Abstract
Background
Food allergy is a common cause of anaphylaxis, but the incidence of fatal food anaphylaxis is not known. The aim of this study was to estimate the incidence of fatal food anaphylaxis for people with food allergy and relate this to other mortality risks in the general population.
Methods
We undertook a systematic review and meta-analysis, using the generic inverse variance method. Two authors selected studies by consensus, independently extracted data and assessed the quality of included studies using the Newcastle-Ottawa assessment scale. We searched Medline, Embase, PsychInfo, CINAHL, Web of Science, LILACS or AMED, between January 1946 and September 2012, and recent conference abstracts. We included registries, databases or cohort studies which described the number of fatal food anaphylaxis cases in a defined population and time period and applied an assumed population prevalence rate of food allergy.
Results
We included data from 13 studies describing 240 fatal food anaphylaxis episodes over an estimated 165 million food-allergic person-years. Study quality was mixed, and there was high heterogeneity between study results, possibly due to variation in food allergy prevalence and data collection methods. In food-allergic people, fatal food anaphylaxis has an incidence rate of 1.81 per million person-years (95%CI 0.94, 3.45; range 0.63, 6.68). In sensitivity analysis with different estimated food allergy prevalence, the incidence varied from 1.35 to 2.71 per million person-years. At age 0–19, the incidence rate is 3.25 (1.73, 6.10; range 0.94, 15.75; sensitivity analysis 1.18–6.13). The incidence of fatal food anaphylaxis in food-allergic people is lower than accidental death in the general European population.
Conclusion
Fatal food anaphylaxis for a food-allergic person is rarer than accidental death in the general population.
Keywords: anaphylaxis, food allergy, mortality, systematic review
Introduction
Food allergy affects up to 10% of young children and 2–3% of adults, and appears to be increasing in prevalence [1,2]. Food allergy is the commonest cause of anaphylaxis, which can be fatal, but the precise incidence of fatal anaphylaxis for food-allergic people is unknown. Uncertainty about the absolute level of risk associated with food allergy may be an important contributor to wider uncertainties over service provision for food allergy, food labelling legislation and management strategies. We undertook a systematic review to quantify the incidence of fatal anaphylaxis for food-allergic people. We estimated the incidence rate of fatal food anaphylaxis in a food-allergic individual, and compared this with the incidence of other risks for people living in the United States or Europe. In view of previous findings that peanut allergy and young age may be risk factors for fatal food anaphylaxis, we undertook subgroup analyses for young people (aged 0–19) with food allergy and for peanut-allergic people [3]. In view of the uncertainty about food allergy prevalence in different populations, we undertook sensitivity analysis to explore the effect of varying food allergy prevalence estimates, on fatal food anaphylaxis incidence.
Methods
Data sources
This review was undertaken and reported in accordance with MOOSE guidance [4]. We searched Medline, Embase, PsycINFO, Web of Science, Allied and Complementary Medicine (AMED), Latin American and Caribbean Health Science Information database (LILACS) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) for articles published between January 1946 and 5 September 2012 which described food anaphylaxis incidence – the Medline search strategy is shown in the Supporting Information Data S1, and similar searches were used for other databases. We searched abstracts from the 2011 and 2012 meetings of the American Academy of Asthma Allergy and Immunology (AAAAI) and the European Academy of Allergy and Clinical Immunology (EAACI) using ‘food allergy’ and ‘anaphylaxis’. We searched US national data (CDC Wonder, Centers for Disease Control and Prevention) and European Union-27 data (Eurostat, European Commission) for incidences of other fatal and non-fatal events. There was no registered protocol for this review, but the methods and analyses were planned a priori. No language restrictions were made, and we planned to include non-English papers if they met our inclusion criteria.
