Abstract
Introduction
The equivalence of breast-conserving surgery followed by postoperative radiotherapy against mastectomy is now firmly established in patients with early breast cancer. The results of surgery in large-breasted women can be poor, with radiation-induced fibrosis, chronic pain and poor cosmesis contributing to long-term psychological and physical morbidity. Therapeutic mammoplasty offers an alternative management strategy to both enhance the role of breast-conserving surgery and provide better outcomes.
Methods
A retrospective note review was undertaken of all patients undergoing therapeutic mammoplasty for breast malignancy between 2007 and 2011. All cases were performed using a Wise pattern-reduction technique. Histology and pathological outcomes were assessed. Postoperative outcomes reviewed included wound infection, seroma and need for further intervention.
Results
During the study period, 20 patients underwent therapeutic mammoplasty with a mean follow-up duration of 36 months. The mean weight of the lumpectomy specimen was 330g. The average cancer size was 34mm, with a mean margin clearance of 7mm. There was one episode of wound infection and three of delayed wound healing at the T-junction. One patient required a mastectomy for involved margins. There were no recurrences at the most recent follow-up visit.
Conclusions
Therapeutic mammoplasty offers a tailored approach to women with larger breasts and early breast cancers with good cosmetic results and oncological outcomes.
Keywords: Early breast cancer, Oncoplastic surgery, Complications, Large breasts
Breast-conserving surgery with adequate surgical margins followed by radiotherapy has been shown to be oncologically equivalent to a mastectomy. 1–3 Despite this, breast conserving surgery in patients with larger tumours or tumours in unfavourable anatomical locations in the breast can lead to poor aesthetic and psychological outcomes 4,5 following radiotherapy. Larger breasts are at risk of receiving non-homogenous radiation dosage and at an increased risk of postoperative 6 and long-term cosmetic 7 complications. These complications may delay or even preclude the initiation of adjuvant therapy and impact adversely on survival. 8
Therapeutic mammoplasty is the use of breast reduction techniques to perform breast cancer resection. Using oncoplastic techniques such as therapeutic mammoplasty can extend the role of breast-conserving surgery in ptotic or large-breasted patients to facilitate wider resection margins, immediate symmetry and perhaps less radiation toxicity. This article presents the experience of therapeutic mammoplasty in large or ptotic-breasted patients in the management of early breast cancer in a district general hospital setting.
Methods
All patients who underwent therapeutic mammoplasty between 2007 and 2011 at a single district general hospital breast unit were included in the study. Patients were offered therapeutic mammoplasty if they had large breasts with grade 3 or 4 ptosis 9 with a tumour confined to a single quadrant, where the tumour volume was such that standard wide local excision would resect approximately 20% or more of their estimated breast volume (by clinical assessment alone). Patients who were insulin-dependent diabetics or who had multifocal disease were not offered this approach.
Patient records were reviewed for demographic characteristics, co-morbidities, surgical technique, adjuvant treatment, postoperative complications and follow-up duration. Tumour size was based on pathological specimens rather than radiographic imaging. For those patients undergoing neoadjuvant therapy, tumour size was classified on the basis of initial pretreatment mammographic tumour size and not on tumour size at the time of surgery. In this series, patients with objective clinical and radiological response after neoadjuvant therapy were offered breast reduction if appropriate. Hormone receptor and HER2/neu status were also noted for each specimen.
Surgical technique
All patients received preoperative intravenous antibiotics (cefuroxime 1.5g) at induction of anaesthesia with two further postoperative intravenous doses. Standard therapeutic mammoplasty technique (Fig 1) and preoperative oncoplastic breast reduction markings (Fig 2) were used. This involved placement of the new nipple height at the level of the projected inframammary fold, dropping of perpendicular limbs to the meridian and maintaining the nipple viability via an inferior pedicle. After skin incision, skin de-epithelialisation is performed. Skin flaps are then elevated, paying careful attention to leave an appropriate subcutaneous tissue plane to preserve the viability of the skin envelope. Local mobilisation of the breast off the chest wall is followed by formal wide local excision of the lesion along with standard resection of breast tissue. Oncological tissue is orientated and marked, and sent separately to normal breast tissue resected. Because the wide local excision defect is closed, surgical clips are placed to facilitate accurate radiotherapy planning.
Figure 1.

Example operative approach demonstrating technique. Cancer excised in reduction pattern excision on affected side with mirror reduction pattern excision of contralateral breast. Top: preoperative incision marking (dotted line) and example tumour location (star); middle: de-epithelialised pedicle flap (tinted area) with skin flaps closed over defect; bottom: postoperative wound closure and suture lines
Figure 2.
