Abstract
Previous studies have reported that risk of cardiovascular morbidity and mortality substantially increases in hypertensive patients, especially among those with inadequate blood pressure control. Two common antihypertensive drug classes including thiazide diuretics and angiotensin-converting enzyme (ACE) inhibitors affect different enzymes in the renin-angiotensin-aldosterone system (RAAS). In the RAAS, angiotensinogen is converted into angiotensin II which increases blood pressure through vasoconstriction. Using a case-only design with 3448 high-risk hypertensive individuals from the Genetics of Hypertension Associated Treatment (GenHAT) study, we examined whether seven single nucleotide polymorphisms (SNPs) in the angiotensinogen gene (AGT) interact with three classes of antihypertensive drugs including chlorthalidone (a thiazide diuretic), lisinopril (an ACE inhibitor), and amlodipine (a calcium channel blocker) to modify the risk of incident coronary heart disease (CHD) and heart failure (HF) among Caucasian and African American participants, separately. We found no gene by treatment interactions to be statistically significant after correction for multiple testing. However, some suggestive results were found. African American participants with the minor allele of rs11122576 had over two-fold higher risk of CHD when using chlorthalidone compared to using amlodipine, or lisinopril compared to amlodipine (p = 0.006 and p = 0.01, respectively). Other marginal associations are also reported among both race groups. The findings reported here suggest that rs11122576 could contribute to future personalization of antihypertensive treatment among African Americans though more studies are needed.
Keywords: AGT gene, antihypertensive drugs, hypertension, coronary heart disease, heart failure
Introduction
Hypertension is one of the most common conditions in the U.S. affecting about 76.4 million (about 1 out of 3) adults in 2012, and is a large contributor to heart disease and stroke incidence and mortality. Notably, less than half of all hypertensive patients have adequate blood pressure control. In 2008, the overall death rate associated with hypertension was 18.3% (Roger et al., 2012). Additionally, treating hypertension costs the U.S. economy $131 billion annually for direct medical expenses, and another $25 billion indirectly due to the loss of productivity (Heidenreich et al., 2011). Antihypertensive pharmacogenetic research has the potential to discover genetic contributors to variability in antihypertensive response, and tailoring therapy based on an individual's genetic make-up has the potential to mitigate cardiovascular disease (CVD) outcomes among treated hypertensives.
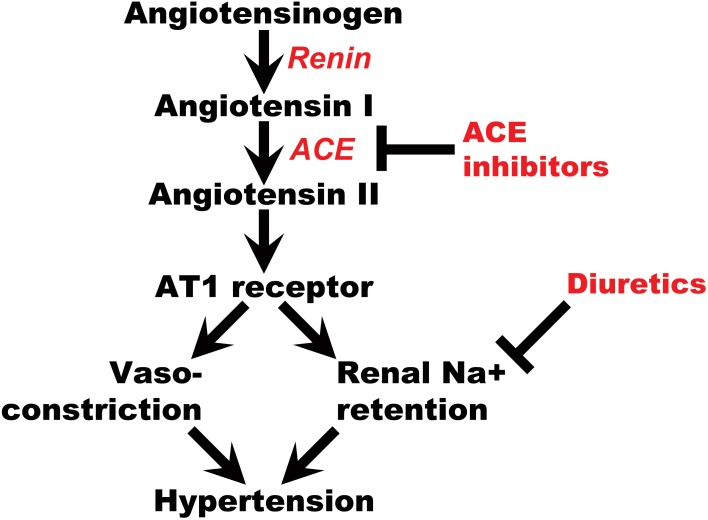
The renin-angiotensin-aldosterone system (RAAS) plays a major role in maintaining salt-water balance and controlling blood pressure (Li et al., 2013). Multiple studies have reported genes belonging to this pathway are associated with hypertension and its sequelae (Jeunemaitre et al., 1992; Mahmood et al., 2003; Pasquale et al., 2005; Al-Najai et al., 2013; Li et al., 2013). Additionally, many antihypertensive drugs act to downregulate this pathway. Therefore, genes belonging to the RAAS may modify antihypertensive treatment response and risk for adverse outcomes (Figure 1). Angiotensinogen is an upstream member of the RAAS encoded by the AGT gene on chromosome 1. It is primarily synthesized in the liver and then cleaved by renin from the kidney to form angiotensin I. Angiotensin I is further cleaved by the angiotensin-converting enzyme (ACE) to form the biologically active form called angiotensin II. In the kidney, angiotensin II induces vessel contraction via the AT1 receptor and Na+ reabsorption in epithelial cells at the proximal tubules via aldosterone release (Craig and Stitzel, 2003). Among the three most widely used antihypertensive drug classes (diuretics, ACE inhibitors, and calcium channel blockers), ACE inhibitors directly affect the RAAS through blocking the conversion of angiotensin I to angiotensin II. Thiazide diuretics can acutely activate RAAS through the inhibition of Na+ reabsorption and reduction of plasma volume (Sica, 2004; Duarte and Cooper-DeHoff, 2010). Polymorphisms in the AGT gene have been found to be associated with CVD, especially coronary heart disease (CHD), and heart failure (HF) (Chen et al., 2012; Li et al., 2013). Therefore, we hypothesize that CHD and HF risk among hypertensive patients treated with these drugs may be modified by variations in the AGT gene. Polymorphisms in the AGT gene may affect this risk directly or through downstream effects on other members of the RAAS pathway.
Figure 1.
Renin-Angiotensin pathway and the roles of ACE inhibitors, diuretics.
In the current study, we evaluated whether seven SNPs in the AGT gene affect risk of CHD and HF in high-risk hypertensive individuals randomized to one of three classes of antihypertensive treatment as part of the Genetics of Hypertension Associated Treatment Study (GenHAT) using a case-only design (Arnett et al., 2002).