Study selection
Two authors (TU, RJB) independently screened titles and abstracts, and selected studies. Disagreements were resolved by discussion. We reviewed reference lists of included studies and review articles to identify other studies. Inclusion criteria were as follows: (i) Study design: Registries, databases or cohort studies including ≥1 case of fatal food anaphylaxis. (ii) Participants: A defined population where an assumed population rate of food allergy could be applied. (iii) Follow-up: To enable calculation of total person-years of observation, we included studies that specified either total population and duration of data collection, or anaphylaxis incidence rate. (iv) Outcomes: We included reports of number of fatal food anaphylaxis events during the follow-up period. Exclusion criteria were as follows: (i) fatalities neither probably nor definitely due to anaphylaxis, in the judgement of the original study authors; (ii) time period not defined; (iii) population in which food anaphylaxis cases occurred could not be quantified. Studies needed to satisfy all 4 inclusion criteria and none of the exclusion criteria, to be included in the review. Where population number was not described in the study, we derived total populations and populations aged 0–19 using regional public health databases. We contacted the author of one study to ask for their original data set [5]. Where more than one study covered the same population during an overlapping time period, we included the study covering the greatest number of person-years.
Data extraction
Two authors (TU, RJB) independently extracted data, and quality assessed the included studies. Differences were resolved by discussion. When available, we separately extracted data for younger people (age 0–19), and those with peanut allergy. We estimated incidences for the total food-allergic population, and subgroups of aged 0–19 years and peanut allergy. Where studies were restricted to a population aged < 19, data were only used for the 0–19 subgroup, and likewise for peanut allergy. For quality assessment, we used the Newcastle-Ottawa quality assessment scale for cohort studies with one modification [6]. Under the criterion ‘was follow-up long enough for outcomes to occur’, we considered studies with at least 20 fatal food anaphylaxis cases to satisfy this criterion. Studies were considered poor quality for Newcastle-Ottawa score < 2.
Data synthesis
For each study, number of events and total person-years of monitoring were extracted, and incidence rate calculated per million person-years [micromort]. Rates were pooled across studies using the natural logarithm of the occurrence rate and its standard error (estimated using 1/√events) based on a random effect model using the generic inverse variance method (STATA version 12 IC; Stata Corporation, College Station, TX, USA). Heterogeneity was assessed using I2. For meta-analyses including ≥10 studies, we assessed publication bias using Funnel plots. We conducted meta-analysis even if significant heterogeneity was seen between study estimates, but explored possible reasons in sensitivity analyses, including study quality. For calculations, we estimated the population food allergy prevalence as 3% overall, 3.9% for a 0–19 year old, 1% for peanut allergy, but explored the effect of different prevalence estimates in sensitivity analyses due to the considerable uncertainty in the literature surrounding food allergy prevalence in different populations [2,7,8]. We assumed that all fatal food anaphylaxis would occur in this population. We made no separate analysis of incidence according to sex, due to insufficient gender-specific data from included studies.
Estimation of comparator risks
We selected sudden unexpected occurrences with recent reliable population-based information as comparator risks using US and EU-27 data. Emergency department attendances for a motor vehicle accident (E810-E819, E958.5, E968.5, E988.5), total emergency department attendance rates and attendances due to an injury (E800-E848, E850-E869, E880-E929) were extracted from National Center for Health Statistics data for 2006–2008 (CDC Wonder, Centers for Disease Control and Prevention). Fatality data including murder (X85-Y09), fire (X00-X09), all accidents (V01-99 or W00-X59), lightning (X33) and all cause mortality were extracted from the same data source for 2005–2007. European data using the same codes and time periods were extracted from Eurostat for the EU-27 population (Eurostat, European Union). The data used were not age adjusted.
Results
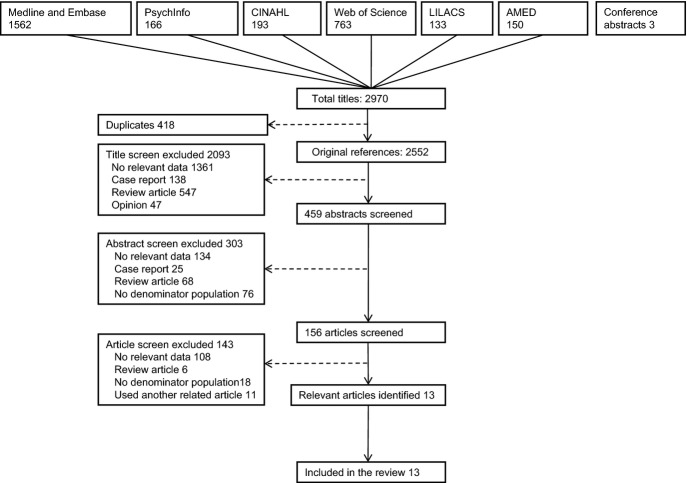
Our search identified 2552 original titles. Title screening yielded 459 abstracts, and 156 full articles were reviewed for potential relevance. We identified 13 studies describing a total of 240 deaths from food anaphylaxis over an estimated 165 million food-allergic person-years [3,5,9–19]. We were unable to include a further 14 population-based studies of anaphylaxis incidence rates (covering an estimated 4 million food-allergic person-years) in our analyses, due to no fatalities recorded (listed in Supporting Information Data S1). Search results are shown in Fig. 1, and characteristics of included studies in Table 1. All included studies contributed to at least one meta-analysis.