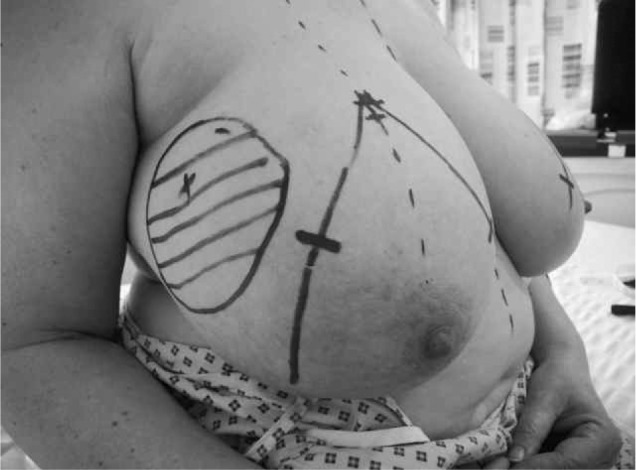
Preoperative photograph of patient with 8cm right upper outer quadrant tumour undergoing therapeutic mammoplasty with preoperative markings
On completion of oncological excision, resection of normal breast tissue, shaping of the breast mound and filling of the defect using mobilised parenchymal tissue is performed. An inferior pedicle breast reduction technique was used in the majority of cases, allowing reshaping of the breast mound and closure of dead space by displacement and rotation of the dermoglandular pedicle, which has a minimum width of 8cm to preserve nipple areolar complex perfusion. The skin is then closed without tension in layers, creating an inverted T scar. The nipple is repositioned at the level of the inframammary fold and re-inset in its new location. The contralateral breast reduction is performed, adjusting for underlying asymmetry in volume of tissue resected to provide symmetry (Fig 3).
Figure 3.
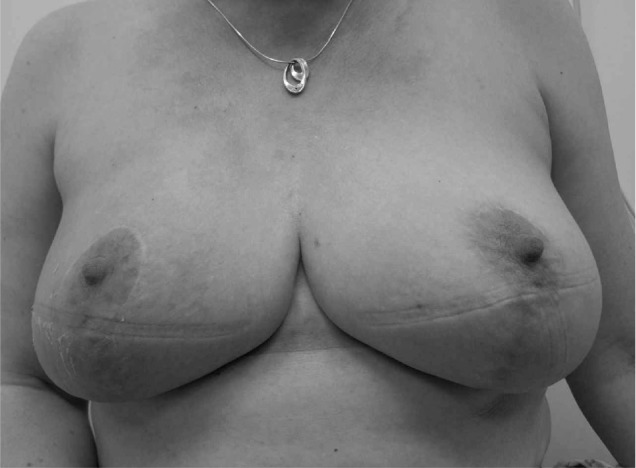
Postoperative photograph of patient at 1 year following therapeutic mammoplasty, radiotherapy and chemotherapy for 8cm right upper outer quadrant tumour
Results
From January 2007 to December 2011, 20 patients undergoing therapeutic mammoplasty were identified and included in our review (Table 1). The median follow-up duration was 36 months (interquartile range [IQR]: 10–45 months). The mean patient age at the time of therapeutic mammoplasty was 50 years. Nine patients (45%) had right-sided breast lesions and the remainder (55%) had left-sided breast lesions. The co-morbidities in our series included hypertension (n=5, 25%), hypercholesterolaemia (n=4, 20%), hypothyroidism (n=1, 5%) and type 2 diabetes mellitus (n=1, 5%) although the vast majority of our patients were healthy without significant medical issues. Five patients (25%) had a history of prior or ongoing smoking. The median body mass index was 28.5kg/m 2 (IQR: 26-33kg/m 2 ).
Table 1.
Characteristics of patients undergoing therapeutic mammoplasty at Kingston Hospital NHS Trust (2007–2011)
| Number of patients | 20 |
| Mean age (range) | 50 years (33–69 years) |
| Mean tumour size (range) | 38mm (7–80mm) |
| Mean weight of specimen (range) | 370g (150–1,414g) |
| Receptor status | |
| ER+/PR+ | 15 |
| ER+/PR- | 1 |
| ER-/PR- | 1 |
| Histological subtype | |
| Infiltrating ductal carcinoma | 13 |
| Infiltrating lobular carcinoma | 4 |
| Phyllodes tumour | 1 |
| B-cell lymphoma | 1 |
| Pseudoangiomatous stromal hyperplasia | 1 |
ER+ = oestrogen receptor positive; ER- = oestrogen receptor negative; PgR+ = progesterone receptor positive; PgR- = progesterone receptor negative
Breast cancer distribution
Pathology specimens and clinical data for the 20 cases were reviewed (Table 1). The mean preoperative imaging lesion size was 35mm (range: 8–70mm). Pathological diagnoses of early breast cancer were seen in 17 cases. Invasive ductal carcinoma was the most common type (n=13), followed by invasive lobular carcinoma (n=4). Three cases received non-breast carcinoma diagnoses (phyllodes tumour, B-cell lymphoma and pseudoangiomatous stromal hyperplasia). These cases had concerning biopsy results for which the multidisciplinary team recommended excision.
