Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax oncoprotein induces growth transformation and is critical for the pathogenesis of the HTLV-1-induced adult T-cell leukemia (ATL). It stimulates the cell cycle and transactivates cellular genes. Here we show that the expression of interleukin-13 (IL-13) is upregulated as a consequence of Tax in HTLV-1-transformed T cells and ATL-derived cultures. IL-13 exerts proliferative and antiapoptotic functions and is linked to leukemogenesis, since it stimulates Hodgkin lymphoma cells by an autocrine mechanism. Overexpression of IL-13 RNA and protein was confirmed in HTLV-1-positive and Tax-transformed cells. Induction of endogenous IL-13 levels in tax-transfected Jurkat cells and in conditional Tax-expressing transformed T lymphocytes suggested that Tax can replace signals required for IL-13 synthesis. For functional analysis, the IL-13 promoter and deletion variants were cloned into luciferase reporter plasmids. Experiments with transfected human T lymphocytes revealed a 16-fold stimulation of the IL-13 promoter by Tax. Experiments with Tax mutants indicated that none of the classical transactivation pathways (SRF, CREB, and NF-κB) is sufficient for the transactivation; at least two different Tax functions are required for full transactivation. The IL-13 promoter is stimulated via two elements; one is a NF-AT binding P element, and the other is a putative AP-1 site. The following observations suggest that IL-13 may stimulate HTLV-1-transformed cells by an autocrine mechanism: (i) the HTLV-1-transformed cells express the IL-13 receptor on their surface, and (ii) STAT6, a downstream effector of IL-13 signaling, is constitutively activated. Thus, in summary, Tax, by transactivating the promoter, induces IL-13 overexpression that possibly leads to an autocrine stimulation of HTLV-1-infected cells.
Human T-cell leukemia virus type 1 (HTLV-1) is the causative agent of a severe and fatal lymphoproliferative disorder of helper T-cell origin, adult T-cell leukemia (ATL), and of the neurodegenerative disease tropical spastic paraparesis/HTLV-1 associated myelopathy (HAM/TSP) (16, 47, 48, 63). These diseases develop after prolonged viral persistence (in the case of ATL, after a minimum of 2 decades). Although HTLV-1 can infect different cell types, such as T cells, macrophages, dendritic cells, or B cells (19), it persists primarily in T cells. Even in nonleukemogenic patients, infected T lymphocytes seem to be stimulated in their growth, since they expand to detectable clones which can persist over many years (12, 14). The capacity of the virus to stimulate permanent T-cell growth in vitro suggests that viral gene functions are involved in this clonal expansion. Such growth-stimulating functions provide a means to replicate the (pro)viral genome without producing virus particles. It is still unclear how HTLV-1 can persist and replicate despite strong virus-specific cytotoxic T-cell and humoral immune responses (3).
As a complex retrovirus, HTLV-1 encodes the regulatory nonstructural proteins Tax and Rex, which are essential for viral replication (19). While Rex acts at a posttranscriptional level (20) to control the expression of the structural proteins, Tax strongly enhances viral gene expression by transactivating the HTLV-1 long terminal repeat promoter. In addition, three accessory proteins, p12, p30, and p13, are expressed; these are important for viral infectivity and replication by influencing cellular signaling and gene expression (4, 9, 10, 29, 33, 46).
Biochemically, Tax can stimulate transcription by binding to the transcriptional activators CREB and SRF and to the coactivators p300/CBP (13, 62). Activation of nuclear factor kappa B (NF-κB) is achieved by binding to and stimulation of IKKγ, a component of the inhibitor of κB kinase (26). Tax also induces activated protein-1 (AP-1), a transcription factor complex composed of members of the Fos/Jun family (2, 25).
Several lines of evidence indicate that Tax also confers the transforming properties of the virus. First, the protein is sufficient for immortalizing T cells (1, 53) and is leukemogenic in transgenic mice (21). Second, Tax interferes with fundamental cellular functions, leading to dysregulated cell cycling (44). In particular, Tax is capable of stimulating the G1 phase by binding to and activating cyclin-dependent kinase holoenzymes (22, 23, 45). Moreover, Tax can stimulate or repress the expression of cellular proteins involved in cell growth and proliferation. Among those are proto-oncogene products (c-Fos and Egr-1), cell cycle and apoptosis regulators (p21WAF1 and Bcl-XL), and cytokines and cytokine receptors (13, 60). The altered gene expression resulting from Tax transmodulation of known and unknown cellular promoters is likely to be a key factor contributing not only to HTLV-1-induced T-cell transformation and leukemogenesis but also to viral spread and immune evasion.
To detect viral strategies related to persistence and pathogenesis by modulating cellular gene expression, we systematically compared the RNA expression patterns of HTLV-1-transformed T lymphocytes with those of their uninfected, nontransformed counterparts. We identified various overexpressed cellular genes that are active in signal transduction (52). Here we show a consistent strong transcriptional induction of the IL-13 gene in HTLV-1-positive T cells, resulting in cytokine overexpression. This upregulation is mediated by Tax transactivation of two different IL-13 promoter elements, a NF-AT binding P element and a putative AP-1 binding site. As a lymphocyte growth factor, IL-13 stimulates Reed-Sternberg cells of Hodgkin lymphoma (57). The observations that HTLV-1-transformed cells express both the cytokine and its receptor and that these cells contain constitutively activated signal transducer of activated T cells 6 (STAT6), a downstream effector of IL-13 signaling, suggest the autocrine stimulation of these cells by IL-13.
MATERIALS AND METHODS
Cell culture.
The HTLV-1-positive T-cell lines C91-PL and MT-2, the CD4+ HTLV-1-negative T-cell line Jurkat, and the HTLV-1-negative, human herpesvirus 8-positive B-cell line Bcbl1 were cultured as previously described (50, 52). The ATL-derived lymphocyte cultures JuanaW, Champ, PaBe, StEd, and ATL-1 and -3 were propagated in 1640 RPMI medium supplemented with 20% fetal calf serum (Invitrogen, Karlsruhe, Germany), 2 mM glutamine, antibiotics, and 20 to 40 U of recombinant IL-2 per ml. The same medium was used for the propagation of Tesi and TAXI-1 cells. These T-lymphocyte lines have been immortalized with a rhadinovirus vector containing an expression cassette for the HTLV-1 Tax protein. In Tesi cells, Tax is under the transcriptional control of the tetracycline operon and can be suppressed by the addition of tetracycline to the medium. Experiments with Tesi cells were carried out after a 10-day cultivation period in the absence and presence of 1 μg of tetracycline per ml.
RNA detection.
