Abstract
Mycophenolic acid (MPA), an inhibitor of IMP dehydrogenase, inhibits reovirus replication and viral RNA and protein production. In mouse L929 cells, antiviral effects were greatest at 30 μg of MPA/ml. At this dosage, MPA inhibited replication of reovirus strain T3D more than 1,000-fold and inhibited replication of reovirus strain T1L nearly 100-fold, compared to non-drug-treated controls. Genetic reassortant analysis indicated the primary determinant of strain-specific differences in sensitivity to MPA mapped to the viral M1 genome segment, which encodes the minor core protein μ2. MPA also inhibited replication of both strains of reovirus in a variety of other cell lines, including Vero monkey kidney and U373 human astrocytoma cells. Addition of exogenous guanosine to MPA-treated reovirus-infected cells restored viral replicative capacity to nearly normal levels. These results suggest the μ2 protein is involved in the uptake and processing of GTP in viral transcription in infected cells and strengthens the evidence that the μ2 protein can function as an NTPase and is likely a transcriptase cofactor.
Viral diseases have historically been considered difficult to treat with selective antiviral chemotherapy. It was believed the viral replication cycle was so closely related to host cell metabolism that any attempt to suppress viral replication would kill or severely harm uninfected cells (15). Only five drugs were licensed for the treatment of viral infections a decade ago; however, due to a greater understanding of viral life cycles and elucidation of virus-specific events as targets for antiviral agents, there are currently more than 30 antiviral drugs approved for treatment of viral infections, and several others are in advanced phase III clinical trials. Half of these agents are for the treatment of human immunodeficiency virus, while the remainder are primarily for hepatitis B virus, influenza viruses, and herpesviruses (16). Unfortunately, we still lack effective therapies for several important viral infections, and current treatments are not always well tolerated. These deficiencies highlight the need for further refinement of antiviral drug design and development.
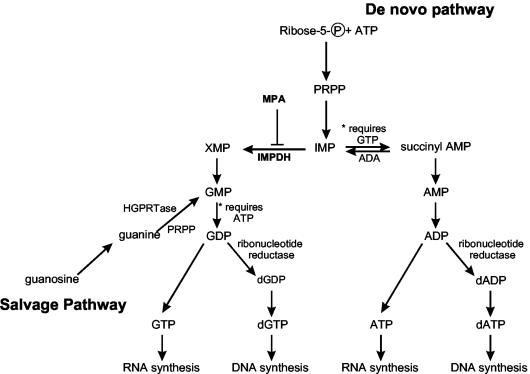
Mycophenolic acid (MPA) is a nonnucleoside, noncompetitive, reversible inhibitor of eukaryotic IMP dehydrogenase (IMPDH) (Fig. 1). IMPDH catalyzes the rate-limiting step in the de novo biosynthesis of purine mononucleotides and is involved in the early steps of GMP synthesis. It is responsible for the conversion of IMP to XMP, which is further converted to GMP, GDP, dGDP, GTP, and dGTP (for review, see reference 1). IMPDH inhibitors are expected to mainly affect viral RNA and/or DNA synthesis when there is an increased need for synthesis, as in the case of virus-infected cells. IMPDH inhibition decreases levels of intracellular guanine nucleotide pools required for adequate RNA and DNA synthesis; therefore, inhibition of IMPDH with MPA has been shown to have antiproliferative (7), immunosuppressive (42), antimicrobial (43), antiviral (63, 70), and antiparasitic (3, 29) effects. Currently, MPA is used clinically to prevent rejection of transplanted kidneys and hearts in combination with steroids and cyclosporine A (2, 33, 66). MPA can inhibit the replication of several viruses in vitro (20, 24, 30, 37, 38, 45, 47, 48) and potentiates the inhibitory effects of acyclic guanosine analogs (such as acyclovir, penciclovir, and ganciclovir) against herpesviruses (47, 48). MPA also potentiates the activity of nucleoside analogs against human immunodeficiency virus (27, 28, 38). This potentiation is thought to occur by an enhancement of antiviral activity caused by depletion of normal dGTP substrate pools, which decreases the competition that nucleoside analogs experience from the normal substrate (dGTP) during the DNA polymerization reaction. Thus, incorporation of the analog and chain termination are increased (15, 19). Despite some progress made in understanding the antiviral properties of MPA, the potential application of its use as a broad-spectrum antiviral agent against both positive- and negative-stranded RNA viruses has not been fully realized.
FIG. 1.
Pathways of purine biosynthesis. MPA inhibits IMPDH, which directly causes depletion of the guanine nucleotides (GMP, GDP, dGDP, GTP, and dGTP) and potentially causes a decrease in succinyl-AMP, which leads to a decrease in adenine nucleotides. The figure was compiled from data in references 1, 14, and 35.
In an effort to further delineate the antiviral mechanism and role of MPA, we examined the effects of MPA on reovirus. Mammalian reoviruses are the prototypic members of the Orthoreovirus genus of the family Reoviridae (50). This family includes a number of important medical (rotavirus) and economic (orbivirus) pathogens (for reviews, see references 23 and 59). Mammalian reoviruses can infect a wide variety of host species, but they cause significant pathology in only a small subset of these hosts, most notably in neonatal mice (68). Despite the apparent inability of reovirus to cause significant human disease, the virus has proven to be a useful model for studying viral pathogenesis. In addition, the segmented nature of the reovirus genome, the known coding capacities of each gene (41, 46), and strain-dependent mobility differences of the genes in polyacrylamide gels allow the generation and identification of intertypic reassortant viruses, which have been used extensively to assign particular functions to many viral proteins (for review, see reference 50).
For this study we examined the antiviral properties of MPA against mammalian reovirus infection. MPA significantly inhibits reovirus replication when used at concentrations less than those when it is used as a clinical immunosuppressive agent. Reovirus inhibition is strain dependent, and genetic reassortant mapping showed the M1 gene, which encodes the minor core protein μ2 that is thought to be a polymerase cofactor, is associated with the antiviral effects of MPA against reoviruses. Studies of RNA and protein production suggest MPA inhibits reovirus replication by preventing both viral RNA and protein production.
MATERIALS AND METHODS
Reagents, cells, and viruses.
MPA and guanosine were purchased commercially (Sigma Chemical Co.). Mouse L929 fibroblast cells were cultured in Joklik modified minimal essential medium (MEM; GIBCO) supplemented to contain 2.5% fetal calf serum (Intergen), 2.5% VSP neonate bovine serum (Biocell), and 2 mM l-glutamine. Vero (African Green monkey kidney) cells and U373 (human astrocytoma) cells were cultured in Dulbecco modified MEM (GIBCO) supplemented to contain 2.5% fetal calf serum, 2.5% VSP neonate bovine serum, 2 mM l-glutamine, and 3.5 g of d-glucose/liter. T1L, T3D, and T1L × T3D reassortant viruses (originally isolated as described elsewhere [5, 11, 21]) and genotypes confirmed as described previously (49) were grown in mouse L929 cell monolayers supplemented to contain 100 U of penicillin per ml, 100 μg of streptomycin sulfate/ml, and 1 μg of amphotericin B/ml as described elsewhere (12).
Virus infections and drug treatments.