Materials and methods
Study population
The GenHAT study (N = 39,114) was initiated to evaluate if several genes associated with hypertension modify the risk of CHD and other CVD outcomes in patients taking different anti-hypertensive drug classes (Arnett et al., 2002). GenHAT is an ancillary study of the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a randomized, double blind, multicenter clinical trial with 42,418 high-risk hypertensive persons 55 years of age or older. Participants were randomized into four groups defined by the class of antihypertensive medication they were assigned to, including chlorthalidone, lisinopril, amlodipine, and doxazosin (an α-adrenergic blocker), in a ratio of 1.7:1:1:1, respectively. Due to the early discontinuation of the doxazosin arm owing to a significant (25%) increase in the incidence of CVD compared to the chlorthalidone arm, we have not included the doxazosin arm in these analyses (ALLHAT Collaborative Research Group, 2000). Participants randomized to chlorthalidone, lisinopril, or amlodipine were followed for an average of 4.9 years. For more details regarding study design and methodology related to GenHAT and ALLHAT, see Arnett et al. (2002) and Davis et al. (1996). In the case-only phase of GenHAT, 9336 participants from three treatment groups affected by fatal CHD, non-fatal myocardial infarction (MI), stroke, HF, coronary revascularization, angina, peripheral arterial disease, end-stage renal disease, or all-cause death, were genotyped for variants in several hypertension-associated genes, including the AGT gene.
The results presented here focus on two outcomes, namely, CHD and HF. The primary outcome for both ALLHAT and GenHAT was CHD, defined as fatal CHD or non-fatal MI (N = 2253). The secondary outcome for this study included HF (treated, fatal, or hospitalized) (N = 1728). Outcomes were reported by the clinical investigators and validated through the review of death certificates and hospitalization records. For a more detailed description of ALLHAT outcomes, see Davis et al. (1996).
This research was approved by the institutional review boards at the University of Alabama at Birmingham, the University of Texas Health Science Center, and the University of Minnesota. Informed consent was collected from all participants in ALLHAT.
Genotyping methods
The DNA of participants from GenHAT was extracted from stored blood using Gentra Purgene Kits (Minneapolis, MN) and 600 loci that were potential candidates for blood pressure regulation or CVD were genotyped by Illumina (San Diego, CA, USA) technology. The amplification success rate was 97%, and the reproducibility was found to be 99.99%. Tag SNPs were selected using the Haploview Program (http://www.broad.mit.edu/mpg/haploview/) and based on two ethnic sources: the Caucasian (CEU) and Yoruban (YRI) population from the International HapMap project (http://www.hapmap.org/). The 10 AGT polymorphisms genotyped in GenHAT were rs7079, rs5051, rs5041, rs3789678, rs34829218, rs2493133, rs2493129, rs2478544, rs11568032, rs11122576. Three AGT polymorphisms (rs5041, rs34829218, rs11568032) with minor allele frequency <5% were not included in the analyses presented.
Statistical methods
To test for baseline differences between treatment groups, we used ANOVA for continuous variables and chi-square tests or Fisher's exact test (i.e., for any cell size < 10 observations) for categorical variables. Principal component analysis (PCA) of 64 ancestry informative markers (AIMs) was used to inform genetically determined Caucasian and African American race (Lynch et al., 2012). Each of the seven AGT SNPs was tested for Hardy Weinberg Equilibrium (HWE) stratified by genetically defined race, which will be referred to as race from here on for simplicity. The linkage disequilibrium (LD) between the seven AGT SNPs was analyzed using HAPLOVIEW (Figure 2) (Copyright © 2003–2006 Broad Institute of MIT and Harvard). Logistic regression was used to test for gene-by-treatment effects among the case groups, with treatment group modeled as the dependent variable and genotype as the independent variable. Three separate comparisons (chlorthalidone vs. amlodipine, chlorthalidone vs. lisinopril, and lisinopril vs. amlodipine) were conducted to test the interaction of genes and treatments. The additive genetic model was tested where the common allele homozygote was treated as the reference group. The dominant genetic model was used for SNPs which have any cell size less than 10 observations. A p-value of 0.05 was considered as suggestive evidence for an association. A Bonferroni correction was used to adjust for multiple testing within each outcome and race: specifically, we considered six independent SNPs (rs5051 and rs2493133 were determined to be in strong LD, described below) and three treatment comparisons; therefore, α was adjusted to 0.05/18 = 0.003. SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Using Quanto V. 1.2 software (Gauderman and Morrison, 2009) for the case-only design with allele frequency is 0.05 or greater, and type 1 error rate set to 0.003, we have 81% power to detect an interaction odds ratio of 1.80 or greater for Caucasians and 80% power to detect an interaction odds ratio of 2.2 or greater for African-Americans.
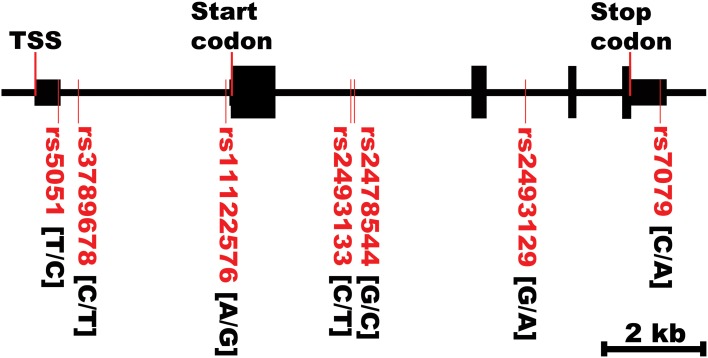
Figure 2.
AGT gene structure and SNPs locations. TSS, transcriptional start site. The first bases in the brackets are the common alleles.
Results
Table 1 shows the baseline characteristics of 3448 subjects included in this analysis. There were no significant differences in the baseline characteristics between three medication arms. Table 2 presents allele frequencies for the seven AGT variants by ethnic subgroups. The allelic frequency of the seven AGT variants was not significantly different between three medication arms in either race group except for rs2493129 among Caucasians. The minor allele frequencies for rs5051 and rs2493331 differed considerably between African Americans and Caucasians, but minor allele frequencies were comparable in both races for all other SNPs.
Table 1.
