Abstract
Neonates have an immature immune system, which cannot adequately protect against infectious diseases. Early in life, immune protection is accomplished by maternal antibodies transferred from mother to offspring. However, decaying maternal antibodies inhibit vaccination as is exemplified by the inhibition of seroconversion after measles vaccination. This phenomenon has been described in both human and veterinary medicine and is independent of the type of vaccine being used. This review will discuss the use of animal models for vaccine research. I will review clinical solutions for inhibition of vaccination by maternal antibodies, and the testing and development of potentially effective vaccines. These are based on new mechanistic insight about the inhibitory mechanism of maternal antibodies. Maternal antibodies inhibit the generation of antibodies whereas the T cell response is usually unaffected. B cell inhibition is mediated through a cross-link between B cell receptor (BCR) with the Fcγ-receptor IIB by a vaccine–antibody complex. In animal experiments, this inhibition can be partially overcome by injection of a vaccine-specific monoclonal IgM antibody. IgM stimulates the B cell directly through cross-linking the BCR via complement protein C3d and antigen to the complement receptor 2 (CR2) signaling complex. In addition, it was shown that interferon alpha binds to the CD21 chain of CR2 as well as the interferon receptor and that this dual receptor usage drives B cell responses in the presence of maternal antibodies. In lieu of immunizing the infant, the concept of maternal immunization as a strategy to protect neonates has been proposed. This approach would still not solve the question of how to immunize in the presence of maternal antibodies but would defer the time of infection to an age where infection might not have such a detrimental outcome as in neonates. I will review successful examples and potential challenges of implementing this concept.
Keywords: maternal antibody, cotton rat, FcγRIIB, B cell receptor, maternal immunization
Introduction
Vaccination of neonates and infants is problematic because of two unsolved problems: the immature immune system of neonates and the presence of inhibitory maternal antibodies. A number of studies have determined that the immaturity of the immune system is most pronounced after birth and is overcome as the child develops. The immaturity (inability to fully respond to an antigenic stimulus) of the neonatal immune system has been observed in humans (1) and a number of other species, e.g., pig (2), cow (3, 4), and horse (5), and in experimental rodent models like mouse (1), rat (6), and cotton rat (7, 8).
Maternal antibodies are transferred from mother to child and protect neonates and infants during the time of maturation of their immune system. The vast majority of maternal antibodies are of the IgG isotype. In humans, maternal antibodies are preferentially transferred before birth transplacentally, and in animals of veterinary importance, preferentially through uptake of IgG in the intestine from colostrum within the first 24 h after birth. These passively acquired antibodies enter the bloodstream of offspring and act as a protective shield throughout the body in the same way as actively produced antibodies. Sometimes IgA antibodies contained in breast milk are also referred to as maternal antibodies. However, there are important differences in the action of passively transferred IgG and IgA antibodies. Upon transfer after birth, IgG antibodies are present in the bloodstream of the neonate in a finite amount that declines over time. These IgG antibodies suppress vaccine-induced immune responses. In contrast, IgA antibodies are continuously supplied through breast milk from the mother and protect the gastro-intestinal tract against pathogens without having an effect on the immune response. For the purpose of this review, the term “maternal antibodies” will be used for passively transferred IgG antibodies.
Maternal antibodies are very effective in protecting neonates and infants against most infectious diseases. The most impressive example is the protection of children with agammaglobulinemia (deficiency in the production of antibody) against bacterial infection for up to 6 months (9). Other documented examples of the ability of maternal antibodies to fully or partially protect are the amelioration of infection with respiratory syncytial virus (RSV) (10) or influenza virus (11) in humans, canine distemper virus in dogs (12), and infection with avian leukosis virus in chickens (13). Over time, maternal antibody titers decline because antibodies are being metabolized and do not protect any longer. However, even low, non-protective titers of maternal antibodies are still able to inhibit vaccination against infectious diseases of humans and animals. It is this phase of decaying maternal antibodies that presents a window of opportunity for infection by pathogens encountering the neonatal child or animal.
Inhibition of Vaccination by Maternal Antibodies
In humans, maternal antibodies wane over a period of 6–12 months (14–17). The kinetics of maternal antibody decline is correlated to the amount of maternal antibody present in the neonate after birth in that higher titers persist for a longer time. In contrast to humans, the duration of maternal antibodies in agriculturally important animal species is usually 3–6 months (18–20) and in chicken only 4–7 days (21). In contrast, maternal antibodies in bats persist similar to humans for 6–12 months (22–24). Maternal antibodies in all species have been reported to reduce or abolish antibody generation after vaccination. The reduction or lack of antibody typically results in reduced or absence of protection against disease (Tables 1 and 2). It is of interest to note that all types of vaccines (live-attenuated, inactivated, subunit, and experimental vaccines) have been reported to be inhibited. For a number of experimental vaccines immunization with variable success in the presence of maternal antibodies has been reported but these reports have to be evaluated based on criteria set out in Section “Evaluation and Development of Experimental Vaccines.”
Table 1.
Inhibition of seroconversion of human vaccines by maternal antibodies.
| Infectious agent | Type of vaccine | Reference |
|---|---|---|
| Tetanus | Combination protein vaccine | (25) |
| Pneumococcus | Combination protein vaccine | (25, 26) |
| Hib | Combination protein vaccine | (25, 27) |
| Pertussis | Combination protein vaccine | (25) |
| Acellular and whole-cell vaccine | (28) | |
| Measles virus | Live-attenuated | (29–31) |
| Mumps virus | Live-attenuated | (32) |
| Hepatitis A virus | Inactivated virus | (33) |
| Hepatitis B virus | Protein vaccine | (34) |
| Rotavirus | Live-attenuated | (35) |
| Poliovirus | Inactivated virus | (36, 37) |
| Live-attenuated vaccine | (38) | |
| Influenza virus | Cold recombinant influenza and trivalent inactivated virus | (39) |
This table lists examples of studies, which document the inhibition of or reduction in seroconversion after immunization with both live and non-live vaccines. Jones et al. (25) document a stronger inhibitory effect of maternal antibodies on tetanus and pneumococcal vaccines than Hib and pertussis vaccines. Most studies indicate that higher levels of maternal antibodies inhibit antibody development more severely than low titers. Two studies (29, 35) indicate that an increase in dose helps to improve antibody responses after immunization in the presence of maternal antibodies.
Table 2.
Inhibition of seroconversion of veterinary vaccines by. maternal antibodies.
| Species | Infectious disease | Type of vaccine | Reference |
|---|---|---|---|
| Dog | Canine parvovirus | Live-attenuated | (40, 41) |
| Canine distemper virus | Live-attenuated | (42, 43) | |
| Cat | Feline panleukopenia virus | Live-attenuated | (44, 45) |
| Feline herpesvirus 1 | Inactivated virus | (44) | |
| Feline calicivirus | Inactivated virus | (44) | |
| Cow | Bovine viral diarrhea virus | Live-attenuated | (46, 47) |
| Foot and mouth disease virus | Inactivated virus | (48) | |
| Bovine respiratory syncytial virus | Live-attenuated | (49–51) | |
| Pig | Erysipelothrix rhusiopathiae | Live-attenuated | (52) |
| Pseudorabies virus | Genetically attenuated | (53) | |
| Classical swine fever virus | Protein vaccine | (54, 55) | |
| Live-attenuated | |||
| Influenza virus | Protein vaccine | (56) | |
| Chicken | Influenza virus | Inactivated virus | (57) |
| Raccoon | Rabies virus | Vaccinia virus expressing rabies glycoprotein | (58) |
| Canine distemper virus | Live-attenuated | (59) | |
| Wolves | Canine distemper virus | Live-attenuated | (60) |
| Ferrets | Canine distemper virus | Live-attenuated | (61) |
This table lists examples of studies, which document the inhibition of or reduction in seroconversion after immunization with both live and non-live vaccines in different species. It also lists an example of a vaccine (against canine distemper virus), which is inhibited in several species. Three studies (46, 51, 53), included in their study T cell measurements and detected T cell responses after immunization in the presence of maternal antibodies although the antibody response was inhibited.
Example: Inhibition of Measles Vaccination by Maternal Antibodies
The best studied example in human and veterinary medicine for the inhibition of vaccination by maternal antibody is measles vaccination. The measles vaccine virus (Edmonston strain) was developed in the late 1950s and early 1960s by attenuating a wild type virus on human and chicken embryo fibroblasts [for review see Ref. (62)]. Today various derivatives of the Edmonston strain are used as vaccine viruses worldwide (63). Immunization by both the standard subcutaneous and more experimental respiratory routes has been successful in protecting seronegative children (64). The protective immune response induced by vaccination consists of neutralizing antibodies against the two glycoproteins, with the (receptor-binding) hemagglutinin protein being the major target and the fusion protein the minor one (65). In addition, CD4 as well as CD8 T cell responses are induced. Case reports describing persistent MV infection in T cell deficient patients support the notion that T cells are required to clear infection but do not protect against infection (66). This role of CD8 T cells in virus clearance has been confirmed in the Rhesus macaques model (67). In cotton rats, CD4 T cells have no role in protecting or clearing virus from the respiratory tract (68). However, in mice they have a role in clearing virus infection from brain tissue through production of IFNγ (69, 70).
During their first year of life, children are protected by neutralizing maternal antibodies against MV infection. Over time, these antibody titers wane and eventually do not protect against wild type infection [for review see Ref. (71)]. Even these low, non-protective antibody titers inhibit seroconversion after both subcutaneous and intranasal immunization in cotton rats as well as humans. The lack of seroconversion severely reduces protection after vaccination with the live-attenuated vaccine virus. In the cotton rat model, this leads to a complete lack of protection (68). In clinical studies, immunization in the presence of maternal antibodies leads to partial reduction in mortality (72) and morbidity (73) (even in seronegative children). However, a broad clinically desirable protective immunity (protective T and B cell responses, and no clinical symptoms after infection rather then reduction in morbidity and mortality) is not established after immunization in the presence of maternal antibodies.
In contrast to the antibody response, MV-specific T cell proliferation is usually measurable after immunization in the presence of maternal antibodies (74–76). In spite of the inhibition of seroconversion, it was observed that vaccination in the presence of maternal antibodies leads to priming of B cells. Although no antibodies were produced, children immunized with measles vaccine in the presence of maternal antibodies responded with a higher immune response to booster vaccination after maternal antibodies had declined than children who had not been vaccinated at all (77). This study indicated that inhibition of B cell responses by maternal antibodies is a temporary effect and not due to the induction of anergy. However, it has also been observed that in children who were immunized in the presence of maternal antibodies long term antibody titers were reduced (even after boosting) compared to children who were seronegative at the time of immunization (76).
Since no current vaccine formulation is fully effective in the presence of maternal antibodies, two approaches have been used clinically to address the problem: one, the use of a high titer vaccine, and the other, the determination of the earliest time point possible for successful vaccination. The high titer vaccine (>104.7 pfu) had a 10- to 50-fold higher viral titer than the normal vaccine and induced some level of protection after immunization in the presence of maternal antibodies (29, 78). However, the use of this vaccine was associated with increased mortality (particularly in females) (79–81), which was attributed to immune suppression by the vaccine and its use was discontinued. A possible explanation for higher mortality in girls might be that girls have been reported to have lower maternal antibody titers (82) that were overwhelmed by a higher vaccine virus dose.