Except where differences have been noted, cells were routinely treated with various concentrations of MPA 1 h prior to infection. Most of the medium was removed and saved (as preadapted medium), and treated cells then were infected with high-titer stocks of T1L, T3D, or T1L × T3D reassortant viruses at various multiplicities of infection (MOIs). A mixture of fresh medium and preadapted medium (3:1; supplemented to contain the same amount of MPA as used during pretreatment) was then added to infected cells, which were incubated at 37°C. Supernatants and cells were harvested at various times (hours) postinfection (hpi) for virus titration by plaque assay as described previously (12). In time course studies, cells were treated with MPA at various times prior to, during, or post-reovirus exposure, and virus was harvested at 72 hpi. In some experiments, infected cells were incubated with 50 μg of guanosine/ml.
Analyses of virus-specific RNA synthesis.
Subconfluent L929 monolayers in P60 dishes were treated with 0, 300 ng, or 3 μg of MPA/ml for 1 h and then infected with T1L or T3D at an MOI of 10 PFU/cell. After virus adsorption for 1 h, fresh medium that contained the same amount of MPA as that used to pretreat cells was added, and infections were labeled by the addition of [32P]orthophosphate (Perkin-Elmer) to a final concentration of 20 μCi/ml. Infections were incubated at 37°C and harvested at 24 or 72 hpi. Immediately after incubation infections were placed on ice, and cell monolayers were scraped from the plate to harvest cells. Infected cells were treated with lysis buffer (140 mM NaCl, 1.5 mM MgCl2, 10 mM Tris [pH 7.4], 0.5% Nonidet P-40), and cellular nuclei and organelles were removed by low-speed centrifugation. Viral double-stranded (dsRNA) genomes were isolated from cell lysates by phenol-chloroform extraction and precipitated overnight at −20°C in 2.5 volumes of ice-cold ethanol. Viral dsRNA pellets were dried and resuspended in agarose electrophoresis sample buffer (0.25% bromophenol blue, 0.25% xylene cyanol FF, 15% Ficoll). Samples were stored at −20°C if not used immediately. Labeled RNA was resolved in 0.9% horizontal agarose gels that contained 0.1% ethidium bromide to allow RNA visualization under UV light and run at 100 V for 1.5 h in 0.5× Tris-borate-EDTA buffer. Nonlabeled purified T3D virions were used as an RNA marker. Gels were dried onto filter paper and then autoradiographed by exposure to Kodak X-AR sheet film (Kodak) at −80°C.
Identification of total viral protein production.
Immunoprecipitations were carried out essentially as described previously (26). Briefly, subconfluent L929 monolayers in P60 dishes were treated with 0, 300 ng, or 3 μg of MPA/ml for 1 h and then infected with T1L or T3D at an MOI of 10 PFU/cell. After virus adsorption for 1 h, fresh medium that contained the same amount of MPA as used to pretreat cells was added and infections were labeled by the addition of [35S]methionine-cysteine (Perkin-Elmer) to a final concentration greater than 20 μCi/ml. Infections were incubated at 37°C and harvested at 24 and 72 hpi. Cytoplasmic extracts were prepared, incubated with anti-T3D reovirus-conjugated protein A-Sepharose beads, and processed as described elsewhere (26). Radiolabeled proteins were dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.24 M Tris-HCl [pH 6.8], 1.5% dithiothreitol, 1% SDS), heated to 95°C for 3 to 5 min, and resolved in 5-to-15% gradient SDS-PAGE gels (16.0 by 12.0 by 0.1 cm) at 5 mA for 18 h. Gels were fixed and impregnated with Enlightning (Perkin-Elmer), dried onto filter paper, and then fluorographed by exposure to Kodak X-AR sheet film (Kodak) at −80°C.
RESULTS
MPA inhibits production of infectious reovirus progeny in L929 fibroblasts.
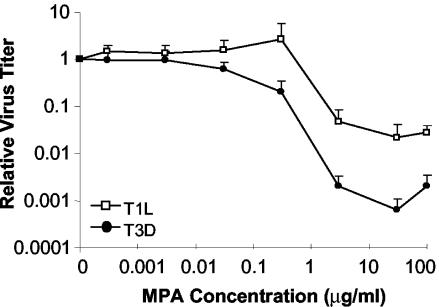
Inhibition of reovirus replication has been achieved with some antiviral compounds, including neplanocin A and some of its derivatives (18, 52, 58), acivicin (31), cicloxolone sodium (13), cyclopentenylcytosine (14, 17), and ribavirin (another IMPDH inhibitor) (9, 34, 55). In an effort to characterize additional antiviral agents that could attenuate reovirus infection, MPA, a molecule that inhibits replication of other viruses (20, 24, 30, 37, 45), was assessed for its ability to inhibit T1L and T3D replication in mouse L929 fibroblasts. The dose response of MPA on the production of infectious viral progeny was determined for concentrations of MPA between 0 and 100 μg/ml (Fig. 2). Concentrations of MPA of ≤30 ng/ml had no significant effect upon the replication of either T1L or T3D when cells were infected at a low MOI of 0.12 PFU/cell. An MPA concentration of 300 ng/ml appeared to produce different effects in T1L infections compared to T3D infections. On average, addition of 300 ng of MPA/ml led to a threefold increase in infectious T1L viral progeny. In some experimental trials, addition of this dose had no significant effect upon T1L production compared to non-drug-treated control infections, whereas in other trials addition of this dose of MPA led to an increase in infectious progeny production that ranged from a one- to eightfold increase. In contrast, application of a dose of 300 ng of MPA/ml to T3D infections consistently led to a decrease in progeny virus production that ranged from 2- to 17-fold (average of 7-fold) less than comparable non-drug-treated control infections. The three- to sevenfold changes in viral replication seen at 300 ng of MPA/ml were not statistically significant when compared to non-drug-treated samples (P > 0.05). Higher concentrations of MPA led to a decrease in virus production for both strains, and these plaque reduction assays demonstrated that decreased production of infectious viral progeny was maximal at an MPA concentration of 30 μg/ml. We observed several strain-dependent differences in responses of T1L and T3D to different doses of MPA, with T3D generally being more sensitive to the antiviral effects of MPA than T1L. In addition to the strain-dependent different responses to 300 ng of MPA/ml noted above, the extent of viral inhibition was also different between T1L and T3D. The inhibition of infectious viral progeny production by MPA was greater in T3D, with a maximum inhibition of 1,560-fold compared to 46-fold for T1L at a dose of 30 μg of MPA/ml. Viral inhibition was not caused by cell toxicity, because there were only small differences in cell viability determined by cell doubling times, trypan blue exclusion, and WST cell toxicity assays at these MPA doses (data not shown). However, cell monolayers started to deteriorate after 24 h of exposure to 100 μg of MPA/ml. The 100-μg/ml MPA dose produced a paradoxical effect: despite the cytotoxic effect seen at this higher drug concentration, production of infectious viral progeny was increased compared to that at lower, less-cytotoxic drug concentrations. For most subsequent experiments, we routinely used a dosage of 3 μg of MPA/ml. This concentration was selected because cytotoxicity was less than at higher MPA concentrations and this concentration would ensure any inhibition seen was due to the inhibitory effects of MPA on the virus rather than cytotoxic effects on the cells. Strain-dependent differences in inhibition were also seen at this concentration of MPA, with near-maximal levels of inhibition. Some studies were also performed using an MPA dosage of 300 ng/ml.
