Abstract
Ion channels play a key role in defining myometrial contractility. Modulation of ion channel populations is proposed to underpin gestational changes in uterine contractility associated with the transition from uterine quiescence to active labour. Of the myriad ion channels present in the uterus, this article will focus upon potassium channels encoded by the KCNQ genes and ether-à-go-go-related (ERG) genes. Voltage-gated potassium channels encoded by KCNQ and ERG (termed Kv7 and Kv11, respectively) are accepted as major determinants of neuronal excitability and the duration of the cardiac action potential. However, there is now growing appreciation that these ion channels have a major functional impact in vascular and non-vascular smooth muscle. Moreover, Kv7 channels may be potential therapeutic targets for the treatment of preterm labour.
New Findings
-
What is the topic of this review?
The topic of this review is how ion channels contribute to the physiology of the uterus, with particular focus on novel potassium channels.
-
What advances does it highlight?
Two families of potassium channels, encoded by KCNQ and KCNH genes, have been identified as important players in the control of myometrial contraction and may represent interesting novel therapeutic targets.
Introduction
The uterus is an incredibly complex organ that displays considerable physiological plasticity, cellular remodelling and robustness during pregnancy. However, perturbations of the precise orchestrations that regulate the contractile state of the uterus can have negative consequences for the mother and fetus. Early activation of contractility that, for example, results in spontaneous preterm birth can be associated with a high risk of neonatal morbidity and mortality, as well as lifelong ill health and socio-economic consequences. Conversely, delayed delivery or dysfunctional labour due to weak or poorly co-ordinated contractions can lead to fetal hypoxia, clinical intervention and a greater risk of postpartum haemorrhage.
If there are to be improvements in clinical management and development of novel therapeutic strategies for complicated pregnancies then a better understanding of the mechanisms that determine normal and pathophysiological uterine contractility is essential. There are many factors that dictate gestational changes in uterine contractility, such as alterations in the steroid hormone environment, inflammation and uterine stretch that is exerted by the growing feto-placental unit. The impact of these stimuli is a fine tuning of the mechanisms controlling uterine smooth muscle contractility at the cellular level, including gap junctions, G-protein-coupled receptors, calcium regulatory proteins and contractile filament interactions, but ultimately, all converge upon a background electrical rhythm generated by the activity of ion channels, much like a good concerto relies on the precise contributions from individual instruments in an orchestra. Understanding the contribution of these individual instruments to the uterine symphony is very much a work in progress, but recent studies have identified KCNQ and KCNH-encoded K+ channels as new and functionally powerful elements that hold promise as major regulatory mechanisms and potential therapeutic targets for the treatment of intrapartum complications.
The purpose of this article is to provide a brief overview of this field of research, with particular focus on two new pieces of the puzzle rather than a comprehensive summary of the many factors implicated in uterine physiology. The reader is recommended to consult a number of more comprehensive reviews for more depth in specific areas (e.g. Taggart & Tribe, 2001; Wray, 2007).
Inherent excitability
Uterine smooth muscle exhibits spontaneous contractility that can be augmented by receptor agonists, such as oxytocin (Wray, 2007). Spontaneous contractions are intimately related to the generation of slow waves, upon which action potentials are superimposed (Casteels & Kuriyama, 2010; Kuriyama & Suzuki, 2004; Bengtsson et al. 2004; Parkington et al. 2002b). As gestation proceeds towards labour, the resting membrane potential of the uterine smooth muscle becomes progressively more depolarized (Kuriyama & Suzuki, 2004; Bengtsson et al. 2004; Parkington et al. 2002b), and this is associated with an increase in the force and frequency of spontaneous contractions. The initiator of the spontaneous activity, however, remains to be identified unequivocally. In the gastrointestinal tract, peristalsis is driven by multibranched, non-contractile cells that express the c-kit receptor (termed interstitial cells of Cajal or ICC). Similar ICC-like cells have been observed in rodent and human myometrial tissue (Ciontea et al. 1965; Duquette et al. 2002; Allix et al. 2006). Moreover, pharmacological blockade of the c-kit receptor with imantanib or deletion of this gene does affect the frequency of contractions in the myometrium of mice. However, the effects are subtle, and imantanib has negligible effect in human myometrium, suggesting that the impact of ICC-like cells is not as clearly defined in the uterus as it is in the gastrointestinal tract. Irrespective of the genesis of the spontaneous contractility, the operation of specific ion channels maintains contractile activity, and elucidation of the nature of the respective depolarizing (excitatory) and hyperpolarizing (inhibitory) channels remains a key challenge for uterine physiologists.
