod Biol. 1985;19:277–279. doi: 10.1016/0028-2243(85)90041-3. [DOI] [PubMed] [Google Scholar]