FIG. 2.
Effect of MPA in L929 cells; effect of MPA on production of infectious reovirus progeny. L929 cells were pretreated with the indicated concentrations of MPA for 1 h before infection with T1L and T3D at an MOI of 0.12 PFU/cell. After virus adsorption, cells were overlaid with fresh MEM that contained the indicated concentrations of MPA and incubated at 37°C. Virus was harvested between 65 and 72 hpi, and viral titer was determined. Results are displayed as the relative titer, with infectious progeny virus produced at each MPA concentration expressed as a proportion of virus produced in the untreated control (0 MPA). The data represent the average of a minimum of three experiments, and the error bars represent 1 standard deviation.
L929 cells also were pretreated with mycophenolate mofetil, the pro-drug version of MPA used clinically. Similar molar concentrations of mycophenolate mofetil led to comparable inhibition of T1L and T3D replication (data not shown). Cells were also pretreated with MPA and then infected with T1L and T3D at various MOIs to determine if the amount of infecting virus affected MPA inhibition. Although there were differences in the amount of inhibition seen at different MOIs, these were not statistically significant when virus was added at an MOI of 0.12, 1.2, or 12 PFU/cell for either T1L or T3D (data not shown).
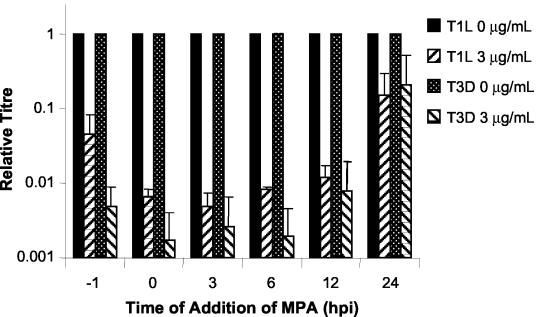
Time of MPA addition affects level of viral growth inhibition.
When MPA was added 1 h preinfection or at 0, 3, 6, 12, or 24 hpi, differences in levels of viral inhibition were seen. Production of infectious viral progeny was reduced the most compared to untreated control cells when MPA was added between 0 and 12 hpi (P < 0.005) (Fig. 3). Viral replication was inhibited less when drug was added 1 h preinfection than when drug was added at the time of infection (T1L, 22-fold compared to >85-fold [P = 0.084]; T3D, 123-fold compared to >580-fold [P = 0.029]). Inhibition of virus production was minimal in cells treated with MPA at 24 hpi and was not significantly different from non-drug-treated control infections. Strain-dependent differences in response to the addition of MPA occurred whether the drug was added 1 h preinfection, at the time of infection, or 3 or 6 hpi. However, the greatest, and statistically significant, difference in viral inhibition between T1L and T3D was seen when cells were pretreated with MPA. Similar results were seen when MPA-treated cells were infected at MOIs of 1.2 and 12 (data not shown).
FIG. 3.
Time course of the effect of MPA on production of infectious virus progeny. L929 cells were infected with T1L and T3D at an MOI of 0.12 PFU/cell and harvested between 65 and 72 hpi. MPA was added at the indicated times with respect to virus inoculation. Results are displayed as the relative titer compared to progeny virus production in the untreated control. The data represent the average of a minimum of two experiments, and the error bars represent 1 standard deviation.
Differences in MPA sensitivity map to the M1 gene segment.
As indicated earlier, MPA has been shown to inhibit replication of several viruses (20, 24, 30, 37, 38, 45, 47, 48). However, viral factors, if any, that play a role(s) in sensitivity to MPA have not yet been delineated. The strain-dependent differences seen in reovirus MPA sensitivity (Fig. 2) provided us a unique opportunity to undertake a genetic approach to identify viral factors involved in the differential effect of MPA on T1L and T3D virus production. A panel of 39 virus clones (the two parents, T1L and T3D, plus 37 reassortant viruses) was investigated to determine the inhibitory effects of MPA (Table 1). All 39 virus clones were tested for their capacities to replicate in the presence or absence of MPA. A few reassortants behaved similarly to T1L, with lower levels of sensitivity to MPA, while others behaved more like T3D with increased sensitivity to MPA. Some reassortants exhibited less sensitivity to MPA than T1L. The different reassortant behaviors prevented clones from being placed into only two distinct groups. Therefore, the relative sensitivities of each clone to MPA were used to facilitate reassortant mapping analyses. These relative sensitivities, expressed as the fold reduction in virus replication for each virus clone, generated a continuum from 26 to approximately 5,000 (Table 1). Wilcoxon rank sum analysis of the ranked clones indicated the viral M1 gene, which encodes minor core protein μ2, was the primary determinant of the strain-dependent difference in MPA sensitivity. The Wilcoxon analysis also suggested the M2, M3, and S1 genome segments contributed to the strain-dependent differences. However, additional statistical analyses (univariate chi-square and multiple logistical regression) indicated the M1 gene was the principle determinant of strain-dependent differences (Table 2). Similarly, reassortant mapping of the strain-dependent differences seen in the presence of 300 ng of MPA/ml implicated the M1 gene (data not shown).
TABLE 1.
Genotypes of T1L × T3D intertypic reassortants tested for MPA sensitivity
| Clone | Parental source for the following gene segmentsa:
|
Fold reductionb | Rankc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | M1 | M2 | M3 | S1 | S2 | S3 | S4 | |||
| H14 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 3 | 1 | 26 | 39 |
| EB143 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 27 | 38 |
| EB129 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 3 | 28 | 37 |
| EB74 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 34 | 36 |
| EB136 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 3 | 3 | 35 | 35 |
| KC36 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 42 | 34 |
| EB120 | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 52 | 33 |
| H24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 58 | 32 |
| EB85 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 1 | 61 | 31 |
| H27 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 62 | 30 |
| KC9 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 76 | 29 |
| H60 | 3 | 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 1 | 81 | 28 |
| EB93 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 90 | 27 |
| T1L | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 91 | 26 |
| EB108 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 3 | 107 | 25 |
| KC10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 109 | 24 |
| EB47 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 122 | 23 |
| G2 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 127 | 22 |
| EB118 | 3 | 3 | 1 | 1 | 3 | 3 | 3 | 3 | 1 | 1 | 128 | 21 |
| KC28 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 3 | 3 | 3 | 130 | 20 |
| EB146 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 140 | 19 |
| EB96 | 1 | 3 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 148 | 18 |
| EB18 | 3 | 3 | 1 | 3 | 3 | 3 | 1 | 1 | 3 | 1 | 149 | 17 |
| G16 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 170 | 16 |
| EB126 | 3 | 3 | 1 | 3 | 1 | 1 | 1 | 3 | 3 | 1 | 203 | 15 |
| EB39 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 217 | 14 |
| EB31 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 3 | 3 | 1 | 249 | 13 |
| H15 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 1 | 273 | 12 |
| KC35 | 1 | 3 | 1 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 290 | 11 |
| E3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 305 | 10 |
| EB113 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 310 | 9 |
| KC19 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 460 | 8 |
| EB13 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 533 | 7 |
| KC55 | 1 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 585 | 6 |
| EB88 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 707 | 5 |
| EB62 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 1 | 757 | 4 |
| T3D | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 844 | 3 |
| EB28 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 1231 | 2 |
| EB97 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 4968 | 1 |
| P valued | 0.2771 | 0.9524 | 0.2561 | 0.00025 | 0.0173 | 0.0028 | 0.0260 | 0.7505 | 0.9960 | 0.3961 | ||
Numbers indicate parental sources of gene: 1, T1L; 3, T3D.
