Abstract
The physiological mechanisms involved in isoproterenol (ISO)-induced chronic heart failure (CHF) are not fully understood. In this study, we investigated local changes in cardiac aldosterone and its synthase in rats with ISO-induced CHF, and evaluated the effects of treatment with recombinant human brain natriuretic peptide (rhBNP). Sprague-Dawley rats were divided into 4 different groups. Fifty rats received subcutaneous ISO injections to induce CHF and the control group (n=10) received equal volumes of saline. After establishing the rat model, 9 CHF rats received no further treatment, rats in the low-dose group (n=8) received 22.5 μg/kg rhBNP and those in the high-dose group (n=8) received 45 μg/kg rhBNP daily for 1 month. Cardiac function was assessed by echocardiographic and hemodynamic analysis. Collagen volume fraction (CVF) was determined. Plasma and myocardial aldosterone concentrations were determined using radioimmunoassay. Myocardial aldosterone synthase (CYP11B2) was detected by quantitative real-time PCR. Cardiac function was significantly lower in the CHF group than in the control group (P<0.01), whereas CVF, plasma and myocardial aldosterone, and CYP11B2 transcription were significantly higher than in the control group (P<0.05). Low and high doses of rhBNP significantly improved hemodynamics (P<0.01) and cardiac function (P<0.05) and reduced CVF, plasma and myocardial aldosterone, and CYP11B2 transcription (P<0.05). There were no significant differences between the rhBNP dose groups (P>0.05). Elevated cardiac aldosterone and upregulation of aldosterone synthase expression were detected in rats with ISO-induced CHF. Administration of rhBNP improved hemodynamics and ventricular remodeling and reduced myocardial fibrosis, possibly by downregulating CYP11B2 transcription and reducing myocardial aldosterone synthesis.
Keywords: Chronic heart failure, Aldosterone, Aldosterone synthase, Recombinant human brain natriuretic peptide
Introduction
Chronic heart failure (CHF) represents the most serious stage of many cardiovascular diseases, and it carries a poor prognosis. Ventricular remodeling plays a role in both the occurrence and development of heart failure. Heart failure is accompanied by significantly increased aldosterone levels in the circulation and locally within the myocardium. This results, directly or indirectly, in the formation of fibrosis and progression of heart failure (1). Aldosterone inhibitors have been shown to improve left ventricular remodeling in heart failure (2).
Aldosterone synthase (CYP11B2) is a key enzyme involved in aldosterone synthesis. A significant elevation in CYPB112 expression has been observed in myocardial tissue in patients with heart failure (3). It has also been demonstrated that the aldosterone synthase inhibitor FAD286 improves left ventricular hemodynamics, remodeling, and cardiac function after myocardial infarction (MI) induced by coronary artery ligation in a rat model of CHF. FAD286 has also been shown to normalize the left ventricular redox status more effectively than spironolactone (4).
Aldosterone synthase inhibitors have become a new option for the treatment of hypertension, heart failure, and renal disorders, as they are able to decrease aldosterone concentrations in plasma and target organs (5). Investigating the changes in aldosterone synthase that accompany CHF is, therefore, an important step to better understanding the mechanism of action of these agents.
CHF induced by isoproterenol (ISO) has been shown to be an ideal animal model of heart failure, which reflects the changes in myocardial cells caused by necrosis in the absence of coronary narrowing or obstruction (6). However, the changes in cardiac aldosterone synthase in this animal model remain unexplored.
Recombinant human brain natriuretic peptide (rhBNP) has a wide range of biological activity. The study of acute clinical effectiveness of nesiritide in a decompensated heart failure (ASCEND-HF) trial showed that short-term treatment with nesiritide improved acute decompensated heart failure. However, no significant prognostic differences could be demonstrated between the nesiritide and placebo groups (7).
A preliminary study in cultured neonatal rat cardiocytes suggests that BNP inhibits CYP11B2 mRNA expression, reduces local cardiac aldosterone synthesis, and suppresses the local cardiac renin-angiotensin-aldosterone system, thereby protecting against cardiac fibrosis (8). However, rhBNP is used primarily for short-term treatment of acute heart failure, and its long-term use in CHF has not been reported.
Based on these findings, it is possible that the transcription level of myocardial aldosterone synthase would be upregulated in rats with ISO-induced CHF and that long-term treatment with rhBNP may improve cardiac function and reduce myocardial fibrosis. The proposed mechanism would involve reduced expression of local myocardial aldosterone synthase in the left ventricle.
We, therefore, assessed the effect of long-term treatment with rhBNP in a rat model of CHF induced by ISO, by evaluating changes in cardiac function, myocardial fibrosis, and levels of left ventricular local myocardial aldosterone and its synthase.
Material and Methods
Experimental animals
Male Sprague-Dawley rats (6-8 weeks old, 200-250 g) were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (China; license #SCXK (Hu) 2003-0003). The rats were housed in a 24±1°C temperature-controlled room with alternating 12:12-h light-dark cycles and were allowed free access to food and water. Rats were raised by designated persons in clean-grade animal houses. Food and bedding were subjected to high-pressure sterilization. All animal experimental procedures were conducted in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals and the Guidelines of the Regulations for the Administration of Affairs Concerning Experimental Animals in Fujian Province.