Total RNA was prepared from various cell cultures by the acidic guanidinium thiocyante method as described previously (52). Prior to reverse transcriptase PCR (RT-PCR), RNA preparations were treated for 2 h with 40 U of DNase I (Roche, Mannheim, Germany) per ml at 37°C and precipitated with ethanol. RNA (5 μg) was subjected to reverse transcription for 50 min at 42°C by using oligo(dT)20-40 primers and SuperScript II (Life Technologies, Karlsruhe, Germany) according to the instructions of the manufacturer. All PCRs were performed in 50 μl of reaction buffer comprising 100 ng of cDNA, 3 U of Taq polymerase (ABI Perkin-Elmer, Weiterstadt, Germany), 100 nM 5′ and 3′ oligonucleotide primers, 200 nM deoxynucleoside triphosphates (Amersham Pharmacia Biotech, Freiburg, Germany), and 1.5 mM MgCl2. The cycling conditions for the amplifications were 94°C for 2 min followed by 35 to 45 cycles of 94°C for 15 s, 64 to 68°C for 30 s, and 72°C for 30 s. The comparability of cDNA preparations was controlled by detection of β-actin RNA. For positive controls, the cDNAs of IL-4 and IL-13Rα2 were cloned into a standard vector to create pIL-4 and pIL-13Rα2. The following oligonucleotide primers were used for the detection of cDNAs: for β-actin cDNA, β-actin-5′ (5′-GGGAAATCGTGCGTGACAT-3′) and β-actin-3′ (5′-GAACTTTGGGGGATGCTCGC-3′); for IL-13 cDNA, IL-13-5′ (5′-ATTGCTCTCACTTGCCTTGGCG-3′) and IL-13-3′ (5′-CATGCAAGCTGGAAAACTGCCC-3′); for IL-13Rα1 cDNA, IL-13Rα1-5′ (5′-GGAGAATACATCTTGTTTCATGG-3′) and IL-13Rα1-3′ (5′-GCGCTTACCTATACTCATTTCTTGG-3′); for IL-13Rα2 cDNA, IL-13Rα2-5′ (5′-AATGGCTTTCGTTTGCTTGG-3′) and IL-13Rα2-3′ (5′-ACGCAATCCATATCCTGAAC-3′); for IL-4Rα cDNA, IL-4Rα-5′ (5′-GACCTGGAGCAACCCGTATC-3′) and IL-4Rα-3′ (5′-CATAGCACAACAGGCAGACG-3′); and for IL-4 cDNA, IL-4-5′ (5′-GCTTCCCCCTCTGTTCTTCC-3′) and IL-4-3′ (5′-TCTGGTTGGCTTCCTTCACA-3′). The PCR products were analyzed by subjecting aliquots to agarose gel electrophoresis. For the verification and quantitation of IL-13 mRNA expression, Northern blot analyses were performed as described previously (52). Briefly, total RNA was separated (10 μg/lane) on 1% formaldehyde denaturing agarose gels and blotted. For the generation of the probe, cloned cDNA inserts (52) were isolated and radioactively labeled with [α-32P]dATP by the random priming method. The hybridized mRNA was quantified by phosphorimaging.
ELISA.
The IL-13 concentration in cell culture supernatants was determined by antigen capture enzyme-linked immunosorbent assay (ELISA) with monoclonal anti-IL-13 capture and biotinylated anti-IL-13 detection antibodies according to the recommendations of the manufacturer (R&D Systems, Wiesbaden, Germany). Briefly, 96-well microtiter plates were coated overnight with the capture antibodies. The plates were incubated with serially diluted cell culture supernatants and biotinylated anti-IL-13 detection antibody (R&D Systems). A standard curve was obtained by using serially dilutions of recombinant human IL-13 (R&D Systems). To detect the cytokine, streptavidin-conjugated horseradish peroxidase (Zymed, San Francisco, Calif.) and substrate solution (H2O2-tetramethylbenzidine [1:1]; Genzyme Diagnostics, Gaithersburg, Md.) were added. After the reaction was terminated with 1 M H2SO4, the optical density was determined by using a microplate reader set to 450 and 560 nm.
Cloning and mutagenesis of the IL-13 promoter.
The IL-13 promoter was amplified by genomic PCR for subsequent restriction site-directed cloning based on the published IL-13 promoter sequence (accession no. U31120). The forward primer sIL13P5′ (5′-GATCGGTACCACCAAGGTAGTTCCCCGCTCCT-3′), containing a KpnI restriction site (underlined), and the reverse primer sIL13Pr2 (5′-GATCGCTAGCGGGTGGCTTTGTG GCCTTGGCG-3′), containing an NheI restriction site, were designed. By using the proofreading Pwo DNA polymerase (Roche), a PCR fragment of 1,556 bp was generated (reaction were conditions according to the manufacturer's instructions). To obtain the IL-13 promoter luciferase construct pGL3-IL-13P, the PCR product was purified from the agarose gel by use of silica gel particles (Qiagen, Hilden, Germany) and inserted into the pGL3 Basic vector (Promega, Mannheim, Germany) via site-directed ligation. The 5′ deletion variants of the promoter were PCR amplified by using the reverse primer for the IL-13 full-length promoter (sIL13Pr2) in combination with various forward primers. The resulting DNA fragments were cloned into pGL3 Basic in analogy to the full-length promoter to obtain plasmids pGL3-IL-13PDel-118, pGL3-IL-13PDel-468, and pGL3-IL-13PDel-908. For the generation of the promoter fragment containing nucleotides (nt) −118 to +9 (pGL3-IL-13PDel-118), the primer sIL13PDel1 (5′-GATCGGTACCAGTAAA ATCAAGATGAGTAA-3′) was used; for the −468 to +9 fragment (pGL3-IL-13PDel-468), the primer sIL13PDel2 (5′-GATCGGTACCATTTAAGAGACTGGTTCATC-3′) was used; and for the −908 to +9 fragment (pGL3-IL-13PDel-908), the primer sIL13PDel3 (5′-GATCGGTACCAAGCCCCAGTGGCACTAGGA-3′) was used. The IL-13 P-element deletion mutant (pGL3-IL-13PD-134/121) was generated via PCR with chimeric oligonucleotides which carry the 5′ and 3′ sequences flanking the deleted region (sCPIL-13P [5′-AACTCTTTCCTTTATGCGAGTAAAATCAAGATGAGT-3′] and asCPIL-13P [5′-ACTCATCTTGATTTTACTCGCATAAAGGAAAGAGTT-3′]). The 5′ flanking region of the deletion was amplified with the asCPIL-13P and sIL13P5′ primers, while the 3′ flanking region was amplified with the sCPIL-13P and sIL13Pr2 primers. The two PCR products were gel purified, and 100 ng of each was subjected to full-length PCR with the outside primers (sIL13P5′ and sIL13Pr2). The purified product was ligated into the pGL3 Basic vector. The IL-13 AP-1 deletion mutant (pGL3-IL-13PD-106/98) and the IL-13P P-element/AP-1 deletion mutant (pGL3-IL-13PDel-134/121/D-106/98) were generated via PCR with chimeric primers (sCP IL-13PDel AP1 [5′-CCACAAAGTAAAATCAAGGATGTGGTTTCTAGATAG-3′] and asCP IL-13PDel AP1 [5′-CTATCTAGAAACCACATCCTTGATTTTACTTTGTGG-3′]) with the templates pGL3-IL-13P and pGL3-IL-13PD-134/121. The IL-13P −126/123 (pGL3-IL-13PD −126/123) and IL-13P −132/129 (pGL3-IL-13PD −132/129) deletion mutants were generated by using the Quick Change site-directed mutagenesis kit from Stratagene (Amsterdam, The Netherlands) with high-pressure liquid chromatography-purified primers (sIL-13PDel −132/129 [5′-CTTTCCTTTATGCGACACTTTTCCACAAAGTAAAAT-3′], asIL-13PDel −132/129 [5′-ATTTTACTTTGTGGAAAAGTGTCGCATAAAGGAA AG-3′], sIL-13PDel −126/123 [5′-TTATGCGACACTGGATTTCAAAGTAAAATCAAGATG-3′], and asIL-13PDel −126/123 [5′-CATCTTGATTTTACTTTGAAATCCAGTGTCGCATAA-3′]). The cycling conditions for all amplifications were 94°C 2 min, followed by 35 to 45 cycles of 94°C for 15 s, 64 to 68°C for 30 s, and 72°C for 30 s. All mutants were sequenced multiply to validate their identity.
Transfection and luciferase assays.
To test the induction of the IL-13 promoter by Tax, luciferase assays were performed. Transient transfections were carried out in 107 Jurkat cells with 20 μg of the luciferase reporter plasmid (pGL3-IL-13P or one of the IL-13 promoter mutants) together with 20 μg of the effector plasmid (Tax wild-type or Tax mutant expression vectors) by electroporation (290 V, 1,500 μF). For Tax expression, the plasmid vector pcTax (for HTLV-1 TaxWT) and the Tax mutants M7, M22, and M47 were used (55). After 48 h, transfected cells were collected by centrifugation, washed with phosphate-buffered saline (PBS) (Roche), and lysed. All lysates were cleared by centrifugation, the luciferase substrate (Roche) was added, and the luminescence was measured. The luciferase activity was normalized to the protein concentration, and the luciferase activity of the IL-13 promoter together with the pcDNA3.1 vector as a control was set at 1.