Excitatory pathways
In its simplest form, contraction of myometrium, like that of all smooth muscle, is mediated by a rise in [Ca2+] leading to activation of myosin light chain kinase, and the subsequent phosphorylation of myosin light chain at serine 19 allows actin–myosin interaction (see Wray, 2007; Taggart & Tribe, 2001). The rise in [Ca2+]i is mediated by an interplay between increased Ca2+ influx through plasmalemmal channels, Ca2+ release from the sarcoplasmic reticulum and Ca2+ sequestration processes. However, the major precipitatory mechanism is the opening of L-type voltage-dependent Ca2+ channels (VDCCs), as evidenced by the marked effect of dihydropyridines, such as nifedipine, on myometrial contraction (Sperelakis et al. 2007; Wray, 2007). There is evidence that T-type VDCCs may also have some role in maintaining spontaneous contractile activity (Taggart & Tribe, 2001). In addition to VDCCs, voltage-gated sodium channels have been recorded from isolated myometrial smooth muscle (Sperelakis et al. 2007; Seda et al. 2010), and the density of these currents increases in late pregnancy. However, little is known about the molecular nature of the sodium channels and how they contribute to functional activity.
Membrane potential is key
If the influx of Ca2+ through VDCCs is a major determinant of myometrial contractility then logically the influence of membrane potential is central to this mechanism (see Tong et al. 1992 for a computational model). An important question, therefore, is what are the principal mechanisms that propel the membrane potential towards voltages that enhance VDCC open probability and, conversely, which specific ion channels ensure repolarization to more negative membrane potential and closure of VDCCs? In most smooth muscle cells, Ca2+-activated Cl− channels (CACCs) provide the major depolarizing impetus, because smooth muscle cells actively accumulate Cl− ions (Chipperfield & Harper, 2008). As a consequence, the activation of CACCs leads to Cl− ion efflux sufficient to produce membrane depolarization (Leblanc et al. 2000) and, subsequently, to further activation of VDCCs. In relationship to uterine smooth muscle, Cl− currents due to CACC activation have been recorded in rat myometrial cells, and inhibitors of this channel, such as niflumic acid, attenuate myometrial contractility (Jones et al. 2009), although these agents are known to have pluripotent effects (Greenwood & Leblanc, 2005). Preliminary data also show that transcripts for TMEM16A (Caputo et al. 1980; Schroeder et al. 2008; Yang et al. 2007), the putative molecular correlate of CACCs, are present in mouse and human myometrium (AJ Davis, RM Tribe & IA Greenwood, unpublished observations) as well as in vascular smooth muscle cells (Davis et al. 2005). It is worth noting that in the gastrointestinal tract, TMEM16A is expressed by the ICCs, not the smooth muscle cells (Hwang et al. 2008). A second mechanism to produce membrane depolarization is to activate non-selective cation channels, and various members of the ORAI/STIM and TRP gene family that encode for proteins associated with store-operated and receptor-operated calcium entry (see Wang et al. 2011 for overview) are present in rodent and human myometrium (Dalrymple et al. 2000; Yang et al. 2008; Babich et al. 2013). Non-selective cation channels also have a degree of inherent Ca2+ permeability that can potentially contribute to the general rise in [Ca2+] and contraction.
