Abstract
Objective
Analyze the long-term outcome of callosotomies with regard to seizure types and frequencies and antiepileptic drug treatment.
Methods
This longitudinal observational study is based on data from the prospective Swedish National Epilepsy Surgery Register. Thirty-one patients had undergone callosotomy in Sweden 1995–2007 and had been followed for 2 and 5 or 10 years after surgery. Data on their seizure types and frequencies, associated impairments, and use of antiepileptic drugs have been analyzed.
Results
The median total number of seizures per patient and month was reduced from 195 before surgery to 110 two years after surgery and 90 at the long-term follow-up (5 or 10 years). The corresponding figures for drop attacks (tonic or atonic) were 190 before surgery, 100 2 years after surgery, and 20 at the long-term follow-up. Ten (56%) of the 18 patients with drop attacks were free from drop attacks at long-term follow-up. Three of the remaining eight patients had a reduction of >75%. At long-term follow-up, four were off medication. Only one of the 31 patients had no neurologic impairment.
Significance
The present population-based, prospective observational study shows that the corpus callosotomy reduces seizure frequency effectively and sustainably over the years. Most improvement was seen in drop attacks. The improvement in seizure frequency over time shown in this study suggests that callosotomy should be considered at an early age in children with intractable epilepsy and traumatizing drop attacks.
Keywords: Epilepsy surgery, Callosotomy, Outcome, Long-term
 |
The most common indication for callosotomy is drop attacks (tonic or atonic), which often lead to severe physical injuries.1,2 Other indications are West syndrome, Lennox-Gastaut syndrome, and recurrent episodes of status epilepticus or complex partial seizures with rapid secondary generalization without any obvious foci.3 Callosotomy is performed in both children and adults. Children seem to have fewer postsurgical neurologic sequelae than adults do, apparently because of the plasticity of their brains.2
A well-known adverse effect of callosotomy is the “disconnection syndrome.” Other adverse effects are language impairments and memory deficits. Many complications are transient, but in some cases they become permanent.2–4 The use of callosotomy is therefore restricted mainly to patients with intractable tonic and atonic seizures with traumatization.3 To spare function some advocate anterior callosotomy, leaving the splenium,4–6 whereas others consider total callosotomy to be more effective, especially in children.2,7–10
The fact that callosotomy is performed in patients of a wide age span, with symptomatic generalized epilepsy syndromes and often with additional impairments such as severe mental retardation, makes evaluation of the procedure difficult, as does also the fact that it is a relatively rare procedure. Most reports are based on retrospective series from single centers.5,7
Previous long-term follow-up studies after callosotomy have shown the best results to be the reduction in the frequency of drop attacks and to some degree also generalized tonic–clonic seizures.2,5,10 In one study there was an improvement in seizure frequency with time.11 In some patients new seizures will emerge after the corpus callosotomy, often of simple partial character.2
The purpose of this prospective longitudinal population-based observational study is to analyze the long-term outcome of the callosotomies performed in Sweden between 1995 and 2007 with regard to seizure types and frequencies and used antiepileptic drugs (AEDs). The patients have been followed for 2 and 5 or 10 years after surgery.
Methods
The Swedish National Epilepsy Surgery Register has collected data since 1990 from all six centers in Sweden that are performing epilepsy surgery. All patients who are accepted for epilepsy surgery in Sweden are included in the register, after having given informed consent. The register is controlled by the Swedish Data Inspection Board and includes information about preoperative evaluations, types of epilepsy, frequencies and types of seizures, surgical methods, complications, previously used AEDs, intellectual levels, and social situations of the patients. Data in the register are regularly and randomly compared to the patient∼s original files to discover possible mistakes in the reports. So far no major mismatches have been found. The register is completely prospective since 1995, with outcome data 2 years after surgery. Five- and 10-year follow-ups have been systematically performed since 2005, hence in this study the patients operated between 2001 and 2007 have been followed 5 years after surgery, and the patients operated between 1995 and 2001 10 years after surgery. The five- and 10-year follow-ups were done as structured telephone interviews with the patients, or the parents and caregivers of the patients. The study was approved by the Regional Board of Medical Ethics at the University of Gothenburg.
