Abstract
Dendritic cells (DCs), which are potent antigen-presenting cells (APCs), are used as adjuvants for the treatment of cancer and infectious diseases in human and nonhuman primates, with documented clinical efficacy. The hepatitis C virus (HCV)-chimpanzee model is the best available model for testing the immunotherapeutic effects of DCs in the setting of a chronic infection, as chimpanzees develop a persistent infection resembling that seen in humans. However, several reports have suggested that DCs derived from chronically infected individuals or nonhuman primates are functionally compromised. As a prelude to clinical studies, we evaluated whether functionally mature DCs could be generated in chimpanzee plasma by good manufacturing practice using CD14+ mononuclear precursors from chronically infected chimpanzees. DCs generated in a medium with HCV-negative plasma and treated with a defined cocktail of cytokines or a CD40 ligand trimer matured fully, as measured by the induction of CD83 expression and the upregulation of costimulatory molecules. Furthermore, the expression of CCR7 was induced, suggesting an acquisition of migration capacity. Mature DCs were capable of stimulating allogeneic T cells, antigen-specific memory CD4+ T cells, and HCV-specific CD8+-T-cell clones. In all cases, there was no evidence of HCV infection in DCs. Furthermore, these DCs maintained their phenotype and APC function after cryopreservation. Finally, no discernible differences were noted between DCs derived from HCV-infected and uninfected chimpanzees. In summary, precursor cells from HCV-infected chimpanzees are fully capable of differentiating into functional, mature DCs, which can now be reproducibly prepared for investigations of their immunotherapeutic potential in the setting of chronic HCV infection.
Hepatitis C virus (HCV) infects an estimated 170 million persons worldwide and is a major cause of chronic liver disease, cirrhosis, and hepatocellular cancer (1). Only a portion of infected individuals resolve the infection (∼30%), with most developing a chronic infection. Acute infections are characterized by high frequencies of HCV-specific CD8+ T cells (31, 52, 54) and HCV-specific CD4+-T-cell responses that can persist for a long time after the clearance of viremia and the resolution of infection (54, 56). On the other hand, individuals who remain chronically infected display weak and restricted CD4+- and CD8+-T-cell responses in both the liver and the blood (7, 12, 27, 29, 43, 49, 52). Significantly, only a small percentage respond to approved therapies, e.g., ribavarin and alpha interferon (IFN-α) therapy.
An understanding of viral persistence in HCV infections is essential for developing new strategies for preventing chronic HCV infections and for developing therapies which promote effective T-cell responses in already chronically infected patients. A promising and frequently used method for inducing or augmenting immune responses is dendritic cell (DC) vaccination. DC-based vaccines and immunotherapy against cancers and simian immunodeficiency virus (SIV) have shown promise in clinical settings (15, 20, 24, 34, 53). We are conducting ongoing human DC-based immunotherapy clinical trials with chronic human immunodeficiency virus (HIV)-infected individuals, using peptides and recombinant canarypox virus as HIV sources. However, these approaches cannot be initiated in humans infected with HCV due to possible adverse effects of immune stimulation, such as hepatopathology. Chimpanzees (Pan troglodytes) provide a factual model for HCV persistence (55), and studies of HCV infections in these primates have led to important observations regarding control of the virus. For example, the removal of either the CD4+- or CD8+-T-cell population led to persistently elevated viral loads in chimpanzees which had previously recovered from their infections and were then rechallenged (22, 50). The development of a safe, DC-based vaccine for HCV-infected chimpanzees that elicits long-term and sustained cellular immunity would facilitate the design of similar vaccine regimens that would be applicable to human patients chronically infected with HCV.
In vivo, HCV targets hepatocytes, probably B cells, and even DCs (30). Indeed, HCV genomic sequences (3, 36, 38) and replicative RNAs have been detected in blood and/or monocyte-derived DCs from chronically infected subjects. Furthermore, the C-type lectin receptor DC-SIGN (DC-specific intercellular adhesion molecule-3-grabbing nonintegrin) is proposed to be an uptake receptor for HCV on monocyte-derived DCs (41). However, the consequences of HCV-DC interactions at the levels of maturation and function are controversial. One study has shown an impaired maturation caused by tumor necrosis factor alpha (TNF-α) of DCs derived from chronic HCV-infected donors (2), another has found an inhibition of DC maturation after exposure to HCV core and NS3 proteins (16), and yet others have found suboptimal T-cell stimulatory functions when alloantigens (2, 26) or recall antigens such as HCV core proteins were presented (25, 26, 48). Interestingly, human DCs transduced with an adenovirus coding for HCV core and E1 proteins mature normally but do not efficiently prime CD4+ T cells in vitro (47), while similarly transduced immature murine DCs were inhibited in their maturation capacity, largely due to the expression of structural proteins (48). In contrast to these studies, other investigators have failed to show a detrimental effect of HCV or its proteins on DCs (35, 37). Furthermore, fully functional DCs can be generated from monocytes of humans with chronic HIV infections, suggesting that high viral loads do not compromise antigen-presenting-cell (APC) activity or at least precursor APC activity (46). The contradictory findings cited above, which may well be due to species or technical differences, emphasize the need to carefully characterize and assess DC function in the setting of HCV infection.
For this study, we directly compared DCs from HCV-infected versus uninfected chimpanzees with respect to yield, phenotype, and function, with the overall goals of first determining whether DC differentiation was compromised and then optimizing DC preparations for immunotherapy. Using good manufacturing practice (GMP) conditions with only autologous products, we noted a significant improvement in the purity and quality of the DCs if they were generated from leukapheresis-derived CD14+ progenitors versus blood-derived monocytes. DCs from infected chimpanzees were phenotypically equivalent to DCs prepared from uninfected animals. Furthermore, they were equally capable of processing and presenting antigens to T cells. Finally, we found no evidence of HCV infection in DCs generated by this approach, thereby indicating a lack of significant infection in progenitors in vivo. Our studies, which are the first to show that functionally intact chimpanzee DCs can be generated under GMP conditions from HCV-infected chimpanzees, pave the way for the initiation of DC-based immune interventions in chronically infected nonhuman primates. Furthermore, they suggest that the observed immune dysfunction of CD8+ T cells is likely due to other factors.
MATERIALS AND METHODS
Animals.
Adult healthy or chronically HCV-infected chimpanzees were used for this study, and the research complied with federal guidelines and institutional policies. Animals were housed in the New Iberia Research Center (New Iberia, La.). The HCV-positive chimpanzees used for this study were all infected with the HCV-H77 genotype 1a virus and included the following animals: 1530, male, age 12; 1534, male, age 15; and CB0507, male, age 12. Noninfected animals were A255B, male, age 12, and TBDX104, male, age 13.
Culture medium and cytokines.
RPMI 1640 culture medium (GIBCO BRL, Gaithersburg, Md.) was supplemented with 20 μg of gentamicin (GIBCO BRL)/ml, 1 mM HEPES (Mediatech, Herndon, Va.), and 1% human plasma, 10% bovine serum, 10% chimpanzee plasma from either uninfected or infected chimpanzees, or 5% pooled human serum (c-Six Diagnostics, Mequon, Wis.). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 200 IU/ml) (Immunex Corporation, Seattle, Wash.) and recombinant human interleukin-4 (IL-4; 400 IU/ml) (Schering-Plough Corporation, Kenilworth, N.J.) were purchased for the generation of DCs in vitro. A monocyte-conditioned medium mimic (MCM mimic) was used to mature DCs and consisted of 5 ng of TNF-α/ml, 5 ng of IL-1β/ml, 150 ng of IL-6 (R&D Systems)/ml, and 1 μg of prostaglandin E2 (PGE2; Sigma)/ml.