In a second approach, children were immunized at different times after birth (in the face of declining maternal antibodies). These studies have shown that a low level of maternal antibody correlates best with vaccination success. At the age of 6 months, maternal antibody titers are still high enough to suppress seroconversion but at the age of 9 months vaccination campaigns are relatively successful (31, 83). However, the complete disappearance of antibody at the age of 12 months seems to be optimal for immunization (32, 74, 84–86). Additional studies into the levels of maternal antibodies demonstrated that strong regional differences exist in that in a number of countries maternal antibodies might have disappeared by 6 months of age, and sometimes immunization at 4.5 months of age might be partially successful (87, 88). Regional differences might be due to a number of factors such as the exposure to wild type virus infection of mothers, which results in higher antibody titers and higher transfer rates into children than in vaccinated mothers (89). Maternal antibody levels in children of vaccinated mothers are lower and decline earlier than in children from naturally infected mothers.
Clinical Relevance of Inhibition of Vaccination by Maternal Antibodies and Practical Solutions
Numerous reports detail the inhibition of antibody responses after vaccination in the presence of maternal antibodies. The important question is whether this is clinically relevant and whether clinical solutions exist to overcome the problem. For measles virus vaccination, maternal antibodies clearly inhibit the generation of neutralizing antibodies and therefore severely restrict protective immunity. However, the clinical outcome might vary depending on the specific vaccine and infectious disease under study. A recent study found that suppression of antibody responses against the tetanus and pneumococcal vaccines was more pronounced than against hemophilus influenza B and pertussis vaccines (25). Theoretically, immunization should be successful when maternal antibodies have declined below the threshold of detection. In practice, it is not feasible to accurately predict this time point as it depends on the amount of maternal antibody transferred, region, gender, nutritional status, and species. Therefore, the measurement of antibody levels would be required before immunization, which is not feasible in a clinical setting. The clinical practice to immunize children and animals repeatedly because most immunizations are based on a prime-boost principle, also ensures that children and animals are immunized when maternal antibodies have been metabolized. Immunization in the presence of maternal antibodies does not interfere with later vaccinations, and this practice ensures that eventually the individual is immunized when maternal antibodies have disappeared. However, a variable period of time without active immune protection will remain and therefore this vaccination schedule is most successful in regions with a low prevalence of infection. The other important question for vaccination is whether antibodies or T cells are a correlate of protection. If T cells play a major role in protection [e.g., Aujeszky virus (90)], early immunization will afford protection because the T cell response is affected very little by maternal antibodies. If, in contrast, neutralizing antibodies are required for protection, early immunization is not likely to succeed.
Type of Placenta Determines Mode of Transfer of Maternal Antibodies
The transfer of maternal antibodies from mother to offspring may occur during pregnancy (from the maternal blood via transplacental transfer) and within 24 h after birth (from colostrum via the small intestine). The amount of antibody transferred by these two mechanisms differs by species depending on the type of placenta (http://placentation.ucsd.edu/placenta.html). Just prior to formation of the placenta, there are a total of six layers of tissue separating maternal and fetal blood. There are three layers of fetal extraembryonic membranes in the chorioallantoic placenta of all mammals, all of which are components of the mature placenta: endothelium lining allantoic capillaries, connective tissue in the form of chorioallantoic mesoderm, and the chorionic epithelium, the outermost layer of fetal membranes derived from trophoblasts. There are also three layers on the maternal side, but the number of these layers that are retained – that is, not destroyed in the process of placentation – varies greatly among species (see Table 3). Overall, species with few layers between maternal and fetal blood have a higher rate of transport of maternal antibodies transplacentally [through Fc-receptors (FcR)]. In species with more layers between maternal and fetal blood, the transport of maternal antibodies preferentially occurs through colostrum. Although the differences between species are important for the study of placentation and transfer of nutrients, for the study of vaccination in the presence of maternal antibodies the structure of the placenta and the mode of transmission of IgG is only relevant if intrapartum studies are performed. Most studies, however, assess the inhibition of vaccination sometime after birth and the relevant parameter in these experimental settings is the amount of IgG being transferred to the offspring.
Table 3.
Type of placenta is species specific and determines route of transfer of maternal antibodies.
| Species | Placenta | Maternal antibody transfer | Transfer mediated by neonatal Fc receptor (FcRn) | Reference |
|---|---|---|---|---|
| Human | Hemochorial | Transplacental | Yes; preferential transport of IgG1 > IgG3 > IgG4 > IgG2 | (91–94) |
| Rodents | Hemochorial | Transplacental/colostrum | Yes | (6, 95–98). |
| Mouse | ||||
| Rat | ||||
| Cotton rat | Hemochorial | Transplacental/colostrum | Unknown | (99, 100) |
| Dogs and cats | Endotheliochorial | Low transplacental/high in colostrum | Unknown | (12, 20, 40, 101–104) |
| Cattle, sheep, pigs, and horses | Epitheliochorial | Colostrum | FcRn present in pig intestine, role in IgG transfer questionable | (94, 105–107) |
| Birds | None | In ovo | FcRY (bird equivalent to FcRn) | (108, 109) |
The three potential maternal layers in a placenta are the endothelium lining of endometrial blood vessels, connective tissue of the endometrium, and endometrial epithelial cells. In humans and rodents, the fetal chorionic epithelium is in direct contact with maternal blood because only the maternal endothelium remains (hemochorial placenta). In contrast, the chorionic epithelium of horse and pig fetuses remains separated from maternal blood by three layers of tissue (epitheliochorial placenta) whereas only two layers remain in dogs and cats (endotheliochorial placenta). The fewer the layers between maternal and fetal blood the higher the rate of transport of maternal antibodies transplacentally. In humans, the majority of maternal IgG is transferred via the placenta. The transfer is an active process during which IgG binds to the neonatal Fc receptor (FcRn), which is located in the placental syncytiotrophoblast. The binding between FcRn and IgG is 100-fold higher at pH 6 than at pH 7.4 (91, 92). After endocytosis of IgG by the placental syncytiotrophoblast IgG binds to FcRn in the endosome at low pH, is transported to the opposite cell membrane and released at physiological pH into the blood stream [reviewed by Palmeira et al. (110)]. This mechanism explains why maternal antibodies are of the IgG isotype. In contrast to this specific transport mechanism, it seems that in agricultural species IgG and other macromolecules are transported from colostrum across the intestinal barrier in a non-specific fashion (111, 112). Within 24-h after birth, the intestine becomes impermeable to macromolecules and IgG transfer ceases. Similar to rodents, e.g., pig intestinal epithelial cells also express FcRn during the neonatal period but it has been argued that the large amount of IgG taken up within 24 h is not consistent with receptor-mediated transport [discussed in Ref. (107)]. In contrast to rodents, pig FcRN also is expressed in the adult period of life (106) and this expression pattern is not correlated with transfer of maternal antibody. In contrast to mammals, birds generate IgY antibodies instead of IgG antibodies and use the IgY Fc-receptor (FcRY) to transport IgY into the egg.
Experimental Models to Study the Effect of Homologous Versus Heterologous Passively Transferred Antibodies on Vaccination
Inhibition of vaccination has been studied in the presence of natural maternal antibodies or in the presence of passively transferred homologous or heterologous antibodies. Overall, inhibition of seroconversion after vaccination has been similar using both methods. For the induction of natural maternal antibodies, dams are immunized during pairing with the male and will transfer maternal antibodies to the pups. In rodents, differences can be found in antibody levels between pups of the same litter, which may occur because of the suckling hierarchy. The supposed advantage of natural maternal antibodies is the fact that antibodies from the same species have a predetermined fit for the FcR present in the species whereas heterologous IgG does not always interact with FcR (113, 114). However, studies in mice and humans have shown that subclasses of homologous IgG differ in their interaction with FcR (115, 116).
Often, the question of inhibition of vaccination by maternal antibodies will have to be separated from the immaturity of the neonatal immune system experimentally. One possible resolution is the transfer of homologous immune serum into adolescent animals (117). Using this experimental model, it was shown that suppression of vaccine responses correlates with amount of antibody present at the time of immunization (118). The injection of antibodies allows us to determine the amount of antibody transferred and ensures equal titers in all animals of the experimental group. In addition, often higher titers of passively transferred antibody can be achieved than would be possible through the induction of natural maternal antibodies. A disadvantage of homologous antibodies (whether natural maternal antibodies or passively transferred) is that they are indistinguishable from antibodies induced actively via immunization. The transfer of heterologous antibodies provides the advantage that actively induced and passively transferred antibodies can be distinguished by ELISA. Heterologous antibodies degrade faster than homologous antibodies, and e.g., in the cotton rat model the half-life of cotton rat IgG was estimated to be 7 days versus a half-life of 3.5 days for injected human IgG (Niewiesk, unpublished). The rate of decay should not matter for vaccination studies, as the amount of antibody at the time of vaccination is important for inhibition, not the duration of antibody levels. However, one should not inject heterologous antibodies repeatedly, as that will lead to the induction of regulatory T cells, which might interfere with the immune response after vaccination (119). Another caveat is warranted when monoclonal antibodies are used. It is important to test whether specific subclasses will interact with the FcγRIIB receptor in the respective species and be able to suppress B cell responses. In cotton rats, for example, mouse IgG1 antibodies do not bind to the FcγIIB receptor and do not interfere with vaccination whereas mouse IgG2a antibodies do (114).
Mechanisms of Inhibition of Vaccination by Maternal Antibodies
Antibody feedback mechanism
Since studies to address the mechanism of inhibition of seroconversion after vaccination in the presence of maternal antibodies have been rare, data from antibody feedback regulation have been used to provide mechanistic insight into B cell inhibition by antibody. Antibody feedback regulation is the phenomenon whereby co-injection of an antigen [usually sheep red blood cells (SRBC)] and a monoclonal antibody specific for this particular antigen into a naive mouse leads to a reduction or inhibition in the generation of antigen-specific antibodies. [It is important to note that this phenomenon is true for large particulate antigens like SRBC and keyhole limpet hemocyanin (KLH)]. The suppression increases with the amount and affinity of antibody given (120, 121) but is independent of the IgG isotype (120, 121). In contrast to the B cell response, a T helper cell response is not inhibited (122). Moreover, a recall response can be induced after the decline of passively transferred antibody. Two major hypotheses might explain this phenomenon: epitope masking and B cell regulation through the Fcγ-receptor IIB (FcγRIIB).
Inhibition of B cell responses through epitope masking
Sheep red blood cell-specific antibodies recognize very few highly repetitive epitopes on SRBC. The theory of epitope masking postulates that antibodies can cover these epitopes and mask them from B cell recognition if enough antibody is used. Originally, it was thought that all epitopes have to be covered by antibodies to prohibit recognition by B cells (epitope-specific suppression). However, it was shown that a monoclonal antibody against one epitope can suppress recognition of a whole particulate antigen by B cells (epitope unspecific suppression) (120, 121, 123). It has been argued that at high antibody concentrations, steric hindrance might explain this form of suppression. But even low antibody concentrations of 0.4 μg (corresponding to 2 × 106 antibody molecules) are able to suppress antibody responses against 108 SRBC (124). Epitope masking as a mechanism of suppression has always been discussed in conjunction with a competing mechanism, the regulation of B cells through the FcγRIIB.