Fold reductions were calculated by comparing each clone's replication in the presence of 3 μg of MPA/ml to replication in the absence of MPA. Values represent the average from two or more trials.
Ranking was used for statistical purposes.
Calculations performed as described at the website http://home.clara.net/sisa/.
TABLE 2.
Statistical analyses of genes associated with differential MPA effects
| Gene | chi squarea | Multiple logistical regression |
|---|---|---|
| L1 | 0.8 | |
| L2 | 0.2 | |
| L3 | 0.3 | |
| M1 | 0.005 | 0.04 |
| M2 | 0.2 | |
| M3 | 0.1 | |
| S1 | 0.7 | |
| S2 | 0.8 | |
| S3 | 0.02 | 0.13 |
| S4 | 0.8 |
Reassortant phenotype data set (from Table 1) divided into halves; univariate chi-square analysis was performed on distribution of each gene within each half.
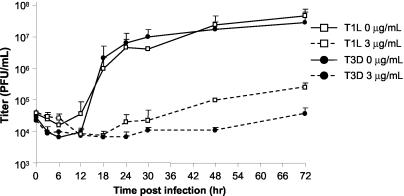
Reovirus growth is inhibited by MPA for up to 48 hpi.
We then investigated the effect of MPA during the course of viral replication to help characterize the effects of MPA at various times throughout the reovirus life cycle. Subconfluent L929 cells were pretreated with MPA at 0 or 3 μg/ml for 1 h, infected with T1L or T3D at an MOI of 0.12 PFU/cell, and harvested at various time points to generate a growth curve (Fig. 4). In non-drug-treated control samples, viral titers increased dramatically after the 12-h viral eclipse phase. In contrast, T1L titers of MPA-treated samples started to increase at 24 h and slowly increased until 72 h, when infections were harvested and titers were about sevenfold greater than input virus titer, indicating a low level of progeny virus production. In contrast, T3D viral titers did not begin to increase until 48 hpi, and the titer at 72 h was not significantly higher than the input titer, suggesting little, if any, progeny virus growth.
FIG. 4.
Virus production over time in the presence of MPA. L929 cells were pretreated with MPA for 1 h before infection with T1L and T3D at an MOI of 0.12 PFU/cell. After virus adsorption, cells were overlaid with fresh MEM that contained the indicated concentrations of MPA and incubated at 37°C. Virus was harvested between 0 and 72 hpi, and viral titer was determined. The data represent the average of a minimum of two experiments, and the error bars represent 1 standard deviation.
Addition of exogenous guanosine rescues viral growth.
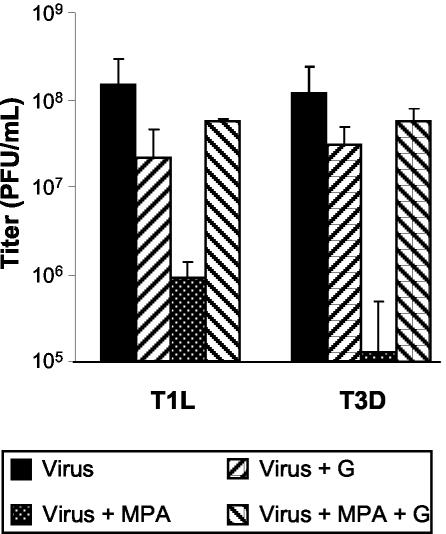
The mechanism of antiviral activity of MPA likely occurs through the depletion of intracellular guanine nucleotide pools (1, 64). Therefore, the addition of excess exogenous guanosine to virus-infected cells treated with MPA should yield viral titers comparable to those in untreated controls. L929 cells were either mock-treated or pretreated for 1 h with 3 μg of MPA/ml, infected with T1L or T3D at an MOI of 0.12 PFU/cell, and harvested between 65 and 72 hpi. Addition of guanosine at a concentration of 50 μg/ml to infections in the presence of MPA restored viral replication to near-normal levels (Fig. 5). These results are in agreement with previous studies (20, 48).
FIG. 5.
Effect of exogenous guanosine on infectious virus progeny production in the presence of MPA. L929 cells were pretreated with MPA for 1 h before infection with T1L and T3D at an MOI of 0.12 PFU/cell. After virus adsorption, cells were overlaid with fresh MEM that contained no supplements, 3 μg of MPA/ml, 50 μg of guanosine/ml, or a combination of MPA plus guanosine. Virus was harvested between 65 and 72 hpi, and viral titer was determined. The data represent the average of a minimum of two experiments, and the error bars represent 1 standard deviation.
MPA inhibits viral RNA and protein production.
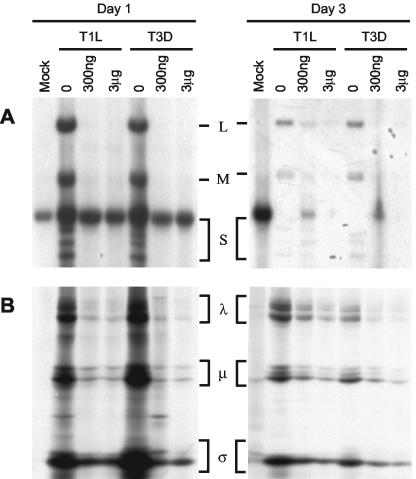
Although the antiviral activity of MPA is due to decreased levels of intracellular GTP, it is not clear which guanosine- or GTP-dependent step in the viral life cycle (e.g., intracellular signaling, translation, or replication) is most susceptible; therefore, we examined earlier stages in the viral life cycle that might be susceptible to MPA inhibition. Since MPA blocked the production of infectious viral progeny but did not decrease levels of virus below input levels, two possible mechanisms of inhibition were hypothesized: (i) MPA inhibited viral RNA synthesis or (ii) MPA inhibited the translation of the infectious viral RNA. We investigated the effect of MPA on both viral RNA production and protein production. To determine whether MPA affects viral RNA production, L929 cells were infected with T1L or T3D virus in the presence or absence of different concentrations of MPA, biosynthetically labeled with [32P]orthophosphate, and incubated at 37°C. Virus was harvested at 24 or 72 hpi, and RNA was extracted and separated in agarose gels. Viral RNA production for both T1L and T3D was significantly decreased compared to the non-drug-treated control at 24 hpi, and the decrease was still evident at 72 hpi (Fig. 6A). However, some progeny RNA production was seen in T1L infections, especially at 300 ng of MPA/ml at later times, consistent with growth curve results (Fig. 4).
FIG. 6.