DCs.
Whole blood or leukapheresis products from uninfected or HCV-infected chimpanzees were obtained from the New Iberia Research Center. Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, N.J.). CD14+ DC progenitor cells were isolated with CD14+ magnetic beads (Miltenyi Biotech, Auburn, Calif.). DCs were generated by culturing the CD14+ cell fraction in six-well plates (∼2 × 106 cells) with 200 IU of GM-CSF (Immunex Corporation)/ml and 400 IU of IL-4 (Schering-Plough Corporation)/ml for 7 days. The cytokines were added to the cultures at days 0, 2, and 4. On day 5, nonadherent cells were collected and moved to new six-well plates, and the cultures were supplemented with a maturation stimulus such as the MCM mimic or 1 μg of CD40LT (soluble trimeric recombinant human CD40L; Immunex Corporation)/ml (42). The CD40LT was endotoxin-free, as measured by standard Limulus amebocyte lysate assays. Mature DCs were collected on day 7. Immature DCs were maintained in culture with GM-CSF and IL-4 for 5 to 7 days and washed extensively prior to use.
Phenotyping of DCs.
Phycoerythrin-conjugated CD1D, CD3, CD14, CD54, CD40, CD80, CD86, HLA ABC, HLA DR, CCR5, CD184 (CXCR4), CCR6, CDx197 (CCR7), and CD207 (Langerin) antibodies; fluorescein isothiocyanate-conjugated immunoglobulin G2A and CD209 antibody (DC-SIGN) (BD Pharmingen, San Jose, Calif.); an unconjugated CD205 antibody (DEC 205); and isotype-matched control antibodies were added to immature or mature DCs, incubated at 4°C for 20 min, and washed. Staining with unconjugated antibodies was followed with phycoerythrin-conjugated secondary goat anti-mouse antibodies (Biosource International, Camarillo, Calif.). Fluorescence-activated cell sorting (FACS) was performed on a FACSort instrument (BD Pharmingen). Data were analyzed with Cell Quest software (BD Pharmingen).
T cells.
Bulk T cells (CD8+ and CD4+ T cells) were isolated by the depletion of contaminating cells, i.e., CD56+, CD19+, and CD14+ cells, with magnetic beads (Miltenyi Biotech). Patr class I-restricted HCV-specific CD8+-T-cell clones were prepared as previously described (18). Clones were used at least 12 days after restimulation in vitro, when they were in a resting state. One CD8+-T-cell clone recognized a peptide from p162A (GAVQNEITL, which is a Patr B1701-restricted epitope and an allele expressed by animals CB0507 and A255B).
Peptides.
The peptide epitope used was GAVQNEITL (Patr B1701 restricted) synthesized by Research Genetics (Huntsville, Ala.).
ELISPOT assay for detection of IFN-γ release from antigen-specific T cells.
Ninety-six-well plates (Millititer; Millipore, Bedford, Mass.) were coated overnight at 4°C with 5 μg of an anti-IFN-γ monoclonal antibody (Mabtech, Stockholm, Sweden)/ml. The antibody-coated plates were washed four times with phosphate-buffered saline and blocked with RPMI containing 5% pooled human serum for 1 h at 37°C. The peptide-pulsed DCs were added to the wells together with the HCV-specific CD8+-T-cell clones and incubated overnight (∼14 to 18 h) at 37°C. The plates were washed, stained, and developed as described previously (28). Only spots with a fuzzy border and a brown color were counted.
Proliferation.
DCs were left unpulsed or were pulsed with 0.1 to 10 μg of tetanus toxoid (Statens Serum Institute, Copenhagen, Denmark)/ml overnight and then harvested. The different DC groups were cultured with autologous T cells in 96-well plates at a concentration of 105 T cells/well and a ratio of 1 DC to 30 T cells. The proliferation assay was pulsed with [3H]thymidine on day 4 or 5 and cells were harvested after 16 to 18 h.
ELISAs.
Chemokines and cytokines released by immature or MCM mimic-stimulated DCs into culture supernatants were monitored by enzyme-linked immunosorbent assays (ELISAs). Supernatants were collected from immature or MCM mimic-matured (24 h) DCs from either HCV-infected or uninfected chimpanzees. Cell-free stimuli in the culture medium and culture medium alone were collected as controls. All samples were stored at −20°C until they were analyzed. RANTES, TNF-α, MCP-3 (CCL7), IL-10, and p70 IL-12 (R&D Systems) ELISAs were performed according to the manufacturer's instructions.
Cryopreservation of mature DCs.
Aliquots of mature DCs were frozen in 8% dimethyl sulfoxide (Sigma) and fetal calf serum (FCS) in cryotubes (Nunc cryovials; NalgeNunc Inc., Rochester, N.Y.). The cryovials were immediately transferred to −80°C in a cryo-freezing container (Nalgene) and were finally transferred to the gas phase of liquid nitrogen until use. Upon use, the frozen cryopreserved DCs were partly thawed in a 37°C water bath and then directly transferred into a tube with culture medium. The tube with the thawed cells was spun down at 4°C. Subsequently, the cells were counted, resuspended in culture medium, and accessed for phenotype and function.
Detection of HCV RNA in DCs.
A real-time quantitative reverse transcription-PCR (RT-PCR)-based assay with a detection sensitivity of 100 to 1,000 copies of HCV RNA per 80 ng of total RNA was performed on total RNA samples derived from chimpanzee DCs from either uninfected or chronically infected animals. Mature DCs from both groups were collected, spun down, washed three times in RPMI, and frozen at −80°C until RNA preparation. The RNAs were purified by use of an RNeasy mini kit (50) (Qiagen Inc., Valencia, Calif.) according to the manufacturer's protocol. Real-time RT-PCR amplification was done with Platinum Quantitative RT-PCR ThermoScript. The One Step system (Invitrogen) was used with an ABI PRISM 7700 sequence detector (Applied Biosystems) to analyze 80 ng of total RNA. The primers specific for the HCV 5′ N-terminal repeat were as follows: 5′CCTCTAGAGCCATAGTGGTCT-3′ (sense; 10 μM), 5′-CCAAATCTCCAGGCATTGAGC-3′ (antisense; 10 μM), and FAM-CACCGGAATTGCCAGGACGACCGG (probe; 10 μM), all purchased from Applied Biosystems. The RT-PCRs were performed as described previously (9). Briefly, RT was done at 50°C for 30 min, followed by Taq polymerase activation at 95°C for 5 min. Fifty cycles of 15 s at 95°C, 40 s at 50°C, and 30 s at 72°C were run. A glyceraldehye-3-phosphate (GAPDH) detection mix (VIC-MGBNFQ; ABI) was included for normalization. Synthetic HCV RNA standards of known concentrations (106, 105, 104, 103, 102, and 101 HCV RNA molecules) were used to calculate a standard curve. The results were analyzed in a multiplex format with SDS software (Applied Biosystems).
RESULTS
Chimpanzee DCs can be propagated from peripheral blood CD14+ progenitor cells.