Fig. 1.
PRISMA flow chart showing results of literature search.
Table 1.
Characteristics of included studies
| Study | Setting | Case identification/numbers | Source of denominator population | Quality score* |
|---|---|---|---|---|
| Bock [9] | Colorado USA 1990–1991 | Emergency department survey – identified cases of probable fatal food anaphylaxis. 1 fatality | Colorado population statistics | 2/4 |
| Foucard & Malmheden Yman [10] | Sweden 1993–1996 | Survey of physicians, with case notes scrutinized by a single investigator. 4 fatalities | Sweden population statistics | 1/4 |
| Calvani et al. [11] | Lazio, Italy 2000–2003 | Hospital and emergency department information system: ICD codes 708.0, 989.5, 995.0, 995.2, 995.3, 995.4, 995.6, 999.4. 1 fatality | Lazio population aged 0–17 cited in paper | 2/4 |
| Pumphrey & Gowland [5]† | United Kingdom 1992–2009 | Prospective registry using national statistics, network of coroners, allergy charity to identify cases. Death certificate coding for anaphylaxis. 104 fatalities | UK population statistics | 3/4 |
| Bock et al. [12] | USA 2001–2006 | Prospective registry. Cases reported by physicians, media and FAAN and confirmed by a structured questionnaire. 31 fatalities | US population statistics 2001–August 2006 | 2/4 |
| Liew et al. [13] | Australia 1997–2005 | National Mortality Database – Death certificate ICD-10 codes T78.0, T78.2, T80.5, T88.6, T78.1, T78.4, X23, X25. Review of cases via Coroner reports and media archives. 7 fatalities | Mid-2001 Australian population figures used, multiplied by 9 | 2/4 |
| Simon & Mulla [14] | Florida, USA 1996–2005 | Death certificate search ICD-9 codes 995.0, 999.4 for 1996–1998; ICD-10 codes T50.9, T63.2, T63.4, T63.6, T63.9, T78.0, T78.2, T80.5, T88.6 for 1999–2005. All death certificates reviewed by a single author. 7 fatalities | Florida population estimates cited in paper | 2/4 |
| Bock [3] | USA 1994–1999 | Prospective registry. Cases reported by relatives, in response to advertising. 32 fatalities | US population statistics | 2/4 |
| Lin et al. [15] | New York State USA 1990–2006 | Statewide hospitalization database: ICD-9 code 995.6. 4 fatalities | New York State population aged 0–19, calculated from anaphylaxis rates cited in paper | 2/4 |
| Salter et al. [16] | Ontario, Canada 1986–2000 | Death certificate search for anaphylaxis as cause of death. 32 fatalities | Ontario population statistics | 3/4 |
| Simons et al. [17] | Canada 2000–2001 | Survey of paediatricians [Canadian Paediatric Surveillance Programme]. 1 fatality | Canada population aged 0–17 in 2000 | 0/4 |
| Levy et al. [18] | Israel 2004–2011 | Discussion with regional allergists, search of national media. 4 fatalities | Israel population estimates cited in paper | 0/4 |
| Tanno et al. [19] | Brazil 2008–2010 | Death certificate search using extensive ICD-10 codes, and review of all records where anaphylaxis was a possible cause of death. 12 fatalities | Brazilian population calculated from anaphylaxis rates cited in paper | 2/4 |
Quality assessment based on Newcastle-Ottawa scale for Cohort Studies [6].
Extended dataset kindly provided by study author.