Potassium channels: nature∼s brakes
Co-ordinated contraction of the myometrium relies on hyperpolarizing influences to limit the extent of membrane depolarization (see Fig. 1) and subsequent contraction. Consequently, potassium channels define the magnitude, duration and periodicity of uterine electrical events. Myometrium expresses a number of genes encoding for different potassium channels, including calcium-activated (BKCa; Anwer et al. 2008; Pérez et al. 1999), SKCa (Brown et al. 2005; Pierce et al. 2003), acid-sensitive twin-pore channel TREK-1 (Bai et al. 1993; Buxton et al. 2007), inwardly rectifying ROMK1 (Lundgren et al. 2005) and various voltage-dependent K+ channels, especially members of the Kv4 family (Song et al. 1996; Smith et al. 1999; Greenwood et al. 2003). In terms of functional impact, inhibitors of BKCa, such as paxilline or iberiotoxin, or blockers of SKCa, such as apamin, have negligible effect on rodent or human myometrial contractility (Aaronson et al. 2006; Brown et al. 2005; Smith et al. 1999; Noble et al. 2008). In comparison, the non-selective Kv inhibitor, 4-aminopyridine, enhances contractility (Aaronson et al. 2006; Smith et al. 1999), and the Kv4.2/4.3 blocker, phrixotoxin-2, induces contractions in non-pregnant, but not pregnant, rat myometrium (Smith et al. 1999). Set against this background, two novel types of Kv channel encoded by members of the KCNQ and KCNH gene families have been identified that appear to act as key regulators of uterine contractility and offer new therapeutic targets.
Figure 1. Schematic representation of the functional role of potassium channels in uterine smooth muscle contraction.
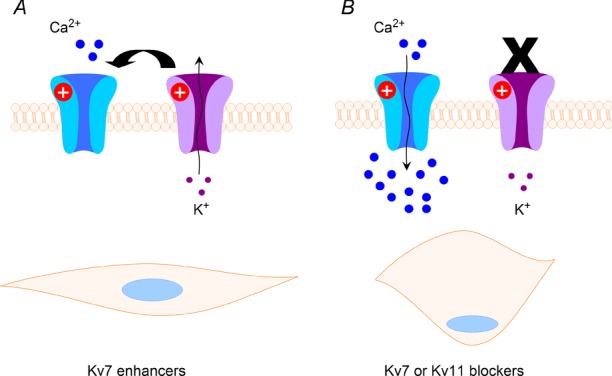
Left-hand panel shows that open K+ channels result in membrane hyperpolarization that indirectly limits the opening of voltage-dependent calcium channels shown in blue. This results in a less contracted smooth muscle. In the right-hand panel, the potassium channels are non-functional due to blockade, loss-of-function mutations or trafficking defects. This leads to membrane depolariziation, and the open probability of the calcium channels increases. The concomitant influx of calcium contributes to smooth muscle contraction.
KCNQ- and ERG-encoded potassium channels
Ether-à-go-go-related genes or ERGs (ERG1, 2 and 3) are members of the KCNH gene family. All genes encode for voltage-dependent K+ channels (Kv11.1–11.3) that assemble as a tetramer to generate a Kv channel with unique voltage-dependent properties due to an over-riding c-type inactivation (Smith et al. 2002). ERG1 (KCNH2) exists mainly as two splice variants (ERG1a and 1b; London et al. 1976) and is expressed predominantly in cardiac myocytes, where it contributes to the late repolarizing phase of the cardiac action potentials; mutations to the underlying gene underpin a major component of hereditary arrhythmias. ERG2 and ERG3 are located in neurones and contribute to the suppression of membrane excitability (Selyanko et al. 2007). The KCNQ gene family contains five members (KCNQ1–5), and each gene encodes a Kv channel (Kv7.1–7.5, respectively) with low activation threshold (V0.5 ≈ −35 mV) and minimal inactivation (Haitin & Attali, 2007). Kv7 channels also exist as tetramers, with Kv7.1 assembling homomerically. Kv7 activity is modulated by local phosphoinositide levels (Hernandez et al. 2009; Haitin & Attali, 2007), calmodulin and association with auxiliary proteins encoded by the KCNE gene family (McCrossan & Abbott, 2009). KCNQ genes have a well-defined pattern of expression, with KCNQ1 located predominantly in the heart as well as the inner ear; KCNQ2, 3 and 5 are mainly neuronal where they comprise the so-called M-channel in neurones (Brown & Adams, 1984; Selyanko et al. 2008); and KCNQ4 is restricted to the inner ear and auditory nerves (Kharkovets et al. 2013). Mutations to KCNQ genes underlie hereditary arrhythmias (KCNQ1), epilepsy (KCNQ2/3) and deafness (KCNQ4).