Inhibition of B cell responses by binding of IgG to the Fcγ-receptor IIB
B cells express the FcγRIIB as the only Fcγ receptor on their surface. It has been shown in vitro that IgG specific for the B cell receptor (BCR) binds to the BCR via its antigen binding domain in the variable region and to FcγRIIB through the constant region. Through the cross-linkage between BCR and FcγRIIB, the tyrosine-based inhibitory motif of FcγRIIB is in close proximity to the tyrosine-based activation motif of the BCR. This proximity leads to inhibition of antigen-specific B cell activation. In vitro, SRBC-specific B cells from spleens of immunized mice can be activated by addition of SRBC to secrete antibody, which can be detected using an ELISPOT system. This activation can be inhibited by the addition of SRBC-specific IgG (124), which forms a complex with SBRC and links the BCR to FcγRIIB. In contrast, the F(ab′)2 fragment, which lacks the constant region (Fc) of IgG, cannot cross-link these receptors and inhibit B cell stimulation. Similarly, mouse B cells with a deletion in the gene coding for the γ chain of the FcγRIIB cannot be inhibited by SRBC-specific IgG (124). These in vitro data strongly argue for a role of FcγRIIB in down-regulating B cell responses. In contrast, data obtained in vivo do not unequivocally support the ability of IgG to suppress B cell responses by FcγRIIB binding. In support of this mechanism are data demonstrating that glycosylation of the constant region (Fc), which is crucial for binding of IgG to the inhibitory FcγRIIB, is necessary for inhibition (125, 126). In addition, some studies have shown that F(ab′)2 fragments, in contrast to complete IgG, do not inhibit antigen-specific responses (120, 127, 128). However, other studies found little difference between F(ab′)2 fragments and complete IgG (120, 127, 128), and in mice with a genetically deleted FcγRIIB (and also deletion of FcγRI and FcγRIII) inhibition still could be induced by IgG (124). Two technical comments must be made in regard to the latter studies. After pepsin digestion, the quality of the F(ab′)2 fragments varies and was not rigorously controlled in these studies. In the study using genetically modified mice, the deletion of the common γ-chain (FcRγ) leads to the absence of FcγRI, FcγRIIB, and FcγRIII. In consequence, these mice displayed a wide array of immunological abnormalities that were not restricted to the B cell compartment (124). After antigen injection, these mice generated a markedly stronger antibody response indicating the lack of a feedback mechanism to regulate B cell responses and antibody titers.
Stimulation of B cell responses by the binding of IgM to the CD21/CD19/CD81/Leu-13 signaling complex
In the study of antibody feedback regulation, it was found that co-injection of antigen and antigen-specific IgM increases antibody responses in the presence of an inhibitory IgG (125). These data suggest that the inhibitory signal through FcγRIIB can be overcome by stimulating signals via the complement receptor 2 (CR2) (CD21/CD19/CD81/Leu-13) complex. In vitro, a complex of antigen, IgM and complement protein C3d cross-links the BCR and CR2 and leads to B cell activation (129, 130). C3d which binds to CD21 is produced through the activation of the classical complement pathway involving the C2 and C4 complement proteins. In mice, the cross-linkage increases the responsiveness of B cells in the presence of antigen-specific IgM and antigen by two orders of magnitude in comparison to antigen alone (131). This immune enhancement by IgM is not seen in CD21 gene-targeted mice (132) or mice in which complement was depleted prior to vaccination (125) or in the absence of antigen. These data support a regulatory role of IgG on B cell activation that can be overcome by stimulation through IgM, and argues against simple epitope masking.
Mechanism of inhibition of seroconversion after vaccination in the presence of maternal antibodies
In addition to mechanisms based on the work performed in the antibody feedback mechanism model (epitope masking and B cell inhibition through FcγRIIB), two additional mechanisms have been hypothesized to play a role in inhibition of vaccination by maternal antibodies: removal of vaccine antigen by macrophages and neutralization of vaccine virus by maternal antibodies.
Antigen removal by macrophages
It has been assumed that macrophages remove antibody–virus complexes from the circulation through binding to FcR to such a degree as to abolish immune responses. There is no experimental evidence to support this hypothesis, and it also does not explain why the B cell response is preferentially inhibited whereas a T cell response is consistently detected after immunization in the presence of maternal antibodies. The only evidence that Fc-receptor-mediated phagocytosis of antigen–antibody complexes redirects the immune response is the finding in mice that it leads to increased IL10 secretion (133). The increase in IL10 could direct T cell responses toward a Th2 response, which, however, would not lead to a decrease in antibody responses. So far, there has been no evidence that the removal of antigen–antibody complexes by macrophages influences antibody responses.
Neutralization of live-attenuated vaccine
An often-suggested explanation for the lack of vaccination success in the presence of maternal antibodies is neutralization of the vaccine virus, which would reduce the amount of viral antigen below a certain (undefined) threshold and thereby interfere with immune recognition. Three facts argue against this hypothesis: maternal antibodies suppress not only replicating live-attenuated vaccines but also (non-replicating) protein vaccines; immunization with vector systems expressing measles virus proteins (which are not sensitive to neutralizing antibodies) are inhibited by maternal antibodies (134–136); and non-neutralizing antibodies block vaccination with a live-attenuated vaccine (114).
B cell inhibition through epitope masking
The idea of epitope masking predicts that B cell epitopes on a vaccine will be covered by antibody and therefore will not be recognized by B cells. In consequence, this effect is dependent on the concentration of antibody present in the circulation, and should be seen with both a complete IgG antibody and an IgG antibody lacking its constant region [so-called F(ab)2 fragment]. However, experimentally we could demonstrate that one antibody at a high concentration is less efficient in inhibiting vaccination than three antibodies at lower concentrations, and that only complete IgG antibody can block vaccination (114). In addition, the inhibition of antibody generation afterward was not specific to the epitope recognized by the inhibitory antibody.
B cell inhibition through cross-link of BCR with FcγRIIB
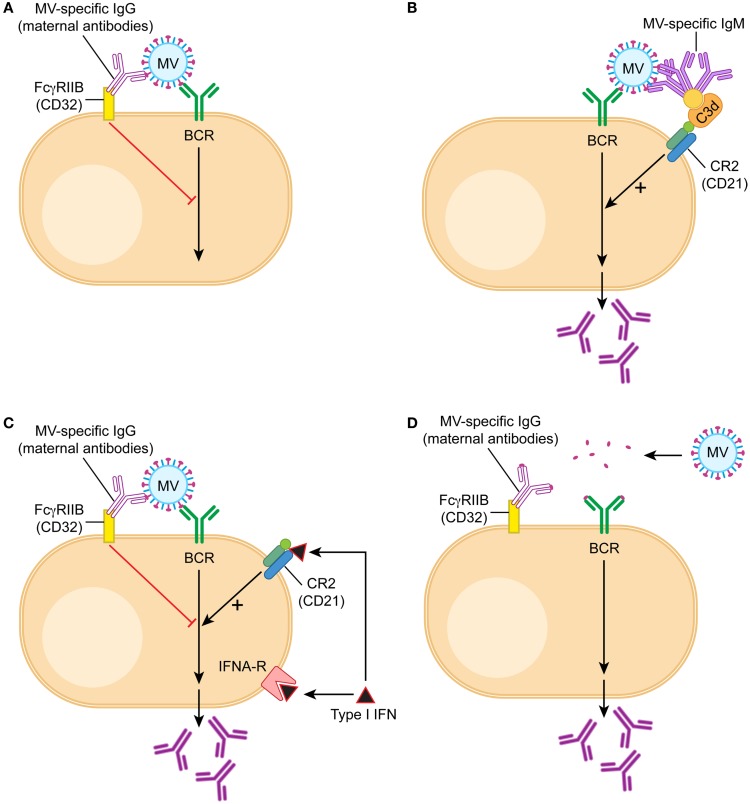
In contrast to these results, which did not support epitope masking as a mechanism, the interaction between FcγRIIB and maternal antibodies proved to be important. Both in vitro and in vivo, IgG can block B cell responses whereas F(ab)2 fragments cannot. A monoclonal antibody with an Fc region that does not bind to cotton rat FcγRIIB cannot inhibit B cell responses (114). These data support a mechanism of inhibition depending on complex formation of the vaccine with IgG antibodies (114). This complex cross-links the BCR (which recognizes the vaccine antigen) to the FcγIIB receptor (which binds the Fc region of the IgG antibody) on the surface of B cells. The cross-link results in a negative signal that inhibits both the proliferation of B cells (137) and the secretion of antibodies (114) (Figure 1A). In evolutionary terms, this mechanism developed to avoid an over-reactive B cell response in order to conserve resources. If IgG antibodies are already present in an organism after infection or vaccination, it is not necessary to produce more antibodies. In essence, maternal antibodies signal that there is no need to produce more antibodies. In contrast to antibody production after an active immune response, however, the passively transferred maternal antibodies decline and the infant is left without an adequate B cell and antibody response.
Figure 1.
Model of B cell activation in the presence of maternal IgG. B cells are being stimulated through three signals, the first one is recognition of antigen by the B cell receptor (BCR), the second the interaction with T cells through CD40/CD40 ligand, and the third cytokines like type I interferon or IL-6. During vaccination in the presence of maternal antibodies T cell responses are generated and therefore the second signal is provided. (A) In the presence of maternal antibodies (IgG), the first signal is downregulated by a cross-link between BCR and FcγRIIB. If MV-specific IgG binds to MV, the constant region is bound by the receptor for the constant region (Fc) of IgG (which is FcγRIIB). FcγRIIB is the only Fc-receptor on B cells and does not bind other immunoglobulins like IgM or IgA. After juxtaposition of the BCR and FcγRIIB, the tyrosine-based inhibitory motif of FcγRIIB is in close proximity to the tyrosine-based activation motif of BCR and delivers a negative signal. (B) If MV-specific IgM binds to MV, it also binds via C3d to CD21 (complement receptor 2), which is part of the positively signaling CD21/CD19/CD83/Leu-13 complex. The opsonin C3d does not bind to IgG. (C) Interferon α (type I interferon) binds to both the interferon receptor and CD21, and the dual receptor usage leads to a strong positive signal. It stimulates antibody secretion by B cells in the presence of maternal antibodies. (D) A possible approach to vaccination in the presence of maternal antibodies is the reduction of the vaccine antigen into small units, which do form antigen–antibody complexes unable to cross-link BCR and FcγRIIB. An example of this approach is experimental vaccination against respiratory syncytial virus in the presence of maternal antibodies (138).
IgM stimulation of inhibited B cells
Further evidence in support of the regulatory model of B cell inhibition is provided by the role of IgM in the stimulation of B cells in vitro and in vivo. The negative feedback regulation of B cells should function in children with similar IgG titers irrespective of whether they have been actively immunized or have received maternal antibodies. However, immunized children will generate additional antibodies after re-immunization whereas children with maternal antibodies will not or at very low titers. This phenomenon can (at least partially) be explained by the presence of IgM that is being generated after active immunization. IgM forms a complex with the vaccine and a complement protein (C3d). This complex cross-links the BCR with the CR2 on the surface of B cells (Figure 1B). The cross-link results in activation of B cells in vitro and can partially overcome the inhibition by the cross-link of the BCR and CD32 in vivo (114). In consequence, some IgG antibody is produced. The important component for the stimulatory effect of CR2 is the CD21 chain that binds to C3d. This finding is consistent with the model of B cell regulation but not with epitope masking.