Baseline characteristics for case-only participants (n = 3448) by treatment group, mean (SD) unless otherwise noted.
| Characteristics | Chlorthalidone | Amlodipine | Lisinopril | P-value |
|---|---|---|---|---|
| Sample size (n, %) | 1511 (43.8) | 1004 (29.1) | 933 (27.1) | |
| Age, mean | 69.6 (8.1) | 69.1 (8.0) | 69.8 (8.1) | 0.13 |
| Caucasiansa, n (col%) | 1000 (60.6) | 649 (58.5) | 597 (58.5) | 0.93 |
| African Americana, n (col%) | 511 (31.0) | 355 (32.0) | 336 (32.9) | |
| Women, n (col %) | 605 (40.0) | 403 (40.1) | 375 (40.2) | 0.99 |
| Previous antihypertensive treatment, n (col%) | 1385 (91.7) | 942 (93.8) | 862 (92.4) | 0.13 |
| Baseline SBP in mmHg, mean (SD) | 147.4 (16.2) | 146.9 (16.5) | 147.9 (16.1) | 0.40 |
| Baseline DBP in mmHg, mean (SD) | 82.4 (10.3) | 82.0 (10.5) | 82.2 (11.0) | 0.70 |
| ELIGIBILITY RISK FACTORS | ||||
| Current cigarette smoker, n (col %) | 319 (21.1) | 202 (20.1) | 210 (22.5) | 0.66 |
| History of MI or stroke, n (%) | 563 (37.3) | 350 (34.9) | 323 (34.6) | 0.31 |
| History of CABG, n (%) | 344 (22.8) | 211 (21.0) | 215 (23.0) | 0.49 |
| Other atherosclerotic CVD, n (%) | 449 (29.7) | 313 (31.2) | 271 (29.1) | 0.57 |
| Major ST depression/T-wave inversion, n (%) | 177 (11.8) | 135 (13.5) | 104 (11.2) | 0.25 |
| Type 2 diabetes, n (%) | 654 (43.3) | 444 (44.2) | 400 (42.9) | 0.82 |
| HDL-C <35 mg/dL, n (%) | 191 (12.6) | 108 (10.8) | 92 (9.9) | 0.09 |
| LVH by electrocardiogram, n (%) | 256 (16.9) | 177 (17.6) | 172 (18.4) | 0.64 |
| Body mass index, mean (SD), kg/m2 | 29.9 (6.6) | 30.2 (6.6) | 29.6 (6.4) | 0.11 |
| Current Aspirin use, n (%) | 668 (44.2) | 443 (44.1) | 424 (45.4) | 0.67 |
| Current Estrogen supplementation,n (%) | 82 (13.6) | 61 (15.1) | 42 (11.2) | 0.35 |
| Fasting glucose, mg/dL | 132.9 (63.8) | 130.4 (63.5) | 127.7 (60.8) | 0.23 |
| Creatinine, mg/dL | 1.1 (0.4) | 1.1 (0.3) | 1.1 (0.3) | 0.62 |
| Total cholesterol, mg/dL | 218.2 (46.8) | 216.9 (44.9) | 216.5 (44.0) | 0.64 |
| HDL cholesterol, mg/dL | 44.5 (14.7) | 45.5 (14.5) | 44.9 (14.1) | 0.24 |
| LDL cholesterol, mg/dL | 138.3 (37.3) | 136.9 (39.1) | 138.3 (38.4) | 0.64 |
| Fasting triglycerides, mg/dL | 188.1 (164.5) | 178.6 (144.9) | 177.9 (137.5) | 0.25 |
| Potassium, mmol/L | 4.4 (0.5) | 4.3 (0.5) | 4.3 (0.5) | 0.74 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MI, myocardial infraction; CABG, coronary artery bypass grafting; CVD, cardiovascular disease; CHD, coronary heart disease; HDL-C, HDL cholesterol; LVH, left ventricular hypertrophy.
Test of differences between genotype groups: ANOVA for continuous variables, chi-square for categorical variables.
Race is genetically determined.
Table 2A.
Alleles frequency of 7 AGT variants and p-values corresponding to the test of allele frequencies differences among treatments for case-only participants among Caucasians (n = 2246).
| AGT variants | Chlorthalidone | Amlodipine | Lisinopril | P-value | |
|---|---|---|---|---|---|
| rs7079 | CC | 437 (45.5%) | 297 (47.7%) | 252 (44.8%) | 0.33 |
| AC | 402 (41.9%) | 258 (41.4%) | 255 (45.4%) | ||
| AA | 121 (12.6%) | 68 (10.9%) | 55 (9.8%) | ||
| rs5051 | TT | 193 (19.8%) | 120 (19.1%) | 108 (18.7%) | 0.22 |
| TC | 435 (44.5%) | 316 (50.2%) | 270 (46.6%) | ||
| CC | 349 (35.7%) | 194 (30.8%) | 201 (34.7%) | ||
| rs3789678 | CC | 779 (79.3%) | 494 (77.8%) | 462 (79.5%) | 0.89 |
| TC | 188 (19.1%) | 132 (20.8%) | 109 (18.8%) | ||
| TT | 16 (1.6%) | 9 (1.4%) | 10 (1.7%) | ||
| rs2493133 | CC | 194 (20.2%) | 117 (18.7%) | 106 (18.5%) | 0.24 |
| TC | 423 (43.9%) | 311 (49.7%) | 268 (46.8%) | ||
| TT | 346 (35.9%) | 198 (31.6%) | 199 (34.7%) | ||
| rs2493129 | GG | 941 (95.1%) | 587 (91.7%) | 551 (93.1%) | 0.01 |
| AG | 46 (4.7%) | 53 (8.3%) | 40 (6.8%) | ||
| AA | 3 (0.3%) | 0 (0%) | 1 (0.2%) | ||
| rs2478544 | GG | 642 (68.4%) | 396 (65.7%) | 386 (68.7%) | 0.36 |
| GC | 263 (28%) | 191 (31.7%) | 154 (27.4%) | ||
| CC | 34 (3.6%) | 16 (2.7%) | 22 (3.9%) | ||
| rs11122576 | AA | 863 (87.6%) | 543 (84.5%) | 493 (83.3%) | 0.07 |
| AG | 112 (11.4%) | 96 (14.9%) | 91 (15.4%) | ||
| GG | 10 (1%) | 4 (0.6%) | 8 (1.4%) | ||
Test of differences between genotype groups: chi-square and Fisher's exact tests. Fisher's exact test was used for SNP which has any cell size less than 10 observations in frequency table.
Table 2B.
