Abstract
Human immunodeficiency virus type 1 (HIV-1) carries virus-encoded and host-derived proteins. Recent advances in the functional characterization of host molecules inserted into mature virus particles have revealed that HIV-1 biology is influenced by the acquisition of host cell membrane components. The CD28/B7 receptor/ligand system is considered one of the fundamental elements of the normal immune response. Two major cell types that harbor HIV-1 in vivo, i.e., monocytes/macrophages and CD4+ T cells, express the costimulatory molecules CD80 (B7.1) and CD86 (B7.2). We investigated whether CD80 and CD86 are efficiently acquired by HIV-1, and if so, whether these host-encoded molecules can contribute to the virus life cycle. Here we provide the first evidence that the insertion of CD80 and CD86 into HIV-1 increases virus infectivity by facilitating the attachment and entry process due to interactions with their two natural ligands, CD28 and CTLA-4. Moreover, we demonstrate that NF-κB is induced by CD80- and CD86-bearing virions when they are combined with the engagement of the T-cell receptor/CD3 complex, an event that is inhibited upon surface expression of CTLA-4. Finally, both CD80 and CD86 were found to be efficiently incorporated into R5- and X4-tropic field strains of HIV-1 expanded in cytokine-treated macrophages. Thus, besides direct interactions between the virus envelope glycoproteins and cell surface constituents, such as CD4 and some specific chemokine coreceptors, HIV-1 may attach to target cells via interactions between cell-derived molecules incorporated into virions and their natural ligands. These findings support the theory that HIV-1-associated host proteins alter virus-host dynamics.
It is now well established that enveloped viruses differ in their abilities to exclude host proteins from their envelope during the budding process (for a review, see reference 22). For example, host constituents are virtually excluded from alphavirus particles, while retroviruses and rhabdoviruses are less selective in the assembly of their envelope and allow the incorporation of a large number of host-cell-derived proteins. The nature of the factor(s) contributing to this process is still obscure, but it may involve the various complex interactions that take place between the viral envelope and the matrix proteins during the formation of the retroviral particle. During the last few years, several studies have aimed to identify cellular proteins that are incorporated into human immunodeficiency virus type 1 (HIV-1) particles and their biological effect(s) on the virus life cycle (reviewed in reference 52). It has been shown that some virion-anchored host molecules positively affect the HIV-1 attachment and entry processes through interactions with their cognate ligands on the target cell surface. For example, HLA-DR increases the HIV-1 infectivity for CD4-expressing cells approximately 2-fold, while acquisition of the adhesion molecule ICAM-1 enhances the virus infectivity for LFA-1-expressing cells up to 10-fold (10, 18). Other studies revealed that the surface expression of LFA-1 in an activated conformational state for ICAM-1 markedly enhances the susceptibility of such target cells to infection by ICAM-1-bearing HIV-1 virions (up to 100-fold) (17, 19). More recently, the presence of host-encoded CD28 in the HIV-1 envelope resulted in a close to 20-fold augmentation in virus infectivity when target cells that expressed high levels of CD80 (B7.1) and CD86 (B7.2), two natural ligands of CD28, were used (23).
Although the exact mechanism(s) governing the incorporation process of host proteins into budding virions is still unclear, the candidate molecule has to be located at the cell surface once the viral entity is extruded from the infected cell in order to be efficiently inserted into HIV-1. Considering that the surface expression levels of several membrane proteins are tightly regulated at different stages of cell activation and that HIV-1 replication is closely linked with the cellular activation state, studies analyzing the biological functions of virion-anchored host proteins should concentrate on surface molecules that are up-regulated upon cell activation or that are not down-regulated after HIV-1 infection.
CD80 and CD86 are the two natural ligands for CD28 and cytolytic-T-lymphocyte-associated antigen 4 (CTLA-4) (reviewed in reference 12). These two type I transmembrane glycoproteins are expressed on several cell types (e.g., B and T lymphocytes, cells of the monocytic lineage, and dendritic cells), allowing them to supply a second antigen-independent signal, called costimulation. The surface expression of both molecules is modulated during the normal immune response by cytokines (e.g., gamma interferon [IFN-γ] or granulocyte-macrophage colony-stimulating factor [GM-CSF]), by bacterial constituents (e.g., lipopolysaccharide), or after signaling events transduced through specific membrane proteins (e.g., the CD40/CD40L interaction, major histocompatibility complex class II molecules, B-cell receptor, etc.) (reviewed in reference 28). For example, the treatment of monocytes with IFN-γ induces CD80 expression while it upregulates CD86 expression on such cells (14). The coengagement of CD3 and CD28 results in the induction of both CD80 and CD86 on the surfaces of CD4+ T lymphocytes (4, 14, 48, 56). Considering that CD4+ T lymphocytes and monocytes/macrophages constitute major cellular reservoirs of HIV-1 in vivo, it is thus tempting to speculate that HIV-1 can incorporate CD80 and CD86. There is actually a paucity of data concerning the incorporation of these two costimulatory molecules into HIV-1 particles. In a qualitative analysis of host-derived antigens found at the surfaces of HIV-1 virions, host CD86 was found to be inserted in viruses produced from infected dendritic cells (DCs) and T-cell cultures as well as in progeny viruses harvested from DC and T-cell cocultures (20). It has also been demonstrated that laboratory and clinical isolates of HIV-1 preferentially incorporate CD86 over CD80 (16). A functional role for virus-embedded host CD86 was provided by a study showing that the binding of CD86-bearing HIV-1 virions, in combination with T-cell receptor (TCR)-mediated biochemical events, leads to nuclear translocation of two transcription factors, NF-κB and NFAT, in an established CD28-expressing human T-cell line (7). However, there is no information on the contribution of virus-anchored CD80 and CD86 to HIV-1 replication.