Type I interferon induction stimulates B cells in the presence of maternal antibodies
Another way to stimulate B cells experimentally in the presence of maternal antibody is the induction of type I interferon. It has been demonstrated that CD21 binds C3d and interferon alpha with the same affinity (139) indicating that interferon alpha might have a functional role in B cell stimulation. Indeed, interferon alpha stimulation leads to the up-regulation of a number of B cell genes in a human B cell line (140). In vivo, B cells use both the interferon receptor and CD21 (which is a chain of the CR2) as a functional interferon receptor to stimulate antibody secretion (137) (Figure 1C). One way of inducing high levels of type I interferon is the combined use of TLR-3 and TLR-9 agonists as adjuvants for immunization. Because of the dual receptor usage, the induction of type I interferon in vivo strongly stimulates B cell responses and restores antibody levels after immunization in the presence of maternal antibodies (137). This finding is consistent with the model of FcγRIIB-mediated inhibition of B cell regulation but not with epitope masking.
In neonates, immunization is not only impaired by the inhibitory action of maternal antibodies, but also by the overall immaturity of the immune system. The induction of type I interferon stimulates immature B cells in neonatal cotton rats, even in the presence of maternal antibodies (141).
Engagement of FcγRIIB is important for inhibition of B cells
Using the cotton rat model of measles virus vaccination (142), we have shown that maternal antibodies inhibit B cells through complex formation with the vaccine and cross-linking of the BCRs and FcγRIIB (114). A mixture of antibodies recognizing three or more epitopes was more inhibitory than one antibody at the same antibody concentration. These data support the current thinking that oligomerization of FcγRIIBs is required for an inhibitory signal interfering with B cell proliferation and antibody secretion (143). However, for effective oligomerization not only the number of different antibodies but also the size of an antigen seems to be important. Passively transferred antibodies specific for di-nitro-phenyl (DNP) groups inhibit immunization with DNP bound to SRBC (4–5 μm) but not immunization with DNP bound to KLH (two subunits of 30 and 33 nm) (144, 145). Similarly, measles virus, which is inhibited by maternal antibodies, is a large antigen [250–450 nm (146)] with multiple epitopes on its surface [at least 13 for hemagglutinin (MV-H) and 6 for fusion protein (MV-F)] (147). These data indicate that an antigen requires a certain size to effectively cross-link (as antibody–antigen-complex) BCRs and FcγRIIBs (Figure 1D). Consistent with this concept is a report that immunization with a small polypeptide containing a neutralizing B cell epitope escaped inhibition by maternal antibodies (138).
Evaluation and Development of Experimental Vaccines
A number of studies claim vaccine efficacy after immunization in the presence of maternal antibodies for both approved vaccines and vaccine candidates. For experimental vaccines successes as well as failures have been reported for a variety of vector systems expressing the vaccine antigen simultaneously with or without different cytokine (combinations). The results from these studies are contradictory and it helps to clearly define the experimental conditions and study design. A vaccine can most easily be proven to be successful if it is used in the presence of low titers of maternal antibodies at the time of immunization, if T cell responses are measured and if surrogate markers like histological changes (in animal models) are used for protective efficacy. To prove convincingly vaccine efficacy in the presence of maternal antibodies, levels of maternal antibodies at the time of vaccination have to be high, neutralizing antibodies should be measured as an immunological parameter, and protection should be measured as clinical efficacy by the absence of clinical symptoms or a significant reduction in viral/bacterial titers. By these standards, very few examples of successful immunization in the presence of maternal antibodies exist, thus necessitating further research into this area. When considering the development and testing of vaccines for immunization in the presence of maternal antibodies, three options seem to emerge as being potentially successful: inoculation at a site with low IgG, continuous expression of antigen, and inoculation with adjuvants stimulating type I interferon secretion. Inhibitory maternal antibodies are of the IgG isotype, and IgG is present in respiratory and ocular fluids and saliva (148, 149) although at lower concentrations than in the blood. Occasionally, vector systems have been used successfully in animal model in the presence of maternal antibodies when alternate routes of immunization (other than subcutaneous or intramuscular) were used (134, 150–152). Experimentally, this approach could be combined with measures of IgG titers at the site of inoculation (e.g., mouth, eye, and nose) to correlate vaccination success with lower antibody titers. The concept of continuous expression of antigen is based on the finding that repeated immunization leads to an antibody response in the presence of maternal antibodies. If a vector system can be found that continuously expresses antigen, B cell responses should be stimulated when antibody titers fall to very low levels. An example of this concept is the expression of infectious bursitis virus (IBV) proteins through an attenuated Marek’s diseases virus (MDV) vector that persists in the chicken and leads to good immunity (153) in the presence of maternal antibodies. The induction of type I interferon is based on the ability of type I interferon to drive B cell responses in tissue culture and in experimental systems in the presence of maternal antibodies. An example of this concept is the immunization of pigs with adjuvants inducing type I interferon (154).
Induction of Maternal Antibodies Through Maternal Immunization as Indirect Immunization of Infants
Because of their relative immunological immaturity, infants are usually not immunized before the age of 2–3 months (depending on country-specific immunization schedules) with the most common exception of neonatal BCG immunization in some countries. In order to provide protection earlier in life, two other vaccination strategies have been discussed and/or used: cocooning and maternal immunization. The method of cocooning relies on immunization of all people in contact with the infant to minimize the risk of pathogen transmission and infection. Although this method can be effective it is sometimes difficult to implement. Another concept that has received more attention recently is maternal immunization. Maternal antibodies have been proposed as a means of protecting the infant during a sensitive time in the development of their immune system. If protective high levels of maternal antibodies can be achieved in infants, they would be protected during the most immature phase of their immune system. In addition, the young organism becomes better adapted to cope with infectious diseases by improved non-immune host responses, e.g., faster regeneration of epithelial cells after gastro-intestinal infection. Maternal immunization has the added advantage that the mother’s immune system is fully mature and will respond well to vaccination, thus providing protection to the mother and high levels of maternal antibodies to the infant. Based on the above named studies about protective levels of maternal antibodies and their kinetics of decline, it should theoretically be possible to induce high levels of antibodies, which are protective for up to 6 months.
Successful examples of maternal immunization
Currently, this concept is supported by data for vaccinations against tetanus, influenza virus, and pertussis. In 1961, a seminal study demonstrated the efficacy of maternal immunization with tetanus toxoid in the prevention of neonatal tetanus and reduction of neonatal mortality (155). Subsequently, the WHO Maternal–Neonatal Tetanus (MNT) elimination program has resulted in the reduction of neonatal death due to tetanus from about 787,000 death in the late 1980s to 59,000 death in 2008 (156). The final goal of the WHO campaign is to eliminate MNT, with elimination being defined at <1 case per 1000 live births.
Another case of successful maternal immunization is influenza vaccination. Influenza virus infection is a serious problem for pregnant women and immunization before, during, and after pregnancy substantially reduces serious clinical outcomes of infection. In addition, studies have demonstrated a protective effect on the child. Immunization of mothers leads to the increased transmission of maternal antibodies (157, 158), a 41–63% reduction in laboratory confirmed cases of influenza virus infection over a period of 6 months (159, 160), and a 39–91% reduction in hospitalizations of newborns (11, 159).
In addition, immunization against pertussis with the acellular pertussis antigen vaccine has proven to increase the level of maternal antibodies (161–163) and protect infants from clinical pertussis (161). A meta-analysis of vaccination studies found that cocooning (immunization of all direct contacts of an infant) and maternal immunization significantly reduced clinical disease and also were found to be cost–effective from a payer’s perspective (164). It should be noted that in these three examples inactivated vaccines were used. In principle, maternal immunization can be applied to other (live-attenuated) vaccines and infectious diseases as well. However, it has been reported that maternal immunization with a pneumococcal (polysaccharide) vaccine does not protect infants against clinical disease (165).
Principal questions surrounding maternal immunization
In order to study the effect of maternal immunization systematically, a number of questions have to be considered. For maternal immunization to be effective, antibodies have to be the immunological correlate of protection for the specific infectious disease and a specified level of protective titers has to be known as a target parameter to have an appreciable effect on clinical outcome. The main task is to determine the best time point and immunization schedule to immunize the mother. There is little information in the literature to guide immunization of mothers in order to obtain the best transfer of maternal antibodies. Current guidelines target immunizations of women with the goal to protect them and the fetus during pregnancy, and are usually divided in immunizations before and during pregnancy. In practical terms, mothers are often being immunized only when they are pregnant and usually immunization with inactivated rather than live-attenuated vaccines is recommended. A number of studies has found that maternal antibody titers in the child are higher than in the serum of the mother when these are low (83, 157) and lower than in the serum of the mother if the mother had high levels of antibodies. The cut-off seems to be a total IgG concentration of 15 g/L (166). This phenomenon can be explained by the transport of IgG via the FcRn receptor across the placental barrier. If the FcRn receptor molecules are saturated, IgG will be degraded by lysosomal enzymes inside the vesicles (167). However, transfer of antibodies does not only depend on the total IgG concentration in the mother’s blood but also on the isotype composition. The binding affinity of FcRn is highest for IgG1, followed by IgG4, IgG3 and is weakest for IgG2 (110). In consequence, more IgG1 is transported transplacentally than e.g., IgG2. This preferential transport mechanism explains why antibodies against specific vaccines are transported more efficiently than others. Usually, antibodies against T cell-dependent antigens (proteins) are of the IgG1 isotype and are more efficiently transported than antibodies against T cell-independent antigens (polysaccharides) of the IgG2 isotype. Based on these considerations, effective vaccines could be used to immunize expecting mothers in order to protect the neonates and infants. However, it is possible that for every vaccine a threshold of maternal antibody titers can be defined that cannot be changed even with very effective vaccines. This hypothesis has to be addressed experimentally in larger clinical studies.
Another factor influencing maternal antibody titers in children is gestational age. IgG transfer from mother to child starts at 13 weeks of age. However, in the third trimester the expression of FcRn receptors increases and subsequently the transfer rate of maternal antibodies improves with the highest amount of IgG (>50%) transferred during the last 4 weeks before birth (168, 169). If relatively ineffective vaccines are available that do not induce long-lasting antibody responses, immunization during the third trimester of pregnancy might be an option. It has been demonstrated that a not very effective vaccine like pertussis might be beneficially applied during this time (170). An additional consideration for maternal immunization is the nutritional status of both mother and child. In general terms, nutrition influences immune responses but has not been shown to influence the level of antibody in the mother. However, it has been shown that undernourished children have lower maternal antibody titers, although the underlying reason is currently unknown (171). Other factors like maternal age, maternal weight, parity, and type of delivery do not influence transplacental antibody transfer (172).
In contrast to humans, immunization of agricultural animals is relatively straightforward as immunization schedules can be easily applied to breeder animals at any time. Overall, the prevailing view is that the time of immunization is not relevant as long as maternal antibody titers are high at birth because transfer occurs during a short window after birth and seems to be solely dependent on antibody titers in colostrum.
Example: How can maternal immunization work against respiratory syncytial virus infection?