Incidence rate of fatal food anaphylaxis in food-allergic people
Assuming a food allergy prevalence rate of 3% (3.9% in those aged 0–19 and 1% for peanut allergy), meta-analysis estimates the incidence rate of fatal food anaphylaxis in a food-allergic person as 1.81 (95% CI 0.94, 3.45; range 0.63, 6.68) per million person-years (micromorts) based on 10 studies [3,5,9,10,12–14,16,18,19], 3.25 (1.73, 6.10; range 0.94, 15.75) micromorts in those aged 0–19 based on 10 studies [3,5,10–13,15–18] and 2.13 (1.09, 4.16; range 1.03, 8.77) micromorts for peanut allergy based on seven studies [3,5,10,12–14,16] (Fig. 2a–c). All meta-analyses showed high levels of heterogeneity. Rates were higher in the smallest series, and this is reflected in the Funnel plot for all ages (Fig. 3a), but overall there was no clear evidence of publication bias in either all ages or those aged 0–19 (Fig. 3b) [9–11]. Rates were also higher in the Canadian study of Salter, for unknown reasons [16]. Our sensitivity analysis excluding poor-quality studies [10,17,18] found decreased fatal food anaphylaxis rate overall – 1.57 (0.75, 3.27; range 0.63, 6.68), in 0–19 year olds – 2.70 (1.31, 5.54; range 0.94, 9.74) and in peanut allergy – 2.04 (1.01, 4.13; range 1.03, 8.77) but heterogeneity of study outcomes remained significant. In 4 of the included studies, data were available for non-fatal food anaphylaxis rates in the same population and time period [9–11,13]. Case fatality rates ranged from 33% of patients admitted to an intensive care unit [10] to 0.14% of patients admitted to hospital [13].
Fig. 2.
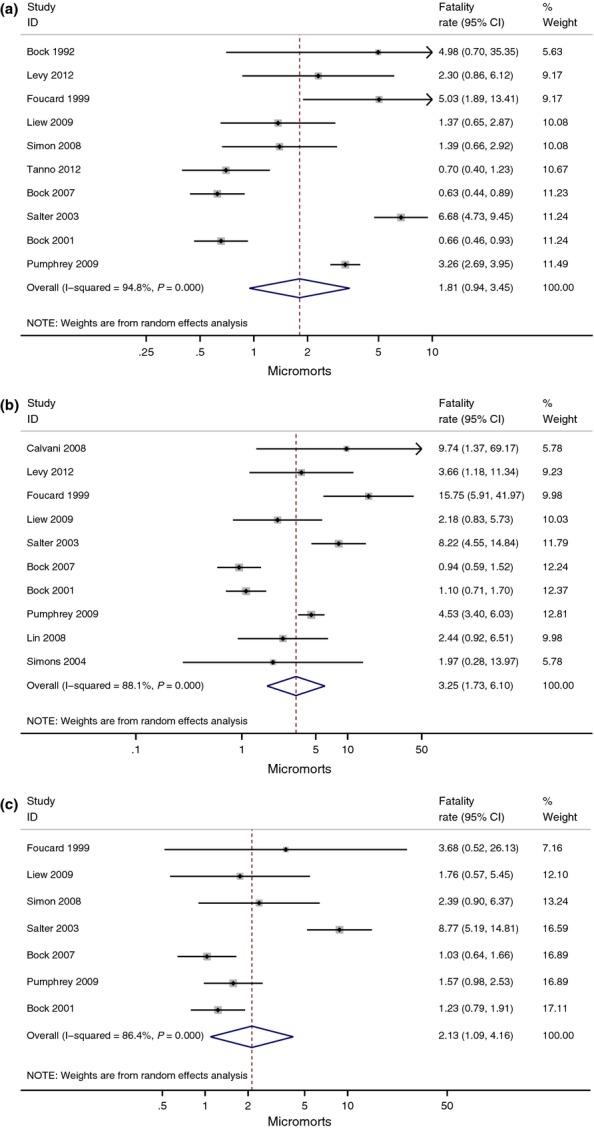
Estimated rate of fatal food anaphylaxis for a food-allergic person (a), a food-allergic person aged 0–19 (b) and a peanut-allergic person (c) expressed as incidence rate per million person-years (micromort).
Fig. 3.