Alleles frequency of 7 AGT variants and p-values corresponding to the test of allele frequencies differences among treatments for case-only participants among African Americans (n = 1202).
| AGT variants | Chlorthalidone | Amlodipine | Lisinopril | P-value | |
|---|---|---|---|---|---|
| rs7079 | CC | 401 (80%) | 296 (85.6%) | 281 (85.2%) | 0.11 |
| AC | 91 (18.2%) | 47 (13.6%) | 42 (12.7%) | ||
| AA | 9 (1.8%) | 3 (0.9%) | 7 (2.1%) | ||
| rs5051 | TT | 353 (70.5%) | 258 (73.7%) | 244 (73.7%) | 0.11 |
| TC | 128 (25.6%) | 88 (25.1%) | 76 (23%) | ||
| CC | 20 (4%) | 4 (1.1%) | 11 (3.3%) | ||
| rs3789678 | CC | 316 (62.8%) | 221 (64.4%) | 196 (59.2%) | 0.43 |
| TC | 153 (30.4%) | 106 (30.9%) | 116 (35.1%) | ||
| TT | 34 (6.8%) | 16 (4.7%) | 19 (5.7%) | ||
| rs2493133 | CC | 319 (64.2%) | 229 (67.6%) | 222 (68.3%) | 0.11 |
| TC | 155 (31.2%) | 103 (30.4%) | 86 (26.5%) | ||
| TT | 23 (4.6%) | 7 (2.1%) | 17 (5.2%) | ||
| rs2493129 | GG | 437 (86.2%) | 296 (84.8%) | 277 (83.4%) | 0.83 |
| AG | 67 (13.2%) | 51 (14.6%) | 52 (15.7%) | ||
| AA | 3 (0.6%) | 2 (0.6%) | 3 (0.9%) | ||
| rs2478544 | GG | 150 (31.9%) | 88 (27.2%) | 107 (34.6%) | 0.26 |
| GC | 222 (47.2%) | 170 (52.5%) | 137 (44.3%) | ||
| CC | 98 (20.9%) | 66 (20.4%) | 65 (21%) | ||
| rs11122576 | AA | 452 (89.2%) | 328 (94%) | 295 (88.3%) | 0.05 |
| AG | 52 (10.3%) | 19 (5.4%) | 37 (11.1%) | ||
| GG | 3 (0.6%) | 2 (0.6%) | 2 (0.6%) | ||
Test of differences between genotype groups: chi-square and Fisher's exact tests. Fisher's exact test was used for SNP which has any cell size less than 10 observations in frequency table.
Many of the variants were not in HWE in the either race group, possibly due to the case-only design as alleles contributing to “case” status may not be random. In the Caucasian group, rs7079 (p = 0.14) and rs2478544 (p = 0.09) were in HWE but five SNPs were not (rs5051, rs3789678, rs2493133, rs2493129, rs11122576, p < 0.05). For the African-American group, five SNPs were in HWE (p > 0.05) but rs7079 (p = 0.006) and rs3789678 (p = 0.003) were not.
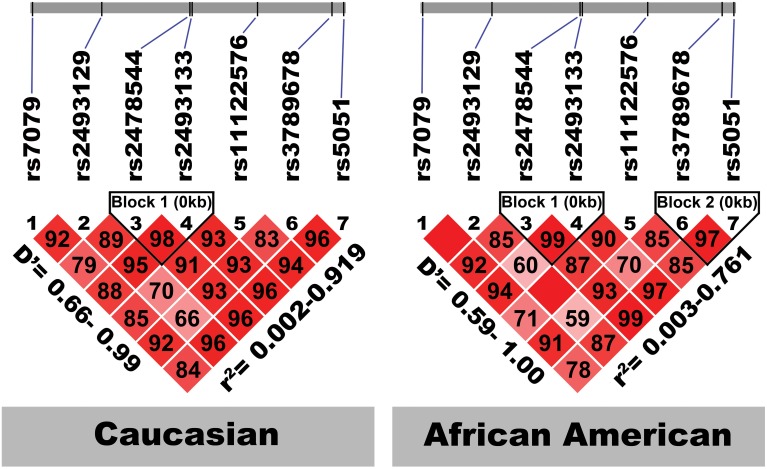
Figure 2 shows the location of the seven SNPs in the AGT gene considered in this study. Five of the seven polymorphisms (rs3789678, rs11122576, rs2493133, rs2478544, rs2493129) were intronic and two (rs5051, rs7079) were in untranslated regions. Two SNPs were in tight LD (i.e., rs5051 and rs2493133) in both race groups, as calculated using HAPLOVIEW (Figure 3). The correlation coefficients for rs5051-rs2493133 were similar in Caucasians (r2 = 0.919) and African-Americans (r2 = 0.761).
Figure 3.
Linkage disequilibrium structure of the seven studied AGT SNPs by race-specific group. The SNPs are shown sequentially as they appear on the chromosome (not to scale). D', coefficient of linkage disequilibrium; r, regression coefficient of linkage disequilibrium.
The race-specific analysis includes 1500 and 1094 cases of CHD and HF, respectively, for the Caucasian group, and 753 and 634 cases of CHD and HF, respectively, for the African-Americans. Tables 3A,B demonstrate the results of the gene by treatment interaction analyses for the seven AGT SNPs. Overall there was little consistency by race. Among African-Americans, those with the minor allele (A) of rs7079 were 90% more likely to have CHD when using chlorthalidone compared to those using amlodipine (p = 0.01). Additionally, African-American participants with the minor allele (G) of rs11122576 were over two times as likely to have CHD when using chlorthalidone compared to amlodipine (OR = 2.5, p = 0.006) and using lisinopril compared to amlodipine (OR = 2.6, p = 0.01). Among Caucasian participants, those with the minor allele (G) of rs11122576 were 40% less likely to have CHD when using chlorthalidone compared to using amlodipine (OR = 0.6, p = 0.01), and were 30% less likely to have CHD when using chlorthalidone compared to using lisinopril (OR = 0.7, p = 0.03).
Table 3A.