In order to answer the question of whether maternal immunization against a specific disease will lead to a reproducible and protective increase in antibody titers in children, one has to consider the total amount of IgG as well as the quality of antibody being induced by immunization. To illustrate these questions, I have chosen the example of immunization against RSV infection. RSV infection leads to severe respiratory infections in adults and is the second most common viral cause of death in the elderly (after influenza virus) (173). RSV infection is also the leading viral infection in lower respiratory disease in children, which is a particular problem in preterm babies and in neonates in their first months of life (174). So far, no vaccine or therapeutic is available. The discussion currently centers on the question of whether a vaccine could be developed that is safe and effective in neonates. Because the result of immunization of neonates is still elusive, an alternate suggestion is to use a to-be-developed vaccine to immunize mothers and provide protection for neonates and infants through maternal antibodies during the first 6 months of life. However, it is controversial as to whether maternal antibodies have a beneficial effect against RSV infection. RSV-specific antibodies are present in children during the first 6 months of life and have an estimated half-life of 1.5 months. Some studies have observed a protective effect of maternal antibodies (10, 175) whereas some did not [for review see Ref. (176)]. Currently, preterm infants (depending on their gestational age) are prophylactically treated with a monoclonal antibody against RSV. This prophylaxis is effective because the antibody has a high affinity (177, 178). In contrast to this antibody, naturally generated antibodies often have lower affinities resulting in lower neutralizing efficacy. In consequence, it will be important to induce high affinity antibodies in mothers through vaccination. Neutralizing antibodies against RSV bind to the fusion protein (F) and the glycoprotein (G). However, it has been demonstrated that vaccination with a RSV lacking the G protein is able to protect against viral challenge (179) and most attention in terms of vaccine development has been focused on the F protein. On the surface of the virion the fusion protein is folded so that the fusion peptide necessary to mediate fusion is protected from the environment (pre-fusion F). Upon triggering, the fusion protein unfolds and initiates fusion with the cellular membrane (post-fusion F). Palivizumab, the antibody that prophylactically protects children against RSV infection binds to both pre- and post-fusion F. Recent publications have defined the structure of the pre- and post-fusion F protein and have demonstrated that the most effective in vitro neutralizing antibodies bind to the pre-fusion F thus leading to the assumption that the goal of vaccination has to be the increase of antibody against pre-fusion F (180–182). Based on this knowledge clinical studies should be devised that not only take the total amount of antibody into account but also measure the affinity of the antibody as well as its target (G protein and pre- as well as post-fusion F protein). Antibodies are being transferred transplacentally based on their ability to bind FcRn and not on their affinity to RSV, and this transfer seems to be saturable depending on antibody level in the maternal blood. If a relatively small proportion of antibody is of high affinity it might not be possible to reach high protective levels of maternal antibodies. Another concern is immunization in the presence of maternal antibodies. As with other infectious diseases, it has been shown that even low levels of maternal antibody inhibit RSV-specific B cell and antibody responses (183, 184), a problem that will still exist after increased transfer of maternal antibodies due to maternal immunization.
Summary and Outlook
Many examples exist that demonstrate the inhibition of both human and veterinary vaccines by maternal antibodies. B cell inhibition is mediated through a cross-link between the BCR with the FcγRIIB by a vaccine–antibody complex. Knowledge of the underlying mechanisms helps us to understand the possibilities and limitations of our current clinical approaches and will help in the development and testing of new vaccines. This knowledge also will help us to evaluate and develop the concept of maternal immunization as a mechanism to protect infants against common infectious diseases. The potential benefit of maternal immunization is protection of the mother and a delayed susceptibility to infection of the child. However, by most estimates children will not be protected for more than 6 months of life whereas full immunological maturity seems to be accomplished only after 12 months. A final unanswered question about maternal immunization, however, is how to deal with immunization in the presence of maternal antibodies. In consequence, the question of vaccinating in the presence of maternal antibodies will remain and still has to be resolved.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol (2009) 9:185–94 10.1038/nri2508 [DOI] [PubMed] [Google Scholar]
- 2.Butler JE, Lager KM, Splichal I, Francis D, Kacskovics I, Sinkora M, et al. The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol (2009) 128:147–70 10.1016/j.vetimm.2008.10.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chattha KS, Firth MA, Hodgins DC, Shewen PE. Expression of complement receptor 2 (CD21), membrane IgM and the inhibitory receptor CD32 (FcgammaRIIb) in the lymphoid tissues of neonatal calves. Vet Immunol Immunopathol (2010) 137:99–108 10.1016/j.vetimm.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 4.Chattha KS, Firth MA, Hodgins DC, Shewen PE. Variation in expression of membrane IgM, CD21 (CR2) and CD32 (Fcgamma RIIB) on bovine lymphocytes with age: a longitudinal study. Dev Comp Immunol (2010) 34:510–7 10.1016/j.dci.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. J Comp Pathol (2007) 137:S27–31 10.1016/j.jcpa.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 6.Heiman HS, Weisman LE. Transplacental or enteral transfer of maternal immunization-induced antibody protects suckling rats from type III group B streptococcal infection. Pediatr Res (1989) 26:629–32 10.1203/00006450-198912000-00023 [DOI] [PubMed] [Google Scholar]
- 7.Svet-Moldavsky GJ, Svet-Moldasky IA. Sarcomas in cotton rats inoculated with Rous virus. Science (1964) 143:54–5 10.1126/science.143.3601.54 [DOI] [PubMed] [Google Scholar]
- 8.Zepeda M, Wilson JM. Neonatal cotton rats do not exhibit destructive immune responses to adenoviral vectors. Gene Ther (1996) 3:973–9 [PubMed] [Google Scholar]
- 9.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) (2006) 85:193–202 10.1097/01.md.0000229482.27398.ad [DOI] [PubMed] [Google Scholar]
- 10.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One (2009) 4:e8088. 10.1371/journal.pone.0008088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis (2010) 51:1355–61 10.1086/657309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakowka S, Long D, Koestner A. Influence of transplacentally acquired antibody on neonatal susceptibility to canine distemper virus in gnotobiotic dogs. J Infect Dis (1978) 137:605–8 10.1093/infdis/137.5.605 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Dong X, Sun X, Li W, Zhao P, Cui Z, et al. Preparation and immunoprotection of subgroup B avian leukosis virus inactivated vaccine. Vaccine (2013) 31:5479–85 10.1016/j.vaccine.2013.08.072 [DOI] [PubMed] [Google Scholar]
- 14.Leuridan E, Hens N, Hutse V, Aerts M, Van Damme P. Kinetics of maternal antibodies against rubella and varicella in infants. Vaccine (2011) 29:2222–6 10.1016/j.vaccine.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 15.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine (2007) 25:6296–304 10.1016/j.vaccine.2007.06.020 [DOI] [PubMed] [Google Scholar]
- 16.Kilic A, Altinkaynak S, Ertekin V, Inandi T. The duration of maternal measles antibodies in children. J Trop Pediatr (2003) 49:302–5 10.1093/tropej/49.5.302 [DOI] [PubMed] [Google Scholar]
- 17.Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, Polprasert N, et al. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg (2003) 69:123–8 [PubMed] [Google Scholar]
- 18.Awa DN, Ngagnou A, Tefiang E, Yaya D, Njoya A. Post vaccination and colostral peste des petits ruminants antibody dynamics in research flocks of Kirdi goats and Foulbe sheep of north Cameroon. Prev Vet Med (2002) 55:265–71 10.1016/S0167-5877(02)00013-2 [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui T, Yamamoto T, Hayama Y, Akiba Y, Nishiguchi A, Kobayashi S, et al. Duration of maternally derived antibodies against Akabane virus in calves: survival analysis. J Vet Med Sci (2009) 71:913–8 10.1292/jvms.71.913 [DOI] [PubMed] [Google Scholar]
- 20.Winters WD, Clouse WJ, Harris SC. Concomitant transfer of BCG-CW and canine virus antibodies from dams to pups. Dev Comp Immunol (1981) 5:321–8 10.1016/0145-305X(81)90039-2 [DOI] [PubMed] [Google Scholar]
- 21.Gharaibeh S, Mahmoud K. Decay of maternal antibodies in broiler chickens. Poult Sci (2013) 92:2333–6 10.3382/ps.2013-03249 [DOI] [PubMed] [Google Scholar]
- 22.Epstein JH, Baker ML, Zambrana-Torrelio C, Middleton D, Barr JA, Dubovi E, et al. Duration of maternal antibodies against canine distemper virus and hendra virus in pteropid bats. PLoS One (2013) 8:e67584. 10.1371/journal.pone.0067584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohayati AR, Hassan L, Sharifah SH, Lazarus K, Zaini CM, Epstein JH, et al. Evidence for Nipah virus recrudescence and serological patterns of captive Pteropus vampyrus. Epidemiol Infect (2011) 139:1570–9 10.1017/S0950268811000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker KS, Suu-Ire R, Barr J, Hayman DTS, Broder CC, Horton DL, et al. Viral antibody dynamics in a chiropteran host. J Anim Ecol (2014) 83:415–28 10.1111/1365-2656.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine (2014) 32:996–1002 10.1016/j.vaccine.2013.11.104 [DOI] [PubMed] [Google Scholar]
- 26.Francis JP, Richmond PC, Pomat WS, Michael A, Keno H, Phuanukoonnon S, et al. Maternal antibodies to pneumolysin but not to pneumococcal surface protein a delay early pneumococcal carriage in high-risk Papua new Guinean infants. Clin Vaccine Immunol (2009) 16:1633–8 10.1128/CVI.00247-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurikka S, Kayhty H, Peltola H, Saarinen L, Eskola J, Makela PH. Neonatal immunization: response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. Pediatrics (1995) 95:815–22 [PubMed] [Google Scholar]
- 28.Englund JA, Anderson EL, Reed GF, Decker MD, Edwards KM, Pichichero ME, et al. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics (1995) 96:580–4 [PubMed] [Google Scholar]
- 29.Whittle H, Hanlon P, O’Neil K, Hanlon L, Marsh V, Jupp E. Trial of high-dose Edmonston-Zagreb measles vaccine in Gambia: antibody response and side-effects. Lancet (1988) 2:811–4 10.1016/S0140-6736(88)92781-X [DOI] [PubMed] [Google Scholar]