CHF model
The model of ISO-induced CHF was produced as described previously (6,9). Briefly, rats were injected subcutaneously with ISO (Jiahe Pharmaceutical Co., Ltd., China, batch No. H31021344) for 15 days at 8:00 am. Doses of 30, 20, and 10 mg/kg were given on days 1, 2, and 3, respectively, followed by 5 mg/kg on days 4 to 15. Rats were raised for an additional 1 month after the 15-day injections, and then transthoracic echocardiography was performed in all survivors. Those with ejection fraction (EF) less than 45% were assigned to the CHF group (6). The rats in the control group received equal volumes of normal saline on days 1 to 15.
Surviving rats with CHF were randomly assigned to the CHF and treatment groups. Based on previous findings (10), the treatment group was further randomly divided into a low-dose group that received rhBNP (Tibetan Pharmaceutical Co., Ltd., China, batch No. S20050033) at a daily dose of 22.5 μg/kg, and a high-dose group that received rhBNP at a daily dose of 45 μg/kg. Rats were injected subcutaneously with rhBNP dissolved in normal saline twice daily at 8:00 am and 5:00 pm. The experiment was terminated after injections for 1 month. Rats in the control and CHF groups were given equal volumes of normal saline for 1 month.
Echocardiography
After 1 month, the rats were weighed and anesthetized with intraperitoneal 75 mg/kg ketamine and 5 mg/kg diazepam. Two-dimensional M-mode transthoracic echocardiographic studies were performed using a Vivid 7 ultrasound system (GE Medical Systems, USA) with an 8-MHz probe. The left ventricular internal diastolic diameter (LVIDd), left ventricular internal systolic diameter (LVIDs), and EF were measured in a horizontal section of the left ventricular short axis of the chordae tendineae.
Hemodynamic measurements
Rats were weighed and anesthetized as described earlier. The left ventricle was cannulated through the right common carotid artery. The following parameters were measured using a multichannel physiological recorder (Chengdu Taimeng Instrument Plant, China) with a pressure transducer: heart rate (HR), left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and the maximal rate of rise and fall of left ventricular pressure (dp/dtmax and dp/dtmin).
Sample collection and pathological analysis
After completion of the hemodynamic measurements, 2 mL blood was drawn from the inferior vena cava for measurement of plasma aldosterone. The rats were then killed by injection of 2 to 3 mL 10% potassium chloride administered through the carotid artery, in order to stop the heart in diastole. The heart was quickly removed via thoracotomy. The hearts were successively cut into 4 sections vertical to the long axis of left ventricle from the apex of heart. Heart tissue from near the base was rapidly frozen in liquid nitrogen for fluorescent quantitative real-time polymerase chain reaction (qPCR) analysis and local myocardial aldosterone measurement. The other three parts were fixed with and stored in 10% formaldehyde in phosphate-buffered saline (PBS). The heart tissues were embedded in paraffin, cut into 5-μm serial sections, and stained with picric acid-Sirius red.
Determination of aldosterone concentrations in plasma and myocardial tissues
The 2-mL blood samples collected prior to killing were transferred into a dried anticoagulant-containing EDTA solution and centrifuged at 1730 g for 15 min at 4°C. The plasma was separated and stored at −20°C for subsequent analysis.
Myocardial tissues (about 50 mg) were weighed and homogenized in 0.5 mL PBS on ice. Tissue homogenates for analysis were obtained after full homogenization and centrifugation at 1730 g for 15 min at 4°C. Aldosterone concentrations in the supernatant liquid of plasma and myocardial tissues were determined by radioimmunoassay (9) (Beckman Coulter Co., USA). The experiments were performed in a GC-1500r radioimmunoassay counter (Anhui Ustc Zonkia Scientific Equipment Company, China), following the manufacturer's instructions.
Picric acid-Sirius red staining
Paraffin-embedded heart sections were dewaxed. The sections were washed with 70% alcohol for 2 min and were then washed in distilled water 3 times, prior to being stained in picric acid-Sirius red (Guangdong Taishan Petrochemical Plant, China) for 30 min.
The slides were rinsed twice in absolute ethanol to remove excess dye, and were cleared in xylene. After they were air-dried, the slides were fixed and enveloped with neutral balata (Sinopharm Chemical Reagent Co., Ltd., China). Myocardial cells stained yellow and collagen stained red by picric acid-Sirius red (11). Collagen volume fraction (CVF) was determined using the image analysis software (Image-Pro Plus, version 4.5, Media Cybernetics, Inc., USA). CVF was calculated as the percentage of the area of stained collagen relative to the total area of the field of vision.
Three sections at the levels of apex, papillary muscle, and mitral valve were collected and used for staining. Ten high-magnification fields were randomly selected from each section, and the mean was calculated (12).