Genotype-by-treatment interaction results for genetic model (additive and dominant) among Caucasians.
| Out-come | AGT variant | Number of cases (col %) | ORs and p-values for genetic model, Caucasians | |||||
|---|---|---|---|---|---|---|---|---|
| CHL | AML | LIS | CHL vs. AML | CHL vs. LIS | LIS vs. AML | |||
| CHD (n = 1500) | rs7079 | CC | 293 (43.9%) | 191 (47.6%) | 163 (44.2%) | p = 0.13; OR = 1.2; CI(0.96–1.4) | p = 0.62; OR = 1.0; CI(0.9–1.3) | p = 0.36; OR = 1.1; CI(0.9–1.4) |
| AC | 289 (43.3%) | 170 (42.4%) | 166 (45.0%) | |||||
| AA | 85 (12.7%) | 40 (10.0%) | 40 (10.8%) | |||||
| rs5051 | TT | 135 (19.9%) | 75 (18.7%) | 65 (17.1%) | p = 0.26; OR = 1.1; CI(0.9–1.3) | p = 0.49; OR = 0.9; CI(0.8–1.1) | p = 0.10; OR = 1.2; CI(1.0–1.4) | |
| TC | 296 (43.7%) | 205 (51.1%) | 175 (46.1%) | |||||
| CC | 247 (36.4%) | 121 (30.2%) | 140 (36.8%) | |||||
| rs3789678 | CC | 542 (79.7%) | 315 (78.0%) | 312 (80.6%) | p = 0.50; OR = 0.9; CI(0.7–1.2) | p = 0.72; OR = 1.0; CI(0.8–1.4) | p = 0.36; OR = 0.9; CI(0.6–1.2) | |
| TC&CC | 138 (20.3%) | 89 (22%) | 75 (19.4%) | |||||
| rs2493133 | CC | 136 (20.4%) | 76 (19.0%) | 63 (16.9%) | p = 0.43; OR = 1.1; CI(0.9–1.3) | p = 0.28; OR = 0.9; CI(0.8–1.1) | p = 0.09; OR = 1.2; CI(1.0–1.5) | |
| TC | 291 (43.6%) | 201 (50.1%) | 170 (45.6%) | |||||
| TT | 240 (36.0%) | 124 (30.9%) | 140 (37.5%) | |||||
| rs2493129 | GG | 652 (95.2%) | 378 (92.2%) | 363 (93.3%) | p = 0.04; OR = 0.6; CI(0.4–1.0) | p = 0.20; OR = 0.7; CI(0.4–1.2) | p = 0.54; OR = 0.8; CI(0.5–1.4) | |
| AG&AA | 33 (4.8%) | 32 (7.8%) | 26 (6.7%) | |||||
| rs2478544 | GG | 452 (69.1%) | 258 (68.1%) | 254 (68.7%) | p = 0.73; OR = 1.0; CI(0.7–1.3) | p = 0.88; OR = 1.0; CI(0.7–1.3) | p = 0.87; OR = 1.0; CI(0.7–1.3) | |
| GC&CC | 202 (30.9%) | 121 (31.9%) | 116 (31.3%) | |||||
| rs11122576 | AA | 603 (88.7%) | 342 (83.4%) | 328 (84.1%) | p = 0.01; OR = 0.6; CI(0.5–0.9) | p = 0.03; OR = 0.7; CI(0.5–1.0) | p = 0.79; OR = 1.0; CI(0.7–1.4) | |
| AG&GG | 77 (11.3%) | 68 (16.6%) | 62 (15.9%) | |||||
| HF (n = 1094) | rs7079 | CC | 208 (47.8%) | 160 (48.0%) | 121 (43.8%) | p = 0.90; OR = 1.0; CI(0.8–1.2) | p = 0.98; OR = 1.0; CI(0.8–1.3) | p = 0.92; OR = 1.0; CI(0.8–1.3) |
| AC | 173 (40.0%) | 133 (40.0%) | 132 (47.8%) | |||||
| AA | 54 (12.4%) | 40 (12.0%) | 23 (8.3%) | |||||
| rs5051 | TT | 90 (20.4%) | 68 (20.0%) | 56 (19.5%) | p = 0.50; OR = 1.1; CI(0.9–1.3) | p = 0.84; OR = 1.0; CI(0.8–1.3) | p = 0.67; OR = 1.0; CI(0.8–1.3) | |
| TC | 197 (44.6%) | 166 (48.8%) | 136 (47.4%) | |||||
| CC | 155 (35.1%) | 106 (31.2%) | 95 (33.1%) | |||||
| rs3789678 | CC | 351 (78.9%) | 266 (77.8%) | 222 (78.7%) | p = 0.71; OR = 0.9; CI(0.7–1.3) | p = 0.96; OR = 1.0; CI(0.7–1.4) | p = 0.78; OR = 0.9; CI(0.6–1.4) | |
| TC&TT | 94 (21.1%) | 76 (22.3%) | 60 (21.3%) | |||||
| rs2493133 | CC | 87 (20.0%) | 64 (18.9%) | 56 (19.8%) | p = 0.56; OR = 1.1; CI(0.9–1.3) | p = 0.55; OR = 1.1; CI(0.9–1.3) | p = 0.96; OR = 1.0; CI(0.8–1.2) | |
| TC | 192 (44.0%) | 166 (49.1%) | 135 (47.7%) | |||||
| TT | 157 (36.0%) | 108 (32.0%) | 92 (32.5%) | |||||
| rs2493129 | GG | 429 (95.6%) | 314 (91.3%) | 269 (92.4%) | p = 0.02; OR = 0.5; CI(0.3–0.9) | p = 0.08; OR = 0.6; CI(0.3–1.1) | p = 0.60; OR = 0.9; CI(0.5–1.5) | |
| AG&AA | 20 (4.4%) | 30 (8.7%) | 22 (7.6%) | |||||
| rs2478544 | GG | 284 (67.6%) | 212 (64.1%) | 187 (68%) | p = 0.31; OR = 0.9; CI(0.6–1.2) | p = 0.92; OR = 1.0; CI(0.7–1.4) | p = 0.31; OR = 0.8; CI(0.6–1.2) | |
| GC&CC | 136 (32.4%) | 119 (35.9%) | 88 (32.1%) | |||||
| rs11122576 | AA | 384 (85.9%) | 295 (85.3%) | 241 (83.1%) | p = 0.80; OR = 0.9; CI(0.6–1.4) | p = 0.30; OR = 0.8; CI(0.5–1.2) | p = 0.46; OR = 1.2; CI(0.8–1.8) | |
| AG&GG | 63 (14.1%) | 51 (14.8%) | 49 (16.9%) | |||||
CHL, chlorthalidone; AML, amlodipine; LIS, lisinopril; CHD, coronary heart disease; HF, heart failure.
Dominant model was used for SNP which has any cell size less than 10 observations by collapsing minor allele homozygotes with heterozygotes which was compared to common allele homozygotes. Additive model coded common homozygotes as 0, heterozygotes as 1; rare homozygotes as 2; and assumed linearity (1-degree of freedom test).