- 30.Garly ML, Bale C, Martins CL, Monteiro M, George E, Kidd M, et al. Measles antibody responses after early two dose trials in Guinea-Bissau with Edmonston-Zagreb and Schwarz standard-titre measles vaccine: better antibody increase from booster dose of the Edmonston-Zagreb vaccine. Vaccine (2001) 19:1951–9 10.1016/S0264-410X(00)00431-X [DOI] [PubMed] [Google Scholar]
- 31.Borras E, Urbiztondo L, Costa J, Batalla J, Torner N, Plasencia A, et al. Measles antibodies and response to vaccination in children aged less than 14 months: implications for age of vaccination. Epidemiol Infect (2012) 140:1599–606 10.1017/S0950268811002184 [DOI] [PubMed] [Google Scholar]
- 32.Gans H, Dehovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine (2003) 21:3398–405 10.1016/S0264-410X(03)00341-4 [DOI] [PubMed] [Google Scholar]
- 33.Letson GW, Shapiro CN, Kuehn D, Gardea C, Welty TK, Krause DS, et al. Effect of maternal antibody on immunogenicity of hepatitis A vaccine in infants. J Pediatr (2004) 144:327–32 10.1016/j.jpeds.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Wu Q, Xu B, Zhou Z, Wang Z, Zhou YH. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine (2008) 26:6064–7 10.1016/j.vaccine.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 35.Appaiahgari MB, Glass R, Singh S, Taneja S, Rongsen-Chandola T, Bhandari N, et al. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine (2014) 32:651–6 10.1016/j.vaccine.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 36.Sormunen H, Stenvik M, Eskola J, Hovi T. Age- and dose-interval-dependent antibody responses to inactivated poliovirus vaccine. J Med Virol (2001) 63:305–10 [DOI] [PubMed] [Google Scholar]
- 37.Simoes EA, Padmini B, Steinhoff MC, Jadhav M, John TJ. Antibody response of infants to two doses of inactivated poliovirus vaccine of enhanced potency. Am J Dis Child (1985) 139:977–80 [DOI] [PubMed] [Google Scholar]
- 38.Warren RJ, Lepow ML, Bartsch GE, Robbins FC. The relationship of maternal antibody. Pediatrics (1964) 34:4–13 [PubMed] [Google Scholar]
- 39.Piedra PA, Glezen WP, Mbawuike I, Gruber WC, Baxter BD, Boland FJ, et al. Studies on reactogenicity and immunogenicity of attenuated bivalent cold recombinant influenza type A (CRA) and inactivated trivalent influenza virus (TI) vaccines in infants and young children. Vaccine (1993) 11:718–24 10.1016/0264-410X(93)90255-V [DOI] [PubMed] [Google Scholar]
- 40.Pollock RV, Carmichael LE. Maternally derived immunity to canine parvovirus infection: transfer, decline, and interference with vaccination. J Am Vet Med Assoc (1982) 180:37–42 [PubMed] [Google Scholar]
- 41.Waner T, Naveh A, Wudovsky I, Carmichael LE. Assessment of maternal antibody decay and response to canine parvovirus vaccination using a clinic-based enzyme-linked immunosorbent assay. J Vet Diagn Invest (1996) 8:427–32 10.1177/104063879600800404 [DOI] [PubMed] [Google Scholar]
- 42.Iida H, Fukuda S, Kawashima N, Yamazaki T, Aoki J, Tokita K, et al. [Effect of maternally derived antibody levels on antibody responses to canine parvovirus, canine distemper virus and infectious canine hepatitis virus after vaccinations in beagle puppies] article in Japanese. Jikken Dobutsu (1990) 39:9–19 [DOI] [PubMed] [Google Scholar]
- 43.Chappuis G. Control of canine distemper. Vet Microbiol (1995) 44:351–8 10.1016/0378-1135(95)00028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Digangi BA, Levy JK, Griffin B, Reese MJ, Dingman PA, Tucker SJ, et al. Effects of maternally-derived antibodies on serologic responses to vaccination in kittens. J Feline Med Surg (2012) 14:118–23 10.1177/1098612X11432239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakel V, Cussler K, Hanschmann KM, Truyen U, Konig M, Kamphuis E, et al. Vaccination against Feline Panleukopenia: implications from a field study in kittens. BMC Vet Res (2012) 8:62. 10.1186/1746-6148-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downey ED, Tait RG, Jr, Mayes MS, Park CA, Ridpath JF, Garrick DJ, et al. An evaluation of circulating bovine viral diarrhea virus type 2 maternal antibody level and response to vaccination in Angus calves. J Anim Sci (2013) 91:4440–50 10.2527/jas.2012-5890 [DOI] [PubMed] [Google Scholar]
- 47.Endsley JJ, Roth JA, Ridpath J, Neill J. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals (2003) 31:123–5 10.1016/S1045-1056(03)00027-7 [DOI] [PubMed] [Google Scholar]
- 48.Patil PK, Sajjanar CM, Natarajan C, Bayry J. Neutralizing antibody responses to foot-and-mouth disease quadrivalent (type O, A, C and Asia 1) vaccines in growing calves with pre-existing maternal antibodies. Vet Microbiol (2014) 169(3–4):233–5 10.1016/j.vetmic.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 49.Ellis JA, Gow SP, Goji N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J Am Vet Med Assoc (2010) 236:991–9 10.2460/javma.236.9.991 [DOI] [PubMed] [Google Scholar]
- 50.Ellis JA, Gow SP, Mahan S, Leyh R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J Am Vet Med Assoc (2013) 243:1602–8 10.2460/javma.243.11.1602 [DOI] [PubMed] [Google Scholar]
- 51.van der Sluijs MT, Kuhn EM, Makoschey B. A single vaccination with an inactivated bovine respiratory syncytial virus vaccine primes the cellular immune response in calves with maternal antibody. BMC Vet Res (2010) 11(6):2. 10.1186/1746-6148-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pomorska-Mol M, Markowska-Daniel I, Pejsak Z. Effect of age and maternally-derived antibody status on humoral and cellular immune responses to vaccination of pigs against Erysipelothrix rhusiopathiae. Vet J (2012) 194:128–30 10.1016/j.tvjl.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 53.Pomorska-Mol M, Markowska-Daniel I, Pejsak Z. Evaluation of humoral and antigen-specific T-cell responses after vaccination of pigs against pseudorabies in the presence of maternal antibodies. Vet Microbiol (2010) 144:450–4 10.1016/j.vetmic.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 54.Klinkenberg D, Moormann RJ, De Smit AJ, Bouma A, De Jong MC. Influence of maternal antibodies on efficacy of a subunit vaccine: transmission of classical swine fever virus between pigs vaccinated at 2 weeks of age. Vaccine (2002) 20:3005–13 10.1016/S0042-207X(02)00283-X [DOI] [PubMed] [Google Scholar]
- 55.Suradhat S, Damrongwatanapokin S. The influence of maternal immunity on the efficacy of a classical swine fever vaccine against classical swine fever virus, genogroup 2.2, infection. Vet Microbiol (2003) 92:187–94 10.1016/S0378-1135(02)00357-7 [DOI] [PubMed] [Google Scholar]
- 56.Markowska-Daniel I, Pomorska-Mol M, Pejsak Z. The influence of age and maternal antibodies on the postvaccinal response against swine influenza viruses in pigs. Vet Immunol Immunopathol (2011) 142:81–6 10.1016/j.vetimm.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 57.Forrest HL, Garcia A, Danner A, Seiler JP, Friedman K, Webster RG, et al. Effect of passive immunization on immunogenicity and protective efficacy of vaccination against a Mexican low-pathogenic avian H5N2 influenza virus. Influenza Other Respir Viruses (2013) 7:1194–201 10.1111/irv.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fry TL, Vandalen KK, Shriner SA, Moore SM, Hanlon CA, Vercauteren KC. Humoral immune response to oral rabies vaccination in raccoon kits: problems and implications. Vaccine (2013) 31:2811–5 10.1016/j.vaccine.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 59.Pare JA, Barker IK, Crawshaw GJ, Mcewen SA, Carman PS, Johnson RP. Humoral response and protection from experimental challenge following vaccination of raccoon pups with a modified-live canine distemper virus vaccine. J Wildl Dis (1999) 35:430–9 10.7589/0090-3558-35.3.430 [DOI] [PubMed] [Google Scholar]
- 60.Maia OB, Gouveia AM. Serologic response of maned wolves (Chrysocyon brachyurus) to canine distemper virus and canine parvovirus vaccination. J Zoo Wildl Med (2001) 32:78–80 [DOI] [PubMed] [Google Scholar]
- 61.Welter J, Taylor J, Tartaglia J, Paoletti E, Stephensen CB. Vaccination against canine distemper virus infection in infant ferrets with and without maternal antibody protection, using recombinant attenuated poxvirus vaccines. J Virol (2000) 74:6358–67 10.1128/JVI.74.14.6358-6367.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz SL, John F. Enders and measles virus vaccine–a reminiscence. Curr Top Microbiol Immunol (2009) 329:3–11 [DOI] [PubMed] [Google Scholar]
- 63.Parks CL, Lerch RA, Walpita P, Wang HP, Sidhu MS, Udem SA. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J Virol (2001) 75:910–20 10.1128/JVI.75.2.910-920.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Low N, Kraemer S, Schneider M, Restrepo AM. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine (2008) 26:383–98 10.1016/j.vaccine.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 65.Griffin DE, Pan C-H. Measles: old vaccines and new vaccines. In: Griffin DE, Oldstone MBA, editors. Measles – Pathogenesis and Control. Heidelberg: Springer Verlag; (2009). p. 191–212 [Google Scholar]
- 66.Griffin DE, Ward BJ, Esolen LM. Pathogenesis of measles virus infection: an hypothesis for altered immune responses. J Infect Dis (1994) 170(Suppl 1):S24–31 10.1093/infdis/170.Supplement_1.S24 [DOI] [PubMed] [Google Scholar]
- 67.Permar SR, Klumpp SA, Mansfield KG, Kim WK, Gorgone DA, Lifton MA, et al. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol (2003) 77:4396–400 10.1128/JVI.77.7.4396-4400.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pueschel K, Tietz A, Carsillo M, Steward M, Niewiesk S. Measles virus-specific CD4 T-cell activity does not correlate with protection against lung infection or viral clearance. J Virol (2007) 81:8571–8 10.1128/JVI.00160-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finke D, Liebert UG. CD4+ T cells are essential in overcoming experimental murine measles encephalitis. Immunology (1994) 83:184–9 [PMC free article] [PubMed] [Google Scholar]
- 70.Weidinger G, Henning G, Ter Meulen V, Niewiesk S. Inhibition of major histocompatibility complex class II-dependent antigen presentation by neutralization of gamma interferon leads to breakdown of resistance against measles virus-induced encephalitis. J Virol (2001) 75:3059–65 10.1128/JVI.75.7.3059-3065.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNulty J, Nair JJ, Griffin C, Pandey S. synthesis and biological evaluation of fully functionalized seco-pancratistatin analogues. J Nat Prod (2008) 71:357–63 10.1021/np0705460 [DOI] [PubMed] [Google Scholar]
- 72.Aaby P, Martins CL, Garly ML, Andersen A, Fisker AB, Claesson MH, et al. Measles vaccination in the presence or absence of maternal measles antibody: impact on child survival. Clin Infect Dis (2014) 59(4):484–92 10.1093/cid/ciu354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samb B, Aaby P, Whittle HC, Seck AM, Rahman S, Bennett J, et al. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr Infect Dis J (1995) 14:203–9 10.1097/00006454-199503000-00007 [DOI] [PubMed] [Google Scholar]