Quantitative real-time PCR (qPCR)
Total RNA was extracted using TRIzol reagent (Gibco/BRL, USA). Tissues stored in liquid nitrogen (about 100 mg) were ground to powder in liquid nitrogen using a mortar prechilled in liquid nitrogen. After the liquid nitrogen had evaporated completely, the powder was transferred into a 15-mL centrifuge tube and homogenized for 5 min at high speed in the presence of 8 mL of TRIzol reagent containing a cell lysis agent, RNase inhibitor, and an ion protective agent. After the solution was left to stand for 30 min at room temperature, 1.6 mL of chloroform was added. The reaction mixture was stirred rapidly for 15 s and left to stand for 5 min at room temperature.
Phase separation was performed by centrifugation at 12,000 g for 15 min at 4°C. The supernatant was collected, transferred into another clean centrifuge tube containing 4.0 mL isopropanol, and mixed gently by inverting 5 times. Centrifugation at 12,000 g for 10 min at 4°C was performed after the solution had been left to stand for 10 min at room temperature.
After the supernatant was removed, the RNA pellet was washed with 3 mL 75% ethanol and centrifuged at 10,000 g for 10 min. The supernatant was again removed, and the pellet was partly air-dried, with the tube inverted to complete the drying process. The pellet was then dissolved in 200 µL diethypyrocarbonate-treated water and transferred to a 1.5 mL Eppendrof tube. The ratio of OD260/OD280 was measured. RNA was identified by electrophoresis and stored at −80°C.
Reverse transcription
Reverse transcription was performed using a Reverse Transcription System Kit (Promega, USA) according to the manufacturer's instructions. Briefly, 2 µL of random primers (50 mmol/µL) and 4 µg of total RNA were added to 32 µL ddH2O. The mixture was denatured at 70°C for 5 min and cooled on ice. Then, 10 µL of 5× M-MLV buffer, 2.5 µL dNTP (2.5 mM), 2.5 µL M-MLV (Promega, 200 U/µL), and 1.5 μL RNase inhibitor (40 U/µL; TaKaRa Dalian Biotechnology Co., Ltd., China) were added to the reaction tube. The tube was incubated for 2 h at 37°C and frozen at −20°C until further use.
Quantitative real-time PCR (qPCR)
The primers were designed and synthesized by TaKaRa (TaKaRa Dalian Biotechnology Co., Ltd.). Primers used for qPCR were as follows: forward CYP11B2: TTGCTAAGGACTGGGTGGTTGT; reverse CYP11B2: AACTTTTCGCCCTACCGACTTG; forward GAPDH: TCCTGCACCACCAACTGCTTAG; reverse GAPDH: AGTGGCAGTGATGGCATGGACT.
qPCR was performed in a total volume of 25 μL, containing 2.0 μL cDNA, 0.5 μL of each primer (10 μM), 0.5 μL ROX reference dye (TaKaRa Dalian Biotechnology Co., Ltd.), 12.5 μL SYBR Premix Ex Taq (TaKaRa Dalian Biotechnology Co., Ltd.), and deionized water. The reaction conditions were 95°C for 10 min, followed by 45 cycles of 94°C for 15 s, and 60°C for 1 min.
Baseline fluorescence was defined as the level of fluorescence measured before cycle 15. The threshold level was set at 10 times the standard deviation (SD) of baseline fluorescence measured during qPCR cycles 6 to 10. The parameter threshold cycle (Ct) was defined as the cycle number at which the fluorescence passed the fixed threshold, normalized to GAPDH.
All samples were analyzed in triplicate, and the mean Ct value was determined. GAPDH served as an endogenous internal reference. ΔCt was calculated by subtracting the average Ct of the target gene from the average Ct of GAPDH. The mean relative expression of each target gene was expressed as 2-ΔCt, and a high value of 2-ΔCt was correlated to the increase in expression of target genes.
Statistical analysis
Statistical analyses were performed using the SPSS version 13.0 statistical software (SPSS Inc., USA), and data are reported as means±SD. One-way analysis of variance and the Student-Neuman-Kuels-q or Dunnett t-tests were used to compare the differences between groups. P<0.05 was considered to be statistically significant.
Results
Survival
Sixty rats were used for the experiment. Ten rats were randomly assigned to the control group (no deaths were observed during the experiment). The other 50 rats were injected subcutaneously with ISO, and 30 rats (60%) survived for 15 days. The 30 survivors were raised for a further 30 days, and five deaths occurred. After modeling, 25 rats survived, with an overall survival rate of 50%. All survivors were randomly assigned to the CHF group (n=9), low-dose rhBNP group (n=8), and high-dose rhBNP group (n=8). During the treatment period, no deaths occurred in any of the groups.
Echocardiography
Echocardiograph parameters are reported in Table 1 and Figure 1. LVIDd and LVIDs were significantly higher in the rats in the CHF group than in the control group (P<0.01), and EF was significantly lower than in the control group (P<0.01). The results suggested that rats with ISO-induced CHF displayed manifestations similar to those seen in dilated cardiomyopathy.