Five patients with breast cancer had stage I disease (29%) and 12 patients had stage II disease (71%). The mean cancer size was 26mm (range: 8–39mm). The tumour size was 38mm for the phyllodes case. The B-cell lymphoma case had a tumour of 70mm and the pseudoangiomatous stromal hyperplasia mass was 80mm.
Among the cancer cases, 16 were oestrogen receptor positive, 15 were progesterone receptor positive and there were no cases of HER2/neu positivity. Three patients had positive lymph nodes on preoperative testing or sentinel node dissection. All of these subsequently underwent completion axillary node dissection. There were no episodes of recurrence at the most recent follow-up visit.
Adjuvant therapy
In our cohort, all breast cancer patients were treated with adjuvant radiotherapy. Five patients received chemotherapy, four of whom received therapy in the neoadjuvant setting. All patients with hormone receptor positive invasive cancer (16/17) received adjuvant endocrine therapy.
Outcomes
Breast conservation with clear initial margins was successful in 16 of the 17 malignant cases. One patient underwent a completion mastectomy following positive margins with subsequent adjuvant chemotherapy.
There were no major postoperative complications, defined as requiring inpatient hospitalisation or an unplanned return to the operating theatre. There were four cases of minor complications including one wound infection requiring oral antibiotics alone and three cases of delayed wound healing at the T-junction. All of these were treated conservatively in the outpatient setting.
Discussion
This series demonstrates that therapeutic mammoplasty is a valuable alternative surgical option for early breast cancer in patients with large tumours and ptotic breasts to avoid poor outcomes from breast-conserving surgery and, in some cases, to avoid mastectomy. Low rates of minor complications and the absence of major complications in this series mean this is a suitable approach in selected cases. Therapeutic mammoplasty is a technique developed from cosmetic breast reduction surgery. In high-volume centres it has shown improved cosmetic results, and similar five-year survival and local recurrence rates to breast-conserving surgery. 10–12
In our study, therapeutic mammoplasty was associated with a low rate of postoperative complications, which compares favourably with complication rates seen in other studies. 13,14 Trials that evaluate wound complications of therapeutic mammoplasty for breast cancer have a relatively small number of cases. A systematic review from 2012 examining 1,702 patients in 25 cases series worldwide found wide-ranging complication rates from 10% to 91%. 13 However, the vast majority of these were minor and adjuvant therapy was delayed in only 6% of patients.
Although our study population was relatively healthy with few co-morbidities, a quarter of the patients had a history of prior or ongoing tobacco use, which did not seem to be a significant risk factor for the development of complications. All the major complications occurred in the early postoperative period, prior to the start of adjuvant radiation therapy. Although all of the patients received adjuvant radiation therapy, no later postoperative complications during or after radiation therapy were seen.
This finding is particularly compelling when considering therapeutic mammoplasty as an alternative to other breast-conserving surgery such as wide local excision in women with larger breasts who will require adjuvant radiotherapy. Radiation dose homogeneity is far more difficult to achieve in large breasts. 15 It is common to have areas in the breast that receive 10–15% more dose and radiation fibrosis, chronic pain and poor cosmesis often result. 16 Therapeutic mammoplasty offers a way to achieve robust wide local excision margins and facilitate smaller, symmetrical breasts that more easily tolerate radiation.
In the literature, the most common complications following therapeutic mammoplasty were minor wound infections and haematoma formation, followed by delayed wound healing and minor wound dehiscence. 13 The rate of nipple–areola complex necrosis was 1–2%. Wound dehiscence occurs most frequently at the T-junction areas (Fig 1). Closure of skin under tension or poor attention to skin envelope viability risks delayed wound healing, particularly in the T-junction. All the minor delayed wound healing seen in this series occurred in this T-junction area. Smoking did not appear to be a factor in complications in our series. Conversely, in the series reported by Munhoz et al, smoking was associated with a significantly increased risk of developing surgical complications following therapeutic mammoplasty. 17 Additionally, Munhoz et al demonstrated a significant increase in complications in obese patients. However, other studies have not seen that association. 18
Quality of life and patient satisfaction have been shown to be improved significantly by the use of therapeutic mammoplasty in managing early breast cancer. 19 Data from 2010 have demonstrated that the use of reduction mammoplasty techniques for reconstruction of partial mastectomy defects improves patient self-esteem and mental health when compared with patients undergoing breast-conserving surgery without reconstruction. 20 Nevertheless, this study is limited by the use of non-validated quality-of-life assessment tools.