Effect of MPA on viral RNA and protein production. (A) Agarose gel analysis of [32P]orthophosphate-labeled viral RNA. Pretreated L929 cells were either mock infected (M) or infected with T1L or T3D in the presence of 0, 300 ng, or 3 μg of MPA/ml, labeled with [32P]orthophosphate, and incubated at 37°C as detailed in Materials and Methods. At 24 or 72 hpi, dsRNA was purified and agarose gels were run at 125 V for 2 h, dried, and exposed to X-ray film. The locations of the L, M, and S gene segments are indicated between the day 1 and day 3 panels. (B) Immunoprecipitation fluorograph of [35S]methionine-cysteine-labeled cell extracts mock infected (M) or infected with T1L or T3D and treated as described for panel A. Extracts were precipitated with anti-T3D polyvalent antiserum conjugated to protein A-Sepharose. Labeled proteins were resolved in 5-to-15% gradient SDS-PAGE gels (16.0 by 12.0 by 0.1 cm) at 5 mA for 18 h, and the gels were fixed, dried, and exposed to X-ray film. The location of the major λ, μ, and σ classes of reovirus proteins are indicated between the day 1 and day 3 panels.
To determine whether MPA affected translation, L929 cells were infected with T1L or T3D in the presence or absence of MPA and biosynthetically labeled with [35S]methionine-cysteine. Infections were incubated at 37°C for 24 or 72 h and harvested, and viral proteins were immunoprecipitated and separated by SDS-PAGE. Synthesis of viral proteins in infections treated with MPA was substantially decreased in cells harvested at both 24 and 72 h for both T1L and T3D (Fig. 6B). The identities of viral proteins were confirmed by comparison with in-house viral markers.
MPA inhibits production of infectious reovirus progeny in other cell lines.
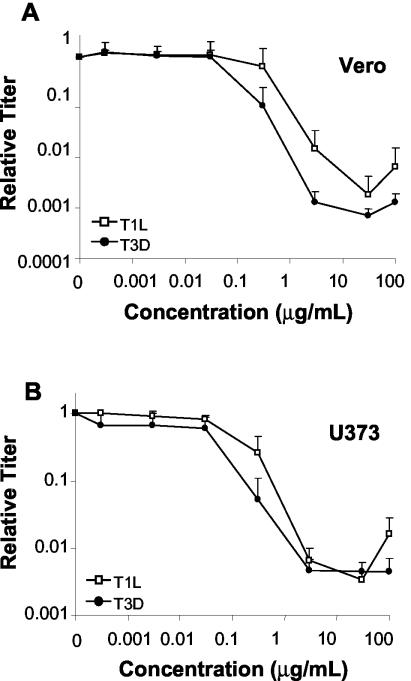
In order to be clinically useful as a broad-spectrum antiviral agent, a compound might need to exert its effects in a variety of tissues. Since the sensitivity of virus-cell culture systems to ribavirin, another IMPDH inhibitor, and MPA are often dependent on the host cell line being used (47, 55, 57), we examined the effects of MPA on the production of infectious reovirus progeny in other cell lines. Vero and U373 cells, which are known to support reovirus replication (6, 32), were pretreated with various concentrations of MPA for 1 h before infection with T1L or T3D at an MOI of 0.12 PFU/cell. Cells were incubated in the presence of various MPA concentrations at 37°C and harvested between 65 and 72 hpi, and the viral titer was determined (Fig. 7).
FIG. 7.
Effect of MPA on production of infectious reovirus progeny in other cell lines. Vero (A) and U373 (B) cells were pretreated with the indicated concentrations of MPA for 1 h before infection with T1L and T3D at an MOI of 0.12 PFU/cell as described in the legend to Fig. 2. Virus was harvested between 65 and 72 hpi, and viral titer was determined. Results are displayed as the relative titer compared to progeny virus production in the untreated control. The data represent the average of a minimum of two experiments, and the error bars represent 1 standard deviation.
Results of infections in MPA-treated Vero cells were similar to results in MPA-treated L929 cells (Fig. 7A). Concentrations of ≤30 ng of MPA/ml had no significant effect on either T1L or T3D virus production. An MPA concentration of 300 ng/ml appeared to produce a different effect in T1L infections compared to those with T3D. Addition of 300 ng of MPA/ml had no significant inhibitory effect on T1L production compared to non-drug-treated controls, whereas it caused a 10-fold decrease in production of T3D infectious viral progeny compared to untreated controls. Higher concentrations of MPA led to a decrease in virus production for both strains, with maximal inhibition of viral production at 30 μg/ml. Viral production was decreased 535- and 1,379-fold for T1L and T3D, respectively, compared to non-drug-treated controls. In addition to strain-dependent different responses at 300 ng/ml, we also observed that T3D was more sensitive to the effects of MPA than T1L. These differences were greatest at 3 μg of MPA/ml, unlike in L929 cells where strain-dependent differences were greatest at 30 μg/ml (Fig. 2).
The effects of MPA on reovirus infection in U373 cells were similar to those in L929 and Vero cells, in that MPA concentrations of ≤30 ng/ml had no significant effects on viral titer compared to non-drug-treated controls (Fig. 7B). However, in contrast to L929 and Vero cells, strain-dependent differences in viral inhibition were minimal, with T3D slightly more sensitive than T1L to the inhibitory effects of MPA at a concentration of 300 ng/ml. Maximum inhibition of virus production for both T1L and T3D was around 250-fold and occurred at 3 and 30 μg of MPA/ml.
DISCUSSION
MPA is currently used as an immunosuppressive agent to prevent organ rejection in kidney and heart transplants. In this capacity, it is believed to act through inhibition of T-cell proliferation. The proliferative response of human lymphocytes is highly dependent on de novo purine biosynthesis, whereas the major salvage pathway catalyzed by hypoxanthine-guanine phosphoribosyltransferase is not required for lymphocyte proliferation (Fig. 1). Thus, inhibition of IMPDH, which results in a depletion of the intracellular GTP and dGTP pools, would be more potent on lymphocytes than on other cell types (for review, see reference 1). Previous studies have reported MPA inhibited several viruses in vitro in a guanosine-dependent manner, likely by a similar mechanism (20, 24). However, we are not aware of previous studies that examined viral factors that contribute to this inhibition and, although some previous studies indicated that MPA inhibits reovirus T1L infection (20, 24), those data are not generally available.
In this study, we investigated the ability of MPA, a nonnucleoside inhibitor of IMPDH, to inhibit reovirus replication in L929, Vero, and U373 cells. Using plaque reduction assays, growth studies, and biosynthetic labeling, we demonstrated MPA inhibits reovirus replication, prevents an increase in reovirus titer beyond input titer, and inhibits production of reovirus progeny by reducing levels of viral RNA and protein. Our data convincingly show MPA is an antireovirus agent and, although it cannot prevent reovirus infection, it can attenuate reovirus replication. In the presence of 3 μg of MPA/ml, viral replication was suppressed for at least 24 and 48 hpi in T1L and T3D infections, respectively, compared to non-drug-treated controls, where viral titers increased dramatically after 12 hpi (Fig. 4). To test whether this represented a decrease in infectious viral progeny or a true decrease in viral production, we investigated the effect of MPA on RNA and protein production. Both RNA and protein production of both virus clones were suppressed in the presence of 3 μg of MPA/ml, and only T3D progeny RNA and protein production were substantially reduced in the presence of 300 ng of MPA/ml, when infections were harvested at 72 hpi (Fig. 6). The inhibitory effect of MPA was not due to cell toxicity and was reversed by the addition of exogenous guanosine (Fig. 5). These observations support the interpretation that the major mechanism of action of MPA is to deplete intracellular GTP and inhibit viral polymerase activity, and they are consistent with reports of in vitro antiviral activity in other virus systems (20, 24, 30, 45, 65, 70).