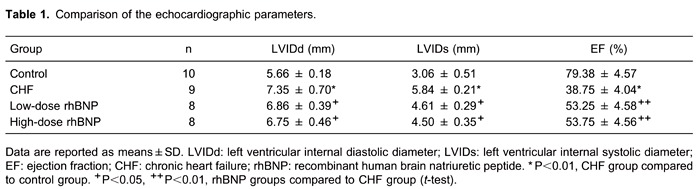
Figure 1. Echocardiography findings (M-mode, horizontal section of LV short axis). A, Control group; B, CHF group; C, low-dose rhBNP group; D, high-dose rhBNP group. CHF: chronic heart failure; rhBNP: recombinant human brain natriuretic peptide.
LVIDd and LVIDs were lower in the low- and high-dose rhBNP groups than in the CHF group (P<0.05), and EF was higher than in the CHF group (P<0.01), indicating that treatment with rhBNP for 1 month reduced LV end-diastolic diameter and improved systolic function. There were no significant differences in LVIDd, LVIDs, or EF between the low- and high-dose rhBNP groups (P>0.05).
Hemodynamic variables
Hemodynamic parameters are summarized in Table 2. LVEDP was significantly higher in the CHF group than in the control group (P<0.01), while LVSP, dp/dtmax, and dp/dtmin were significantly lower than in the control group (P<0.01). LVEDP was significantly lower (P<0.05) in the low- and high-dose rhBNP groups than in the CHF group, and dp/dtmax (P<0.05) and dp/dtmin (P<0.01) were significantly higher than in the CHF group. LVSP remained at a relatively stable level in the low-dose rhBNP group. There were no significant differences in these variables between low- and high-dose rhBNP groups and no obvious between-group differences were found for HR.
These findings suggest that, in rats with ISO-induced changes, myocardial fibers impaired systolic and diastolic cardiac function and that administration of rhBNP partly reversed these effects.
Myocardial fibrosis
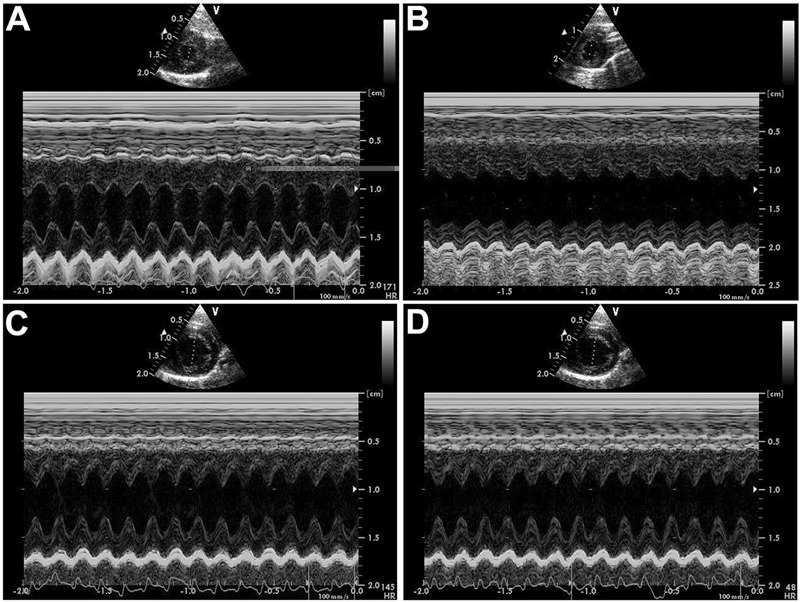
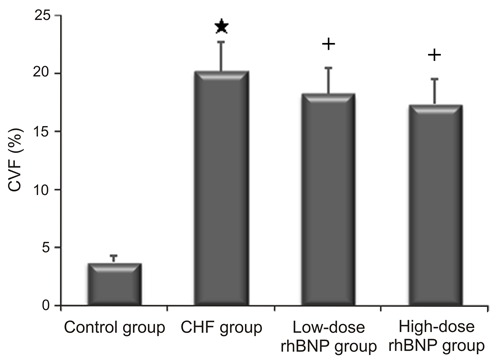
A high degree of collagen deposition was found in the myocardium of rats with ISO-induced heart failure (Figure 2). Consequently, CVF was significantly higher in the CHF group than in the control group (P<0.01). CVF in both the low- and high-dose rhBNP groups was significantly lower than in the CHF group (P<0.05). No significant differences were found in the CVF between low- and high-dose rhBNP (P>0.05; Figure 3). These findings suggest that rhBNP may reduce collagen deposition and improve myocardial fibrosis.
Figure 2. Changes in myocardial fibrosis (picric acid-Sirus red staining). Red indicates collagen fiber and yellow indicates myocardium. A, Control group. B, There was a significant increase in fibrous tissue in the CHF group. C, Fibrous tissue in the low-dose rhBNP group. D, Fibrous tissue in the high-dose rhBNP group. Fibrous tissue in the low-dose and high-dose rhBNP groups was reduced compared to the CHF group. CHF: chronic heart failure; rhBNP: recombinant human brain natriuretic peptide.