Immunoblots and immunoprecipitation.
Cells were collected by centrifugation, washed with Tris-buffered saline (TBS), and lysed in Western blot lysis buffer (150 mM NaCl, 10 mM Tris [pH 7], 10 mM EDTA, 1% Triton, 2 mM dithiothreitol) containing protease inhibitors (20 μg of leupeptin per ml and 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (100 mM sodium fluoride and 2 mM sodium vanadate). The protein concentration was determined by the Bradford assay (Bio-Rad, Munich, Germany). For Western blotting, 40 μg of total protein was loaded per lane. For the immunoprecipitation of STAT6, cell lysate containing 1 mg of protein was incubated with 4 μg of anti-STAT6 antibodies (Upstate Biotechnologie, Hamburg, Germany) and 25 μl of pansorbin cells (Calbiochem). The precipitate was washed six times, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto a nylon membrane (Millipore, Eschborn, Germany) overnight. The membrane was blocked with 5% bovine serum albumin in TBS for 1 h, and the primary antibody (2 μg of STAT6 antibody per ml or 0.34 μg of phosphotyrosine antibody [clone 4G10] per ml [Upstate Biotechnologie]) was added and left for 1 h. The membrane was washed for 30 min with 0.2% Tween in TBS, the secondary horseradish peroxidase-conjugated antibody (Amersham Pharmacia) was added for 30 min, and subsequently the membrane was washed again for 30 min. The immunoblots were then developed by using the ECL system (Amersham Pharmacia Biotechnologies) and with a charge-coupled device camera.
Flow cytometry.
Expression of IL-13, IL-13Rα1, and IL-4Rα was analyzed by flow cytometry with monoclonal anti-IL-13 (clone JES10-5A2; Becton Dickinson), anti-IL-13Rα1 (clone B-K19; Notabene), and anti-IL-4Rα (clone X2/45-12; Bioscience) antibodies. All analyses were carried out with isotype matched control antibodies (Becton Dickinson). Cells were fixed with 2% formaldehyde solution in PBS, and for the IL-13 staining, the cells were permeabilized with 0.2% saponin in PBS containing 5% fetal calf serum and 0.01% sodium azide (fluorescence-activated cell sorting [FACS] buffer). The cells were then incubated with 0.5 to 1 μg of the antibody for 1 h and washed with FACS buffer. In case of IL-13Rα1 and IL-4Rα antibodies, which are not directly labeled, cells were incubated with 50 ng of phycoerythrin-labeled secondary antibody (Dako A/S) per μl, washed again, and then measured in the cytometer (FACSCalibur; Becton Dickinson).
RESULTS
Stimulation of IL-13 expression in HTLV-infected, Tax-expressing human lymphocytes.
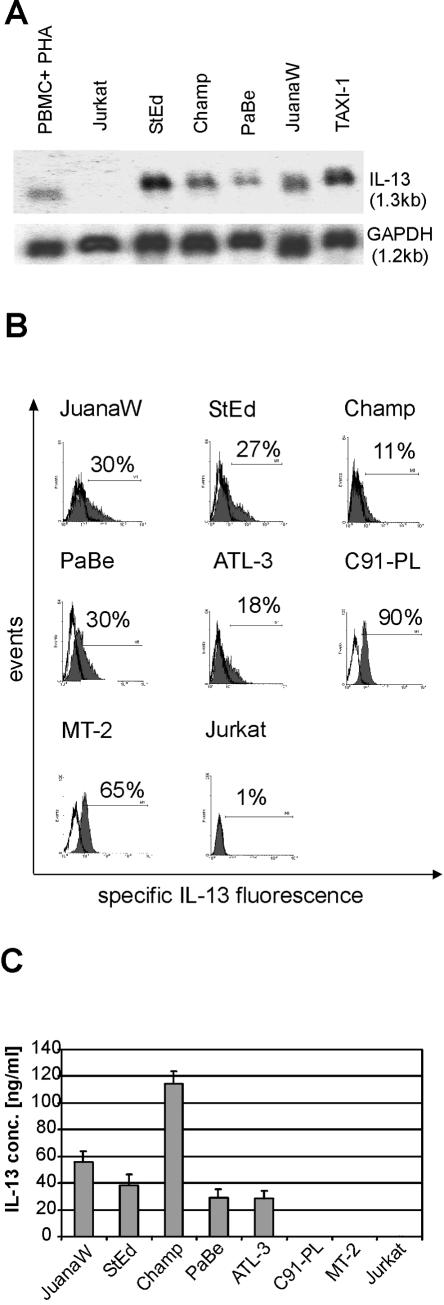
To identify cellular genes associated with HTLV-1-transformation, a systematic comparison of RNA expression patterns of infected and uninfected nontransformed lymphocytes had been performed with an RT-PCR-based assay (52). This had resulted in the cloning of several cDNAs derived from potentially overexpressed genes, including an IL-13 cDNA fragment. This cytokine acts as a growth factor for lymphoid cells, including Reed-Sternberg cells of Hodgkin lymphoma, and exerts important immunomodulatory and anti-inflammatory functions, and thus it could be relevant for HTLV-1 pathogenesis. We therefore investigated whether the IL-13 mRNA upregulation is a general feature of HTLV-1-infected cells. RNAs from ATL-derived cells (StEd, Champ, PaBe, and JuanaW) and cells transformed by the rhadinoviral transduction of the tax gene (TAXI-1) were isolated and analyzed in Northern blots (Fig. 1A). These blots revealed large amounts of the 1.3-kb IL-13 mRNA in all HTLV-1- and Tax-positive cells. The transcript has been shown to encode the functional cytokine (41). Six independent experiments revealed a 5- to 17-fold upregulation of the IL-13 gene expression in all four ATL-derived cell cultures (StEd, Champ, PaBe, and JuanaW) and in vitro Tax-transformed (TAXI-1) cells compared to phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells. In contrast, Jurkat cells did not contain detectable amounts of IL-13 transcripts.
FIG. 1.
Overexpression of IL-13 in HTLV-1-infected human T cells. IL-13 expression in HTLV-1-positive, ATL-derived cultures (JuanaW, StEd, Champ, PaBe, and ATL-3), in HTLV-1-transformedT-cell lines (C91-PL and MT-2), and in uninfected controls (Jurkat cells and PHA-treated peripheral blood mononuclear cells [PBMC+PHA]) was analyzed. (A) Total RNA was extracted and subjected to Northern blot analyses. Equal loading was controlled by reprobing the blot with a probe detecting RNA of glyceraldehyde phosphate dehydrogenase (GAPDH) (B) Cells were permeabilized by addition of 0.2% saponin, and intracellular IL-13 was stained with specific antibodies (grey curves) and analyzed by flow cytometry. The white curves represent staining with an unspecific isotype-matched control antibody. (C) Tissue culture supernatants of the cultures were subjected to ELISA for detection of secreted IL-13. The results represent the means from three experiments. Error bars show standard deviations.
To analyze whether the increase of mRNA synthesis results in elevated protein expression, the HTLV-1-infected cells were permeabilized and the intracellular contents of IL-13 were detected by direct immunofluorescent staining and flow cytometry (Fig. 1B). The experiments revealed large amounts of protein in the ATL-derived cultures and even larger amounts within the in vitro-HTLV-1-transformed cells (MT-2 and C91-PL). To demonstrate secretion of the expressed cytokine, the supernatant of the cultured cells was analyzed by ELISA (Fig. 1C). High concentrations of the IL-13 protein could be found in the ATL-derived cultures. The moderate relative differences between intracellular and secreted IL-13 in some of the cell lines is most probably due to different proliferation rates. At the end of the 48-h incubation period, faster-proliferating cultures (e.g., Champ) contained up to twice the number of secreting cells as did slower-replicating cultures (ATL-3 and PaBe). Despite fast replication, the cell lines MT-2 and C91-PL contained only low concentrations of IL-13 (close to the detection limit) in the supernatant. These cell lines have acquired numerous changes due to long-term cultivation; among them is the loss of IL-2 dependence for proliferation. The requirement for IL-2 is a consistent feature of all freshly HTLV-1-immortalized human T cells. The lack of increased IL-13 protein concentrations in the supernatants of C91-PL and MT-2 cells may thus indicate a secretory defect of IL-13 in long-term-cultured HTLV-1-transformed cells. Taken together, these experiments indicate that the upregulation of IL-13 synthesis is a consistent feature of HTLV-1- and Tax-expressing human T lymphocytes.