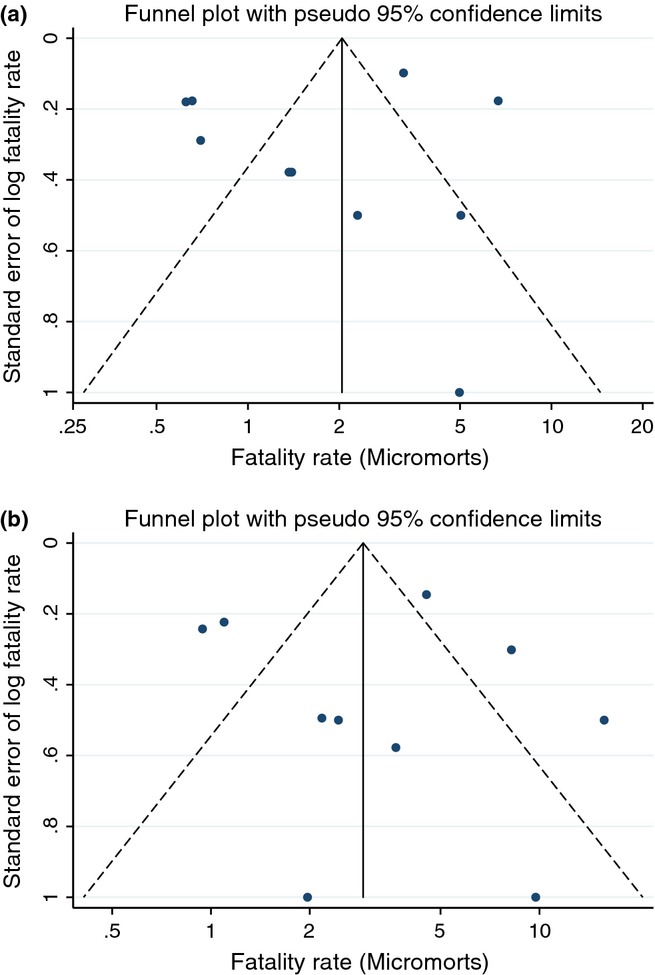
Funnel plots to assess risk of publication bias in analysis of overall fatal food anaphylaxis incidence rate (a) and fatal food anaphylaxis incidence rate at age 0–19 (b).
Comparison of food allergy risks with other age-specific risks
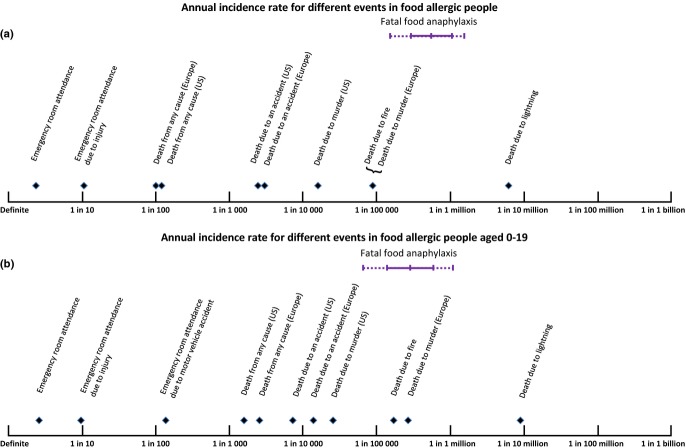
Figure 4 shows the estimated incidence of fatal food anaphylaxis for a food-allergic person (Fig. 4a) and a food allergic 0–19 year old (Fig. 4b), in comparison with incidences of other events for the general population in US or Europe. Incidence of fatal food anaphylaxis for a food-allergic person is ≥100 times lower than incidence of death due to any accident in the general population, and at age 0–19, the incidence is ≥10 times lower than accidental death incidence in the general population.
Fig. 4.
Estimated risk of fatal food anaphylaxis for a food-allergic person (a) or food-allergic person aged 0–19 (b), compared with other population risks. Continuous bar represents mean with 95% confidence interval; dotted bar is the range of point estimates from individual studies. Where reference risks vary markedly between European and United States populations, they are stated separately. Otherwise, reference risks are for a United States population.
Sensitivity of fatal food anaphylaxis incidence rate to changes in food allergy prevalence
Table 2 shows how fatal food anaphylaxis incidence rate varies with different estimated food allergy prevalence rates. For the highest estimates of population food allergy prevalence, the pooled incidence rate of fatal food anaphylaxis for a food-allergic person falls to 1.35 (0.71, 2.59), 1.18 (0.63, 2.22) at aged 0–19 and 0.73 (0.38, 1.43) for peanut allergy. For the lowest estimates of population food allergy prevalence, the pooled incidence rate of fatal food anaphylaxis rises to 2.71 (1.42, 5.17), 6.13 (3.25, 11.56) at aged 0–19 and 4.25 (2.17, 8.31) for peanut allergy.