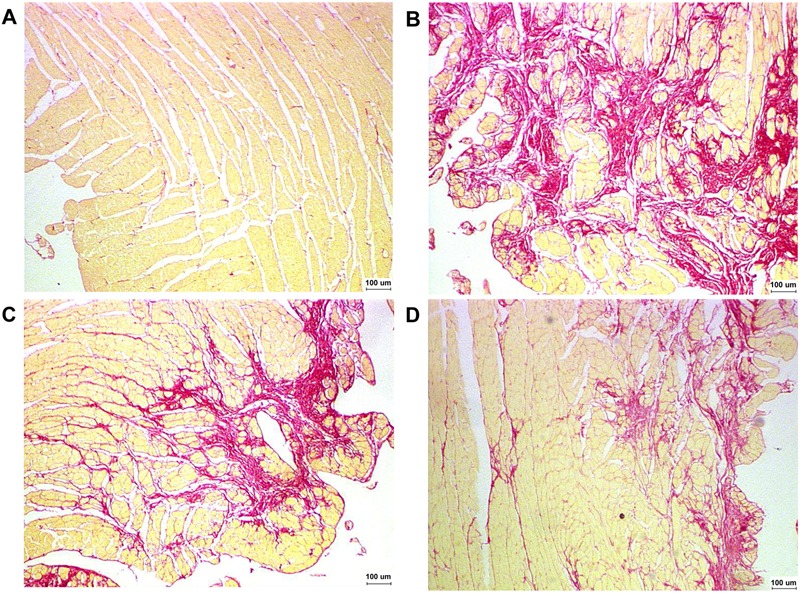
Figure 3. Collagen volume fraction (CVF) values of rats in different groups. Data are reported as means±SD. CHF: chronic heart failure (CHF); rhBNP: recombinant human brain natriuretic peptide *P<0.01 vs control group; +P<0.05 vs CHF group (t-test).
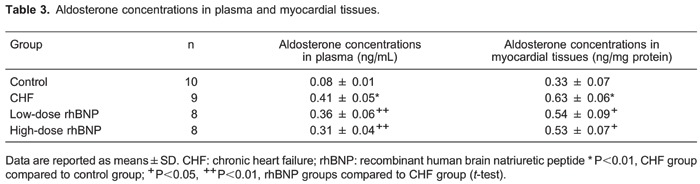
Aldosterone concentrations in the plasma and myocardial tissues
Aldosterone concentrations in plasma and myocardial tissues were significantly higher in the CHF group than in the control group (P<0.01). In addition, aldosterone concentrations in plasma and myocardial tissues in the low-dose and high-dose rhBNP groups were significantly lower than in the CHF group (P<0.01; Table 3). There were no significant differences in the aldosterone concentrations in the plasma and myocardial tissues between low- and high-dose rhBNP groups. These results indicate that rhBNP decreased aldosterone concentrations in plasma and in myocardial tissues.
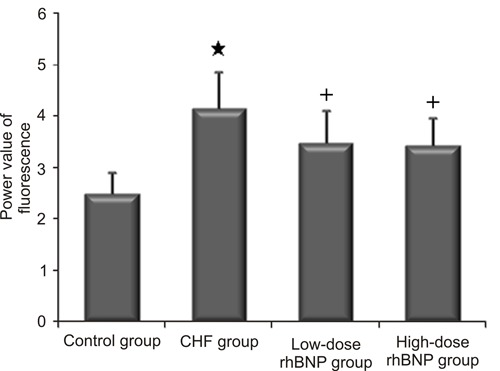
Transcription level of CYP11B2
Significantly elevated CYP11B2 transcription levels were detected in the rats with CHF induced by ISO (Figure 4), and the corresponding degree of fluorescence was significantly higher than in the control group (P<0.01). CYP11B2 transcription was significantly reduced following treatment with low- and high-dose rhBNP for 1 month. There were no significant differences between low- and high-dose rhBNP groups in terms of CYP11B2 transcription (Figure 5). These results suggest that rhBNP downregulated CYP11B2 transcription.
Figure 4. Amplification and dissolution curves for GAPDH and CYP11B2. A, GAPDH amplification curve; B, GAPDH melting curve; C, CYP11B2 amplification curve; D, CYP11B2 melting curve.
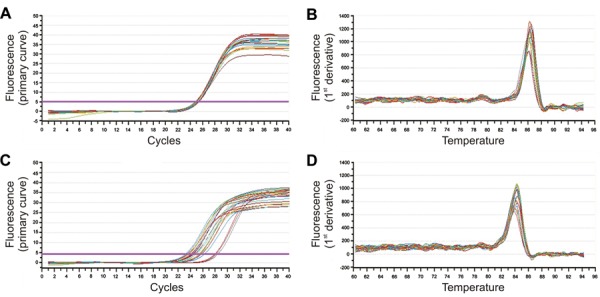
Figure 5. Power values of CYP11B2 fluorescence measurement in each group of rats. Data are reported as means±SD. CHF: chronic heart failure; rhBNP: recombinant human brain natriuretic peptide *P<0.01 vs control group; +P<0.05 vs CHF group (t-test).