In this prospective and longitudinal study, all patients who have undergone corpus callosotomy in Sweden between 1995 and 2007 and have been followed 2 and 5 or 10 years after surgery were included. In this report the 5- and 10-year follow-ups have been merged due to the limited sample size, and they have been described as long-term follow-up. The register data have been analyzed concerning the following variables preoperatively and postoperatively: seizure type and frequency, used AEDs and intellectual level, and other neurologic deficits.
The classification of seizure types has been made according to the International League Against Epilepsy (ILAE).12 The partial seizures have been divided into simple partial, complex partial, and secondary generalized seizures. The generalized seizures are divided into atypical absences, myoclonic seizures, clonic, tonic–clonic, tonic, and atonic seizures. The estimated mean number of seizures throughout the year preceding surgery and at follow-up has been reported as mean number of seizures per month. The number of drop attacks is reported in more detail.
At follow-up the seizure outcome was graded as follows: seizure-free since surgery, >75% reduction, 50–75% reduction, 0–50% reduction in seizure frequency, and increased seizure frequency after surgery.
The use of AEDs has been reported as the total number of drugs tried before the preoperative evaluation and the number of AEDs taken preoperatively and at the time of follow-up.
The intellectual level is based on a preoperative neuropsychological assessment and categorized into three groups; IQ < 50 (severe mental retardation), IQ 50–69 (mild mental retardation), and IQ > 70 (normal). Motor and visual impairments were documented.
The results are described by medians of frequencies and percentages. The numbers were considered too small for further statistical analyses.
Results
In all, 803 surgical procedures were performed and reported to the Swedish National Epilepsy Surgery Register between 1995 and 2007. Thirty-three of these were corpus callosotomies (4.1% of all procedures). Two callosotomies were done in the same patient: an anterior callosotomy followed by a total callosotomy 2 years later. One patient was excluded because the callosotomy was a reoperation after a hemispherectomy (Fig. 1).
Figure 1.
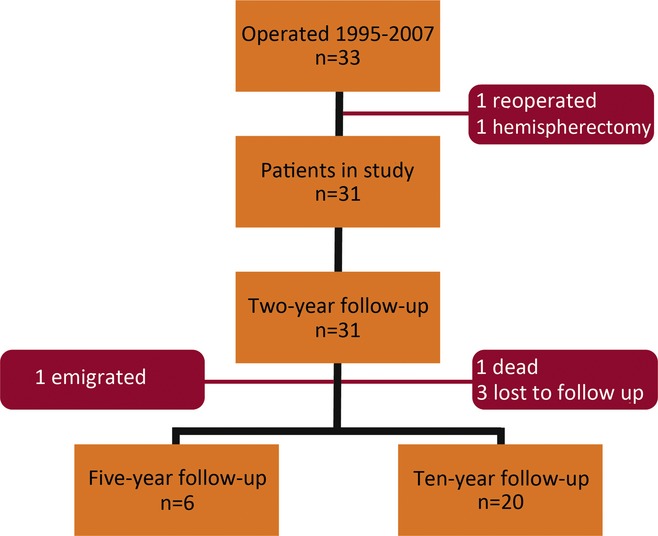
Flow chart of 33 callosotomies in Sweden 1995–2007. n, number.
Data from 31 patients were analyzed. Eleven of the patients underwent total callosotomy, 19 anterior callosotomy, and one posterior callosotomy. Two of the patients who had a total callosotomy had previously undergone anterior callosotomy (one before the study period and the other one during the period). Two of the patients undergoing anterior callosotomy had previously had a frontal lobe resection. Two patients had cysto/ventriculoperitoneal shunts prior to epilepsy surgery: one due to an arachnoidal cyst and one due to hydrocephalus. Two patients had a vagus nerve stimulation (VNS) implant. No surgical complications after the callosotomies have been reported.
All 31 patients were followed 2 years after surgery. Six of the patients had a 5-year follow-up (one patient was lost to follow-up due to emigration). Twenty were followed 10 years postoperatively (three patients were not reachable at the 10-year follow-up). One patient died 7 years after surgery (non–epilepsy-related death). In all, 26 patients (84%) had long-term follow-up 5 or 10 years after surgery (Fig. 1). The median age at long-term follow-up was 18.6 years (10.3–52.8).