Table 2.
Fatal food anaphylaxis incidence rates – calculated using different estimated food allergy prevalence rates
| Estimated food allergy prevalence* | Mean (95% CI) mortality rate (micromorts) | |
|---|---|---|
| All fatal food anaphylaxis | Food allergy prevalence 4% [2] | 1.35 (0.71, 2.59) |
| Food allergy prevalence 3% [2] | 1.81 (0.94, 3.45) | |
| Food allergy prevalence 2% [2] | 2.71 (1.42, 5.17) | |
| Fatal food anaphylaxis in young people (age 0–19) | Food allergy prevalence 10.4% [1] | 1.18 (0.63, 2.22) |
| Food allergy prevalence 3.9% [7] | 3.25 (1.73, 6.10) | |
| Food allergy prevalence 2% [2] | 6.13 (3.25, 11.56) | |
| Fatal peanut anaphylaxis | Peanut allergy prevalence 2.9% [1] | 0.73 (0.38, 1.43) |
| Peanut allergy prevalence 1% [2] | 2.13 (1.09, 4.16) | |
| Peanut allergy prevalence 0.5% [2] | 4.25 (2.17, 8.31) |
Discussion
In this systematic review, we evaluated the incidence rate of fatal food anaphylaxis in people with food allergy, and compared this with general population incidence rates for other unexpected medical events. In all studies and all subgroups evaluated, the incidence rate of fatal food anaphylaxis in food-allergic people is lower than the rate of accidental death in the general population. While it is possible that specific groups of food-allergic people have a higher incidence of fatal food anaphylaxis, such groups cannot yet be easily identified in clinical practice.
Fatal food anaphylaxis is a rare, dramatic event which often involves young people and commands wide media attention – as with other high profile, rare events the public perception of risk may be significantly greater than the true risk [20]. Given the importance of anxiety as a contributor to the quality of life impact of food allergy [21], our finding that fatal food anaphylaxis incidence is relatively low may be important information for food-allergic people and their carers. Some people with food allergy and their families have restricted lives because of fear of anaphylaxis and fatal outcomes, which they may estimate to be more likely to occur than reality. The extent to which such lifestyle restrictions are effective for preventing fatal outcomes is unclear. Our findings allow for a more accurate appraisal of fatal food anaphylaxis incidence and will inform debate over clinical practice, healthcare provision, school and community policies and food labelling regulation in relation to food allergy.
The limitations of this systematic review mainly relate to variations between studies in method of data capture and case definition of fatal food anaphylaxis, and limited information about food allergy prevalence in the populations studied. We are also unable to exclude the possibility of a systematic bias operating across different studies, in either the acquisition and coding of fatal food anaphylaxis data or the estimation of food allergy prevalence. Given the potential difficulty of identifying all those with fatal food anaphylaxis, there was reasonable consistency between studies in the estimated incidence rate in US, UK, Italy, Australia, Sweden, Brazil, Israel and Canada over 3 decades. The heterogeneity between study results may be partly explained by varying method of data capture – studies relied on a mixture of reporting through patient charities, professional bodies and networks, media reports and coroner reports. Variations in medical management of anaphylaxis may have also contributed to varying fatality rates. However, all studies found the incidence rate to be low – less than once every 100 000 person-years in the highest quality studies. We estimated food allergy as 3% overall, 3.9% at age 0–19 and 1% for peanut allergy. In reality, estimates of food allergy prevalence vary considerably between studies. Our sensitivity analysis showed fatal food anaphylaxis incidence rate varies with varying food allergy prevalence estimates, but under all assumptions, the rate remains less than once every 100 000 person-years. Recently, Australian and US data found a higher prevalence of food allergy and peanut allergy in children than previously reported, and many studies of food allergy prevalence have failed to systematically identify allergy to all possible food allergens, suggesting that fatal food anaphylaxis incidence may be towards the lower end of our range of estimates [1,22].