Table 3B.
Genotype-by-treatment interaction results for genetic model (additive and dominant) among African Americans.
| Out-come | AGT variant | Number of cases (col %) | ORs and p-values for genetic model, African Americans | |||||
|---|---|---|---|---|---|---|---|---|
| CHL | AML | LIS | CHL vs. AML | CHL vs. LIS | LIS vs. AML | |||
| CHD (n = 753) | rs7079 | CC | 259 (78.3%) | 175 (87.1%) | 175 (85.0%) | p = 0.01; OR = 1.9; CI(1.1–3.0) | p = 0.06; OR = 1.6; CI (1.0–2.5) | p = 0.54; OR = 1.2; CI(0.7–2.1) |
| AC&AA | 72 (21.7%) | 26 (12.9%) | 31 (15%) | |||||
| rs5051 | TT | 237 (71.6%) | 150 (73.2%) | 162 (78.3%) | p = 0.69; OR = 1.1; CI(0.7–1.6) | p = 0.09; OR = 1.4; CI(1.0–2.1) | p = 0.23; OR = 0.8; CI(0.5–1.2) | |
| TC&CC | 94 (28.4%) | 55 (26.8%) | 45 (21.7%) | |||||
| rs3789678 | CC | 215 (64.6%) | 139 (69.9%) | 126 (60.9%) | p = 0.21; OR = 1.3; CI(0.9–1.9) | p = 0.39; OR = 0.9; CI(0.6–1.2) | p = 0.06; OR = 1.5; CI(1.0–2.2) | |
| TC&TT | 118 (35.4%) | 60 (30.1%) | 81 (39.1%) | |||||
| rs2493133 | CC | 211 (64.5%) | 134 (68.7%) | 145 (71.1%) | p = 0.33; OR = 1.2; CI(0.8–1.8) | p = 0.12; OR = 1.4; CI(0.9–2.0) | p = 0.61; OR = 0.9; CI(0.6–1.4) | |
| TC&TT | 116 (35.5%) | 61 (31.3%) | 59 (28.9%) | |||||
| rs2493129 | GG | 288 (86.0%) | 164 (82.4%) | 180 (86.5%) | p = 0.27; OR = 0.8; CI(0.5–1.2) | p = 0.85; OR = 1.0; CI(0.6–1.7) | p = 0.25; OR = 0.7; CI(0.4–1.3) | |
| AG&AA | 47 (14%) | 35 (17.6%) | 28 (13.5%) | |||||
| rs2478544 | GG | 96 (31.3%) | 48 (25.7%) | 63 (32.1%) | p = 0.31; OR = 0.9; CI(0.7–1.1) | p = 0.97; OR = 1.0; CI(0.8–1.3) | p = 0.34; OR = 0.9; CI(0.7–1.2) | |
| GC | 144 (47.0%) | 96 (51.3%) | 89 (45.4%) | |||||
| CC | 67 (21.8%) | 43 (23.0%) | 44 (22.5%) | |||||
| rs11122576 | AA | 294 (87.5%) | 191 (94.6%) | 181 (87.0%) | p = 0.006; OR = 2.5; CI(1.2–4.9) | p = 0.87; OR = 1.0; CI(0.6–1.6) | p = 0.01; OR = 2.6; CI(1.2–5.4) | |
| AG&GG | 42 (12.5%) | 11 (5.5%) | 27 (13%) | |||||
| HF (n = 634) | rs7079 | CC | 190 (80.9%) | 181 (86.2%) | 154 (86.0%) | p = 0.13; OR = 1.5; CI(0.9–2.5) | p = 0.16; OR = 1.5; CI(0.9–2.5) | p = 0.96; OR = 1.0; CI(0.6–1.8) |
| AC&AA | 45 (19.1%) | 29 (13.9%) | 25 (14%) | |||||
| rs5051 | TT | 157 (67.1%) | 160 (76.2%) | 125 (69.8%) | p = 0.03; OR = 1.6; CI(1.0–2.4) | p = 0.55; OR = 1.1; CI(0.7–1.7) | p = 0.16; OR = 1.4; CI(0.9–2.2) | |
| TC&CC | 77 (32.9%) | 50 (23.8%) | 54 (30.2%) | |||||
| rs3789678 | CC | 147 (62.8%) | 124 (60.5%) | 100 (55.9%) | p = 0.59; OR = 0.9; CI(0.7–1.3) | p = 0.16; OR = 0.8; CI(0.6–1.1) | p = 0.38; OR = 1.2; CI(0.8–1.6) | |
| TC | 76 (32.5%) | 70 (34.2%) | 68 (38.0%) | |||||
| TT | 11 (4.7%) | 11 (5.4%) | 11 (6.2%) | |||||
| rs2493133 | CC | 145 (62.5%) | 142 (69.3%) | 117 (66.5%) | p = 0.14; OR = 1.4; CI(0.9–2.0) | p = 0.41; OR = 1.2; CI(0.8–1.8) | p = 0.56; OR = 1.1; CI(0.7–1.8) | |
| TC&TT | 87 (37.5%) | 63 (30.7%) | 59 (33.5%) | |||||
| rs2493129 | GG | 207 (87.7%) | 189 (89.2%) | 143 (80.3%) | p = 0.64; OR = 1.2; CI(0.6–2.1) | p = 0.04; OR = 0.6; CI(0.3–1.0) | p = 0.02; OR = 2.0; CI(1.1–3.6) | |
| AG&AA | 29 (12.3%) | 23 (10.9%) | 35 (19.7%) | |||||
| rs2478544 | GG | 72 (32.6%) | 56 (28.4%) | 63 (38.7%) | p = 0.56; OR = 0.9; CI(0.7–1.2) | p = 0.35; OR = 1.1; CI(0.9–1.5) | p = 0.14; OR = 0.8; CI(0.6–1.1) | |
| GC | 105 (47.5%) | 102 (51.8%) | 69 (42.3%) | |||||
| CC | 44 (20.0%) | 39 (19.8%) | 31 (19.0%) | |||||
| rs11122576 | AA | 214 (91.1%) | 196 (92.9%) | 161 (89.0%) | p = 0.48; OR = 1.3; CI(0.6–2.6) | p = 0.47; OR = 0.8; CI(0.4–1.5) | p = 0.18; OR = 1.6; CI(0.8–3.3) | |
| AG&GG | 21 (8.9%) | 15 (7.2%) | 20 (11%) | |||||
CHL, chlorthalidone; AML, amlodipine; LIS, lisinopril; CHD, coronary heart disease; HF, heart failure.