Our present results revealed that reovirus strains T1L and T3D differed in their sensitivities to MPA in L929 cells, with T3D more sensitive to the antiviral effects of MPA than T1L. Maximum inhibition was seen at a level of 30 μg of MPA/ml, where inhibition of T3D was 1,560-fold compared to 46-fold for T1L in L929 cells (Fig. 2). Genetic reassortant analysis of strain-dependent differences suggested the M1 gene, which encodes the μ2 protein, as the primary determinant of the difference in inhibition between T1L and T3D in L929 cells (Table 1 and 2). Some reassortant clones (e.g., EB129 and KC19, among others) appeared to be exceptions to the presumed M1 mapping, and a few other viral genes (M2, M3, and S1) appeared to contribute to the phenotypic differences. Interestingly, other studies (for example, references 61 and 71) have also found that some phenotypes do not map unambiguously to the M1 gene, necessitating statistical evaluation. To determine the statistical significance of each reovirus gene in the differential MPA sensitivity phenotype, a variety of statistical tests were performed. Division of the reassortant data set into halves and a univariate chi-square analysis of the distribution of each gene in each half indicated that the M1 gene was the most significant single determinant (P = 0.005) and that the S3 gene also was significant, but to a lesser extent (P = 0.02) (Table 2). A Wilcoxon rank sum analysis (that assigns weight to the placement of each gene within the data set) indicated the M1 gene was the most significant determinant (P = 0.00025) but also suggested that the M2 (P = 0.017), M3 (P = 0.0028), and S1 (P = 0.026) genes contributed to the phenotypic differences. Multiple logistical regression analyses were also performed to determine whether the above genes truly contributed to the phenotypic differences or whether the apparent contribution was caused by nonrandom gene distributions (49) in the available reassortants (Table 2). Irrespective of whether multiple logistical regression was performed on the complete data set of all individual experiments (data not shown) or whether it was performed on the average fold reductions (values in Table 1), the M1 gene was found to be the only major determinant of MPA phenotypic differences (P = 0.04).
The reovirus M1 gene is 2,304 bp long (67, 69, 72) and encodes the minor reovirus structural protein μ2. It is a minor component of the inner capsid, present in only 20 to 24 copies per particle (10). Protein μ2 has not been definitively localized within the reovirus particles but is thought to associate with the RNA-dependent RNA polymerase and reside near the icosahedral five-fold axes (22, 56). The functions of μ2 are only partially understood. The M1 gene segment is genetically associated with viral strain differences in the severity of cytopathic effect in mouse L929 cells, the frequency of myocarditis in mice, the levels of viral growth in cardiac myocytes and endothelial cells, the degree of organ-specific virulence in SCID mice, and the level of interferon induction in cardiac myocytes (25, 39, 44, 61, 62). The μ2 protein has also been shown to bind RNA and to be involved in formation of viral inclusions through microtubule binding in infected cells (4, 40, 53). Other genetic studies have associated the M1 gene with viral strain differences in the in vitro transcriptase and nucleoside triphosphatase activities of viral core particles (51, 71), which have been used to suggest that μ2 is a transcriptase cofactor, but μ2 remains the most poorly understood of the eight proteins found in reovirus virions. The complete sequence of the M1 gene segment has been reported for both T1L and T3D (53, 69, 72). The T1L and T3D M1 sequences are among the most highly conserved among different reovirus strains, showing about 98% nucleotide identity and >98.5% amino acid identity, which indicates 1 or more of the 10 amino acid differences found between the strains' μ2 proteins are responsible for the phenotypic differences. Computer-based comparisons of the M1 gene and μ2 protein sequences to others in GenBank have failed to show significant homology to other proteins, so that no clear indications of the function of μ2 have been revealed. However, small regions of sequence similarity to NTP-binding motifs have been identified near the middle of μ2.
The results of this study provide further insight into the reovirus minor core protein μ2, which remains the most functionally and structurally enigmatic of the reovirus proteins. The strain-specific difference in antiviral activity of MPA was genetically associated with the M1 gene segment (Table 1). Based on the mechanism of action of MPA, this suggests the μ2 protein plays a role in the uptake and processing of GTP in viral transcription and strengthens the evidence that the μ2 protein can function as an NTPase and is likely a transcriptase cofactor. Results from work with an MPA-resistant Sindbis virus mutant suggested that an alteration of the RNA guanylyltransferase was responsible for the MPA-resistant phenotype (60), and it is possible the reovirus μ2 protein may also contribute to guanylyltransferase activity. Previous work has indicated that the core spike protein λ2 has guanylyltransferase activity (8, 36, 54) and, while the location of μ2 is unknown, it is presumed to be in close proximity to, or interact with, protein λ2 (50). Generation and study of an MPA-resistant reovirus mutant may help elucidate additional roles played by minor core protein μ2 in reovirus replication.
Acknowledgments
We thank Joanne Embree, George Zhanel, Wanhong Xu, Jieyuan Jiang, Ita Hadzisejdic, Tammy Stuart, Lindsay Noad, and Anh Tran for critical reviews of the study, Archibald Nartey for maintenance of cultured cells, Mary Cheang for performing the statistical analyses depicted in Table 2, and Christopher Robertson and Paul Hazelton for helpful discussions.
This work was supported by grants MT-11630 and GSP-48371 from the Canadian Institutes of Health Research. L.L.H. is the recipient of a Canadian Institutes of Health Research MD/PhD studentship.
REFERENCES
- 1.Allison, A. C., and E. M. Eugui. 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47:85-118. [DOI] [PubMed] [Google Scholar]
- 2.Behrend, M., R. Lueck, and R. Pichlmayr. 1997. Long-term experience with mycofenolate mofetil in the prevention of renal allograft rejection. Transplant. Proc. 29:2927-2929. [DOI] [PubMed] [Google Scholar]
- 3.Berman, J. D., and H. K. Webster. 1982. In vitro effects of mycophenolic acid and allopurinol against Leishmania tropica in human macrophages. Antimicrob. Agents Chemother. 21:887-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentano, L., D. L. Noah, E. G. Brown, and B. Sherry. 1998. The reovirus protein μ2, encoded by the M1 gene, is an RNA-binding protein. J. Virol. 72:8354-8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, E. G., M. L. Nibert, and B. N. Fields. 1983. The L2 gene of reovirus serotype 3 controls the capacity to interfere, accumulate deletions, and establish persistent infection, p. 275-287. In R. W. Compans and D. H. L. Bishop (ed.), Double-stranded RNA viruses. Elsevier Biomedical, New York, N.Y.