Discussion
It has been reported previously that ISO causes diffuse myocardial necrosis and cardiac fibrosis, which are similar to that observed in patients with MI and remodeling necrosis. However, unlike with MI, the effects of ISO are observed with a patent coronary circulation (6). In the rat model, changes in myocardial cells progressed to cause heart failure. This was the result of reduced levels of oxygen, microcirculatory disturbance, changes in the permeability of myocardial cell membranes, Ca2+ overload, and the toxic effects of the oxidative products of ISO, which resulted in myocardial ischemia-reperfusion injury (6).
It has been previously reported that the rat model of heart failure could be induced by two subcutaneous injections of either 85, 170, or 340 mg/kg ISO, with resulting survival rates of 45, 30, and 18%, respectively. In the present study, rats were injected subcutaneously with progressively decreasing doses of ISO for 15 days, for a total dose of 120 mg/kg. This resulted in a survival rate of 50%, demonstrating that multiple low-dose injections of ISO improved survival rate by avoiding the acute toxicity of high-dose ISO. The EF in surviving rats was <45%. It has been reported that chronic injection of low-dose ISO for different times (1.2 mg·kg−1·day−1 for 3 days to 16 weeks) only resulted in left ventricular hypertrophy at 3 days and at 1 and 2 weeks. However, systolic failure was induced at a longer time of 4 weeks, indicating that there was ongoing injury of cardiomyocytes by ISO (13). In our study, rats received continuous subcutaneous injection of ISO for 2 weeks and waited for 1 month to establish the chronic heart failure model. Then, the survival rate of rats was observed to reflect the impact of ISO on cardiomyocytes.
Our results showed that CYP11B2 mRNA transcription, aldosterone concentrations, and myocardial fibrosis in left ventricular myocardial tissues in the CHF group were significantly higher than those in the control group (65.9, 412.5, and 439.8%, respectively). These findings suggest that both myocardial aldosterone synthase (CYP11B2) transcription and aldosterone synthesis increased significantly in the left ventricle of rats with ISO-induced CHF.
Traditionally, aldosterone has been thought to be produced solely by zona glomerulosa cells in the adrenal cortex in response to angiotensin II, making it an important component of the circulating renin-angiotensin-aldosterone system. Recently, aldosterone has also been reported to be produced in extraadrenal tissues, including the heart, blood vessels, and brain (14-16). Aldosterone concentrations in blood from patients' coronary sinus (from the heart), anterior interventricular vein (AIV; from the left ventricle), and aortic root were tested and compared by Yamamoto et al. (17) and Mizuno et al. (18). Results showed that the aldosterone concentrations in coronary sinus or AIV blood were higher than those in aortic root blood, which indicated that myocardial tissues had the ability to synthesize aldosterone. The underlying mechanism may be associated with the autocrine and/or paracrine role of cardiomyocytes, through which aldosterone concentrations in coronary sinus blood are higher than those in aortic root blood (17,18). In this study, aldosterone concentrations, which were directly tested in the left ventricle of ISO-induced heart failure rats, were significantly higher than in the control; meanwhile, such aldosterone concentrations were decreased with BNP treatment, both of which indicated that aldosterone synthesis levels were increased in the left ventricle of heart failure rats. Similar results were reported by Gomez-Sanchez et al. (19). However, it has not been reported whether aldosterone in the left ventricle was specifically produced in myocardial cells, highly specialized contractile cells, the endothelium, fibroblasts, or vascular smooth muscle cells. We speculated that it might be produced mainly by cardiomyocytes.
BNP has been shown to induce arterial and venous vasodilatation via vascular smooth muscle relaxation. It also has diuretic effects and is able to reduce pulmonary capillary wedge pressure and right atrial pressure in patients with CHF (20,21). These studies have suggested that BNP decreases the preload of heart and reduces peripheral vascular resistance, systolic blood pressure, and mean arterial pressure. Although BNP has no definite positive inotropic action, it significantly increases cardiac index and EF, relieves dyspnea, and reduces the occurrence of ventricular arrhythmias (22).
Short-term administration of the BNP agonist nesiritide has been shown to have beneficial effects in patients with acute decompensated heart failure, but is unable to significantly improve their prognosis (7). A study in dogs with experimentally induced CHF indicated that 10 days of repeated short-term administration of BNP resulted in increased cardiac output and decreased systemic vascular resistance and pulmonary capillary wedge pressure. However, there was no evidence of increased plasma renin activity (10). Neutral endopeptidase (NEP), which metabolizes both bradykinin and the natriuretic peptides, protected atrial natriuretic peptide and BNP from degradation in vivo and enhanced their biological activities. Subcutaneous injection with the selective NEP inhibitor 28603 stimulated urinary excretion of cyclic guanosine monophosphate (cGMP) and sodium in a dose-related manner in angiotensin converting enzyme inhibition in dogs with tachycardia-induced heart failure (23). Vasopeptidase inhibitors (VPI) are angiotensin converting enzyme and NEP inhibitors. Chen et al. (24) reported that acute VPI intravenous injection could increase plasma BNP concentration and potentiates the cardiorenal actions of subcutaneous administration of BNP in experimental CHF. Our results showed that 1 month of subcutaneous administration of low- or high-dose rhBNP improved hemodynamics and significantly increased EF in rats with experimentally induced CHF. These beneficial effects may be due to its vasodilator and diuretic effects.