Immature DCs can be generated from blood progenitor cells from normal chimpanzees in the presence of GM-CSF and IL-4, as previously described (6). However, this method relies on the presence of 10% FCS in the culture medium, which cannot be used to prepare DCs for in vivo injections because of the potential induction of bovine-specific immune responses, including allergic phenomena. Furthermore, no studies have addressed whether immature chimpanzee DCs differentiate in response to stimuli which promote the maturation of human DCs. While immature DCs are efficient at acquiring antigens, mature DCs are required for the processing and presentation of antigens to T cells (51). Finally, the preparation of chimpanzee DCs for immunotherapeutic purposes should meet the standards of GMP conditions, as mandated for human DCs used in clinical studies. To address these issues, we prepared DCs from PBMCs isolated from leukapheresis products from healthy and chronically infected HCV-positive chimpanzees. The advantage of leukapheresis is that it provides a large number of prevaccination PBMCs which can be used to (i) simultaneously generate DCs for multiple vaccinations, (ii) analyze the extent of prevaccination immunity, and (iii) provide a reserve of cells for subsequent clinical interventions, as needed.
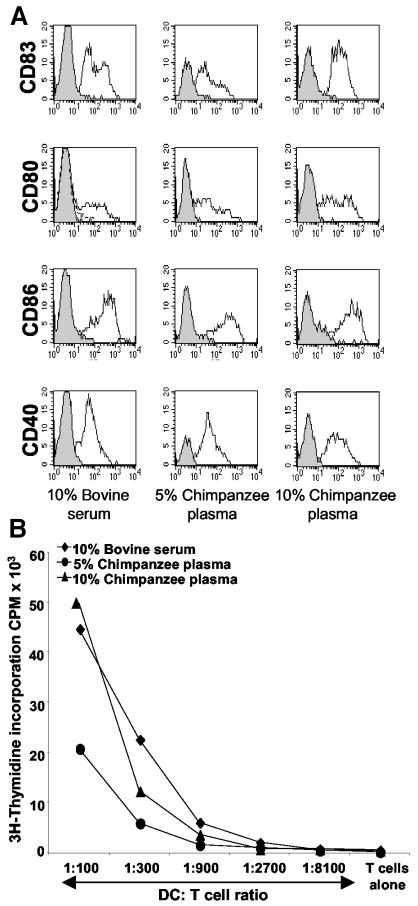
PBMCs from leukapheresis samples were used to obtain CD14+ cell-enriched fractions by magnetic bead separation (Miltenyi), and they were cultured for 5 days in medium supplemented with GM-CSF, IL-4, and different doses of chimpanzee plasma (5 or 10% plasma obtained from uninfected chimpanzees) or 10% FCS. A cocktail of IL-1, IL-6, TNF-α, and PGE2 (MCM mimic), normally used to mature human DCs for use in clinical trials, was added to DC cultures on day 5. The MCM mimic induces a homogeneous maturation of human DCs and yields high numbers of differentiated cells (32). Preliminary studies established that the pre-enrichment of CD14+ DC precursors from PBMCs instead of an adherence step led to significantly higher yields of DCs. The 10% plasma supported the highest yield of phenotypically and functionally mature chimpanzee DCs. Yields of ∼12% mature DCs were obtained from the starting population of CD14+ cells, similar to the yield for cells cultured in 10% FCS (data not shown). Furthermore, the purity of the cells obtained approached 100% (data not shown). The expression of CD83, a maturation-associated marker, and the costimulatory molecules CD40, CD80, and CD86 was examined at day 7 (Fig. 1A). Mature DCs cultured in 10% plasma or 10% FCS were phenotypically equivalent, whereas fewer CD83-positive DCs were seen in cultures containing 5% plasma. The functional advantages of DCs generated in cultures containing 10% versus 5% plasma were confirmed in a standard allogeneic mixed leukocyte reaction (Fig. 1B).
FIG. 1.
DCs are efficiently propagated in culture medium containing 10% chimpanzee plasma. (A) CD14+ DC progenitors (from HCV-infected chimpanzee 1530) were cultured with IL-4 and GM-CSF in RPMI supplemented with 10% bovine serum or 5 or 10% chimpanzee plasma obtained from an uninfected chimpanzee (A255B or TBDX104). On day 5, a cocktail of cytokines (MCM mimic) was added, and the cells were harvested after 48 h of culture. FACS histograms show profiles for isotype controls (gray areas with solid lines) and monoclonal antibodies (white areas with solid lines). The gates were set to include cells with large amounts of forward scatter (FSC) and side scatter (SSC). The data shown are representative of four experiments. (B) The different groups of DCs (prepared from chimpanzee 1530) were added to 2 × 105 allogeneic T cells in graded doses in triplicate. On day 4, [3H]thymidine was added, and the cultures were incubated for 16 h. The results are expressed in counts per minute. One representative of three experiments is shown.
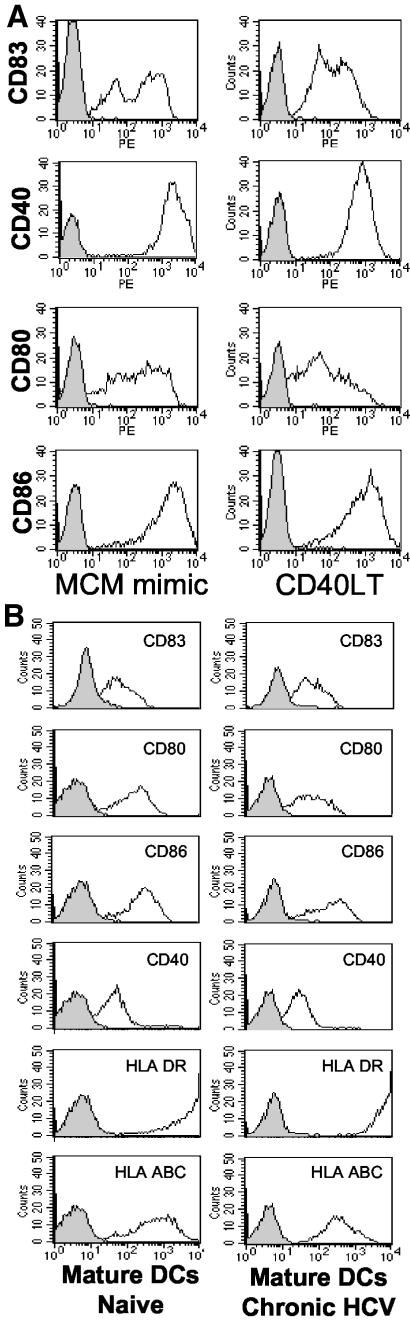
We next examined the differentiation of DCs obtained from HCV-infected chimpanzees by using two different clinical-grade maturation stimuli, i.e., the MCM mimic and CD40LT. The surface phenotypes were virtually identical for DCs matured with CD40LT versus the MCM mimic (Fig. 2A) and were comparable to what is observed with human DCs (32). Therefore, the MCM mimic is a viable alternative to CD40LT, which is currently not readily available in a GMP-grade form for clinical trials.
FIG.2.