- 74.Gans H, Yasukawa L, Rinki M, Dehovitz R, Forghani B, Beeler J, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis (2001) 184:817–26 10.1086/323346 [DOI] [PubMed] [Google Scholar]
- 75.Gans HA, Yasukawa LL, Alderson A, Rinki M, Dehovitz R, Beeler J, et al. Humoral and cell-mediated immune responses to an early 2-dose measles vaccination regimen in the United States. J Infect Dis (2004) 190:83–90 10.1086/421032 [DOI] [PubMed] [Google Scholar]
- 76.Njie-Jobe J, Nyamweya S, Miles DJ, Van Der Sande M, Zaman S, Touray E, et al. Immunological impact of an additional early measles vaccine in Gambian children: responses to a boost at 3 years. Vaccine (2012) 30:2543–50 10.1016/j.vaccine.2012.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertley FM, Ibrahim SA, Libman M, Ward BJ. Measles vaccination in the presence of maternal antibodies primes for a balanced humoral and cellular response to revaccination. Vaccine (2004) 23:444–9 10.1016/j.vaccine.2004.06.021 [DOI] [PubMed] [Google Scholar]
- 78.Aaby P, Jensen T, Hansen H. Trial of high dose Edmonston-Zagreb measles vaccine in Guinea-Bissau: protective efficacy. Lancet (1988) 2:809–11 10.1016/S0140-6736(88)92780-8 [DOI] [PubMed] [Google Scholar]
- 79.Garenne M, Leroy O, Beau J, Sene I. Child mortality after high titer measles vaccine: prospective study in Senegal. Lancet (1991) 338:903–7 10.1016/0140-6736(91)91771-L [DOI] [PubMed] [Google Scholar]
- 80.Halsey N. Increased mortality following high titer measles vaccines: too much good thing. Pediatr Infect Dis J (1993) 12:462–5 10.1097/00006454-199306000-00002 [DOI] [PubMed] [Google Scholar]
- 81.Seng R, Samb B, Simondon F, Cisse B, Soumare M, Jensen H, et al. Increased long term mortality associated with rash after early measles vaccination in rural Senegal. Pediatr Infect Dis J (1999) 18:48–52 10.1097/00006454-199901000-00012 [DOI] [PubMed] [Google Scholar]
- 82.Martins C, Bale C, Garly ML, Rodrigues A, Lisse IM, Andersen A, et al. Girls may have lower levels of maternal measles antibodies and higher risk of subclinical measles infection before the age of measles vaccination. Vaccine (2009) 27:5220–5 10.1016/j.vaccine.2009.06.076 [DOI] [PubMed] [Google Scholar]
- 83.Techasena W, Sriprasert P, Pattamadilok S, Wongwacharapipoon P. Measles antibody in mothers and infants 0-2 years and response to measles vaccine at the age of 9 and 18 months. J Med Assoc Thai (2007) 90:106–12 [PubMed] [Google Scholar]
- 84.Gans HA, Arvin AM, Galinus J, Logan L, Dehovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA (1998) 280:527–32 10.1001/jama.280.6.527 [DOI] [PubMed] [Google Scholar]
- 85.Johnson CE, Darbari A, Darbari DS, Nalin D, Whitwell J, Chui LW, et al. Measles vaccine immunogenicity and antibody persistence in 12 vs 15-month old infants. Vaccine (2000) 18:2411–5 10.1016/S0264-410X(99)00574-5 [DOI] [PubMed] [Google Scholar]
- 86.Martins CL, Garly ML, Bale C, Rodrigues A, Ravn H, Whittle HC, et al. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. Br Med J (2008) 337:a661. 10.1136/bmj.a661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gagneur A, Pinquier D, Aubert M, Balu L, Brissaud O, De Pontual L, et al. Kinetics of decline of maternal measles virus-neutralizing antibodies in sera of infants in France in 2006. Clin Vaccine Immunol (2008) 15:1845–50 10.1128/CVI.00229-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oyedele OO, Odemuyiwa SO, Ammerlaan W, Muller CP, Adu FD. Passive immunity to measles in the breastmilk and cord blood of some nigerian subjects. J Trop Pediatr (2005) 51:45–8 10.1093/tropej/fmh073 [DOI] [PubMed] [Google Scholar]
- 89.Szenborn L, Tischer A, Pejcz J, Rudkowski Z, Wojcik M. Passive acquired immunity against measles in infants born to naturally infected and vaccinated mothers. Med Sci Monit (2003) 9:CR541–6. [PubMed] [Google Scholar]
- 90.Pomorska-Mol M, Markowska-Daniel I. Interferon-gamma secretion and proliferative responses of peripheral blood mononuclear cells after vaccination of pigs against Aujeszky’s disease in the presence of maternal immunity. FEMS Immunol Med Microbiol (2010) 58:405–11 10.1111/j.1574-695X.2010.00651.x [DOI] [PubMed] [Google Scholar]
- 91.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol (1984) 99:159s–64s 10.1083/jcb.99.1.159s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature (1989) 337:184–7 10.1038/337184a0 [DOI] [PubMed] [Google Scholar]
- 93.Costa-Carvalho BT, Vieria HM, Dimantas RB, Arslanian C, Naspitz CK, Sole D, et al. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res (1996) 29:201–4 [PubMed] [Google Scholar]
- 94.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients (2011) 3(4):442–74 10.3390/nu3040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodewald R. Selective antibody transport in the proximal small intestine of the neonatal rat. J Cell Biol (1970) 45:635–40 10.1083/jcb.45.3.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest (1972) 51:2916–27 10.1172/JCI107116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol (1973) 58:189–211 10.1083/jcb.58.1.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol (1980) 85:18–32 10.1083/jcb.85.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Favaron PO, Carter AM, Ambrosio CE, Morini AC, Mess AM, De Oliveira MF, et al. Placentation in Sigmodontinae: a rodent taxon native to South America. Reprod Biol Endocrinol (2011) 9:55. 10.1186/1477-7827-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun (1983) 42:81–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winters WD. Time dependent decreases of maternal canine virus antibodies in newborn pups. Vet Rec (1981) 108:295–9 10.1136/vr.108.14.295 [DOI] [PubMed] [Google Scholar]
- 102.Omata Y, Oikawa H, Kanda M, Mikazuki K, Dilorenzo C, Claveria FG, et al. Transfer of antibodies to kittens from mother cats chronically infected with Toxoplasma gondii. Vet Parasitol (1994) 52:211–8 10.1016/0304-4017(94)90113-9 [DOI] [PubMed] [Google Scholar]
- 103.Casal ML, Jezyk PF, Giger U. Transfer of colostral antibodies from queens to their kittens. Am J Vet Res (1996) 57:1653–8 [PubMed] [Google Scholar]
- 104.Claus MA, Levy JK, Macdonald K, Tucker SJ, Crawford PC. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J Feline Med Surg (2006) 8:184–91 10.1016/j.jfms.2006.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourne FJ, Curtis J. The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology (1973) 24:157–62 [PMC free article] [PubMed] [Google Scholar]
- 106.Stirling CM, Charleston B, Takamatsu H, Claypool S, Lencer W, Blumberg RS, et al. Characterization of the porcine neonatal Fc receptor – potential use for trans-epithelial protein delivery. Immunology (2005) 114:542–53 10.1111/j.1365-2567.2004.02121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cervenak J, Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet Immunol Immunopathol (2009) 128:171–7 10.1016/j.vetimm.2008.10.300 [DOI] [PubMed] [Google Scholar]
- 108.Cutting JA, Roth TF. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim Biophys Acta (1973) 298:951–5 10.1016/0005-2736(73)90398-2 [DOI] [PubMed] [Google Scholar]
- 109.West AP, Jr, Herr AB, Bjorkman PJ. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity (2004) 20:601–10 10.1016/S1074-7613(04)00113-X [DOI] [PubMed] [Google Scholar]
- 110.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol (2012) 2012:985646. 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Staley TE, Bush LJ. Receptor mechanisms of the neonatal intestine and their relationship to immunoglobulin absorption and disease. J Dairy Sci (1985) 68:184–205 10.3168/jds.S0022-0302(85)80812-2 [DOI] [PubMed] [Google Scholar]
- 112.Lecce JG, Morgan DO. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J Nutr (1962) 78:263–8 [DOI] [PubMed] [Google Scholar]
- 113.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol (2001) 13:1551–9 10.1093/intimm/13.12.1551 [DOI] [PubMed] [Google Scholar]
- 114.Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood (2011) 117:6143–51 10.1182/blood-2010-11-320317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood (2009) 113:3716–25 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 116.Willcocks LC, Smith KG, Clatworthy MR. Low-affinity Fcgamma receptors, autoimmunity and infection. Expert Rev Mol Med (2009) 11:e24. 10.1017/S1462399409001161 [DOI] [PubMed] [Google Scholar]
- 117.Prince GA, Horswood RL, Camargo E, Suffin SC, Chanock RM. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect Immun (1982) 37:1074–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol (1988) 62:3907–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Groot AS, Moise L, Mcmurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood (2008) 112:3303–11 10.1182/blood-2008-02-138073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bruggemann M, Rajewsky K. Regulation of the antibody response against hapten-coupled erythrocytes by monoclonal antihapten antibodies of various isotypes. Cell Immunol (1982) 71:365–73 10.1016/0008-8749(82)90270-2 [DOI] [PubMed] [Google Scholar]
- 121.Heyman B, Wigzell H. Immunoregulation by monoclonal sheep erythrocyte-specific IgG antibodies: suppression is correlated to level of antigen binding and not to isotype. J Immunol (1984) 132:1136–43 [PubMed] [Google Scholar]
- 122.Karlsson MC, Wernersson S, Diaz , De Stahl T, Gustavsson S, Heyman B. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proc Natl Acad Sci U S A (1999) 96:2244–9 10.1073/pnas.96.5.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Getahun A, Heyman B. Studies on the mechanism by which antigen-specific IgG suppresses primary antibody responses: evidence for epitope masking and decreased localization of antigen in the spleen. Scand J Immunol (2009) 70:277–87 10.1111/j.1365-3083.2009.02298.x [DOI] [PubMed] [Google Scholar]
- 124.Karlsson MC, Getahun A, Heyman B. FcgammaRIIB in IgG-mediated suppression of antibody responses: different impact in vivo and in vitro. J Immunol (2001) 167:5558–64 10.4049/jimmunol.167.10.5558 [DOI] [PubMed] [Google Scholar]
- 125.Heyman B, Pilstrom L, Shulman MJ. Complement activation is required for IgM-mediated enhancement of the antibody response. J Exp Med (1988) 167:1999–2004 10.1084/jem.167.6.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heyman B, Nose M, Weigle WO. Carbohydrate chains on IgG2b: a requirement for efficient feedback immunosuppression. J Immunol (1985) 134:4018–23 [PubMed] [Google Scholar]
- 127.Sinclair NR, Lees RK, Elliott EV. Role of the Fc fragment in the regulation of the primary immune response. Nature (1968) 220:1048–9 10.1038/2201048a0 [DOI] [PubMed] [Google Scholar]
- 128.Sinclair NR. Regulation of the immune response. J Exp Med (1969) 129:1183–201 10.1084/jem.129.6.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lyubchenko T, Dal Porto J, Cambier JC, Holers VM. Coligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J Immunol (2005) 174:3264–72 10.4049/jimmunol.174.6.3264 [DOI] [PubMed] [Google Scholar]
- 130.Thornton BP, Vetvicka V, Ross GD. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin Exp Immunol (1996) 104:531–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol (2000) 18:393–422 10.1146/annurev.immunol.18.1.393 [DOI] [PubMed] [Google Scholar]