It is well known that aldosterone plays a vital role in heart failure, causing retention of sodium and water, promotion of myocardial fibrosis, reduced vascular compliance, and the development of malignant ventricular arrhythmia. Recent evidence suggests that aldosterone is not only synthesized and secreted in the zona glomerulosa of the adrenal cortex, but also within the heart itself.
Aldosterone synthase is regulated by two genes, CYP11B1 and CYP11B2. As CYP11B2 is the final enzyme needed for aldosterone production, its activity can be used to evaluate aldosterone synthesis. Previous research has confirmed that gene expression of CYP11B2 is present in heart muscle and that expression is markedly increased during heart failure (3), which in turn increases local cardiac aldosterone levels (17).
Clinical trials have shown that intravenous injection of BNP significantly reduces plasma levels of renin, aldosterone, norepinephrine, and endothelin-1 (25). Ito et al. (8) reported that both exogenous and endogenous BNP could suppress the expression of CYP11B2 mRNA in cultured mouse embryonic cardiomyocytes. However, the mechanisms were not fully understood. It has been reported that natriuretic peptide receptor A (NPR-A) is present on cardiomyocytes and isolated fibroblasts. Chen et al. (26) reported that BNP has potent anti-hypertrophic and antifibrotic properties via NPR-A. cGMP is the main second messenger of BNP. A cGMP-dependent protein kinase (PKG or cGK) represents the principal intracellular mediator of cGMP signals (27,28). This is probably a common mechanism underlying the suppression of CYP11B2 by rhBNP.
In the present study, long-term subcutaneous administration of rhBNP significantly decreased CYP11B2 transcription and reduced myocardial aldosterone and myocardial fibrosis, in addition to improving hemodynamics and myocardial systolic function. One explanation for the reduced levels of myocardial fibrosis is that rhBNP may be able to decrease left ventricular CYP11B2 transcription, thereby suppressing local cardiac aldosterone synthesis. However, no significant differences were observed between low-dose and high-dose rhBNP groups, which may be related to the minor differences in dosage.
BNP can reduce the level of myocardial fibrosis, and its mechanism is related not only to the ability of BNP to reduce aldosterone synthesis in the left ventricle under heart failure but also to the ability of BNP to upregulate matrix metalloproteinases (MMPs), which promotes the degradation of fibers. It has been reported by Tsuruda et al. (29) that BNP exists in in vitro cultured cardiac fibroblasts and decreases collagen synthesis and increases MMPs via cGMP protein kinase G signaling (25). Further studies are needed to determine whether BNP in vivo plays such a role.
BNP was approved for use by the United States Food and Drug Administration in August 2001 (29) and was included in the European Society of Cardiology guidelines for the treatment of CHF in 2005 (30). In clinical practice, BNP is mainly used for acute heart failure and is primarily given intravenously. Studies in rabbits have shown that subcutaneous administration of BNP prolongs its duration of action relative to that achieved with intravenous administration. Moreover, subcutaneous administration offers a simpler treatment approach for long-term treatment of CHF (31). For these reasons we used subcutaneous injection in the current study. However, pivotal clinical trial evidence is required before this route of administration can be adopted in clinical practice.
The present study found that cardiac aldosterone and aldosterone synthase transcription were elevated in rats with ISO-induced CHF. Long-term subcutaneous administration of rhBNP improved hemodynamics and cardiac function and reduced myocardial fibrosis, possibly by downregulating CYP11B2 transcription and reducing myocardial aldosterone synthesis.
Acknowledgments
Research supported by Key Clinical Specialty Discipline Construction Program of Fujian and Nation, P.R.C. and Medical Innovation Subject of Fujian, P.R.C. (#2007-CX-12).
Footnotes
First published online July 11, 2014.