- 6.Butler, M., A. Burgener, M. Patrick, M. Berry, D. Moffatt, N. Huzel, N. Barnabe, and K. Coombs. 2000. Application of a serum-free medium for the growth of Vero cells and the production of reovirus. Biotechnol. Prog. 16:854-858. [DOI] [PubMed] [Google Scholar]
- 7.Carter, S. B., T. J. Franklin, D. F. Jones, B. J. Leonard, S. D. Mills, R. W. Turner, and W. B. Turner. 1969. Mycophenolic acid: an anti-cancer compound with unusual properties. Nature 223:848-850. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland, D. R., H. Zarbl, and S. Millward. 1986. Reovirus guanylyltransferase is L2 gene product lambda 2. J. Virol. 60:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly, J. L., and T. S. Dermody. 2002. Virion disassembly is required for apoptosis induced by reovirus. J. Virol. 76:1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombs, K. M. 1998. Stoichiometry of reovirus structural proteins in virus, ISVP, and core particles. Virology 243:218-228. [DOI] [PubMed] [Google Scholar]
- 11.Coombs, K. M., B. N. Fields, and S. C. Harrison. 1990. Crystallization of the reovirus type 3 Dearing core. Crystal packing is determined by the lambda 2 protein. J. Mol. Biol. 215:1-5. [DOI] [PubMed] [Google Scholar]
- 12.Coombs, K. M., S. C. Mak, and L. D. Petrycky-Cox. 1994. Studies of the major reovirus core protein sigma 2: reversion of the assembly-defective mutant tsC447 is an intragenic process and involves back mutation of Asp-383 to Asn. J. Virol. 68:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dargan, D. J., C. B. Galt, and J. H. Subak-Sharpe. 1992. The effect of cicloxolone sodium on the replication in cultured cells of adenovirus type 5, reovirus type 3, poliovirus type 1, two bunyaviruses and Semliki Forest virus. J. Gen. Virol. 73:407-411. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq, E. 1993. Antiviral agents: characteristic activity spectrum depending on the molecular target with which they interact. Adv. Virus Res. 42:1-55. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq, E. 2001. 2001 ASPET Otto Krayer Award Lecture. Molecular targets for antiviral agents. J. Pharmacol. Exp. Ther. 297:1-10. [PubMed] [Google Scholar]
- 16.De Clercq, E. 2001. Antiviral drugs: current state of the art. J. Clin. Virol. 22:73-89. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq, E., R. Bernaerts, Y. F. Shealy, and J. A. Montgomery. 1990. Broad-spectrum antiviral activity of carbodine, the carbocyclic analogue of cytidine. Biochem. Pharmacol. 39:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Clercq, E., M. Cools, J. Balzarini, V. E. Marquez, D. R. Borcherding, R. T. Borchardt, J. C. Drach, S. Kitaoka, and T. Konno. 1989. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A, and their 5′-nor derivatives. Antimicrob. Agents Chemother. 33:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Clercq, E., L. Naesens, L. De Bolle, D. Schols, Y. Zhang, and J. Neyts. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381-395. [DOI] [PubMed] [Google Scholar]
- 20.Diamond, M. S., M. Zachariah, and E. Harris. 2002. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology 304:211-221. [DOI] [PubMed] [Google Scholar]
- 21.Drayna, D., and B. N. Fields. 1982. Genetic studies on the mechanism of chemical and physical inactivation of reovirus. J. Gen. Virol. 63:149-159. [DOI] [PubMed] [Google Scholar]
- 22.Dryden, K. A., D. L. Farsetta, G. Wang, J. M. Keegan, B. N. Fields, T. S. Baker, and M. L. Nibert. 1998. Internal structures containing transcriptase-related proteins in top component particles of mammalian orthoreovirus. Virology 245:33-46. [DOI] [PubMed] [Google Scholar]
- 23.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1786. In D. M. Knipe and P. M. Howley (ed)., Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 24.Gong, Z. J., S. De Meyer, C. Clarysse, C. Verslype, J. Neyts, E. De Clercq, and S. H. Yap. 1999. Mycophenolic acid, an immunosuppressive agent, inhibits HBV replication in vitro. J. Viral Hepat. 6:229-236. [DOI] [PubMed] [Google Scholar]
- 25.Haller, B. L., M. L. Barkon, G. P. Vogler, and H. W. Virgin IV. 1995. Genetic mapping of reovirus virulence and organ tropism in severe combined immunodeficient mice: organ-specific virulence genes. J. Virol. 69:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazelton, P. R., and K. M. Coombs. 1995. The reovirus mutant tsA279 has temperature-sensitive lesions in the M2 and L2 genes: the M2 gene is associated with decreased viral protein production and blockade in transmembrane transport. Virology 207:46-58. [DOI] [PubMed] [Google Scholar]
- 27.Heredia, A., D. Margolis, D. Oldach, R. Hazen, N. Le, and R. Redfield. 1999. Abacavir in combination with the inosine monophosphate dehydrogenase (IMPDH)-inhibitor mycophenolic acid is active against multidrug-resistant HIV-1. J. Acquir. Immune Defic. Syndr. 22:406-407. [DOI] [PubMed] [Google Scholar]
- 28.Hossain, M. M., J. J. Coull, G. L. Drusano, and D. M. Margolis. 2002. Dose proportional inhibition of HIV-1 replication by mycophenolic acid and synergistic inhibition in combination with abacavir, didanosine, and tenofovir. Antivir. Res. 55:41-52. [DOI] [PubMed] [Google Scholar]
- 29.Hupe, D. J., B. A. Azzolina, and N. D. Behrens. 1986. IMP dehydrogenase from the intracellular parasitic protozoan Eimeria tenella and its inhibition by mycophenolic acid. J. Biol. Chem. 261:8363-8369. [PubMed] [Google Scholar]
- 30.Ichimura, H., and J. A. Levy. 1995. Polymerase substrate depletion: a novel strategy for inhibiting the replication of the human immunodeficiency virus. Virology 211:554-560. [DOI] [PubMed] [Google Scholar]
- 31.Keast, D., and A. R. Vasquez. 1992. Inhibition in vitro of the replication of murine cytomegalovirus or reovirus type 3 by the glutamine analogue acivicin. Arch. Virol. 124:235-244. [DOI] [PubMed] [Google Scholar]
- 32.Keirstead, N. D., and K. M. Coombs. 1998. Absence of superinfection exclusion during asynchronous reovirus infections of mouse, monkey, and human cell lines. Virus Res. 54:225-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobashigawa, J., L. Miller, D. Renlund, R. Mentzer, E. Alderman, R. Bourge, M. Costanzo, H. Eisen, G. Dureau, R. Ratkovec, M. Hummel, D. Ipe, J. Johnson, A. Keogh, R. Mamelok, D. Mancini, F. Smart, H. Valantine, et al. 1998. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Transplantation 66:507-515. [DOI] [PubMed] [Google Scholar]
- 34.Labrada, L., G. Bodelon, J. Vinuela, and J. Benavente. 2002. Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction. J. Virol. 76:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehninger, A. L., D. L. Nelson, and M. M. Cox. 1993. Principles of biochemistry, p. 688-735. Worth Publishers, New York, N.Y.