- 132.Applequist SE, Dahlstrom J, Jiang N, Molina H, Heyman B. Antibody production in mice deficient for complement receptors 1 and 2 can be induced by IgG/Ag and IgE/Ag, but not IgM/Ag complexes. J Immunol (2000) 165:2398–403 10.4049/jimmunol.165.5.2398 [DOI] [PubMed] [Google Scholar]
- 133.Anderson CF, Mosser DM. Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J Immunol (2002) 168:3691–701 10.4049/jimmunol.168.8.3697 [DOI] [PubMed] [Google Scholar]
- 134.Schlereth B, Buonocore L, Tietz A, Ter Meulen V, Rose JK, Niewiesk S. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J Gen Virol (2003) 84:2145–51 10.1099/vir.0.19050-0 [DOI] [PubMed] [Google Scholar]
- 135.Weidinger G, Ohlmann M, Schlereth B, Sutter G, Niewiesk S. Vaccination with recombinant modified vaccinia virus Ankara protects against measles virus infection in the mouse and cotton rat model. Vaccine (2001) 19:2764–8 10.1016/S0264-410X(00)00531-4 [DOI] [PubMed] [Google Scholar]
- 136.Schlereth B, Germann PG, ter Meulen V, Niewiesk S. DNA vaccination with the hemagglutinin and the fusion proteins, but not the nucleocapsid protein protects against experimental measles virus infection. J Gen Virol (2000) 81:1321–5 [DOI] [PubMed] [Google Scholar]
- 137.Kim D, Niewiesk S. Synergistic induction of interferon alpha through TLR-3 and TLR-9 agonists identifies CD21 as interferon alpha receptor for the B Cell response. PLoS Pathog (2013) 9:e1003233. 10.1371/journal.ppat.1003233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siegrist C-A, Plotnicky-Gilquin H, Cordova M, Berney M, Bonnefoy J-Y, Nguyen TN, et al. Protective efficacy against respiratory syncytial virus following murine neonatal immunization with BBG2Na vaccine: influence of adjuvants and maternal antibodies. J Infect Dis (1999) 179:1326–33 10.1086/314778 [DOI] [PubMed] [Google Scholar]
- 139.Delcayre AX, Salas F, Mathur S, Kovats K, Lotz M, Lernhardt W. Epstein Barr virus/complement C3d receptor is an interferon alpha receptor. EMBO J (1991) 10:919–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Asokan R, Hua J, Young KA, Gould HJ, Hannan JP, Kraus DM, et al. Characterization of human complement receptor type 2 (CR2/CD21) as a receptor for IFN-alpha: a potential role in systemic lupus erythematosus. J Immunol (2006) 177:383–94 10.4049/jimmunol.177.1.383 [DOI] [PubMed] [Google Scholar]
- 141.Kim D, Niewiesk S. Synergistic induction of interferon a through TLR-3 and TLR-9 agonists stimulates immune responses against measles virus in neonatal cotton rats. Vaccine (2014) 32(2):265–70 10.1016/j.vaccine.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niewiesk S. Current animal models: cotton rat. In: Griffin DE, Oldstone MBA, editors. Measles – Pathogenesis and Control. Heidelberg: Springer Verlag; (2009). p. 89–110 [Google Scholar]
- 143.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol (2008) 8:34–47 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 144.Enriquez-Rincon F, Klaus GG. Differing effects of monoclonal anti-hapten antibodies on humoral responses to soluble or particulate antigens. Immunology (1984) 52:129–36 [PMC free article] [PubMed] [Google Scholar]
- 145.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron (1999) 30:597–623 10.1016/S0968-4328(99)00036-0 [DOI] [PubMed] [Google Scholar]
- 146.Daikoku E, Morita C, Kohno T, Sano K. Analysis of morphology and infectivity of measles virus particles. Bull Osaka Med Coll (2007) 53:107–14 [Google Scholar]
- 147.Bouche FB, Ertl OT, Muller CP. Neutralizing B cell responses in measles. Viral Immunol (2002) 15:451–72 10.1089/088282402760312331 [DOI] [PubMed] [Google Scholar]
- 148.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun (1997) 65:1387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoshida M, Masuda A, Kuo TT, Kobayashi K, Claypool SM, Takagawa T, et al. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Semin Immunopathol (2006) 28:397–403 10.1007/s00281-006-0054-z [DOI] [PubMed] [Google Scholar]
- 150.Vincent AL, Ma W, Lager KM, Richt JA, Janke BH, Sandbulte MR, et al. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol (2012) 86:10597–605 10.1128/JVI.01439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sarfati-Mizrahi D, Lozano-Dubernard B, Soto-Priante E, Castro-Peralta F, Flores-Castro R, Loza-Rubio E, et al. Protective dose of a recombinant Newcastle disease LaSota-avian influenza virus H5 vaccine against H5N2 highly pathogenic avian influenza virus and velogenic viscerotropic Newcastle disease virus in broilers with high maternal antibody levels. Avian Dis (2010) 54:239–41 10.1637/8735-032509-Reg.1 [DOI] [PubMed] [Google Scholar]
- 152.Blasco E, Lambot M, Barrat J, Cliquet F, Brochier B, Renders C, et al. Kinetics of humoral immune response after rabies VR-G oral vaccination of captive fox cubs (Vulpes vulpes) with or without maternally derived antibodies against the vaccine. Vaccine (2001) 19:4805–15 10.1016/S0264-410X(01)00211-0 [DOI] [PubMed] [Google Scholar]
- 153.Zhou X, Wang D, Xiong J, Zhang P, Li Y, She R. Protection of chickens, with or without maternal antibodies, against IBDV infection by a recombinant IBDV-VP2 protein. Vaccine (2010) 28:3990–6 10.1016/j.vaccine.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 154.Polewicz M, Gracia A, Garlapati S, Van Kessel J, Strom S, Halperin SA, et al. Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies. Vaccine (2013) 31:3148–55 10.1016/j.vaccine.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 155.Schofield FD, Tucker VM, Westbrook GR. Neonatal tetanus in New Guinea. Br Med J (1961) 2:785–9 10.1136/bmj.2.5255.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.WHO. Maternal and Neonatal Tetanus (MNT) elimination [Online]. (2014). Available from: http://www.who.int/immunization/diseases/MNTE_initiative/en/
- 157.Wutzler P, Schmidt-Ott R, Hoyer H, Sauerbrei A. Prevalence of influenza A and B antibodies in pregnant women and their offspring. J Clin Virol (2009) 46:161–4 10.1016/j.jcv.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 158.Rohner GB, Meier S, Bel M, Combescure C, Othenin-Girard V, Swali RA, et al. Influenza vaccination given at least 2 weeks before delivery to pregnant women facilitates transmission of seroprotective influenza-specific antibodies to the newborn. Pediatr Infect Dis J (2013) 32:1374–80 10.1097/01.inf.0000437066.40840.c4 [DOI] [PubMed] [Google Scholar]
- 159.Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med (2011) 165:104–11 10.1001/archpediatrics.2010.192 [DOI] [PubMed] [Google Scholar]
- 160.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med (2008) 359:1555–64 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 161.Cohen P, Scadron SJ. The effects of active immunization of the mother upon the offspring. J Pediatr (1946) 29:609–19 10.1016/S0022-3476(46)80128-8 [DOI] [PubMed] [Google Scholar]
- 162.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol (2011) 204(334):e331–5 10.1016/j.ajog.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 163.Leuridan E, Hens N, Peeters N, De Witte L, Van Der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J (2011) 30:608–10 10.1097/INF.0b013e3182093814 [DOI] [PubMed] [Google Scholar]
- 164.Westra TA, De Vries R, Tamminga JJ, Sauboin CJ, Postma MJ. Cost-effectiveness analysis of various pertussis vaccination strategies primarily aimed at protecting infants in the Netherlands. Clin Ther (2010) 32:1479–95 10.1016/j.clinthera.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 165.Lopes CR, Berezin EN, Ching TH, Canuto Jde S, Costa VO, Klering EM. Ineffectiveness for infants of immunization of mothers with pneumococcal capsular polysaccharide vaccine during pregnancy. Braz J Infect Dis (2009) 13: 104–6 [PubMed] [Google Scholar]
- 166.Michaux JL, Heremans JF, Hitzig WH. Immunoglobulin levels in cord-blood serum of negroes and caucasians. Trop Geogr Med (1966) 18:10–4 [PubMed] [Google Scholar]
- 167.Saji F, Koyama M, Matsuzaki N. Current topic: human placental Fc receptors. Placenta (1994) 15:453–66 10.1016/S0143-4004(05)80415-1 [DOI] [PubMed] [Google Scholar]
- 168.Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod (1999) 4:81–9 10.1530/ror.0.0040081 [DOI] [PubMed] [Google Scholar]
- 169.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol (1996) 36:248–55 10.1111/j.1600-0897.1996.tb00172.x [DOI] [PubMed] [Google Scholar]
- 170.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis (2013) 56:539–44 10.1093/cid/cis923 [DOI] [PubMed] [Google Scholar]
- 171.Cavalcante RS, Kopelman BI, Costa-Carvalho BT. Placental transfer of Haemophilus influenzae type b antibodies in malnourished pregnant women. Braz J Infect Dis (2008) 12:47–51 10.1590/S1413-86702008000100011 [DOI] [PubMed] [Google Scholar]
- 172.Doroudchi M, Samsami Dehaghani A, Emad K, Ghaderi A. Placental transfer of rubella-specific IgG in fullterm and preterm newborns. Int J Gynaecol Obstet (2003) 81:157–62 10.1016/S0020-7292(02)00442-3 [DOI] [PubMed] [Google Scholar]
- 173.Elliot AJ, Fleming DM. Influenza and respiratory syncytial virus in the elderly. Expert Rev Vaccines (2008) 7:249–58 10.1586/14760584.7.2.249 [DOI] [PubMed] [Google Scholar]
- 174.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet (2010) 375:1545–55 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roca A, Abacassamo F, Loscertales MP, Quinto L, Gomez-Olive X, Fenwick F, et al. Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J Med Virol (2002) 67:616–23 10.1002/jmv.10148 [DOI] [PubMed] [Google Scholar]
- 176.Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother (2013) 9:1263–7 10.4161/hv.24096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis (1997) 176:1215–24 10.1086/514115 [DOI] [PubMed] [Google Scholar]
- 178.Wu H, Pfarr DS, Tang Y, An LL, Patel NK, Watkins JD, et al. Ultra-potent antibodies against respiratory syncytial virus: effects of binding kinetics and binding valence on viral neutralization. J Mol Biol (2005) 350:126–44 10.1016/j.jmb.2005.04.049 [DOI] [PubMed] [Google Scholar]
- 179.Widjojoatmodjo MN, Boes J, Van Bers M, Van Remmerden Y, Roholl PJ, Luytjes W. A highly attenuated recombinant human respiratory syncytial virus lacking the G protein induces long-lasting protection in cotton rats. Virol J (2010) 7:114. 10.1186/1743-422X-7-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science (2013) 342:592–8 10.1126/science.1243283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science (2013) 340:1113–7 10.1126/science.1234914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature (2013) 501:439–43 10.1038/nature12442 [DOI] [PubMed] [Google Scholar]
- 183.Murphy BR, Alling DW, Snyder MH, Walsh EE, Prince GA, Chanock RM, et al. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol (1986) 24:894–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shinoff JJ, O’brien KL, Thumar B, Shaw JB, Reid R, Hua W, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis (2008) 198:1007–15 10.1086/591460 [DOI] [PubMed] [Google Scholar]