Eleven of the patients were female and 20 male. Age at surgery ranged from 2.5 to 41.8 years (mean 13.3, median 10.8 years). For clinical characteristics, see Table 1. Twelve patients were diagnosed with cerebral palsy (CP) and six had other motor impairments (all with some degree of mental retardation). Six patients were visually impaired. Only one of the 31 patients had no neurologic impairment. Twenty-eight patients had an IQ < 70: 13 had mild (IQ 50–69) and 15 had severe (IQ < 50) mental retardation. Three patients had an IQ of 70 or more.
Table 1.
Clinical characteristics of the 31 patients preoperatively
| Mean | Median | Range | |
|---|---|---|---|
| Age at onset of epilepsy in years | 1.9 | 1.3 | 0.0–11.4 |
| Age at surgery in years | 13.3 | 10.8 | 2.5–41.8 |
| Duration of epilepsy in years | 10.9 | 9.4 | 1.0–31.0 |
| Total number of seizures per month | 593 | 195 | 3–4,000 |
| Number of AEDs at time of surgery | 2.2 | 2 | 0–4 |
| Number of AEDs previously tried | 4.8 | 4 | 2–9 |
AED, antiepileptic drug.
All except six patients had two different types of seizures or more. The median monthly frequency of seizures preoperatively was 195 (range 3–4,000). Eighteen of the patients had drop attacks: 11 had atonic drop attacks and three had tonic drop attacks; four had both atonic and tonic drop attacks. Fourteen had clonic or tonic–clonic seizures. Five had the diagnosis of Lennox-Gastaut syndrome and three had infantile spasms.
There was no relationship between the long-term seizure outcome and the preoperative IQ level, see Table 2.
Table 2.
Seizure outcome related to IQ categories at long-term follow-up
| Seizure-free n | >75% reduction n | 50–75% reduction n | 0–50% reduction n | More seizures n | Total n | |
|---|---|---|---|---|---|---|
| IQ < 50 | 0 (6) | 8 (2) | 1 (2) | 4 (0) | 0 (0) | 13 (10) |
| IQ 50–69 | 2 (4) | 2 (1) | 1 (0) | 1 (0) | 4 (0) | 10 (5) |
| IQ ≥ 70 | 0 (0) | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 2 (1) |
| Total | 2 (10) | 11 (3) | 3 (3) | 5 (0) | 4 (0) | 25a (16b) |
All seizure types versus drop attacks (in brackets).
n, number.
One patient with IQ 50–69 had unknown seizure frequency preoperatively.
Two patients with drop attacks were lost to long-term follow-up, one with IQ < 50 and one with IQ 50–69.
Seizure outcome—all seizure types
Of the 31 patients, 2 were seizure-free 2 years postoperatively, 5 patients had a >75% reduction in seizure frequency, 5 patients had 50–75%, and 16 patients had 0–50% reduction in seizure frequency. Three patients had more seizures after surgery (Fig. 2). The median number of seizures per patient and month was reduced by 44% from 195 before surgery to 110, at 2 years after surgery.
Figure 2.
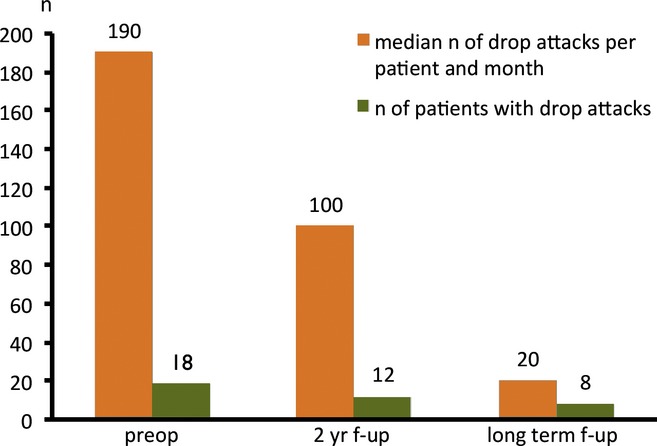
Drop attacks preoperatively, 2 years after surgery and at long-term follow-up. n, number; preop, preoperatively; f-up, follow-up.