KCNQ- and ERG-encoded potassium channels and smooth muscle
The impact of ERG- and KCNQ-encoded K+ channels on cardiac and neuronal physiology was established over 10 years ago. However, both gene families have been ascribed new roles of late through their identification as key players in the regulation of smooth muscle activity.
Expression of KCNQ in smooth muscle was first identified in rat stomach by Ohya et al. (2010a). Since then, KCNQ transcripts have been identified in mouse, rat and human blood vessels (e.g. Ohya et al. 2002a; Yeung et al. 2008; Makie et al. 2011; Ng et al. 2008), as well as in the gastrointestinal tract, urinary tract and airways (see Jepps et al. 2011 for comprehensive overview). KCNQ channel blockers, such as linopirdine or XE991, evoke contractions in the quiescent smooth muscles, such as arteries, or enhance spontaneous contractility (e.g. Yeung & Greenwood, 2002, Jepps et al. 2009, Rode et al. 1993; Ipavec et al. 2008; Anderson et al. 1999). Serendipitously, there are also activators of KCNQ-encoded channels, such as the novel anticonvulsant retigabine, that relax smooth muscles (see Jepps et al. 2011).
Expression of ERG has been determined in the gastrointestinal tract (Akbarali et al. 2006; Ohya et al. 2010a; Farrelley et al. 2010; Parr et al. 2003), mouse portal vein (Ohya et al. 2011b) and bovine epididymis (Mewe et al. 2004), where the smooth muscles exhibit phasic contractions. In these tissues, ERG channel blockers, such as dofetilide or E4031, augment spontaneous contractions tremendously and often cause individual events to fuse into a tonic contraction.
In terms of the myometrium, all KCNQ isoforms are expressed in non-pregnant mice, with KCNQ1 being dominant, and the transcript level for all isoforms remains stable throughout the oestrus cycle (McCallum et al. 1997). In pregnant mice, the expression of all KCNQ genes drops dramatically at early stages of gestation but recovers to robust levels by late stages (McCallum et al. 1997), suggesting that their main role is to regulate contractility at the end of pregnancy rather than to induce quiescence in early pregnancy. Transcripts for all KCNQ genes except for KCNQ5 have also been detected in myometrium from women undergoing Caesarean section at term (McCallum et al. 1997). Of the three ERG genes, only ERG1 is expressed in mouse (Greenwood et al. 2003) and human myometrium (R. M. Tribe & I. A. Greenwood, unpublished observations). In the BALB/c mouse myometrium, both splice variants of ERG1 were detected, with the longer C-terminal ‘a’ isoform dominant (Greenwood et al. 2003), and the expression of this gene did not vary throughout mouse gestation or following parturition (Greenwood et al. 2003). All members of the KCNE gene family whose expression products alter the membrane insertion capabilities and biophysical properties of KCNQ- and ERG-encoded channels (McCrossan & Abbott, 2009) are also expressed in virgin and pregnant mouse myometrium (Greenwood et al. 2003; McCallum et al. 1997). Moreover, transcripts for KCNE2 and KCNE4 increased markedly in mouse myometrium throughout pregnancy (Greenwood et al. 2003; McCallum et al. 1997), an observation that was mirrored at the protein level (Greenwood et al. 2003).