Conclusions
Therapeutic mammoplasty can provide an effective, oncologically safe operation in the district general hospital setting with limited complications while also achieving good aesthetic outcomes. It should therefore be considered as a surgical approach in early breast cancer for women with larger tumours and ptotic breasts.
References
- 1.Poggi MM, Danforth DN, Sciuto LCet al Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003; 98: 697–702. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant Jet al Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1,233–1,241. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino J, Redmond Cet al Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993; 328: 1,581–1,586. [DOI] [PubMed] [Google Scholar]
- 4.Fitzal F, Mittlboeck M, Trischler Het al Breast-conserving therapy for centrally located breast cancer. Ann Surg 2008; 247: 470–476. [DOI] [PubMed] [Google Scholar]
- 5.Poulsen B, Graversen HP, Beckmann J, Blichert-Toft M. A comparative study of post-operative psychosocial function in women with primary operable breast cancer randomized to breast conservation therapy or mastectomy. Eur J Surg Oncol 1997; 23: 327–334. [DOI] [PubMed] [Google Scholar]
- 6.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer 2008; 113: 3,108–3,115. [DOI] [PubMed] [Google Scholar]
- 7.Hershman DL, Wang X, McBride Ret al Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys 2006; 65: 1,353–1,360. [DOI] [PubMed] [Google Scholar]
- 8.Mikeljevic JS, Haward R, Johnston Cet al Trends in postoperative radiotherapy delay and the effect on survival in breast cancer patients treated with conservation surgery. Br J Cancer 2004; 90: 1,343–1,348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCulley SJ, Macmillan RD. Planning and use of therapeutic mammoplasty – Nottingham approach. Br J Plast Surg 2005; 58: 889–901. [DOI] [PubMed] [Google Scholar]
- 10.Chang EI, Peled AW, Foster RDet al Evaluating the feasibility of extended partial mastectomy and immediate reduction mammoplasty reconstruction as an alternative to mastectomy. Ann Surg 2012; 255: 1,151–1,157. [DOI] [PubMed] [Google Scholar]
- 11.Rietjens M, Urban CA, Rey PCet al Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast 2007; 16: 387–395. [DOI] [PubMed] [Google Scholar]
- 12.Clough KB, Lewis JS, Couturaud Bet al Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003; 237: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntosh J, O’Donoghue JM. Therapeutic mammaplasty – a systematic review of the evidence. Eur J Surg Oncol 2012; 38: 196–202. [DOI] [PubMed] [Google Scholar]
- 14.Hernanz F, Regaño S, Vega A, Gómez Fleitas M. Reduction mammaplasty: an advantageous option for breast conserving surgery in large-breasted patients. Surg Oncol 2010; 19: e95–e102. [DOI] [PubMed] [Google Scholar]
- 15.Moody AM, Mayles WP, Bliss JMet al The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol 1994; 33: 106–112. [DOI] [PubMed] [Google Scholar]
- 16.Smith ML, Evans GR, Gürlek Aet al Reduction mammaplasty: its role in breast conservation surgery for early-stage breast cancer. Ann Plast Surg 1998; 41: 234–239. [PubMed] [Google Scholar]
- 17.Munhoz AM, Montag E, Arruda EGet al Critical analysis of reduction mammaplasty techniques in combination with conservative breast surgery for early breast cancer treatment. Plast Reconstr Surg 2006; 117: 1,091–1,103. [DOI] [PubMed] [Google Scholar]
- 18.Gulcelik MA, Dogan L, Camlibel Met al Early complications of a reduction mammoplasty technique in the treatment of macromastia with or without breast cancer. Clin Breast Cancer 2011; 11: 395–399. [DOI] [PubMed] [Google Scholar]
- 19.Patel KM, Hannan CM, Gatti ME, Nahabedian MY. A head-to-head comparison of quality of life and aesthetic outcomes following immediate, staged-immediate, and delayed oncoplastic reduction mammaplasty. Plast Reconstr Surg 2011; 127: 2,167–2,175. [DOI] [PubMed] [Google Scholar]
- 20.Veiga DF, Veiga-Filho J, Ribeiro LMet al Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg 2010; 125: 811–817. [DOI] [PubMed] [Google Scholar]