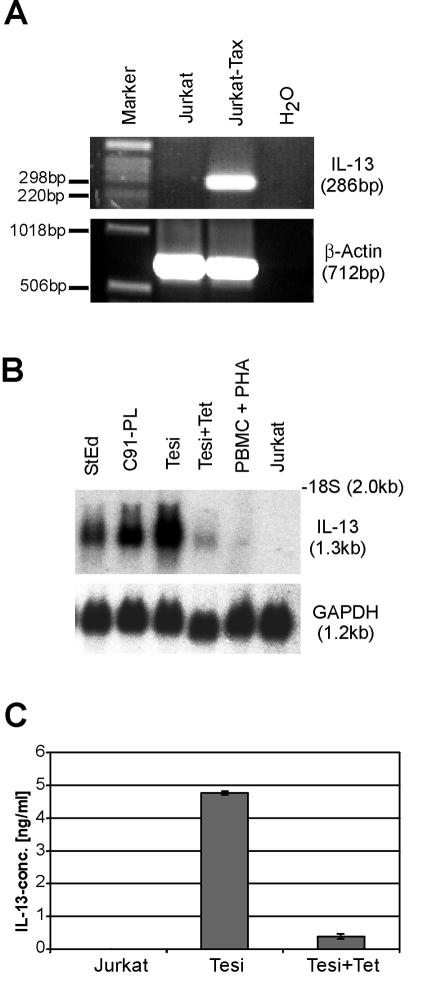
To determine whether the increased IL-13 expression in Tax-positive cells is caused by the viral transcriptional transactivator, Jurkat T cells were transfected with a Tax expression plasmid, and RNA was analyzed by RT-PCR (Fig. 2A). In these cells Tax reproducibly resulted in the synthesis of significant amounts of IL-13 RNA, whereas in untransfected Jurkat cells no IL-13 transcripts were detectable (Fig. 2A). The impact of Tax on IL-13 transcription could also be documented in conditionally Tax-expressing Tesi T cells (Fig. 2B). This cell line was obtained by infecting human cord blood lymphocytes with a rhadinovirus vector expressing HTLV-1 Tax under the control of the tetracycline repressor system (53). To investigate a possible influence of Tax on IL-13 gene expression, Tax synthesis was shut off by culturing the Tesi cells in the presence of tetracycline (Tesi+Tet cells). As opposed to the case for normal Tesi cells, hardly any IL-13 transcripts and protein could be detected in Tesi+Tet cells (Fig. 2B and C). In contrast, for untreated Tesi cells, IL-13 secretion and a similarly increased IL-13 mRNA level as in HTLV-1-infected control cells (StEd and C91-PL) were seen. Thus, the increased IL-13 levels consistently correlate with the expression of the viral transactivator Tax. This result provides a primary indication for a Tax-mediated transactivation of the IL-13 gene.
FIG. 2.
Correlation of IL-13 upregulation with HTLV-1 Tax synthesis. The impact of Tax on endogenous IL-13 expression in Jurkat T cells and in Tax-immortalized primary human T cells (Tesi) was investigated. (A) Jurkat cells were transfected with a Tax expression plasmid; RNA was reverse transcribed and analyzed by PCR. As an internal standard, β-actin PCRs were performed with the same cDNA. (B) Human T cells transformed with a repressible Tax gene (Tesi) were kept under conditions of induced (Tesi) and repressed (Tesi+Tet) Tax expression. Total RNA was analyzed by Northern blotting with an IL-13-specific probe. C91-PL and StEd, HTLV-1-infected lymphocytes; PBMC + PHA and Jurkat, uninfected T lymphocytes. (C) Tissue culture supernatants of Jurkat, Tesi, and Tesi+Tet cells were subjected to IL-13 ELISA. The results represent the means from three experiments. Error bars show standard deviations.
Tax-mediated stimulation of the IL-13 promoter depends on NF-AT and AP-1 binding sequences.
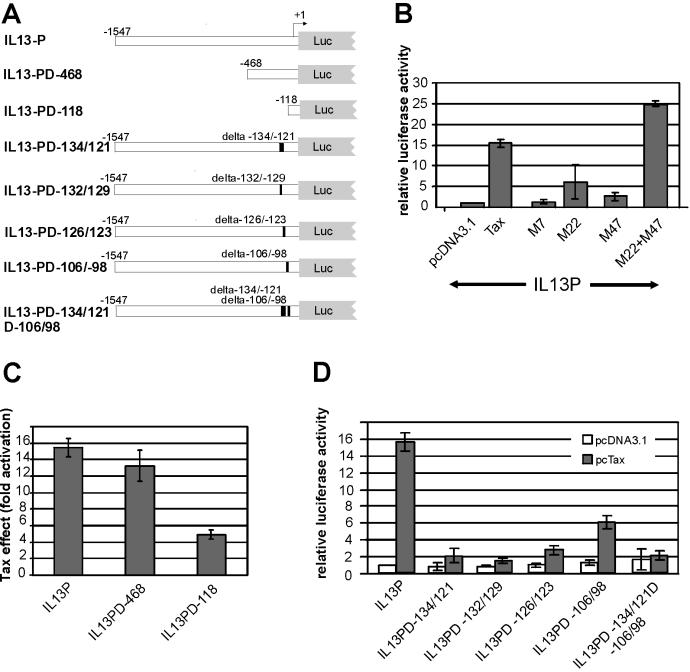
To test whether Tax stimulates the IL-13 promoter, corresponding sequences (−1547 to +9) were PCR amplified from genomic DNA and cloned into a luciferase reporter plasmid (Fig. 3A). Luciferase activity determined from transfected Jurkat T lymphocytes revealed a 16-fold stimulation in the presence of Tax (Fig. 3B). To narrow down signaling pathways relevant to transactivation, Tax mutants M7, M22, and M47 (59) were cotransfected along with the IL-13 promoter construct and luciferase activities were determined. As shown in Fig. 3B, M7, which is inactive in transactivation, had no effect on the IL-13 promoter. A slight to moderate (sixfold) increase of activity was observed with mutant M22. This mutant activates the CREB transcriptional pathway (59) but is impaired in the stimulation of NF-κB transcription factors due to a defect in associating with IKKγ (26). Similarly, Tax mutant M47 was found to slightly increase the promoter activity (two to threefold). This mutant can activate NF-κB but is impaired in stimulating the CREB and SRF pathways due to its inability to interact with the p300- and CBP-associated factor (24, 27). The mutation in M22 completely abolishes the transactivation via AP-1, and the mutation in M47 reduces it to twofold (25). The capacity to stimulate SRF is retained in both mutants, however, at a moderately reduced level. The consensus binding site of SRF, the CArG box [CC(A/T)6GG] is not present in the IL-13 promoter. The combination of both mutants, M22 and M47, resulted in a huge transactivation of the promoter, which even extended the activity of TaxWT (Fig. 3B). These results clearly indicate that at least two Tax functions cooperate to stimulate the IL-13 promoter.
FIG. 3.