A functional role for both KCNQ- and ERG-encoded K+ channels has been determined in isometric tension and single-cell electrophysiological studies. Linopirdine and XE991 are specific inhibitors of all KCNQ channel isoforms that increase contractile activity in either non-pregnant or pregnant mouse myometrium, mainly through an increase in the frequency of contractions (McCallum et al. 1997, 1997). These agents have similar effects on term non-labouring samples of human myometrium (McCallum et al. 1997). In line with a working hypothesis that increased K+ channel activity limits membrane depolarization and suppresses voltage-dependent Ca2+ influx, the KCNQ-encoded K+ channel activators, flupirtine and retigabine, produce rapid inhibition of spontaneous and oxytocin-driven contractility in mouse and human myometrium (McCallum et al. 1997, 1997). This tocolytic activity is more marked in myometrium from late pregnant mice compared with early pregnant mice (McCallum et al. 1997).
Specific blockers of ERG-encoded channels, such as dofetilide or E4031, have a more striking effect on spontaneous contractility of mouse myometrium than KCNQ channel blockers (mean integral of tension increases by ∼300%, in comparison to ∼50% seen with XE991) that is usually manifest as an increase in the amplitude and duration of individual contractions (Greenwood et al. 2003). Inhibitors of ERG-encoded channels also have a dramatic effect on oxytocin-mediated contractions in mouse myometrium, with tissues often generating sustained contractions of considerable magnitude (Greenwood et al. 2003). Activators of ERG-encoded K+ channels (NS1643 or PD118057) also attenuate contractions in mouse uterus. However, in contrast to KCNQ channel modulators, the effects of channel blockers and activators is lost in the final stages of mouse pregnancy (Greenwood et al. 2003). This is associated with an inability to record dofetilide-sensitive K+ currents in isolated myometrial smooth muscle cells that are present in cells from non-pregnant animals (Greenwood et al. 2003). Modulators of ERG channels become effective again in tissues harvested only 3 h after delivery (Greenwood et al. 2003). Currently, the effects of ERG inhibitors in human myometrial tissues have only been studied in samples obtained from non-labouring woman at term (end of pregnancy), so it is not yet confirmed whether a similar molecular mechanism exists in humans. However, this redundancy in the functional impact of ERG-encoded channels in late mouse pregnancy represents a potential pivot point in the switch from a quiescent system to an excitable system able to generate considerable rhythmic contraction in order to facilitate fetal delivery.
Conclusion
The uterus remains an enigma. Despite much research, there is still much to ascertain with regard to the mechanisms that drive the switch from quiescence to contractile activity preceding labour, and little is known about the stimulus for induction of preterm labour. Furthermore, existing therapies are far from being the ideal tocolytics. The recent findings that KCNQ- and (ERG) KCNH-encoded K+ channels have a major impact on myometrial contractility and that the functional impact of KCNH-encoded channels diminishes in an animal model of term pregnancy represent progression towards answering some of these questions.
Additional Information
Competing interests
None declared.
Funding
Research has been supported by Action Medical Research, BBSRC, MRC and the British Heart Foundation.
References
- Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, Watson S, Liu B, Tribe RM. A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol. 147:815–824. doi: 10.1038/sj.bjp.0706644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarali HI, Thatte H, He XD, Giles WR, Goyal RK. Role of HERG-like K+ currents in opossum esophageal circular smooth muscle. Am J Physiol Cell Physiol. 277:C1284–C1290. doi: 10.1152/ajpcell.1999.277.6.C1284. [DOI] [PubMed] [Google Scholar]
- Allix S, Reyes-Gomez E, Aubin-Houzelstein G, Noël D, Tiret L, Panthier JJ, Bernex F. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod. 2006;79:510–517. doi: 10.1095/biolreprod.107.066373. [DOI] [PubMed] [Google Scholar]
- Anderson UA, Carson C, Johnston L, Joshi S, Gurney AM, McCloskey KD. Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br J Pharmacol. 1999;169:1290–1304. doi: 10.1111/bph.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol Cell Physiol. 2008;265:C976–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2013;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, Taggart MJ, Fyfe GK. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 1993;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Chow EM, Marshall JM. Activity of circular muscle of rat uterus at different times in pregnancy. Am J Physiol Cell Physiol. 2004;246:C216–C223. doi: 10.1152/ajpcell.1984.246.3.C216. [DOI] [PubMed] [Google Scholar]
- Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2005;292:C832–C840. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1984;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Singer CA, Tichenor JN. Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLoS One. 2007;5:e12372. doi: 10.1371/journal.pone.0012372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 1980;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Casteels R, Kuriyama H. Membrane potential and ionic content in pregnant and non-pregnant rat myometrium. J Physiol. 2010;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2008;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- Ciontea SM, Radu E, Regalia T, Ceafalan L, Cretoiu D, Gherghiceanu M, Braga RI, Malincenco M, Zagrean L, Hinescu ME, Popescu LM. C-kit immunopositive interstitial cells (Cajal-type) in human myometrium. J Cell Mol Med. 1965;9:407–420. doi: 10.1111/j.1582-4934.2005.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Beech DJ, Poston L, Tribe RM. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2000;8:946–951. doi: 10.1093/molehr/8.10.946. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol. 2005;299:C948–C959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette RA, Shmygol A, Vaillant C, Mobasheri A, Pope M, Burdyga T, Wray S. Vimentin-positive, c-KIT-negative interstitial cells in human and rat uterus: a role in pacemaking. Biol Reprod. 2002;72:276–283. doi: 10.1095/biolreprod.104.033506. [DOI] [PubMed] [Google Scholar]
- Farrelly AM, Ro S, Callaghan BP, Khoyi MA, Fleming N, Horowitz B, Sanders KM, Keef KD. Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol Gastrointest Liver Physiol. 2010;284:G883–G895. doi: 10.1152/ajpgi.00394.2002. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Leblanc N. Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. Trends Pharmacol Sci. 2005;28:1–5. doi: 10.1016/j.tips.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Yeung SY, Tribe RM, Ohya S. Loss of functional K+ channels encoded by ether-à-go-go-related genes in mouse myometrium prior to labour onset. J Physiol. 2003;587:2313–2326. doi: 10.1113/jphysiol.2009.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitin Y, Attali B. The C-terminus of Kv7 channels: a multifunctional module. J Physiol. 2007;586:1803–1810. doi: 10.1113/jphysiol.2007.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CC, Zaika O, Shapiro MS. A carboxy-terminal inter-helix linker as the site of phosphatidylinositol 4,5-bisphosphate action on Kv7 (M-type) K+ channels. J Gen Physiol. 2009;132:361–381. doi: 10.1085/jgp.200810007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O∼Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2008;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipavec V, Martire M, Barrese V, Taglialatela M, Currò D. KV7 channels regulate muscle tone and nonadrenergic noncholinergic relaxation of the rat gastric fundus. Pharmacol Res. 2008;64:397–409. doi: 10.1016/j.phrs.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2009;297:G107–G115. doi: 10.1152/ajpgi.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Olesen SP, Greenwood IA. One man∼s side effect is another man∼s therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders. Br J Pharmacol. 2011;168:19–27. doi: 10.1111/j.1476-5381.2012.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Shmygol A, Kupittayanant S, Wray S. Electrophysiological characterization and functional importance of calcium-activated chloride channel in rat uterine myocytes. Pflugers Arch. 2009;448:36–43. doi: 10.1007/s00424-003-1224-7. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2013;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H, Suzuki H. Effects of prostaglandin E2 and oxytocin on the electrical activity of hormone-treated and pregnant rat myometria. J Physiol. 2004;260:335–349. doi: 10.1113/jphysiol.1976.sp011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O∼Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2000;83:541–556. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- London B, Trudeau MC, Newton KP, Beyer AK, Copeland NG, Gilbert DJ, Jenkins NA, Satler CA, Robertson GA. Two isoforms of the mouse ether-a-go-go-related gene co-assemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1976;81:870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med. 2005;216:57–64. doi: 10.3181/00379727-216-44156. [DOI] [PubMed] [Google Scholar]