Maturation of DCs cultured from a chronically HCV-infected chimpanzee is achieved by both MCM mimic and CD40LT. (A) MCM mimic (TNF-α, IL-1β, IL-6, and PGE2) or CD40LT (1 μg/ml) was added in a medium supplemented with 10% plasma from a noninfected chimpanzee to immature DCs from a chronically infected chimpanzee (CB0507) on day 5 of culture. On day 7, the DCs were harvested and labeled with monoclonal antibodies as indicated. FACS histograms show profiles for isotype controls (gray areas with solid lines) and monoclonal antibodies (white areas with solid lines). (B) MCM mimic was added to immature DCs from an uninfected (A255B) or chronically infected (CB0507) chimpanzee on day 5 of culture. On day 7, the DCs were harvested and labeled with the indicated monoclonal antibodies. FACS histograms show profiles for isotype controls (gray areas with solid lines) and monoclonal antibodies (white areas with solid lines). For both panels, the gates were set to include cells with large amounts of FSC and SSC. The data shown are for one representative experiment out of four.
DCs derived from chronically infected humans may exhibit impairment in their differentiation (2). However, when they were compared simultaneously, DCs from uninfected and chronically infected chimpanzees had similar phenotypic profiles after the addition of the MCM mimic (Fig. 2B) (n = 4).
Altogether, the data cited above indicate that DCs generated from CD14+ precursors from infected chimpanzees can fully differentiate and are phenotypically identical to their counterparts from uninfected chimpanzees.
Phenotypic characterization of mature DCs generated from chronically infected chimpanzees.
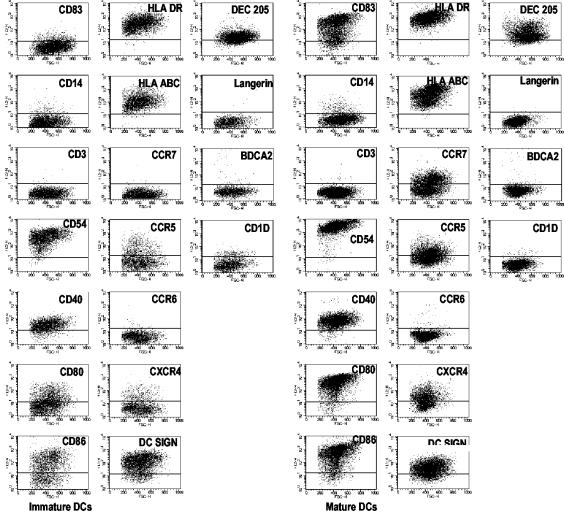
We next undertook a more extensive phenotypic analysis of immature and MCM mimic-matured DCs from chronically infected chimpanzees. This included measuring the expression of lineage markers, maturation markers, costimulatory molecules, adhesion molecules, and chemokine receptors (Fig. 3). The immature and mature DCs were negative for the monocyte/macrophage marker CD14, as expected. CD83, a maturation marker, was only expressed by mature DCs. Both immature and mature DCs expressed adhesion molecules (CD54), costimulatory molecules (CD80 and CD86), and HLA class I and II molecules, but mature DCs expressed higher levels of these markers. Mature DCs also displayed the chemokine receptors CCR5, CXCR4, and CCR7, but they were negative for CCR6 (Fig. 3). The levels of CCR5, CXCR4, and CCR7, however, varied from a low level of expression to up to 80% of the DCs for different donors. Notably, immature DCs expressed an absence of or lower levels of these chemokine receptors. Of the DC-associated markers (e.g., DC-SIGN, BDCA-2, Langerin, and CD1d), only DC-SIGN was significantly expressed, as was previously shown (41). Importantly, the phenotypes were similar for DCs generated from HCV-infected and uninfected animals, verifying our observations that DC precursors from the former animals are not impaired with respect to their phenotypic differentiation (not shown).
FIG. 3.
Extensive phenotypic characterization of mature DCs from chronically HCV-infected chimpanzee. The MCM mimic (TNF-α, IL-1β, IL-6, and PGE2) was added to immature DCs from chronically infected animals on day 5 of culture. On day 7, the DCs were harvested and labeled with isotype controls and the indicated monoclonal antibodies. FACS dot plots for a chronically HCV-infected animal (CB0507) show the degree of expression of various markers and receptors. The horizontal bars mark the extents of staining by matched isotype controls. Gates were set to include cells with large amounts of FSC and SSC. The data shown are for one representative experiment out of four.
Altogether, these results indicate that DCs from chronically infected HCV-positive chimpanzees mature normally and express all of the markers associated with maturation, including CCR7, which is essential for migration towards CCL19 and CCL21 in the lymph nodes and on high endothelial cell venules, respectively. This is the first time that the expression of the chemokine receptors CCR5, CCXR4, and CCR7 has been documented for chimpanzee DCs.
Cytokine and chemokine profiles of chimpanzee DCs.
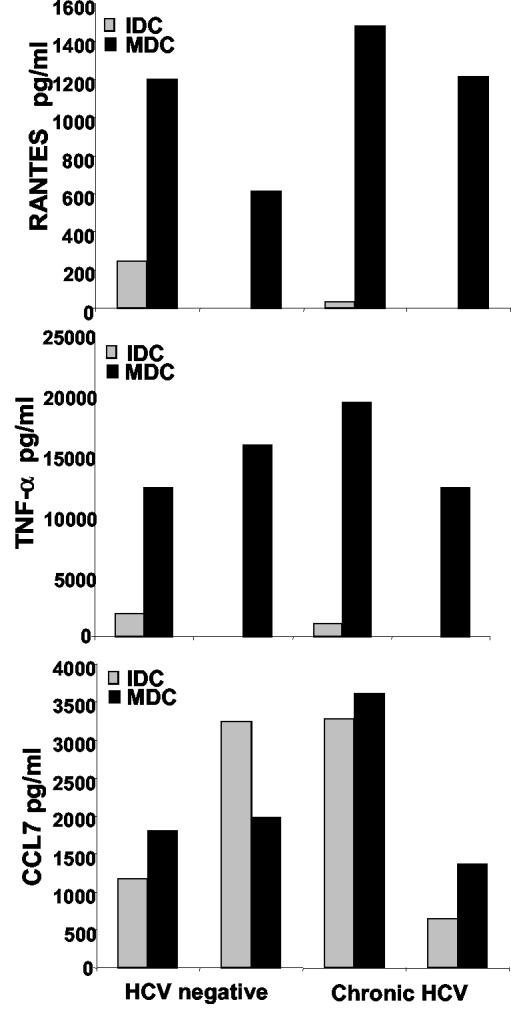
DCs produce and respond to distinct chemokines and cytokines depending on their maturation state and the maturation stimuli used to activate them in vitro (45). ELISA analyses of cell culture supernatants were used to determine the secreted chemokine and cytokine profiles of immature and mature DCs. CCL5 (RANTES), CCL7 (MCP-3), TNF-α, IL-10, and IL-12 were analyzed from samples collected 24 h after stimulation with or without the MCM mimic. We found that MCM mimic-stimulated chimpanzee DCs produced significant amounts of the inflammatory chemokines RANTES and CCL7 as well as the cytokine TNF-α (Fig. 4). Notably, the TNF-α levels were higher than could be accounted for by the addition of the MCM mimic, which contains TNF-α. The MCM mimic failed to induce IL-12 p70 secretion from chimpanzee DCs, which is also the case for human DCs (32; also data not shown). The levels of RANTES were similar between mature DCs from healthy and HCV-infected chimpanzees (Fig. 4). Furthermore, IL-10 and IL-18 were secreted at low levels in both immature and mature DCs, and no significant differences were seen between infected and uninfected animals. In conclusion, the cytokine and chemokine secretion profiles for immature versus mature chimpanzee DCs correspond to those of their human counterparts and no impairment in these functions was observed when the DCs were derived from infected animals.