Transactivation of the IL-13 promoter by the HTLV-1 Tax protein. The IL-13 promoter was inserted into a luciferase indicator construct (pGL3 Basic). (A) Overview of the IL-13 promoter deletion variants used. The deleted sequences are indicated. (B) Tax transactivation of the IL-13 promoter by at least two separate pathways. Jurkat T lymphocytes were transfected with the IL-13 promoter (IL13P) in the presence of an empty expression vector (pcDNA3.1), TaxWT, a single Tax mutant (M7, M22, or M47), or a combination of two mutants (M22+M47). Luciferase activity was determined and normalized to the negative control (pcDNA3.1). Neither M22 nor M47 alone was able to fully transactivate the promoter, but the combination of both was able to do so. The bars represent the means from eight independent experiments and the standard deviations. (C) Stimulation of promoter variants with 5′ deletions by Tax. The fold stimulation of luciferase activity in the presence of Tax is indicated. The bars represent the means and standard deviations from four independent experiments. (D) Requirement of two promoter regions for Tax stimulation. The indicated internal promoter deletion variants were cotransfected with pcTax or an empty vector (pcDNA3.1); the resulting luciferase activity was normalized to IL13P. Deletion of nt −134 to −121 resulted in a severe reduction of transactivation, deletions within nt −106 to −98 resulted in a moderate reduction, and the variant with a double deletion was not inducible by Tax. The bars represent the means and standard deviations from six independent experiments.
To understand the mechanism of transactivation, IL-13 promoter mutants were generated. First, promoter variants with 5′ deletions (Fig. 3A) which retain the sequences from the transcriptional start site to nt −468 (IL-13-PD-468) or to nt −118 (IL-13-PD-118) were prepared. Transfection of these mutants in the presence and absence of Tax and subsequent luciferase assays revealed that IL-13-PD-468 is stimulated by Tax to almost the same extent as the full-length promoter, whereas the activity of IL-13-PD-118 is only moderately stimulated (Fig. 3C). This indicated the presence of relevant promoter elements within the segment from nt −118 to −468 as well as in the retained segment from nt −118 to −1. The sequences from nt −1547 to −468 are not important for the transactivation of this promoter by Tax.
To identify more precisely sequences involved in Tax transactivation, promoter variants with internal deletions were prepared. The 14-nt deletion in IL-13-PD-134/121 removes the P element of the IL-13 promoter (11), which has been described to be important for its stimulation by T-cell activation signals. The element contains a binding site for NF-AT transcription factors. The −134/121 deletion did not affect the basal promoter activity but greatly affected its stimulation by Tax (Fig. 3D). Two smaller deletions (IL13PD-132/129 and IL-13-PD-126/123), which affect only parts of the P element, also were able to abolish most of the promoter's capacity to be transactivated. These results clearly indicated that the P element is critical for the transactivation of the IL-13 gene by Tax. As a second region, sequences between nt 106 and 98 were deleted (Fig. 3A). These contain an AP-1 site, which might be responsible for the residual Tax activation of the −118 promoter mutant. As shown in Fig. 3D, the deletion of these nucleotides halved the amount of Tax stimulation of the IL-13 promoter. In addition, a double mutant with a deleted P element and AP-1 site (IL13PD-134/121D106/98) was tested for transactivation (Fig. 3A and D). This construct was not stimulated, thus indicating that both promoter elements account for the Tax transactivation of the IL-13 promoter. In summary, these results indicate that the P element and the AP-1 sequence cooperate to allow full Tax transactivation of the IL-13 promoter, in which the P element accounts for the majority of the Tax effect.
Expression of IL-13 receptors on HTLV-1-immortalized cells.
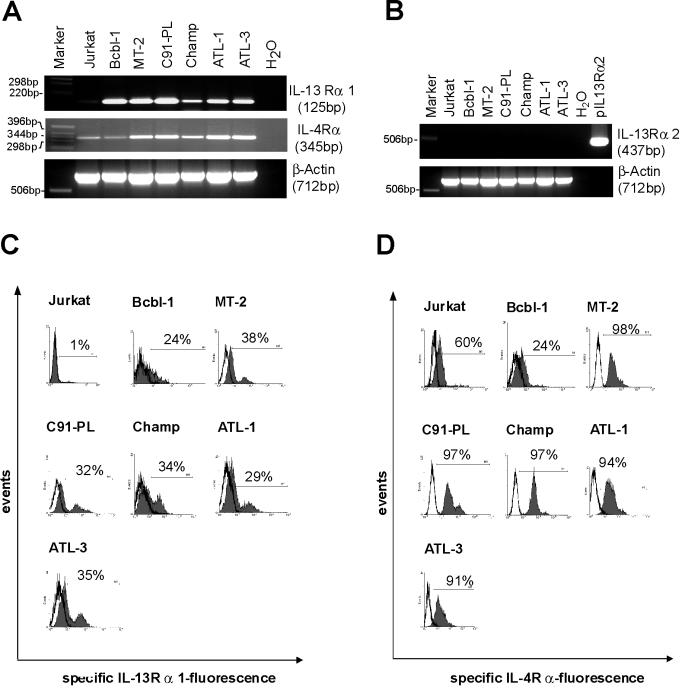
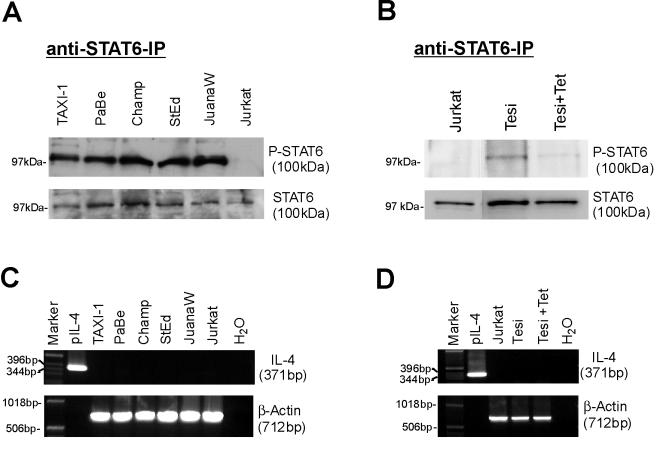
Primary human T cells can respond functionally to IL-13 (28) and by phosphorylating STAT6 (8, 31). In addition, it has been shown that upon activation, human T cells downregulate IL-13Rα1 expression (15), presumably with an attendant loss of IL-13 responsiveness. Functional IL-13 receptors consist of the IL-13Rα1 chain and the IL-4Rα chain. Another IL-13 binding protein, the IL-13Rα2 chain, acts as a decoy receptor (38). To determine whether the HTLV-1-infected cells could in principle respond to IL-13, the expression of IL-13 receptor RNA was investigated by RT-PCR in a series of HTLV-1-positive cells (Fig. 4A and B). These cells contained both IL-4Rα and IL-13Rα1 transcripts but no IL-13Rα2 RNA. Subsequent FACS analysis demonstrated high expression of the IL-4Rα chain and moderate to high expression of the IL-13Rα1 chain on the surfaces of HTLV-1-infected cells (Fig. 4C and D). The heterogeneous distribution of the receptors on the HTLV-1-positive cells may indicate different states of activation. Taken together, these results strongly suggest the presence of functional IL-13 receptors on HTLV-1-infected cells. To determine whether the receptor could be active in transducing signals, the activation state of STAT6 was analyzed. STAT6 becomes phosphorylated on tyrosine residues as a consequence of ligand binding. The STAT6 protein was immunoprecipitated and reacted with phosphotyrosine-specific antibodies in immunoblots. These revealed that in contrast to the HTLV-1-negative Jurkat cells, in the HTLV-1-positive cells, STAT6 is highly tyrosine phosphorylated (Fig. 5A). All cells, however, contain approximately the same amounts of the STAT6 protein. This became clear from probing aliquots of the cell lysates with STAT6 antibodies. To get an impression of whether the Tax expression could induce autocrine IL-13 signaling, the STAT6 phosphorylation of Tesi cells (53) was investigated in the absence and presence of Tax. The results shown in Fig. 5B demonstrate a correlation of Tax and tyrosine phosphorylation of STAT6; in Tax- and IL-13-positive Tesi cells, STAT6 was phosphorylated, while in the Tax-negative Tesi+Tet cells, STAT6 phosphorylation was almost completely absent. To exclude other mechanisms of STAT6 activation, we checked our cultures for IL-4 expression. The binding of this cytokine to the cognate receptors is the other important inducing mechanism of STAT6 phosphorylation. However, RT-PCR analyses could exclude the expression of IL-4 RNA (Fig. 5C and D). In summary, these data suggest that in HTLV-1-transformed cells the Tax-mediated IL-13 overexpression results in autocrine IL-13 receptor stimulation.