- 36.Luongo, C. L., C. M. Contreras, D. L. Farsetta, and M. L. Nibert. 1998. Binding site for S-adenosyl-L-methionine in a central region of mammalian reovirus lambda2 protein. Evidence for activities in mRNA cap methylation. J. Biol. Chem. 273:23773-23780. [DOI] [PubMed] [Google Scholar]
- 37.Malinoski, F., and V. Stollar. 1981. Inhibitors of IMP dehydrogenase prevent Sindbis virus replication and reduce GTP levels in Aedes albopictus cells. Virology 110:281-289. [DOI] [PubMed] [Google Scholar]
- 38.Margolis, D., A. Heredia, J. Gaywee, D. Oldach, G. Drusano, and R. Redfield. 1999. Abacavir and mycophenolic acid, an inhibitor of inosine monophosphate dehydrogenase, have profound and synergistic anti-HIV activity. J. Acquir. Immune Defic. Syndr. 21:362-370. [PubMed] [Google Scholar]
- 39.Matoba, Y., B. Sherry, B. N. Fields, and T. W. Smith. 1991. Identification of the viral genes responsible for growth of strains of reovirus in cultured mouse heart cells. J. Clin. Investig. 87:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbisa, J. L., M. M. Becker, S. Zou, T. S. Dermody, and E. G. Brown. 2000. Reovirus mu2 protein determines strain-specific differences in the rate of viral inclusion formation in L929 cells. Virology 272:16-26. [DOI] [PubMed] [Google Scholar]
- 41.McCrae, M. A., and W. K. Joklik. 1978. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology 89:578-593. [DOI] [PubMed] [Google Scholar]
- 42.Mitsui, A., and S. Suzuki. 1969. Immunosuppressive effect of mycophenolic acid. J. Antibiot. (Tokyo) 22:358-363. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno, K., M. Tsujino, M. Takada, M. Hayashi, and K. Atsumi. 1974. Studies on bredinin. I. Isolation, characterization and biological properties. J. Antibiot. (Tokyo) 27:775-782. [DOI] [PubMed] [Google Scholar]
- 44.Moody, M. D., and W. K. Joklik. 1989. The function of reovirus proteins during the reovirus multiplication cycle: analysis using monoreassortants. Virology 173:437-446. [DOI] [PubMed] [Google Scholar]
- 45.Morrey, J. D., D. F. Smee, R. W. Sidwell, and C. Tseng. 2002. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir. Res. 55:107-116. [DOI] [PubMed] [Google Scholar]
- 46.Mustoe, T. A., R. F. Ramig, A. H. Sharpe, and B. N. Fields. 1978. Genetics of reovirus: identification of the dsRNA segments encoding the polypeptides of the mu and sigma size classes. Virology 89:594-604. [DOI] [PubMed] [Google Scholar]
- 47.Neyts, J., G. Andrei, and E. De Clercq. 1998. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother. 42:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neyts, J., and E. De Clercq. 1998. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antivir. Res. 40:53-56. [DOI] [PubMed] [Google Scholar]
- 49.Nibert, M. L., R. L. Margraf, and K. M. Coombs. 1996. Nonrandom segregation of parental alleles in reovirus reassortants. J. Virol. 70:7295-7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nibert, M. L., and L. A. Schiff. 2001. Reoviruses and their replication, p. 1679-1728. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 51.Noble, S., and M. L. Nibert. 1997. Core protein μ2 is a second determinant of nucleoside triphosphatase activities by reovirus cores. J. Virol. 71:7728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obara, T., S. Shuto, Y. Saito, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq, and A. Matsuda. 1996. New neplanocin analogues. 7. Synthesis and antiviral activity of 2-halo derivatives of neplanocin A. J. Med. Chem. 39:3847-3852. [DOI] [PubMed] [Google Scholar]
- 53.Parker, J. S., T. J. Broering, J. Kim, D. E. Higgins, and M. L. Nibert. 2002. Reovirus core protein μ2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J. Virol. 76:4483-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu, T., and C. L. Luongo. 2003. Identification of two histidines necessary for reovirus mRNA guanylyltransferase activity. Virology 316:313-324. [DOI] [PubMed] [Google Scholar]
- 55.Rankin, J. T., Jr., S. B. Eppes, J. B. Antczak, and W. K. Joklik. 1989. Studies on the mechanism of the antiviral activity of ribavirin against reovirus. Virology 168:147-158. [DOI] [PubMed] [Google Scholar]
- 56.Reinisch, K. M., M. L. Nibert, and S. C. Harrison. 2000. Structure of the reovirus core at 3.6 Å resolution. Nature 404:960-967. [DOI] [PubMed] [Google Scholar]
- 57.Richman, D. D., R. S. Kornbluth, and D. A. Carson. 1987. Failure of dideoxynucleosides to inhibit human immunodeficiency virus replication in cultured human macrophages. J. Exp. Med. 166:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robins, M. J., S. F. Wnuk, X. Yang, C. S. Yuan, R. T. Borchardt, J. Balzarini, and E. De Clercq. 1998. Inactivation of S-adenosyl-L-homocysteine hydrolase and antiviral activity with 5′,5′,6′,6′-tetradehydro-6′-deoxy-6′-halohomoadenosine analogues (4′-haloacetylene analogues derived from adenosine). J. Med. Chem. 41:3857-3864. [DOI] [PubMed] [Google Scholar]
- 59.Roy, P. 2001. Orbiviruses, p. 1835-1870. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 60.Scheidel, L. M., and V. Stollar. 1991. Mutations that confer resistance to mycophenolic acid and ribavirin on Sindbis virus map to the nonstructural protein nsP1. Virology 181:490-499. [DOI] [PubMed] [Google Scholar]
- 61.Sherry, B., and B. N. Fields. 1989. The reovirus M1 gene, encoding a viral core protein, is associated with the myocarditic phenotype of a reovirus variant. J. Virol. 63:4850-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherry, B., J. Torres, and M. A. Blum. 1998. Reovirus induction of and sensitivity to beta interferon in cardiac myocyte cultures correlate with induction of myocarditis and are determined by viral core proteins. J. Virol. 72:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 64.Sintchak, M. D., and E. Nimmesgern. 2000. The structure of inosine 5′-monophosphate dehydrogenase and the design of novel inhibitors. Immunopharmacology 47:163-184. [DOI] [PubMed] [Google Scholar]
- 65.Smee, D. F., M. Bray, and J. W. Huggins. 2001. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir. Chem. Chemother. 12:327-335. [DOI] [PubMed] [Google Scholar]
- 66.Sollinger, H. W., et al. 1995. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 60:225-232. [DOI] [PubMed] [Google Scholar]
- 67.Swanson, M. I., Y. M. She, W. Ens, E. G. Brown, and K. M. Coombs. 2002. Mammalian reovirus core protein micro 2 initiates at the first start codon and is acetylated. Rapid Commun. Mass Spectrom. 16:2317-2324. [DOI] [PubMed] [Google Scholar]
- 68.Tyler, K. L. 2001. Mammalian reoviruses, p. 1729-1745. In D. M. Knipe and P. M. Howley (ed.) Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 69.Wiener, J. R., J. A. Bartlett, and W. K. Joklik. 1989. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein mu 2 and the major nonstructural protein mu NS, respectively. Virology 169:293-304. [DOI] [PubMed] [Google Scholar]
- 70.Williams, R. H., D. H. Lively, D. C. DeLong, J. C. Cline, and M. J. Sweeny. 1968. Mycophenolic acid: antiviral and antitumor properties. J. Antibiot. (Tokyo) 21:463-464. [DOI] [PubMed] [Google Scholar]
- 71.Yin, P., M. Cheang, and K. M. Coombs. 1996. The M1 gene is associated with differences in the temperature optimum of the transcriptase activity in reovirus core particles. J. Virol. 70:1223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou, S., and E. G. Brown. 1992. Nucleotide sequence comparison of the M1 genome segment of reovirus type 1 Lang and type 3 Dearing. Virus Res. 22:159-164. [DOI] [PubMed] [Google Scholar]