FIG. 4.
DCs from uninfected and HCV-infected chimpanzees exhibit the same cytokine and chemokine production. Immature DCs from the uninfected TBDX104 and chronically HCV-infected CB0507 animals were stimulated with the MCM mimic for 24 h. The supernatants from immature (IDC) or MCM mimic-matured (24 h) DCs (MDC) were collected and stored at −20°C before being analyzed by ELISAs. Samples were thawed and tested. RANTES, TNF-α, and CCL7 were assayed by using R&D kits. Data for two sets of supernatants from uninfected and chronically HCV-infected chimpanzee DCs are shown.
Stimulation of antigen-specific CD4+ and CD8+ T cells by DCs prepared from chronically infected chimpanzees.
Most DC-based vaccines for humans utilize cells pulsed ex vivo with major histocompatibility complex (MHC) class I- or II-restricted peptides. We therefore examined the ability of mature chimpanzee DCs to bind and present class I peptides to well-characterized HCV-specific CD8+-T-cell clones. The CD8+-T-cell clone used for this experiment was previously characterized and shown to respond specifically to the GAVQNEITL peptide and not to an irrelevant peptide (13). Mature DCs were pulsed with the Patr B1701-restricted nonamer GAVQNEITL and added to the corresponding CD8+-T-cell clone. After 24 h, T-cell clone activation was assessed by a IFN-γ ELISPOT assay. DCs from both an uninfected chimpanzee (A255B) and a chronically infected chimpanzee (CB0507) efficiently activated the HCV-specific CD8+-T-cell clone (Fig. 5A and B). The DCs from the chronically infected animal activated higher numbers of IFN-γ-producing cells. In addition, an allogeneic mixed lymphocyte reaction to compare the T-cell stimulation capacities of DCs from uninfected (A255B) and HCV-infected (CB0507) chimpanzees found their activation capacities to be equivalent (Fig. 5C).
FIG. 5.
Peptide-pulsed DCs from uninfected and chronically HCV-infected chimpanzees activate T-cell responses. Mature DCs from an uninfected (A255B) (A) and a chronically infected (CB0507) (B) chimpanzee were pulsed with increasing doses of the HCV p162A-derived epitope GAVQNEITL, which is B1701 restricted. The peptide-pulsed DCs were added to 10,000 peptide-specific T-cell clones at a ratio of 1:2 or 1:4 in triplicate. The activation of T cells was measured as the numbers of spot-forming cells (SFC) in an IFN-γ ELISPOT assay after 14 h. (C) Mature DCs from an uninfected (TBDX104) and a chronically infected (1534) chimpanzee were added to 2 × 105 allogeneic T cells in graded doses in triplicate. On day 4, [3H]thymidine was added, and the cultures were incubated for 16 h. The results are expressed in counts per minute. (D) Immature (IDC) and mature DCs (MDC) from HCV-infected chimpanzee CB0507 were incubated for 1 h with increasing doses of tetanus toxoid (0 to 10 μg/ml). The DCs were added to 200,000 autologous T cells in triplicate in 96-well plates at a ratio of 1:30. After 5 days, T-cell proliferation was measured by [3H]thymidine incorporation.
Barratt-Boyes et al. have shown that DCs derived from healthy chimpanzees are superior to monocytes and PBMCs for presenting tetanus toxoid to T cells (6). However, in that study the DCs were not exposed to maturation stimuli prior to the assay. We therefore compared the efficiencies of immature and mature DCs generated from a chronically infected animal (CB0507) for activating tetanus toxoid-specific memory CD4+ T cells. As expected, DCs were capable of activating tetanus toxoid-specific CD4+ T cells and the mature DCs induced a higher degree of proliferation (Fig. 5D) (n = 3). Therefore, DCs prepared from precursors of HCV-infected chimpanzees can process and present antigens to T cells.
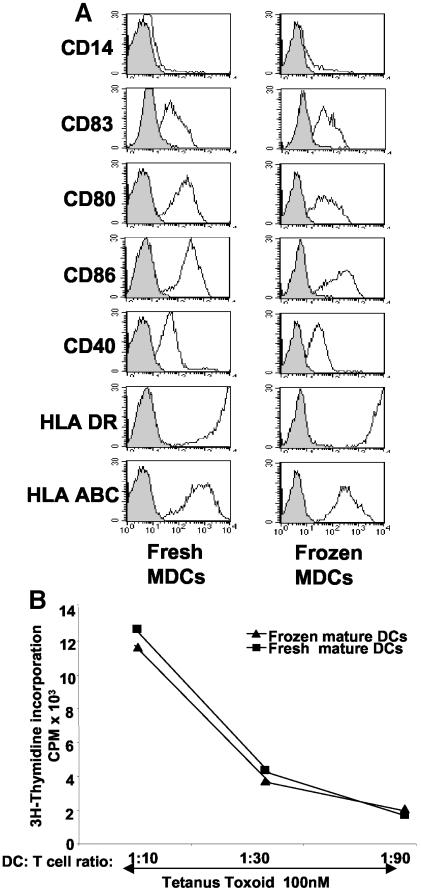
Cryopreserved mature DCs retain their phenotype and function.
In a vaccine setting, it would be advantageous to have large quantities of antigen-pulsed DCs prepared, frozen in aliquots, and thawed only at designated time points for injection. This would simplify the effort required to prepare DCs and ensure that DCs given in a series of vaccinations are identical. We compared the phenotypes (Fig. 6A) and functions (Fig. 6B) of cryopreserved mature DCs from an infected chimpanzee with those of freshly cultured DCs. The freezing and cryopreservation did not affect the DC phenotype, as it was essentially identical to that of freshly prepared cells (Fig. 6A). In addition, we were able to preserve cell numbers so that yields from thawed specimens ranged between 70 and 90%. The abilities of cryopreserved and fresh DCs to activate tetanus toxoid-specific T cells were then compared (Fig. 6B) (n = 3). As expected, the stimulation of tetanus toxoid-specific memory CD4+-T-cell responses was similar for freshly prepared and cryopreserved DCs. Therefore, we can recoup phenotypically and functionally mature DCs from frozen and thawed specimens without a substantial loss in cell numbers. This process should facilitate the undertaking of DC-based clinical trials in HCV-infected chimpanzees.
FIG. 6.
Cryopreserved mature DCs keep their phenotype and function. (A) Cryopreserved, mature DCs (MDC) (CB0507) were thawed and phenotypically compared to freshly matured DCs. The DCs were labeled with isotype controls and the indicated monoclonal antibodies. FACS histograms show profiles for isotype controls (gray areas with solid lines) and monoclonal antibodies (white areas with solid lines). The gates were set to include cells with large amounts of FSC and SSC. The data shown are representative of four experiments. (B) Freshly prepared and cryopreserved DCs (CB0507) were incubated for 1 h with 1 μg of tetanus toxoid/ml. Tetanus toxoid-pulsed DCs were added in graded doses to 2 × 105 autologous T cells. After 5 days, T-cell proliferation was measured by [3H]thymidine incorporation. The results are expressed in counts per minute. One representative experiment of three is shown.
DCs propagated from chronic HCV-positive chimpanzees are negative for HCV RNA.