Dominant model was used for SNP which has any cell size less than 10 observations by collapsing minor allele homozygotes with heterozygotes which was compared to common allele homozygotes. Additive model coded common homozygotes as 0, heterozygotes as 1; rare homozygotes as 2; and assumed linearity (1-degree of freedom test).
With respect to the risk of HF, an association was observed among the African-American group for rs5051 when comparing chlorthalidone to amlodipine, rs2493129 when comparing chlorthalidone to lisinopril, and rs2493129 when comparing lisinopril to amlodipine with OR = 1.6, OR = 0.6, and OR = 2.0, respectively. A suggestive gene by treatment interaction was also observed among the Caucasian group at the rs2493129 locus with OR = 0.5 (p = 0.02) when comparing chlorthalidone to amlodipine. However, none of the tested models demonstrated statistical significance after the Bonferroni correction for multiple testing.
Discussion
In this study, we evaluated whether AGT variants interact with antihypertensive treatments to effect the risk of CHD and HF during follow-up in GenHAT. Due to its high prevalence, the annual healthcare costs of hypertension are sharply on the rise (Heidenreich et al., 2011). Personalized antihypertensive treatment may benefit patients by maximizing blood pressure control and mitigating CVD morbidity and mortality resulting in reduced health care costs. Our results demonstrate that variation in the AGT gene might have a role in CHD and HF among hypertensive patients.
Other groups have considered the association between AGT polymorphisms and blood pressure response to antihypertensive agents. For instance, Frazier et al. (2004) found that the A allele of AGT G6A (rs5051) has a significant effect on systolic blood pressure reduction in response to 25 mg of hydrochlorothiazide (a thiazide diuretic) in African American women. Another study in 1447 hypertensive Chinese patients demonstrated that patients with the minor allele (A) of the AGT rs7079 SNP had a better blood pressure response to ACE inhibitors (benazepril) when compared to ACE users with the common allele (Su et al., 2007). Another group recently reported no difference in blood pressure response to hydrochlorothiazide associated with rs7079 (Huang et al., 2011). In sum, these studies suggest that variations in the AGT gene may have an influence on blood pressure response to antihypertensive therapies.
Other reports have found associations between SNPs in AGT and CVD events. For example, in a cohort study of 4097 hypertensive patients, ACE-inhibitor users with the minor allele (T) of M235T (rs699) had higher risk of having MI and stroke compared to non-users with the common allele (Schelleman et al., 2007). Another prospective study, the Thrombogenic Factor and Coronary Events (THROMBO) study, failed to show a significant association between M235T and recurrent coronary events in ACE inhibitor users (Goldenberg et al., 2006). Though these models are not directly comparable to our own, they support a role for the AGT gene in CVD risk during antihypertensive treatment.
Suggestive findings for rs11122576 (associated with a greater than two-fold risk of CHD) for two different treatment comparisons were observed in African Americans. Although these findings did not meet our significance criteria, they provide insight into genetic underpinnings of variation in response and outcomes. The pharmacogenetic effect of rs11122576 comparing chlorthalidone to amlodipine was in the opposite direction for Caucasians (OR = 0.6, p = 0.01). This may be due to differences in LD patterns in African Americans compared to Caucasians, but suggests the SNP could be tagging some important variant in both races. A recent study found a significant association of the minor allele of rs11122576 with CHD mortality in a cohort of 1186 CHD participants mostly of European ancestry-after 3-years of follow-up. The minor allele of rs1122576 was also associated with of higher risk of renal disease, type 2 diabetes, and higher body mass index in that study (Ellis et al., 2013). Similarly to the CHD results, there were more marginally significant associations among African Americans compared to Caucasians for the HF outcome, however none were close to statistical significance.
The GenHAT study is a large, ethnically diverse sample ancillary to a large clinical trial which facilitates pharmacogenetic studies among well-defined treatment groups with extensive adjudication of CVD outcomes. Despite this strength, our study also has some limitations. First, ALLHAT recruited hypertensive patients aged 55 years and older with a large proportion of diabetes mellitus (36.0%) and/or evidence of atherosclerotic cardiovascular disease (46.9%). Therefore, it may not be valid to generalize the findings of this study to healthier and younger populations. Second, the case-only design did not allow the evaluation of the main effects of treatment or genotype on the outcome (Khoury and Flanders, 1996). Third, we only had sufficient genotyped data for seven AGT SNPs, so this study cannot completely evaluate the antihypertensive pharmacogenetic effects of the AGT gene. Finally, and most importantly, we recognize most genomic findings are greatly strengthened by replication studies. However, due to specific drug regimens and SNP selections in GenHAT comparable study populations are difficult to find.
The findings reported here for antihypertensive treatment effects of seven AGT SNPs in 2253 CHD cases and 1728 HF cases suggest that a patient's AGT variants might help predict which hypertensive medication(s) can lower cardiovascular disease risk. Our results highlight rs11122576 for African American participants where the minor allele of rs11122576 was associated with over two-fold higher risk of CHD for chlorthalidone treatment compared to amlodipine treatment and lisinopril treatment compared to amlodipine treatment (p = 0.006 and p = 0.01, respectively). Although marginally significant, our results demonstrate biological relevance in a large, racially diverse population. These findings need to be investigated further and confirmed in independent sample of hypertensive patients.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported in part by grant HL63082 (GenHAT) from the NIH/NHLBI and grant N01-HC-35130 (ALLHAT) from the NIH/NHLBI.