FIG. 4.
Expression of both chains of a functional IL-13 receptor on HTLV-1-infected T cells. The functional IL-13 receptor consists of IL-13Rα1 and IL-4Rα. Its expression in ATL-derived cultures (Champ, ATL-1, and ATL-3), in in vitro-HTLV-1-transformed T-cell lines (C91-PL and MT-2), and in Jurkat T cells was analyzed. Bcbl1, a B-cell line, served as positive control for IL-13Rα1 expression. (A) RNA from HTLV-1-infected IL-13 producing T cells was analyzed by RT-PCR with specific primers for IL-13Rα1 and IL-4Rα. IL-4Rα was expected to be expressed on all T cells and B cells. (B) Absence of the decoy receptor IL-13Rα2. RT-PCR of the same cDNAs as in panel A is shown; pIL-13Rα2 is a positive control containing cloned receptor cDNA. The first nine lanes of the β-actin gel are identical to those in panel A. (C) Surface expression of IL-13Rα1. The cells indicated were stained with specific antibodies and analyzed by flow cytometry. (D) Surface expression of IL-4Rα. The cells indicated were stained with specific antibodies and analyzed by flow cytometry.
FIG. 5.
Activation of STAT6 in HTLV-1-infected and Tax-transformed lymphocytes. The cells were lysed, and STAT6 protein was immunoprecipitated. (A) Tyrosine phosphorylation of STAT6 in HTLV-1-infected T cells. To detect the activated STAT6, which is phosphorylated on tyrosine residues, a Western blot was probed with a phosphotyrosine-specific antibody. To control for equal amounts of STAT6 protein, the cell lysates were probed with a STAT6-specific antibody. (B) Correlation of Tax expression and STAT6 phosphorylation. Tesi cells were kept under conditions of induced (Tesi) and repressed (Tesi+Tet) Tax expression and were analyzed for STAT6 tyrosine phosphorylation as described above. (C and D) Absence of IL-4 production in HTLV-1-infected T cells, Tesi cells, and Tesi+Tet cells. To exclude the possibility that STAT6 was activated by IL-4, the cells were checked for IL-4 expression by RT-PCR.
DISCUSSION
Here we show that the expression of IL-13, a cytokine with immune regulatory and antiapoptotic function, is upregulated in HTLV-1-infected cells due to stimulation of the cognate promoter by the viral transactivator Tax. This upregulation may result in autocrine stimulation of the infected cells, since these cells express both chains of the IL-13 receptor and contain activated STAT6 protein, a hallmark of IL-13 signaling.
The increase of protein and RNA synthesis in the presence of Tax correlated well with the upregulation of the IL-13 promoter, thus indicating that Tax acts by stimulating the initiation of mRNA synthesis. The IL-13 promoter is not active in unstimulated T cells but can be activated upon T-cell stimulation (11, 41). Even in unstimulated Jurkat T cells, Tax strongly increased promoter activity, suggesting that it can replace signals generated from activated T cells. None of the classical Tax pathways is fully sufficient for the stimulation of the IL-13 promoter: the consensus binding site of SRF (13), the CArG box [CC(A/T)6GG], is not present in the IL-13 promoter, and neither the CREB pathway-deficient mutant M47 nor the NF-κB activation-deficient mutant M22 was able to fully activate the promoter. However, M47 and M22 together resulted in full activation. Thus, the signaling pathways that stimulate the IL-13 promoter rely on more than one Tax function. Most probably Tax acts indirectly by stimulating necessary cellular factors. The inability of both the M47 and the M22 mutants to stimulate the promoter resembles their effect on a CD28 response element (CD28RE), which is well activated by Tax and can bind NF-AT transcription factors (17). Interestingly, it has been shown that CD28 signaling can contribute to IL-13 transcription (40, 41).
Experiments with deletion mutants indicated that two promoter elements, a P element and an element with homology to an AP-1 site, cooperate in the complete transactivation of the IL-13 promoter. The P element accounts for most of the Tax effect. This site is also important for the activation of IL-13 transcription by T-cell activation signals (11). It most probably acts as a mediator of NF-AT activity and was shown to bind NF-ATp. Activated NF-AT transcription factors were detected in the nuclei of HTLV-1-transformed T cells (55), and NF-AT factors are relevant for the Tax stimulation of the promoters of IRF-4, Fas ligand, and IL-2 (17, 51). Moreover, it has been shown that Tax can induce NF-AT containing nuclear protein complexes binding to CD28RE (17). Thus, it is likely that the IL-13 promoter is induced by Tax-mediated NF-AT activation. Additionally, in HTLV-1-infected cells, NF-AT activity could also be enhanced by a second mechanism, the binding of the viral protein p12I to calcineurin (30). NF-AT activation by Tax may be limited by competing for CBP/p300 transcriptional coactivators (54, 56). This negative control mechanism could also provide an explanation for the higher induction of the promoter by a combination of the Tax mutants M22 and M47 compared to TaxWT. Since M47, in contrast to TaxWT and M22, cannot bind p300/CBP, transfection of both mutants has half of the CBP-squelching capacity of TaxWT. The high induction with M22 plus M47 (Fig. 3B) resembles the behavior of the FasL promoter in the presence of the same Tax mutants. (6).
The deleted AP-1 sequence overlaps with one of three GATA-3 transcription factor binding sites, which are relevant for the IL-13 promoter activity in T cells (32). Several lines of evidence support the idea that the reduced transactivation of the promoter variant with the AP-1 site deleted involves AP-1 and not GATA-3: (i) AP-1 is required for the Tax transactivation of several promoters (25, 34, 42), (ii) the AP-1-defective Tax mutant M22 has strongly decreased IL-13 promoter stimulation, (iii) the potential recognition sequences of AP-1 are conserved in the human and murine IL-13 promoters (39), and (iv) AP-1 has been suggested to be important for physiologic IL-13 induction in human intestinal mast cells (36).
Tax may replace cellular signals required for the induction of IL-13 synthesis. This is suggested by induction of endogenous IL-13 synthesis in Jurkat cells by transfected Tax expression plasmids and in the Tesi Tax-repressible system. Thus, it is likely that HTLV-1 also induces IL-13 expression in vivo. This is supported by our preliminary observation that IL-13 protein is more frequently detected in the sera of HAM/TSP patients, which are known to have high proviral loads (43), than in the sera of uninfected individuals or HTLV-1 carriers with low proviral loads (data not shown). This notion is corroborated by a recently published study which demonstrates IL-13 protein production in Tax-positive HAM/TSP patient lymphocytes ex vivo (7).
RNA and protein analyses demonstrate the expression of the IL-13Rα1 and IL-4Rα chains in HTLV-1-infected T cells. Together these constitute functional IL-13 receptors (5). T cells, like many other cells types, can express the IL-13 receptor (15, 18); in these cells IL-13 induces tyrosine phosphorylation of Janus kinase 3 and STAT6, which results in STAT6 DNA binding complexes (8, 31, 64). The tyrosine phosphorylation of STAT6 found in HTLV-1-infected cells, thus, could originate from IL-13 interaction with its ligand. This notion is corroborated by the observation that IL-4, an alternative inducer of STAT6 activation, is not produced by these cultures. Together with the highly induced expression of the cytokine, these data provide a strong argument for an autocrine activation of the IL-13 receptor in HTLV-1-transformed cells. In this respect HTLV-1-infected lymphocytes resemble Hodgkin lymphoma cells, which have been shown to be autostimulated by IL-13 (58).