- McCallum LA, Greenwood IA, Tribe RM. Expression and function of Kv7 channels in murine myometrium throughout oestrous cycle. Pflugers Arch. 1997;457:1111–1120. doi: 10.1007/s00424-008-0567-5. [DOI] [PubMed] [Google Scholar]
- McCallum LA, Pierce SL, England SK, Greenwood IA, Tribe RM. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med. 1997;15:577–586. doi: 10.1111/j.1582-4934.2010.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2009;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2011;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewe M, Wulfsen I, Schuster AM, Middendorff R, Glassmeier G, Schwarz JR, Bauer CK. Erg K+ channels modulate contractile activity in the bovine epididymal duct. Am J Physiol Regul Integr Comp Physiol. 2004;294:R895–R904. doi: 10.1152/ajpregu.00521.2007. [DOI] [PubMed] [Google Scholar]
- Noble K, Floyd R, Shmygol A, Shmygol A, Mobasheri A, Wray S. Distribution, expression and functional effects of small conductance Ca-activated potassium (SK) channels in rat myometrium. Cell Calcium. 2008;47:47–54. doi: 10.1016/j.ceca.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol. 2008;162:42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2010;282:G277–G287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- Ohya S, Horowitz B, Greenwood IA. Functional and molecular identification of ERG channels in murine portal vein myocytes. Am J Physiol Cell Physiol. 2011;283:C866–C877. doi: 10.1152/ajpcell.00099.2002. [DOI] [PubMed] [Google Scholar]
- Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current. Circ Res. 2002a;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Tonta MA, Brennecke SP, Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol. 2002b;181:1445–1451. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- Parr E, Pozo MJ, Horowitz B, Nelson MT, Mawe GM. ERG K+ channels modulate the electrical and contractile activities of gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G392–G398. doi: 10.1152/ajpgi.00325.2002. [DOI] [PubMed] [Google Scholar]
- Pérez GJ, Toro L, Erulkar SD, Stefani E. Characterization of large-conductance, calcium-activated potassium channels from human myometrium. Am J Obstet Gynecol. 1999;168:652–660. doi: 10.1016/0002-9378(93)90513-i. [DOI] [PubMed] [Google Scholar]
- Pierce SL, Kresowik JD, Lamping KG, England SK. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod. 2003;78:1058–1063. doi: 10.1095/biolreprod.107.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode F, Svalø J, Sheykhzade M, Rønn LC. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur J Pharmacol. 1993;638:121–127. doi: 10.1016/j.ejphar.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seda M, Pinto FM, Wray S, Cintado CG, Noheda P, Buschmann H, Candenas L. Functional and molecular characterization of voltage-gated sodium channels in uteri from nonpregnant rats. Biol Reprod. 2010;77:855–863. doi: 10.1095/biolreprod.107.063016. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Delmas P, Hadley JK, Tatulian L, Wood IC, Mistry M, London B, Brown DA. Dominant-negative subunits reveal potassium channel families that contribute to M-like potassium currents. J Neurosci. 2008;22:RC212. doi: 10.1523/JNEUROSCI.22-05-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Delmas P, Buckley NJ, London B, Brown DA. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J Neurosci. 2007;19:7742–7756. doi: 10.1523/JNEUROSCI.19-18-07742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 2002;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 1999;5:41. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 1996;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Inoue Y, Ohya Y. Fast Na+ channels and slow Ca2+ current in smooth muscle from pregnant rat uterus. Mol Cell Biochem. 2007;114:79–89. [PubMed] [Google Scholar]
- Taggart MJ. Cellular ionic mechanisms controlling uterine contraction: effects of gestational state. In: Savineau JP, Tribe RM, editors. New Frontiers in Smooth Muscle Biology and Physiology. 2001. [Google Scholar]
- Tong WC, Choi CY, Kharche S, Holden AV, Zhang H, Taggart MJ. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PLoS One. 1992;29:e18685. doi: 10.1371/journal.pone.0018685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. STIM, Orai and TRPC channels in the control of calcium entry signals in smooth muscle. Clin Exp Pharmacol Physiol. 2011;35:1127–1133. doi: 10.1111/j.1440-1681.2008.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Insights into the uterus. Exp Physiol. 2007;92:621–631. doi: 10.1113/expphysiol.2007.038125. [DOI] [PubMed] [Google Scholar]
- Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod. 2002;67:988–994. doi: 10.1095/biolreprod.102.004119. [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]