The persistence of the HCV viral genome in mature monocyte-derived DCs was examined by a quantitative PCR-based assay. DCs from uninfected and chronically infected chimpanzees were cultured in plasma from a noninfected animal and frozen until the samples were analyzed by RT-PCR. We found no evidence of HCV genome persistence in DCs from chronically HCV-infected chimpanzees (Fig. 7). It is possible that if the DCs contained any HCV RNA, the level was below the detection sensitivity of this assay, which was 100 to 1,000 copies of HCV RNA. We looked for HCV RNA in 80 ng of RNA, which corresponds to 40,000 to 50,000 DCs, and we should have been able to detect ∼100 copies of HCV RNA in these cells if it was there. Our data differ from those of earlier studies which found that DCs derived from HCV-positive, chronically infected humans may contain HCV genomes and may even be productively infected by HCV (21). Whether these differences are due to the animals examined or to species differences needs to be further addressed.
FIG. 7.
DCs propagated from HCV-positive, chronically infected chimpanzees are negative for HCV RNA. DCs prepared from uninfected and chronically infected chimpanzees were cultured in 10% plasma from an uninfected animal and frozen until the samples were analyzed by RT-PCR. Eighty nanograms of total RNA from DCs (∼40,000 to 50,000 cells) was used for the quantitative PCRs. The controls were serially diluted HCV cRNA (106, 105, 104, 103, 102, and 101 HCV RNA molecules). DC 1 to DC 4, RNA samples from chronically HCV-infected chimpanzee (CB0507) DCs; DC5, RNA sample from uninfected chimpanzee (A255B) DCs. Serially diluted cRNAs were spiked with total RNAs from DCs (CB0507), GAPDH RNA was measured as an internal standard (not shown), and H2O was measured as a no-template control (NTC).
In summary, our data show unequivocally that phenotypically and functionally mature DCs without any evidence of HCV infection can be reproducibly generated from chronically infected chimpanzees.
DISCUSSION
The pivotal role of DCs in the activation of naïve T cells and the generation of primary T-cell responses is being explored in clinical trials of DC-based immunotherapy in humans. The in vitro generation of autologous DCs, followed by the injection of antigen-loaded DCs for the generation or boosting of antigen-specific T-cell-mediated immunity in vivo has been the most common approach to date (20, 24, 34, 53). The chimpanzee is the most suitable animal model for studying infections of and immunity to HCV, in addition to immunotherapeutic interventions, as the data obtained from these studies can be extrapolated to humans (55). The objective of our study was to determine whether functionally competent DCs could be differentiated from blood precursors of HCV-infected chimpanzees, as previous studies have indicated that these cells are compromised, possibly by exposure to high levels of HCV. A compromise in function would inherently rule out the use of DCs as vaccine adjuvants in the chimpanzee model. Therefore, we first isolated DC precursors from leukapheresis products and assessed whether they could be differentiated under the GMP conditions that are required for vaccine preparations, analogous to the preparation of human DCs. The parameters examined were yields, purity, maturation stimuli, phenotype, cytokine and chemokine secretion, APC function, and function of cryopreserved cells.
In earlier studies, DCs were propagated from chimpanzee PBMCs by the adhesion of DC progenitors to culture plates (5). In the case of human PBMCs, this method provides >90% pure DCs, whereas in the case of chimpanzees the purity is <70% (8; also data not shown) and the yields are reportedly low (44). To obtain DCs of a higher level of purity that would fulfill GMP standards, we first positively selected CD14+ progenitors from PBMCs. The advantages of the CD14+ selection step are that it applies the same conditions of sterility used by the Clinimacs systems (Miltenyi) for obtaining human CD14+ human monocytes and it yields reproducibly higher numbers and purities of DCs (yields of up to 12% of the CD14+ population and purities in excess of 95%). Furthermore, we found that DCs could be successfully propagated in culture medium containing plasma from chimpanzees versus FCS, thereby avoiding exposure to foreign proteins which are potentially immunogenic.
Previous studies of chimpanzee DCs have largely focused upon immature DCs. In one study, the subcutaneous injection of in vitro-propagated immature chimpanzee DCs revealed that a significant proportion migrated rapidly to the draining lymph nodes (4). Importantly, the injected cells retained a high level of expression of MHC class II molecules, CD40, and CD86. However, in another study, when they were pulsed with peptides from OVA or the MUC-1 tumor antigen, although DCs induced antibody responses, these were no higher than those from a direct injection of antigen with adjuvant (6). T-cell responses were seen for only one of the animals (6). The lack of a significant induction of immunity may well be due to the use of immature DCs, which may be tolerogenic (14), versus mature DCs, which are immunostimulatory (15). For these reasons, we compared the ability of two standard maturation stimuli currently in clinical use to induce the maturation of chimpanzee DCs and assessed the phenotypic and functional differences between immature and mature DCs. Importantly, the MCM mimic, now used in several human clinical trials, was equivalent to CD40LT for differentiating immature DCs from infected chimpanzees. No significant phenotypic or functional differences were apparent between the two stimuli. Since CD40LT is no longer available for clinical use to most investigators, the MCM mimic serves as a suitable and simple alternative.
An extensive analysis of the phenotypes and the cytokine and chemokine secretion profiles of immature versus mature chimpanzee DCs revealed a close correspondence to their human counterparts, with the induction of CD83 and CCR7 expression and the upregulation of costimulatory molecules CD40, CD80, and CD86 and MHC class I and II molecules. Interestingly, Rollier et al. reported the induction of CD83 by chimpanzee DCs generated in GM-CSF and IL-13 versus GM-CSF and IL-4 (44). Whether these DCs were equivalent to the mature DCs we obtained after culturing in GM-CSF and IL-4, followed by the MCM mimic, will require further evaluation. Our studies also revealed for the first time the expression of the chemokine receptors CCR5, CCXR4, and CCR7 on chimpanzee DCs. This phenotype of mature chimpanzee DCs (in particular for CCR7) suggests that they should be capable of migrating to draining lymph nodes after injection and of priming and activating immune responses. In addition, the expression of CCR5 suggests that mature chimpanzee DCs will respond to RANTES within the local environment. Finally, we also demonstrated that DCs from HCV-infected chimpanzees retain their capacity to secrete CCL7, TNF-α, and RANTES at levels that are equal to those for DCs from uninfected chimpanzees. The TNF-α levels were substantially higher than could be accounted for by the addition of the MCM mimic, suggesting that there is endogenous cytokine secretion by the mature DCs. Mature DCs therefore have the capacity to attract other DCs or T cells into the local environment and thereby amplify local immune responses.
Whether DCs are functionally compromised due to HCV exposure remains highly controversial. A number of studies have described the impairment of DC maturation or function when cells are derived from either HCV-positive chronically infected humans or chimpanzees (2, 3, 26). One study showed a resistance to maturation by TNF-α, (2) and another found normal DC maturation in response to lipopolysaccharide (23). These differences could be due to the fact that TNF-α may not be a potent enough maturation stimulus on its own, as it ordinarily does not induce the complete maturation of human DCs (11, 32). As noted above, we discerned no significant impairment in the maturation of DCs from HCV-infected chimpanzees, as their phenotype and secretion profiles were identical to those for DCs from uninfected animals. Significantly, these DCs were equally capable of activating allogeneic T cells, similar to the recent findings of Rollier et al. (44). However, we also show for the first time that mature DCs from chronically infected chimpanzees efficiently process and present tetanus toxoid to autologous T cells and stimulate HCV-specific CD8+-T-cell clones. Because our DCs were derived from subjects who were infected with HCV for many years (>8 years), it appears at least from the small cohort studied here that a long-term exposure to HCV does not obviously compromise DC precursor function. DCs derived from monocyte precursors of chronically infected subjects have been shown to display suboptimal T-cell stimulatory functions when HCV core proteins are presented (25, 26). One reason may be that these subjects came to clinical attention when they were obviously ill, which our chimpanzee cohort is not, and that very significant virus levels or a virus-induced pathology is needed to compromise DC functions. However, it should be kept in mind that DCs from chronically infected individuals may be unable to present HCV antigens to autologous T cells because of dysfunctional T-cell responses rather than defects in the DCs themselves.