Among the IL-13 functions observed in T cells are inhibition of gamma interferon production, enhancement of cytolytic potential (64), inhibition of chemotactic migration of T lymphocytes (28), and stimulation of IL-13Rα1 expression (15). At least some of these immune-modulating functions could result in perturbing the host response to HTLV-1 and could support viral persistence. Since interaction of IL-13 with its receptor leads to activation of an antiapoptotic phosphatidylinositol 3-kinase-dependent signaling cascade (61), inhibition of apoptosis might be an outcome of autocrine IL-13 stimulation in T cells. Inhibition of apoptosis by IL-13 has been demonstrated in different cell types, including B cells, synoviocytes, and colon carcinoma cells. For example, IL-13 inhibits apoptosis in B cells, especially in combination with the CD40 ligand (35, 49, 61). In particular, the cell death induced by cytokines such as tumor necrosis factor alpha and CD95 is suppressed (37, 61). Thus, IL-13 upregulation may be relevant for the survival of infected and virally transformed lymphocytes in vivo and may hence contribute to persistence and transformation.
Acknowledgments
We thank Steven Jacobson (National Institutes of Health, Bethesda, Md.) for providing ATL patient blood samples and Graham Taylor (St. Mary's Hospital, London, United Kingdom) for serum probes from HAM/TSP patients. The technical assistance of Kirsten Fraedrich is greatly appreciated.
This work was supported by the DFG (grant SFB466TP-C3).
REFERENCES
- 1.Akagi, T., H. Ono, H. Nyunoya, and K. Shimotohno. 1997. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene 14:2071-2078. [DOI] [PubMed] [Google Scholar]
- 2.Arnulf, B., A. Villemain, C. Nicot, E. Mordelet, P. Charneau, J. Kersual, Y. Zermati, A. Mauviel, A. Bazarbachi, and O. Hermine. 2002. Human T-cell lymphotropic virus oncoprotein Tax represses TGF-beta 1 signaling in human T cells via c-Jun activation: a potential mechanism of HTLV-I leukemogenesis. Blood 100:4129-4138. [DOI] [PubMed] [Google Scholar]
- 3.Bangham, C. R. 2000. The immune response to HTLV-I. Curr. Opin. Immunol. 12:397-402. [DOI] [PubMed] [Google Scholar]
- 4.Bartoe, J. T., B. Albrecht, N. D. Collins, M. D. Robek, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 74:1094-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callard, R. E., D. J. Matthews, and L. Hibbert. 1996. IL-4 and IL-13 receptors: are they one and the same? Immunol. Today 17:108-110. [DOI] [PubMed] [Google Scholar]
- 6.Chen, X., V. Zachar, M. Zdravkovic, M. Guo, P. Ebbesen, and X. Liu. 1997. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J. Gen. Virol. 78:3277-3285. [DOI] [PubMed] [Google Scholar]
- 7.Chung, H. K., H. A. Young, P. K. Goon, G. Heidecker, G. L. Princler, O. Shimozato, G. P. Taylor, C. R. Bangham, and D. Derse. 2003. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 102:4130-4136. [DOI] [PubMed] [Google Scholar]
- 8.Curiel, R. E., R. Lahesmaa, J. Subleski, M. Cippitelli, R. A. Kirken, H. A. Young, and P. Ghosh. 1997. Identification of a Stat-6-responsive element in the promoter of the human interleukin-4 gene. Eur. J. Immunol. 27:1982-1987. [DOI] [PubMed] [Google Scholar]
- 9.D'Agostino, D. M., L. Ranzato, G. Arrigoni, I. Cavallari, F. Belleudi, M. R. Torrisi, M. Silic-Benussi, T. Ferro, V. Petronilli, O. Marin, L. Chieco-Bianchi, P. Bernardi, and V. Ciminale. 2002. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J. Biol. Chem. 277:34424-34433. [DOI] [PubMed] [Google Scholar]
- 10.Ding, W., B. Albrecht, R. E. Kelley, N. Muthusamy, S. J. Kim, R. A. Altschuld, and M. D. Lairmore. 2002. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 76:10374-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolganov, G., S. Bort, M. Lovett, J. Burr, L. Schubert, D. Short, M. McGurn, C. Gibson, and D. B. Lewis. 1996. Coexpression of the interleukin-13 and interleukin-4 genes correlates with their physical linkage in the cytokine gene cluster on human chromosome 5q23-31. Blood 87:3316-3326. [PubMed] [Google Scholar]
- 12.Etoh, K., S. Tamiya, K. Yamaguchi, A. Okayama, H. Tsubouchi, T. Ideta, N. Mueller, K. Takatsuki, and M. Matsuoka. 1997. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 57:4862-4867. [PubMed] [Google Scholar]
- 13.Fujii, M., T. Chuhjo, T. Minamino, N. Masaaki, K. Miyamoto, and M. Seiki. 1995. Identification of the Tax interaction region of serum response factor that mediates the aberrant induction of immediate early genes through CArG boxes by HTLV-I Tax. Oncogene 11:7-14. [PubMed] [Google Scholar]
- 14.Gabet, A. S., F. Mortreux, A. Talarmin, Y. Plumelle, I. Leclercq, A. Leroy, A. Gessain, E. Clity, M. Joubert, and E. Wattel. 2000. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene 19:4954-4960. [DOI] [PubMed] [Google Scholar]
- 15.Gauchat, J. F., E. Schlagenhauf, N. P. Feng, R. Moser, M. Yamage, P. Jeannin, S. Alouani, G. Elson, L. D. Notarangelo, T. Wells, H. P. Eugster, and J. Y. Bonnefoy. 1997. A novel 4-kb interleukin-13 receptor alpha mRNA expressed in human B, T, and endothelial cells encoding an alternate type-II interleukin-4/interleukin-13 receptor. Eur. J. Immunol. 27:971-978. [DOI] [PubMed] [Google Scholar]
- 16.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 17.Good, L., S. B. Maggirwar, and S. C. Sun. 1996. Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 15:3744-3750. [PMC free article] [PubMed] [Google Scholar]
- 18.Graber, P., D. Gretener, S. Herren, J. P. Aubry, G. Elson, J. Poudrier, S. Lecoanet-Henchoz, S. Alouani, C. Losberger, J. Y. Bonnefoy, M. H. Kosco-Vilbois, and J. F. Gauchat. 1998. The distribution of IL-13 receptor alpha1 expression on B cells, T cells and monocytes and its regulation by IL-13 and IL-4. Eur. J. Immunol. 28:4286-4298. [DOI] [PubMed] [Google Scholar]
- 19.Green, P. L., and I. S. Y. Chen. 2001. Human T-cell leukemia virus type 1 and 2, p. 1941-1961. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 20.Grone, M., C. Koch, and R. Grassmann. 1996. The HTLV-1 Rex protein induces nuclear accumulation of unspliced viral RNA by avoiding intron excision and degradation. Virology 218:316-325. [DOI] [PubMed] [Google Scholar]
- 21.Grossman, W. J., J. T. Kimata, F. H. Wong, M. Zutter, T. J. Ley, and L. Ratner. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 92:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haller, K., T. Ruckes, I. Schmitt, D. Saul, E. Derow, and R. Grassmann. 2000. Tax-dependent stimulation of G1 phase-specific cyclin-dependent kinases and increased expression of signal transduction genes characterize HTLV type 1-transformed T cells. AIDS Res. Hum. Retroviruses 16:1683-1688. [DOI] [PubMed] [Google Scholar]
- 23.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrod, R., Y. Tang, C. Nicot, H. S. Lu, A. Vassilev, Y. Nakatani, and C. Z. Giam. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 18:5052-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwai, K., N. Mori, M. Oie, N. Yamamoto, and M. Fujii. 2001. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology 279:38-46. [DOI] [PubMed] [Google Scholar]