Life-threatening asthma is associated with food allergy, and fatal food anaphylaxis may present with acute wheezing [23]. A subgroup of fatal asthma cases occurs rapidly with less marked eosinophilic airway inflammation [24,25] – if some of these cases are fatal food anaphylaxis, then we may have underestimated fatal food anaphylaxis incidence due to miscoding as fatal asthma. A Swedish study found 11 of 37 (30%) fatal asthma cases at ages 1–34 were triggered by ingestion of a food allergen, raising this possibility [26]. However, the study included ICD codes for anaphylaxis (995 and T78.0) in the original search, and it is unclear how many of the 11 fatal food anaphylaxis cases had actually been classified as fatal asthma. Other studies have failed to identify miscoding of fatal food anaphylaxis as fatal asthma. A Finnish study evaluating the validity of death certificate coding of asthma deaths [27] from 1976 to 1998 did not reveal any miscoded fatal food anaphylaxis cases, nor did a similar analysis of UK asthma deaths in young people over 1 year [5].
We were unable to identify a group of food-allergic people with markedly different outcomes, however previous work has identified that those with asthma, previous severe reaction, IgE binding to a diverse range of epitopes, deficient platelet-activating factor acetyl-hydrolase or serum angiotensin-converting enzyme may have more severe food-allergic reactions [28–30]. One might expect patient education, the frequency with which a food is used as an ingredient, and allergen avoidance behaviour to influence an individual∼s risk, and there is some evidence to support this for non-fatal food anaphylaxis [31]. Those with food allergy secondary to inhalant sensitization, without asthma, and with previous mild reactions may be at lower incidence. Asthma is commonly cited as a risk factor for fatal food anaphylaxis, but while 85–96% of fatal food anaphylaxis occurs in people with asthma [3,5], asthma is present in 29–76% of all food-allergic people [7,32]. Hence, the predictive value of an asthma diagnosis for risk of fatal food anaphylaxis is poor. More work is needed to identify tools for risk stratification and reduction in food-allergic people [33].
In conclusion, we have quantified the incidence rate of fatal food anaphylaxis for food-allergic people. Although fatal food anaphylaxis is a rapid and frightening event, it appears to be very rare, such that for most food-allergic people, the incidence of fatal food anaphylaxis is likely to add relatively little to their overall mortality risk. This information should not belittle the concerns of food-allergic people and their families, and appropriate education, food labelling, allergen avoidance and anaphylaxis management strategies remain important. Our findings do, however, put the level of risk in perspective and may provide some reassurance to those affected by food allergy.
Acknowledgments
We would like to thank Dr Richard Pumphrey for providing original data for this project, and Liz Callow for assistance with running the literature searches.
Declaration of all sources of funding
Thisanayagam Umasunthar received salary support from Lincoln Medical. John Warner is supported by a National Institute for Health Research Senior Investigator Award. This work was supported by a National Institute for Health Research Biomedical Research Centre.
Author contributions
TU undertook the literature search and wrote the first draft of the manuscript, RJB designed the study, assisted with data collection and synthesis and wrote the final draft of the manuscript. JLB assisted with study design and data analysis. All authors reviewed and commented on earlier versions of the manuscript.