We report here that virus attachment, internalization, and production are all increased for HIV-1 particles bearing host CD80 or CD86 compared to those in isogenic viruses lacking these cell membrane constituents. We further demonstrate that the potentiating effect is due to interactions between virally embedded CD80 and CD86 and cell surface CD28 and CTLA-4. We confirm that CD86-bearing viruses have the capacity to form a synergy with the TCR/CD3 complex, leading to the activation of the nuclear transcriptional factor NF-κB, and extend this property to CD80-bearing HIV-1 particles. In addition, we demonstrate that the coexpression of CTLA-4 at the T-cell surface abolishes this HIV-1 costimulatory property. Finally, CD80 and CD86 were detected in several clinical strains of HIV-1 bearing distinct coreceptor profiles that were produced from M-CSF-treated macrophages. These data confirm the notion that the biology of HIV-1 is influenced not only by virus-encoded proteins but also by host cell constituents that are incorporated into mature virions.
MATERIALS AND METHODS
Cells.
The parental lymphoid T-cell Jurkat line (clone E6.1) was obtained from the American Type Culture Collection (Manassas, Va.). Jurkat cells, which lack expression of endogenous CTLA-4, were stably transfected with a vector, pBIG2i, encoding the CTLA-4 molecule (CD152). This plasmid contains a hybrid bidirectional tetracycline-responsive promoter element to direct the expression of both CTLA-4 and the rtTAN tetracycline-responsive transactivator. The expression of CTLA-4 is achieved by adding doxycycline (100 ng/ml) to the cell culture (6). The human lymphoblastoid T-cell line CEM-T4 is a natural isolated subclone of the CEM line that expresses a high surface level of CD4 (obtained through the AIDS Repository Reagent Program, Rockville, Md.). Flow cytometric analyses revealed that these cell lines are all positive for CD28 (data not shown). The RAJI-CD4 cell line has been described previously (9) and expresses neither CD28 nor CTLA-4 (data not shown). DT30 cells are murine mastocytoma P815 cells stably expressing human cell surface CD80 proteins (2). Such cells also express murine Fcγ receptors and are thus capable of binding and cross-linking soluble antibodies. DT30 cells were fixed briefly in 1% paraformaldehyde, washed extensively with phosphate-buffered saline (PBS), and then stored frozen at −85°C. Since such cells are fixed, they do not grow or secrete factors that could mediate signal transduction in the studied target cells. In our experiments, DT30 cells were used to stimulate Jurkat cells at a 1:1 ratio. Jurkat, CEM-T4, RAJI-CD4, and DT30 cells were maintained in a complete culture medium made of RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Invitrogen), glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 μg/ml). 293T cells are human embryonic kidney cells that express the simian virus 40 large T antigen. These cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% FBS, l-glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 μg/ml). Flow cytometric studies indicated that 293T cells are negative for CD80 and CD86 (data not shown). Primary human peripheral blood lymphocytes (PBLs) from healthy donors were isolated by Ficoll-Hypaque density gradient centrifugation. Cells were maintained at 106 cells/ml in RPMI 1640 supplemented with 20% FBS in the presence of phytohemagglutinin-L (1 μg/ml) and recombinant interleukin-2 (50 U/ml) for 2 days before HIV-1 infection.
Antibodies.
The anti-CD3 hybridoma OKT3 (specific for the ɛ chain of the CD3 complex) was obtained from the American Type Culture Collection. Antibodies from this hybridoma were purified by the use of mAbTrap protein G affinity columns according to the manufacturer's instructions (Pharmacia Biotechnology AB, Uppsala, Sweden). The monoclonal antibody 9.3 is specific for human CD28 and inhibits interactions between CD28 and CD80/CD86 (32, 33) (kindly provided by J. A. Ledbetter, Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, N.J.). The monoclonal antibody 10A8 is directed against human CTLA-4 (34) and was supplied by Robert Peach (Bristol-Myers Squibb Pharmaceutical Research Institute). The monoclonal antibody L307.4 is specific for CD80 and was purchased from Pharmingen (Mississauga, Ontario, Canada), while BU-63 is known to be specific for CD86 and was supplied by D. L. Hardie (The University of Birmingham, Birmingham, United Kingdom) (15).