In the final analysis, we tested whether DCs derived from HCV-infected chimpanzees contained evidence of HCV infection. Recently, it was found that blood DCs from HCV-infected individuals contained HCV and that DCs from some of these patients had replicative HCV RNA (21). Furthermore, Navas et al. showed that immature and matured monocyte-derived DCs can be infected by HCV genotype 1 and can support at least the first step of the viral cycle in vitro, although at very low levels (38). This was done in a system in which the DCs were cultured in the presence of HCV-positive serum. In our case, when we examined the presence of HCV RNA in DCs derived from chronically infected chimpanzees, we did not detect HCV RNA, i.e., DCs from chimpanzees do not contain and are not infected with HCV. For verification of our findings, a larger group of HCV-infected animals will need to be examined.
Overall, our data are consistent with the interpretation of others (10) that blood monocytes are not infected in vivo, even in animals that are infected for long periods of time. In many ways, our findings mirror the situation for other chronic viral infections, e.g., HIV-1 infections, for which it is possible to readily obtain DCs from monocyte precursors which are not obviously infected or functionally compromised even in the setting of relatively high virus loads (46). It is important to note that our studies do not address whether circulating blood DCs are compromised in vivo, which would be difficult to examine due to the low levels of DCs in the total PBMC population (<1%). Therefore, we cannot definitely conclude that T-cell dysfunctions in the chronic infection setting are not due to a blood or tissue DC dysfunction. It is possible that, as in untreated and acute HIV-1 infections, DC numbers may decline substantially (17, 40), an option that we are currently exploring.
The use of DCs in immunotherapy in which repetitive injections are performed requires the generation of DCs for each DC vaccination, either from fresh blood samples or from frozen PBMC aliquots (15, 39, 53). Feuerstein et al. have shown that human DCs can be generated in large quantities and cryopreserved and that the thawed DCs still function in a similar manner as fresh cells (19). We similarly examined the function of cryopreserved chimpanzee DCs versus freshly prepared DCs from the same donor. Analogous to the experience with human DCs, we found cryopreserved cells to be phenotypically and functionally similar to fresh cells. This is promising both for in vitro experiments and in vivo clinical applications, as it ensures that DCs used at different time points are identical in both source and function. Furthermore, the preparation of a large quantity of DCs at one time and subsequent cryopreservation are a more practical approach. We are currently investigating whether cryopreserved DCs also present HCV antigens to autologous T cells.
In summary, we and other groups (33, 44) have shown that monocyte-derived DCs from chronically infected humans or chimpanzees are phenotypically and functionally intact when generated in vitro. The ability to generate standardized and significant numbers of DCs from blood CD14+ progenitors, as shown here, facilitates the possibility of testing their adjuvant capacity in vivo. Because mature DCs can prime CD4 and CD8 immune responses effectively, we predict that they will have the potential to reverse the ineffective T-cell responses observed in chronic infections.
Acknowledgments
We thank Dana Hasselschwert and Neil Smith at the New Iberia Research Center for their assistance with obtaining chimpanzee blood samples and for their help with animal welfare.
These studies were supported by grants to C. Walker (R01 AI47367 and U19 AI48231), M. Larsson (FAIR award and NYU CFAR award), C. Rice (CA57973 and A140034; Greenberg Medical Research Institute), A. Grakoui (postdoctoral fellowship from The Cancer Research Institute), and N. Bhardwaj (AI 44628, a Burroughs Wellcome Clinical Investigator Award and a Doris Duke Distinguished Clinical Scientist Award). N. Bhardwaj is an Elizabeth Glaser Scientist.
REFERENCES
- 1.Alter, M. J. 1996. Epidemiology of hepatitis C. Eur. J. Gastroenterol. Hepatol. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 2.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 3.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 4.Barrat-Boyes, S. M., S. C. Watkins, and O. J. Finn. 1997. In vivo migration of dendritic cells differentiated in vitro. A chimpanzee model. J. Immunol. 158:4543-4547. [PubMed] [Google Scholar]
- 5.Barratt-Boyes, S. M., R. A. Henderson, and O. J. Finn. 1996. Chimpanzee dendritic cells with potent immunostimulatory function can be propagated from peripheral blood. Immunology 87:528-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barratt-Boyes, S. M., H. Kao, and O. J. Finn. 1998. Chimpanzee dendritic cells derived in vitro from blood monocytes and pulsed with antigen elicit specific immune responses in vivo. J. Immunother. 21:142-148. [DOI] [PubMed] [Google Scholar]
- 7.Battegay, M., J. Fikes, A. M. Di Bisceglie, P. A. Wentworth, A. Sette, E. Celis, W. M. Ching, A. Grakoui, C. M. Rice, and K. Kurokohchi. 1995. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J. Virol. 69:2462-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender, A., M. Sapp, G. Schuler, R. M. Steinman, and N. Bhardwaj. 1996. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods 196:121-135. [DOI] [PubMed] [Google Scholar]
- 9.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boisvert, J., X. S. He, R. Cheung, E. B. Keeffe, T. Wright, and H. B. Greenberg. 2001. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J. Infect. Dis. 184:827-835. [DOI] [PubMed] [Google Scholar]
- 11.Buelens, C., V. Verhasselt, D. De Groote, K. Thielemans, M. Goldman, and F. Willems. 1997. Human dendritic cell responses to LPS and CD40 ligation are differentially regulated by IL-10. Eur. J. Immunol. 27:1848-1852. [DOI] [PubMed] [Google Scholar]
- 12.Cerny, A., J. G. McHutchison, C. Pasquinelli, M. E. Brown, M. A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F. V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Investig. 95:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar, M. V., R. M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhodapkar, M. V., R. M. Steinman, M. Sapp, H. Desai, C. Fossella, J. Krasovsky, S. M. Donahoe, P. R. Dunbar, V. Cerundolo, D. F. Nixon, and N. Bhardwaj. 1999. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Investig. 104:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolganiuc, A., K. Kodys, A. Kopasz, C. Marshall, T. Do, L. Romics, P. Mandrekar, M. Zapp, and G. Szabo. 2003. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J. Immunol. 170:5615-5624. [DOI] [PubMed] [Google Scholar]
- 17.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 18.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 19.Feuerstein, B., T. G. Berger, C. Maczek, C. Roder, D. Schreiner, U. Hirsch, I. Haendle, W. Leisgang, A. Glaser, O. Kuss, T. L. Diepgen, G. Schuler, and B. Schuler-Thurner. 2000. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods 245:15-29. [DOI] [PubMed] [Google Scholar]