References
- 1.Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res. 2000;47:1–3. doi: 10.1016/S0008-6363(00)00092-4. [DOI] [PubMed] [Google Scholar]
- 2.Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure. J Am Coll Cardiol. 2006;48:A56–A66. doi: 10.1016/j.jacc.2006.07.007. [DOI] [Google Scholar]
- 3.Yoshimura M, Nakamura S, Ito T, Nakayama M, Harada E, Mizuno Y, et al. Expression of aldosterone synthase gene in failing human heart: quantitative analysis using modified real-time polymerase chain reaction. J Clin Endocrinol Metab. 2002;87:3936–3940. doi: 10.1210/jcem.87.8.8731. [DOI] [PubMed] [Google Scholar]
- 4.Mulder P, Mellin V, Favre J, Vercauteren M, Remy-Jouet I, Monteil C, et al. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J. 2008;29:2171–2179. doi: 10.1093/eurheartj/ehn277. [DOI] [PubMed] [Google Scholar]
- 5.Azizi M, Amar L, Menard J. Aldosterone synthase inhibition in humans. Nephrol Dial Transplant. 2013;28:36–43. doi: 10.1093/ndt/gfs388. [DOI] [PubMed] [Google Scholar]
- 6.Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ Res. 1994;75:105–113. doi: 10.1161/01.RES.75.1.105. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Yoshimura M, Nakamura S, Nakayama M, Shimasaki Y, Harada E, et al. Inhibitory effect of natriuretic peptides on aldosterone synthase gene expression in cultured neonatal rat cardiocytes. Circulation. 2003;107:807–810. doi: 10.1161/01.CIR.0000057794.29667.08. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XQ, Hong HS, Li YH. Changes of myocardial aldosterone and the natriuretic peptide receptors and in rats with chronic heart failure induced by isoproterenol. Chinese J Geriatrics. 2011;1:168–171. [Google Scholar]
- 10.Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC., Jr Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol. 2000;36:1706–1712. doi: 10.1016/S0735-1097(00)00911-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang BY, Li YS, Huang GS. Pathology techniques. Beijing: People's Medical Publishing House; 2000. p. 144. [Google Scholar]
- 12.Wei S, Chow LT, Sanderson JE. Effect of carvedilol in comparison with metoprolol on myocardial collagen postinfarction. J Am Coll Cardiol. 2000;36:276–281. doi: 10.1016/S0735-1097(00)00671-9. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita D, Shimizu J, Kitagawa Y, Yamashita D, Tohne K, Nakajima-Takenaka C, et al. Isoproterenol-induced hypertrophied rat hearts: does short-term treatment correspond to long-term treatment? J Physiol Sci. 2008;58:179–188. doi: 10.2170/physiolsci.RP004508. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology. 1997;138:3369–3373. doi: 10.1210/endo.138.8.5326. [DOI] [PubMed] [Google Scholar]
- 15.Slight SH, Joseph J, Ganjam VK, Weber KT. Extra-adrenal mineralocorticoids and cardiovascular tissue. J Mol Cell Cardiol. 1999;31:1175–1184. doi: 10.1006/jmcc.1999.0963. [DOI] [PubMed] [Google Scholar]
- 16.Silvestre JS, Heymes C, Oubenaissa A, Robert V, Aupetit-Faisant B, Carayon A, et al. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.CIR.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Yasue H, Mizuno Y, Yoshimura M, Fujii H, Nakayama M, et al. Aldosterone is produced from ventricles in patients with essential hypertension. Hypertension. 2002;39:958–962. doi: 10.1161/01.HYP.0000015905.27598.E9. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno Y, Yoshimura M, Yasue H, Sakamoto T, Ogawa H, Kugiyama K, et al. Aldosterone production is activated in failing ventricle in humans. Circulation. 2001;103:72–77. doi: 10.1161/01.CIR.103.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of aldosterone in the rat heart. Endocrinology. 2004;145:4796–4802. doi: 10.1210/en.2004-0295. [DOI] [PubMed] [Google Scholar]
- 20.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 21.Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 22.Burger AJ, Horton DP, LeJemtel T, Ghali JK, Torre G, Dennish G, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002;144:1102–1108. doi: 10.1067/mhj.2002.125620. [DOI] [PubMed] [Google Scholar]
- 23.Seymour AA, Abboa-Offei BE, Smith PL, Mathers PD, Asaad MM, Rogers WL. Potentiation of natriuretic peptides by neutral endopeptidase inhibitors. Clin Exp Pharmacol Physiol. 1995;22:63–69. doi: 10.1111/j.1440-1681.1995.tb01920.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen HH, Lainchbury JG, Harty GJ, Burnett JC., Jr Maximizing the natriuretic peptide system in experimental heart failure: subcutaneous brain natriuretic peptide and acute vasopeptidase inhibition. Circulation. 2002;105:999–1003. doi: 10.1161/hc0802.104282. [DOI] [PubMed] [Google Scholar]
- 25.Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005;11:30–38. doi: 10.1111/j.1527-5299.2005.03794.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–129. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Rosenkranz AC, Woods RL, Dusting GJ, Ritchie RH. Antihypertrophic actions of the natriuretic peptides in adult rat cardiomyocytes: importance of cyclic GMP. Cardiovasc Res. 2003;57:515–522. doi: 10.1016/S0008-6363(02)00667-3. [DOI] [PubMed] [Google Scholar]
- 29.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. doi: 10.1161/01.RES.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 30.Grines CL. Safety and effectiveness of dofetilide for conversion of atrial fibrillation and nesiritide for acute decompensation of heart failure: a report from the cardiovascular and renal advisory panel of the Food and Drug Administration. Circulation. 2000;101:E200–E201. doi: 10.1161/01.CIR.101.21.e200. [DOI] [PubMed] [Google Scholar]
- 31.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]