- 26.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, H., H. Lu, R. L. Schiltz, C. A. Pise-Masison, V. V. Ogryzko, Y. Nakatani, and J. N. Brady. 1999. PCAF interacts with Tax and stimulates Tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 19:8136-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinquan, T., J. Frydenberg, N. Mukaida, J. Bonde, C. G. Larsen, K. Matsushima, and K. Thestrup-Pedersen. 1995. Recombinant human growth-regulated oncogene-alpha induces T lymphocyte chemotaxis. A process regulated via IL-8 receptors by IFN-gamma, TNF-alpha, IL-4, IL-10, and IL-13. J. Immunol. 155:5359-5368. [PubMed] [Google Scholar]
- 29.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, S. J., W. Ding, B. Albrecht, P. L. Green, and M. D. Lairmore. 2003. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J. Biol. Chem. 278:15550-15557. [DOI] [PubMed] [Google Scholar]
- 31.Kohler, I., P. Alliger, A. Minty, D. Caput, P. Ferrara, B. Holl-Neugebauer, G. Rank, and E. P. Rieber. 1994. Human interleukin-13 activates the interleukin-4-dependent transcription factor NF-IL4 sharing a DNA binding motif with an interferon-gamma-induced nuclear binding factor. FEBS Lett. 345:187-192. [DOI] [PubMed] [Google Scholar]
- 32.Lavenu-Bombled, C., C. D. Trainor, I. Makeh, P. H. Romeo, and I. Max-Audit. 2002. Interleukin-13 gene expression is regulated by GATA-3 in T cells: role of a critical association of a GATA and two GATG motifs. J. Biol. Chem. 277:18313-18321. [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre, L., A. Vanderplasschen, V. Ciminale, H. Heremans, O. Dangoisse, J. C. Jauniaux, J. F. Toussaint, V. Zelnik, A. Burny, R. Kettmann, and L. Willems. 2002. Oncoviral bovine leukemia virus G4 and human T-cell leukemia virus type 1 p13(II) accessory proteins interact with farnesyl pyrophosphate synthetase. J. Virol. 76:1400-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, X., X. Chen, V. Zachar, C. Chang, and P. Ebbesen. 1999. Transcriptional activation of human TR3/nur77 gene expression by human T-lymphotropic virus type I Tax protein through two AP-1-like elements. J. Gen. Virol. 80:3073-3081. [DOI] [PubMed] [Google Scholar]
- 35.Lomo, J., H. K. Blomhoff, S. E. Jacobsen, S. Krajewski, J. C. Reed, and E. B. Smeland. 1997. Interleukin-13 in combination with CD40 ligand potently inhibits apoptosis in human B lymphocytes: upregulation of Bcl-xL and Mcl-1. Blood 89:4415-4424. [PubMed] [Google Scholar]
- 36.Lorentz, A., I. Klopp, T. Gebhardt, M. P. Manns, and S. C. Bischoff. 2003. Role of activator protein 1, nuclear factor-kappaB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J. Allergy Clin. Immunol. 111:1062-1068. [DOI] [PubMed] [Google Scholar]
- 37.Manna, S. K., and B. B. Aggarwal. 1998. IL-13 suppresses TNF-induced activation of nuclear factor-kappa B, activation protein-1, and apoptosis. J. Immunol. 161:2863-2872. [PubMed] [Google Scholar]
- 38.McKenzie, A. N., and P. G. Fallon. 2003. Decoy receptors in the regulation of T helper cell type 2 responses. J. Exp. Med. 197:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie, A. N., X. Li, D. A. Largaespada, A. Sato, A. Kaneda, S. M. Zurawski, E. L. Doyle, A. Milatovich, U. Francke, N. G. Copeland, et al. 1993. Structural comparison and chromosomal localization of the human and mouse IL-13 genes. J. Immunol. 150:5436-5444. [PubMed] [Google Scholar]
- 40.Minty, A., S. Asselin, A. Bensussan, D. Shire, N. Vita, A. Vyakarnam, J. Wijdenes, P. Ferrara, and D. Caput. 1997. The related cytokines interleukin-13 and interleukin-4 are distinguished by differential production and differential effects on T lymphocytes. Eur. Cytokine Netw. 8:203-213. [PubMed] [Google Scholar]
- 41.Minty, A., P. Chalon, J. M. Derocq, X. Dumont, J. C. Guillemot, M. Kaghad, C. Labit, P. Leplatois, P. Liauzun, B. Miloux, et al. 1993. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 362:248-250. [DOI] [PubMed] [Google Scholar]
- 42.Mori, N., N. Mukaida, D. W. Ballard, K. Matsushima, and N. Yamamoto. 1998. Human T-cell leukemia virus type I Tax transactivates human interleukin 8 gene through acting concurrently on AP-1 and nuclear factor-kappaB-like sites. Cancer Res. 58:3993-4000. [PubMed] [Google Scholar]
- 43.Nagai, M., K. Usuku, W. Matsumoto, D. Kodama, N. Takenouchi, T. Moritoyo, S. Hashiguchi, M. Ichinose, C. R. Bangham, S. Izumo, and M. Osame. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4:586-593. [DOI] [PubMed] [Google Scholar]
- 44.Neuveut, C., and K. T. Jeang. 2002. Cell cycle dysregulation by HTLV-I: role of the tax oncoprotein. Front. Biosci. 7:d157-d163. [DOI] [PubMed] [Google Scholar]
- 45.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. 2001. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823-829. [DOI] [PubMed] [Google Scholar]
- 47.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 48.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Relic, B., J. Guicheux, F. Mezin, E. Lubberts, D. Togninalli, I. Garcia, W. B. van den Berg, and P. A. Guerne. 2001. Il-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J. Immunol. 166:2775-2782. [DOI] [PubMed] [Google Scholar]
- 50.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera, I., E. W. Harhaj, and S. C. Sun. 1998. Involvement of NF-AT in type I human T-cell leukemia virus Tax-mediated Fas ligand promoter transactivation. J. Biol. Chem. 273:22382-22388. [DOI] [PubMed] [Google Scholar]
- 52.Ruckes, T., D. Saul, J. Van Snick, O. Hermine, and R. Grassmann. 2001. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98:1150-1159. [DOI] [PubMed] [Google Scholar]
- 53.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serfling, E., F. Berberich-Siebelt, S. Chuvpilo, E. Jankevics, S. Klein-Hessling, T. Twardzik, and A. Avots. 2000. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim. Biophys. Acta 1498:1-18. [DOI] [PubMed] [Google Scholar]
- 55.Sharma, S., Y. Mamane, N. Grandvaux, J. Bartlett, L. Petropoulos, R. Lin, and J. Hiscott. 2000. Activation and regulation of interferon regulatory factor 4 in HTLV type 1-infected T lymphocytes. AIDS Res. Hum. Retroviruses 16:1613-1622. [DOI] [PubMed] [Google Scholar]
- 56.Sisk, T. J., T. Gourley, S. Roys, and C. H. Chang. 2000. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J. Immunol. 165:2511-2517. [DOI] [PubMed] [Google Scholar]
- 57.Skinnider, B. F., U. Kapp, and T. W. Mak. 2001. Interleukin 13: a growth factor in Hodgkin lymphoma. Int. Arch. Allergy Immunol. 126:267-276. [DOI] [PubMed] [Google Scholar]
- 58.Skinnider, B. F., U. Kapp, and T. W. Mak. 2002. The role of interleukin 13 in classical Hodgkin lymphoma. Leuk. Lymphoma 43:1203-1210. [DOI] [PubMed] [Google Scholar]
- 59.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 60.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 61.Wright, K., G. Kolios, J. Westwick, and S. G. Ward. 1999. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. Evidence for an interleukin-13-driven phosphatidylinositol 3-kinase-dependent survival mechanism. J. Biol. Chem. 274:17193-17201. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida, M., M. Seiki, K. Yamaguchi, and K. Takatsuki. 1984. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 81:2534-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu, C. R., R. A. Kirken, M. G. Malabarba, H. A. Young, and J. R. Ortaldo. 1998. Differential regulation of the Janus kinase-STAT pathway and biologic function of IL-13 in primary human NK and T cells: a comparative study with IL-4. J. Immunol. 161:218-227. [PubMed] [Google Scholar]