- 20.Fong, L., and E. G. Engleman. 2000. Dendritic cells in cancer immunotherapy. Annu. Rev. Immunol. 18:245-273. [DOI] [PubMed] [Google Scholar]
- 21.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Clin. Investig. 187:1951-1957. [DOI] [PubMed] [Google Scholar]
- 22.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302:659-662. [DOI] [PubMed] [Google Scholar]
- 23.Jinushi, M., T. Takehara, T. Kanto, T. Tatsumi, V. Groh, T. Spies, T. Miyagi, T. Suzuki, Y. Sasaki, and N. Hayashi. 2003. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J. Immunol. 170:1249-1256. [DOI] [PubMed] [Google Scholar]
- 24.Jonuleit, H., A. Giesecke-Tuettenberg, T. Tuting, B. Thurner-Schuler, T. B. Stuge, L. Paragnik, A. Kandemir, P. P. Lee, G. Schuler, J. Knop, and A. H. Enk. 2001. A comparison of two types of dendritic cells as adjuvants for the induction of melanoma-specific T cell responses in humans following intranodal injection. Int. J. Cancer 93:243-251. [DOI] [PubMed] [Google Scholar]
- 25.Kakumu, S., A. Okumura, T. Ishikawa, K. Iwata, M. Yano, and K. Yoshioka. 1997. Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 108:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 162:5584-5591. [PubMed] [Google Scholar]
- 27.Koziel, M. J., D. Dudley, J. T. Wong, J. Dienstag, M. Houghton, R. Ralston, and B. D. Walker. 1992. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 149:3339-3344. [PubMed] [Google Scholar]
- 28.Larsson, M., X. Jin, B. Ramratnam, G. S. Ogg, J. Engelmayer, M. A. Demoite, A. J. McMichael, W. I. Cox, R. M. Steinman, D. Nixon, and N. Bhardwaj. 1999. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of pol specific CD8+ T cells in chronically infected HIV-1 positive individuals. AIDS 13:767-777. [DOI] [PubMed] [Google Scholar]
- 29.Lauer, G. M., K. Ouchi, R. T. Chung, T. N. Nguyen, C. L. Day, D. R. Purkis, M. Reiser, A. Y. Kim, M. Lucas, P. Klenerman, and B. D. Walker. 2002. Comprehensive analysis of CD8+-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J. Virol. 76:6104-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 31.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, A. W., T. Truong, K. Bickham, J. F. Fonteneau, M. Larsson, I. Da Silva, S. Somersan, E. K. Thomas, and N. Bhardwaj. 2002. A clinical grade cocktail of cytokines and PGE(2) results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine 20(Suppl. 4):A8-A22. [DOI] [PubMed] [Google Scholar]
- 33.Longman, R. S., A. L. Talal, I. M. Jacobson, M. L. Albert, and C. M. Rice. 2003. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood 103:1026-1029. [DOI] [PubMed] [Google Scholar]
- 34.Lu, W., X. Wu, Y. Lu, W. Gou, and J. M. Andrieu. 2003. Therapeutic dendritic cell vaccine for simian AIDS. Nat. Med. 9:27-32. [DOI] [PubMed] [Google Scholar]
- 35.Matsui, M., O. Moriya, N. Abdel-Aziz, Y. Matsuura, T. Miyamura, and T. Akatsuka. 2002. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells transduced with replication-defective recombinant adenovirus. Vaccine 21:211-220. [DOI] [PubMed] [Google Scholar]
- 36.Mellor, J., G. Haydon, C. Blair, W. Livingstone, and P. Simmonds. 1998. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J. Gen. Virol. 79:705-714. [DOI] [PubMed] [Google Scholar]
- 37.Moriya, O., M. Matsui, M. Osorio, H. Miyazawa, C. M. Rice, S. M. Feinstone, S. H. Leppla, J. M. Keith, and T. Akatsuka. 2001. Induction of hepatitis C virus-specific cytotoxic T lymphocytes in mice by immunization with dendritic cells treated with an anthrax toxin fusion protein. Vaccine 20:789-796. [DOI] [PubMed] [Google Scholar]
- 38.Navas, M. C., A. Fuchs, E. Schvoerer, A. Bohbot, A. M. Aubertin, and F. Stoll-Keller. 2002. Dendritic cell susceptibility to hepatitis C virus genotype 1 infection. J. Med. Virol. 67:152-161. [DOI] [PubMed] [Google Scholar]
- 39.Nestle, F. O., S. Alijagic, M. Gilliet, Y. Sun, S. Grabbe, R. Dummer, G. Burg, and D. Schadendorf. 1998. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 4:328-332. [DOI] [PubMed] [Google Scholar]
- 40.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 41.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranslow, W., S. Srinivasan, R. Paxton, M. Gibson, M. Spiggs, and R. Armitage. 1994. Structural characteristics of CD40 ligand that determine biological function. Semin. Immunol. 6:267-278. [DOI] [PubMed] [Google Scholar]
- 43.Rehermann, B., K. M. Chang, J. G. McHutchison, R. Kokka, M. Houghton, and F. V. Chisari. 1996. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Investig. 98:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollier, C., J. A. R. Drexhage, B. E. Verstepen, E. J. Verschoor, R. E. Bontrop, G. Koopman, and J. L. Heeney. 2003. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology 38:851-858. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto, F., B. Palermo, D. Lenig, M. Miettinen, S. Matikainen, I. Julkunen, R. Forster, R. Burgstahler, M. Lipp, and A. Lanzavecchia. 1999. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 29:1617-1625. [DOI] [PubMed] [Google Scholar]
- 46.Sapp, M., J. Engelmeyer, M. Larsson, A. Granelli-Piperno, R. M. Steinman, and N. Bhardwaj. 1999. Dendritic cells generated from blood monocytes of HIV-1 patients are not infected and act as competent antigen presenting cells eliciting potent T cell responses. Immunol. Lett. 66:121-128. [DOI] [PubMed] [Google Scholar]
- 47.Sarobe, P., J. J. Lasarte, N. Casares, A. Lopez-Diaz de Cerio, E. Baixeras, P. Labarga, N. Garcia, F. Borras-Cuesta, and J. Prieto. 2002. Abnormal priming of CD4+ T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J. Virol. 76:5062-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarobe, P., J. J. Lasarte, A. Zabaleta, L. Arribillaga, A. Arina, I. Melero, F. Borras-Cuesta, and J. Prieto. 2003. Hepatitis C virus proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J. Virol. 77:10862-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirai, M., H. Okada, M. Nishioka, T. Akatsuka, C. Wychowski, R. Houghten, C. D. Pendleton, S. M. Feinstone, and J. A. Berzofsky. 1994. An epitope in hepatitis C virus core region recognized by cytotoxic T cells in mice and humans. J. Virol. 68:3334-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chein, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinman, R. M. 2000. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mount Sinai J. Med. 68:160-166. [PubMed] [Google Scholar]
- 52.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurner, B., C. Röder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kämpgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223:1-15. [DOI] [PubMed] [Google Scholar]
- 54.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76:12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker, C. M. 1997. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin. Immunopathol. 19:85-98. [DOI] [PubMed] [Google Scholar]
- 56.Wertheimer, A. M., C. Miner, D. M. Lewinsohn, A. W. Sasaki, E. Kaufman, and H. R. Rosen. 2003. Novel CD4+ and CD8+ T-cell determinants within the NS3 protein in subjects with spontaneously resolved HCV infection. Hepatology 37:577-589. [DOI] [PubMed] [Google Scholar]