At the long-term follow-up (5 or 10 years), 2 of the 26 patients were free from seizures. One of these two patients had been seizure-free since the 2-year follow-up. The median monthly number of seizures was 72 (range 2–3,200). This was a reduction of 68% compared to before the operation and of 25% compared to the 2-year follow-up.
Seizure outcome—drop attacks
At the 2-year follow-up, the number of patients with drop attacks (tonic or atonic) was reduced from 18 to 12. The median frequency of drop attacks was reduced from 190 (range 10–3,000) per patient and month to 100 (range 2–1,000) per patient and month.
Of the 12 patients with persistent drop attacks, 2 had a reduction of >75%, 5 had a reduction of 50–75%, 3 had a <50% reduction, and one patient had more drop attacks after surgery.
At long-term follow-up (5 or 10 years), 10 of the 18 patients with preoperative drop attacks were free of these attacks (56%). Of the remaining eight patients, three had a >75% reduction in drop attacks, 3 had a reduction of 50–75%, and 2 patients were lost to long-term follow-up. Two patients had drop attacks as a new type of seizure. The median number of drop attacks among the patients at long-term follow-up was 20 (range 3–150) per patient and month (Fig. 2 and Table S1).
Seizure outcome—clonic/tonic–clonic seizures
The number of patients with clonic/tonic–clonic seizures had decreased from 14 to 11 at the 2-year follow-up. In these patients the number of seizures per patient and month increased from median 23 (range 1–300) to 50 (range 2–400). Nine patients had clonic/tonic–clonic seizures at the long-term follow-up. Median number of seizures per patient and month was 4 (range 1–1,600). Five of these patients had a reduced number of clonic/tonic–clonic seizures at the long-term follow-up. Four had an increase, two of them also had persisting drop attacks (patients 2 and 14 in Table S1), and two were free from drop attacks (patients 7 and 14 in Table S1). Only one of these had no clonic/tonic–clonic seizures at baseline.
AED outcome
At the 2-year follow-up the median number of AEDs per patient was 2 (range 0–5). One patient was completely off medication. At the long-term follow-up, the corresponding figure was 3 (range 0–5), and four patients were off medication. For individual AED outcomes see Table S1.
Two of the 10 patients free of drop attacks at long-term follow-up were off medication, 3 had fewer AEDs, and 2 had no change in medication compared to the 2-year follow-up. Three patients who were free from drop attacks both at the 2-year and the long-term follow-up had an increase in the number of AEDs at the last follow-up.
Discussion
In this prospective longitudinal population-based study the long-term outcome after callosotomy has been investigated. Callosotomy was shown to reduce drop attacks effectively and sustainably over the years. Two years after surgery, 6 of the 18 patients who had drop attacks were completely free from drop attacks, and overall 44% (8/18) had a >75% reduction in the frequency of drop attacks. In our study the patients improved with time. At the long-term follow-up, 10 patients were free from drop attacks and in all 72% (13/18) had a >75% reduction in the frequency of drop attacks. Only one other earlier study has demonstrated an improvement years after the callosotomy.11 In their study of 23 patients with generalized seizures, the number of patients in remission after callosotomy increased from 13 one year after surgery to 16 at long-term follow-up (2–8 years). In a meta-analysis of long-term follow-up after surgery (≥5 years) sustained freedom from drop attacks was found in 35%.13
The use of AEDs did not decrease after surgery (the median number of AEDs being two per patient before and after surgery and three at long-term follow-up), probably due to the severity of the underlying epilepsy and to the fact that very few patients became completely seizure-free. However, three patients who were free from drop attacks at all follow-up had an increase in the number of AEDs at the last follow-up, which probably indicated more problems with other seizure types. Therefore, the freedom from drop attacks was not explained by an increase in AEDs.