Conflict of interests
Thisanayagam Umasunthar was supported by funding from Lincoln Medical; John Warner is a trustee of the Anaphylaxis Campaign; John Warner and Robert Boyle have received research funding from the UK Food Standards Agency; Robert Boyle has received a grant for conference attendance from Meda Pharmaceuticals; the authors report no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. MEDLINE search strategy
References
- Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allergy Clin Immunol. 2007;119:1018–9. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O∼Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–55. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- Venter C, Hasan Arshad S, Grundy J, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–8. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- Bock SA. The incidence of severe adverse reactions to food in Colorado. J Allergy Clin Immunol. 1992;90:683–5. doi: 10.1016/0091-6749(92)90143-p. [DOI] [PubMed] [Google Scholar]
- Foucard T, Malmheden Yman I. A study on severe food reactions in Sweden – is soy protein an underestimated cause of food anaphylaxis? Allergy. 1999;54:261–5. doi: 10.1034/j.1398-9995.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- Calvani M, Di Lallo D, Polo A, Spinelli A, Zappala D, Zicari AM. Hospitalizations for pediatric anaphylaxis. Int J Immunopathol Pharmacol. 2008;21:977–83. doi: 10.1177/039463200802100422. [DOI] [PubMed] [Google Scholar]
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–42. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- Simon MR, Mulla ZD. A population-based epidemiologic analysis of deaths from anaphylaxis in Florida. Allergy. 2008;63:1077–83. doi: 10.1111/j.1398-9995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: new York State, 1990–2006. Ann Allergy Asthma Immunol. 2008;101:387–93. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- Salter J, Mehra S, Cairns JT, Sussman G, Vadas P. A Study of 32 Food-Induced Anaphylaxis Deaths in Ontario; 1986–2000. Anaphylaxis Canada. (8-11-2010) Available from: http://www.anaphylaxis.org/content/programs/programs_research_deaths.asp. [Google Scholar]
- Simons FER, Chad ZH, Gold M. Anaphylaxis in children – real-time reporting from a national network. Allergy Clin Immunol Int. 2004;242(Suppl. 1) [Google Scholar]
- Levy MB, Goldberg MR, Nachshon L, Tabachnik E, Katz Y. Lessons from cases of mortality due to food allergy in Israel: Cow∼s Milk Protein should be considered a potentially fatal allergen. Isr Med Assoc J. 2012;14:29–33. [PubMed] [Google Scholar]
- Tanno LK, Ganem F, Demoly P, Toscano CM, Bierrenbach AL. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD-10. Allergy. 2012;67:783–9. doi: 10.1111/j.1398-9995.2012.02829.x. [DOI] [PubMed] [Google Scholar]
- Hakes JK, Viscusi WK. Dead reckoning: demographic determinants of the accuracy of mortality risk perceptions. Risk Anal. 2004;24:651–64. doi: 10.1111/j.0272-4332.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- King RM, Knibb RC, Hourihane JO. Impact of peanut allergy on quality of life, stress and anxiety in the family. Allergy. 2009;64:461–8. doi: 10.1111/j.1398-9995.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003;112:168–74. doi: 10.1067/mai.2003.1569. [DOI] [PubMed] [Google Scholar]
- Carroll N, Carello S, Cooke C, James A. Airway structure and inflammatory cells in fatal attacks of asthma. Eur Respir J. 1996;9:709–15. doi: 10.1183/09031936.96.09040709. [DOI] [PubMed] [Google Scholar]
- James AL, Elliot JG, Abramson MJ, Walters EH. Time to death, airway wall inflammation and remodelling in fatal asthma. Eur Respir J. 2005;26:429–34. doi: 10.1183/09031936.05.00146404. [DOI] [PubMed] [Google Scholar]
- Bergstrom SE, Boman G, Eriksson L, et al. Asthma mortality among Swedish children and young adults, a 10-year study. Respir Med. 2008;102:1335–41. doi: 10.1016/j.rmed.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Malmstrom K, Kaila M, Kajosaari M, Syvanen P, Juntunen-Backman K. Fatal asthma in Finnish children and adolescents 1976–1998: validity of death certificates and a clinical description. Pediatr Pulmonol. 2007;42:210–5. doi: 10.1002/ppul.20552. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Grimshaw KE, Warner JO, Hourihane JO. The promiscuity of immunoglobulin E binding to peanut allergens, as determined by Western blotting, correlates with the severity of clinical symptoms. Clin Exp Allergy. 2005;35:767–73. doi: 10.1111/j.1365-2222.2005.02252.x. [DOI] [PubMed] [Google Scholar]
- Summers CW, Pumphrey RS, Woods CN, McDowell G, Pemberton PW, Arkwright PD. Factors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist center. J Allergy Clin Immunol. 2008;121:632–8. doi: 10.1016/j.jaci.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- Fleischer DM, Perry TT, Atkins D, et al. Allergic Reactions to Foods in Preschool-Aged Children in a Prospective Observational Food Allergy Study. Pediatrics. 2012;130:E25–32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT, Ewan PW. Good prognosis, clinical features, and circumstances of peanut and tree nut reactions in children treated by a specialist allergy center. J Allergy Clin Immunol. 2008;122:286–9. doi: 10.1016/j.jaci.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Menikou S, Patel MP, Rose KL, et al. Relationship between complotype and reported severity of systemic allergic reactions to peanut. J Allergy Clin Immunol. 2012;129:1398–401. doi: 10.1016/j.jaci.2011.10.049. e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. MEDLINE search strategy