References
- ALLHAT Collaborative Research Group. (2000). Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 283, 1967–1975 10.1038/sj.jhh.1001600 [DOI] [PubMed] [Google Scholar]
- Al-Najai M., Muiya P., Tahir A. I., Elhawari S., Gueco D., Andres E., et al. (2013). Association of the angiotensionogen gene polymorphism with atherosclerosis and its risk traits in the Saudi population. BMC Cardiovasc. Disord. 13:17 10.1186/1471-2261-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett D. K., Boerwinkle E., Davis B. R., Eckfeldt J., Ford C. E., Black H. (2002). Pharmacogenetic approaches to hypertension therapy: design and rationale for the Genetics of Hypertension Associated Treatment (GenHAT) study. Pharmacogenomics J. 2, 309–317 10.1038/sj.tpj.6500113 [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang L., Wang H. W., Wang X. Y., Li X. Q., Zhang L. L. (2012). The M235T polymorphism in the angiotensinogen gene and heart failure: a meta-analysis. J. Renin Angiotensin Aldosterone Syst. 15, 190–195 10.1177/1470320312465455 [DOI] [PubMed] [Google Scholar]
- Craig C. R., Stitzel R. E. (2003). Modern Pharmacology with Clinical Applications. Vol. 2 Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Davis B. R., Cutler J. A., Gordon D. J., Furberg C. D., Wright J. T., Jr., Cushman W. C., et al. (1996). Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am. J. Hypertens. 9(4 Pt 1), 342–360 10.1016/0895-7061(96)00037-4 [DOI] [PubMed] [Google Scholar]
- Duarte J. D., Cooper-DeHoff R. M. (2010). Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev. Cardiovasc. Ther. 8, 793–802 10.1586/erc.10.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. L., Palmer B. R., Frampton C. M., Troughton R. W., Doughty R. N., Whalley G. A., et al. (2013). Genetic variation in the renin-angiotensin-aldosterone system is associated with cardiovascular risk factors and early mortality in established coronary heart disease. J. Hum. Hypertens. 27, 237–244 10.1038/jhh.2012.24 [DOI] [PubMed] [Google Scholar]
- Frazier L., Turner S. T., Schwartz G. L., Chapman A. B., Boerwinkle E. (2004). Multilocus effects of the renin-angiotensin-aldosterone system genes on blood pressure response to a thiazide diuretic. Pharmacogenomics J. 4, 17–23 10.1038/sj.tpj.6500215 [DOI] [PubMed] [Google Scholar]
- Gauderman W. J., Morrison J. M. (2009). QUANTO 1.2: a computer program for power and sample size calculations for genetic-epidemiology studies. Available online at: http://hydra.usc.edu/gxe (Accessed January 21, 2014).
- Goldenberg I., Moss A. J., Ryan D., McNitt S., Eberly S. W., Zareba W. (2006). Polymorphism in the Angiotensinogen Gene, hypertension, and ethnic differences in the risk of recurrent coronary events. Hypertension 48, 693–699 10.1161/01.HYP.0000239204.41079.6b [DOI] [PubMed] [Google Scholar]
- Heidenreich P. A., Trogdon J. G., Khavjou O. A., Butler J., Dracup K., Ezekowitz M. D., et al. (2011). Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944 10.1161/CIR.0b013e31820a55f5 [DOI] [PubMed] [Google Scholar]
- Huang C. C., Chung C. M., Hung S. I., Leu H. B., Wu T. C., Huang P. H., et al. (2011). Genetic variation in renin predicts the effects of thiazide diuretics. Eur. J. Clin. Invest. 41, 828–835 10.1111/j.1365-2362.2011.02472.x [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X., Soubrier F., Kotelevtsev Y. V., Lifton R. P., Williams C. S., Charru A., et al. (1992). Molecular basis of human hypertension: role of angiotensinogen. Cell 71, 169–180 10.1016/0092-8674(92)90275-H [DOI] [PubMed] [Google Scholar]
- Khoury M. J., Flanders W. D. (1996). Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls! Am. J. Epidemiol. 144, 207–213 10.1093/oxfordjournals.aje.a008915 [DOI] [PubMed] [Google Scholar]
- Li X., Li Q., Wang Y., Li Y., Ye M., Ren J., et al. (2013). AGT gene polymorphisms (M235T, T174M) are associated with coronary heart disease in a Chinese population. J. Renin Angiotensin Aldosterone Syst. 14, 354–359 10.1177/1470320312452029 [DOI] [PubMed] [Google Scholar]
- Lynch A. I., Irvin M. R., Boerwinkle E., Davis B. R., Vaughan L. K., Ford C. E., et al. (2012). RYR3 gene polymorphisms and cardiovascular disease outcomes in the context of antihypertensive treatment. Pharmacogenomics J. 13, 330–334 10.1038/tpj.2012.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S. M., Saboohi K., Osman Ali S., Bokhari A. M., Frossard P. M. (2003). Association of the angiotensin-converting enzyme (ACE) gene G2350A dimorphism with essential hypertension. J. Hum. Hypertens. 17, 719–723 10.1038/sj.jhh.1001600 [DOI] [PubMed] [Google Scholar]
- Pasquale D. P., Cannizzaro S., Scalzo S., Maringhini G., Pipitone F., Fasullo S., et al. (2005). Cardiovascular effects of I/D angiotensin-converting enzyme gene polymorphism in healthy subjects. Findings after follow-up of six years. Acta Cardiol. 60, 427–435 10.2143/AC.60.4.2004993 [DOI] [PubMed] [Google Scholar]
- Roger V. L., Go A. S., Lloyd-Jones D. M., Benjamin E. J., Berry J. D., Borden W. B., et al. (2012). Heart disease and stroke statistics-2012 update a report from the American Heart Association. Circulation 125, e2–e220 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelleman H., Klungel O. H., Witteman J. C., Breteler M. M., Yazdanpanah M., Danser A. H., et al. (2007). Angiotensinogen M235T polymorphism and the risk of myocardial infarction and stroke among hypertensive patients on ACE-inhibitors or beta-blockers. Eur. J. Hum. Genet. 15, 478–484 10.1038/sj.ejhg.5201789 [DOI] [PubMed] [Google Scholar]
- Sica D. A. (2004). Current concepts of pharmacotherapy in hypertension: thiazide-type diuretics: ongoing considerations on mechanism of action. J. Clin. Hypertens. (Greenwich) 6, 661–664 10.1111/j.1524-6175.2004.03902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Lee L., Li X., Lv J., Hu Y., Zhan S., Yang H., et al. (2007). Association between angiotensinogen, angiotensin II receptor genes, and blood pressure response to an angiotensin-converting enzyme inhibitor. Circulation 115, 725–732 10.1161/CIRCULATIONAHA.106.642058 [DOI] [PubMed] [Google Scholar]