The reason for the continuing improvement in seizure control over time is not clear. One possibility is changes in medication. On the other hand these patients had all tried many AEDs preoperatively without dramatic improvement. Another possible explanation for the improvement at the long-term follow-up is that there is a spontaneous decline in seizure frequency with increasing age, possibly due to brain maturation. Median age at surgery in this study was 10.8 years, and the median age at long-term follow-up was 18.6 years. There is a lack of and a need for systematic studies of the prognosis in patients with drop attacks who are not subjected to callosotomy.
Nine of the 10 patients free of drop attacks at the long-term follow-up had undergone an anterior callosotomy. This indicates that anterior callosotomy is effective in reducing drop attacks, as has previously been shown by Tanriverdi et al.5
After approval of the VNS in the late 1990s, the VNS came to replace callosotomy in the treatment of therapy-resistant generalized seizures, especially drop attacks. In a comparison between the two procedures, callosotomy was shown to be more effective against all seizure types, but also connected with more complications, albeit mostly transient.14,15 In a recently published meta-analysis, outcomes were compared after VNS and corpus callosotomy in the treatment of Lennox-Gastaut syndrome, showing the latter method to be better than VNS when it came to the reduction in the frequency of atonic seizures.16 In our series there were five patients with Lennox-Gastaut syndrome, four with atonic attacks preoperatively (patients 5, 6, 7, and 15 in Table S1). Three of these had no atonic attacks at long-term follow-up.
A formal cost–benefit analysis of callosotomy versus VNS has not been performed. Nevertheless, in discussions of the accessibility and affordability of epilepsy surgery in developing countries, callosotomy is being advocated as a feasible procedure in a setting with an epilepsy surgery team.3 Long-term seizure outcomes constitute important knowledge for such decision making.
It has been suggested that severe mental retardation is connected to an unfavorable seizure outcome. In a study by Rathore et al.,17 the opposite was shown, when callosotomy was performed in patients with severe mental retardation and led to favorable outcome in 65%. We found no correlation between seizure outcome and IQ: indeed, 6 of the 10 patients who became completely free of drop attacks at long-term follow-up had an IQ < 50.
Strengths and limitations of the study
The fact that the study is prospective and longitudinal is an obvious strength as well as the fact that the register is national and population based.
One limitation of this study is the small number of patients and the wide spread in age in the cohort and underlying epilepsy syndrome or disease. Another limitation is that there are no data in the register on the specific effects of the callosotomy on cognition and behavior.
There are great difficulties in finding the right instruments to assess changes in daily function and quality of life in these severely impaired children and their families. In one study the Child Behavior Check List was used to show better attention after callosotomy in patients with severe epilepsy and attention deficits.18 Parental satisfaction was assessed with a questionnaire in one study, showing a high percentage of improved behavior and alertness in the child after callosotomy.17 In a recently published study on outcome of callosotomy in adults, 6 of 15 adults reported an improvement in quality of life.19 It would be of great interest to look further into the changes in quality of life for this severely impaired group of patients and their families.
Conclusions
This study shows that callosotomy reduces seizure frequency effectively and sustainably over the years. Most improvement was seen in drop attacks. The study also shows that anterior callosotomy is effective in reducing drop attacks and that low IQ does not seem to affect seizure outcome negatively.
Previous studies have shown that callosotomy at an early age leads to an improvement in daily function and family satisfaction as well as in overall quality of life.2,6 The side effects also appear to be less in children.15 This, combined with the improvement in seizure frequency over time shown in this study, suggests that callosotomy should be considered at an early age in children with traumatizing drop attacks.
Acknowledgments
The authors thank epilepsy nurse Gerd Ekstedt for assistance with data, the steering committee of the Swedish National Epilepsy Surgery Register, and all the Swedish epilepsy surgery teams (in Gothenburg, Linköping, Lund, Stockholm, Umeå, Uppsala).
Funding
The study was funded by the Margarethahem Foundation, and by grants from the Swedish Research Council (grant 521-2011-169) and the Sahlgrenska Academy at Gothenburg University through the LUA/ALF agreement (grant ALFGBG137431).
Disclosure
Dr. Malmgren has served on an educational advisory board for UCB and has received speaker∼s honoraria from UCB. The remaining authors have no conflicts of interest. We confirm that we have read the Journal∼s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Individual monthly number of (A) atonic and (B) tonic drop attacks. Number of antiepileptic drugs preoperatively and at follow-up.
References
- 1.Kim DS, Yang KH, Kim TG, et al. The surgical effect of callosotomy in the treatment of intractable seizures. Yonsei Med J. 2004;45:233–240. doi: 10.3349/ymj.2004.45.2.233. [DOI] [PubMed] [Google Scholar]
- 2.Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42:67–71. doi: 10.1046/j.1528-1157.2001.081422.x. [DOI] [PubMed] [Google Scholar]
- 3.Asadi-Pooya AA, Sharan A, Nei M, et al. Corpus callosotomy. Epilepsy Behav. 2008;13:271–278. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Mamelak AN, Barbaro NM, Walker JA, et al. Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurg. 1993;79:688–695. doi: 10.3171/jns.1993.79.5.0688. [DOI] [PubMed] [Google Scholar]
- 5.Tanriverdi T, Olivier A, Poulin N, et al. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients – clinical article. J Neurosurg. 2009;110:332–342. doi: 10.3171/2008.3.17570. [DOI] [PubMed] [Google Scholar]
- 6.Turanli G, Yalnizoğlu D, Genç-Açikgöz D, et al. Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nerv Syst. 2006;22:1322–1327. doi: 10.1007/s00381-006-0045-3. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki M, Uematsu M, Sato Y, et al. Complete remission of seizures after corpus callosotomy. J Neurosurg Pediatr. 2012;10:7–13. doi: 10.3171/2012.3.PEDS11544. [DOI] [PubMed] [Google Scholar]
- 8.Jalilian L, Limbrick DDD, Jr, Steger-May K, et al. Complete versus anterior two-thirds corpus callosotomy in children: analysis of outcome. J Neurosurg Pediatr. 2010;6:257–266. doi: 10.3171/2010.5.PEDS1029. [DOI] [PubMed] [Google Scholar]
- 9.Shim KW, Lee YM, Kim HD, et al. Changing the paradigm of 1-stage total callosotomy for the treatment of pediatric generalized epilepsy. J Neurosurg Pediatr. 2008;2:29–36. doi: 10.3171/PED/2008/2/7/029. [DOI] [PubMed] [Google Scholar]
- 10.Sunaga S, Shimizu H, Sugano H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure. 2009;18:124–128. doi: 10.1016/j.seizure.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Spencer SS, Elguera ED, Williamson PD, et al. Evolution of seizure characteristics after callosotomy. J Epilepsy. 1991;4:149–156. [Google Scholar]
- 12.Commission on Classification and Terminology of the ILAE: proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 13.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 14.Nei M, O∼Conner M, Liporace J, et al. Refractory generalized seizures: response to corpus callosotomy and vagal nerve stimulation. Epilepsia. 2006;47:115–122. doi: 10.1111/j.1528-1167.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong TT, Kwan SY, Chang KP, et al. Corpus callosotomy in children. Childs Nerv Syst. 2006;22:999–1011. doi: 10.1007/s00381-006-0133-4. [DOI] [PubMed] [Google Scholar]
- 16.Lancman G, Virk M, Shao H, et al. Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastuat syndrome: a meta-analysis. Seizure. 2013;22:3–8. doi: 10.1016/j.seizure.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathore C, Abraham M, Rao RM, et al. Outcome after corpus callosotomy in children with injurious drop attacks and severe mental retardation. Brain Dev. 2007;29:577–585. doi: 10.1016/j.braindev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Yonekawa T, Nakagawa E, Takeshita E, et al. Effect of corpus callosotomy on attention deficit and behavioral problems in pediatric patients with intractable epilepsy. Epilepsy Behav. 2011;22:697–704. doi: 10.1016/j.yebeh.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Park MS, Nakagawa E, Schoenberg MR, et al. Outcome of corpus callosotomy in adults. Epilepsy Behav. 2013;28:181–184. doi: 10.1016/j.yebeh.2013.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Individual monthly number of (A) atonic and (B) tonic drop attacks. Number of antiepileptic drugs preoperatively and at follow-up.