Flow cytometry.
The expression of CD80 and CD86 on the surfaces of transiently transfected 293T cells or monocyte-derived macrophages (MDM) was monitored by using L307.4 and BU-63, respectively. Briefly, an aliquot of transfected cells (106) was washed with PBS at pH 7.4. Pelleted cells were incubated for 30 min on ice with a saturating concentration of the primary antibody (L307.4 or BU-63; 1 μg/106 cells) in a final volume of 100 μl of PBS. The cells were washed twice with 500 μl of PBS and incubated for 30 min with a saturating concentration of an R-phycoerythrin-conjugated goat anti-mouse antibody (1 μg/106 cells) in a final volume of 100 μl of PBS. Finally, the cells were washed twice with PBS and resuspended in 300 μl of PBS containing 1% (wt/vol) paraformaldehyde before cytofluorometric analysis (EPICS Elite ESP; Coulter Electronics, Miami, Fla.).
Plasmids and virus preparations.
The commercial pNF-κB-Luc molecular construct contains five consensus NF-κB-binding sequences placed in front of the luciferase gene along with a minimal promoter (Stratagene). The pHXB-Luc vector leads to the production of single-round infectious X4-utilizing viruses, whereas pNL4.3 is a plasmid that encodes fully infectious X4-tropic viruses. pCDLSRα-B7.1 and pCN-B7.2 are molecular constructs coding for the human CD80 and CD86 costimulatory molecules, respectively. Isogenic virus particles differing only by the absence or presence of the host-derived CD80 or CD86 protein on their outer membranes were produced by calcium phosphate transfection in 293T cells as described previously (10, 18, 41). In some experiments, clinical strains of HIV-1 were produced in MDM that were treated with M-CSF. Four field isolates of HIV-1 with distinct coreceptor usage were used for this work: they are 93TH054/R5, 91US056/R5, 92UG046/X4, and 93UG070/X4 (AIDS Repository Reagent Program). To obtain cytokine-treated MDM, we incubated freshly isolated PBLs for 1 h at 37°C in 48-well flat-bottom tissue culture plates (Microtest III; Beckton Dickinson, Lincoln Park, N.J.) (3 × 106 cells/ml, 500 μl/well). The cells were next washed twice with PBS to remove nonadherent cells and were kept in culture for an additional 3 days in RPMI 1640 supplemented with 20% FBS in the presence of M-CSF (100 ng/ml). MDM were infected overnight with the tested clinical isolates of HIV-1 and were washed twice with PBS to remove uninternalized viruses. MDM were kept in culture in the presence of M-CSF (100 ng/ml) and the supernatants were harvested at day 8 postinfection. Free p24 was eliminated from the tested virus preparations by the use of centrifugal filter units (Centricon Plus-20, molecular weight cutoff of 100,000; Millipore Corporation, Bedford, Mass.). Virus stocks were normalized for virion content by an in-house double antibody sandwich enzyme-linked immunosorbent assay specific for the major viral p24 protein (8). The standardization of p24 contents was based on a previous observation indicating that virus preparations harvested from transfected 293T cells contain minimal amounts of p24 that are not associated with infectious progeny virus (18). All virus preparations were stored at −85°C and underwent only one freeze-thaw cycle before the initiation of infection studies.
Virus capture assay.
The presence of virion-bound host CD80 and CD86 proteins was estimated by a previously described virus capture assay (11). Briefly, 12.5 × 106 magnetic beads (BioMag, Fc specific; PerSeptive Diagnostics, Inc., Cambridge, Mass.) that were previously coated with an anti-CD80 (clone L-307.4) or anti-CD86 (clone BU-63) monoclonal antibody were incubated with similar amounts of viral preparations, standardized in terms of the viral core p24 protein (2.5 ng of p24 for NL4-3 and 10 ng of p24 for clinical strains of HIV-1 harvested from MDM), in a final volume of 1 ml of binding medium (PBS plus 0.1% bovine serum albumin). This mixture was incubated for 1 h at 4°C on a rotating plate. The beads were washed three times in binding medium with a magnetic separation unit and were resuspended in 200 μl of binding medium. The amount of captured viruses was defined by measuring the p24 content. Since CD45 has been shown to be excluded from mature HIV-1 particles (16, 40), magnetic beads coated with an antibody specific for human CD45RO (clone UCHL-1) were used as negative controls.
Virus infection studies.
Cells were inoculated with reporter HXB-Luc viruses either lacking or bearing the studied costimulatory molecules CD80 and CD86. Similar amounts of each virus stock (10 ng of p24) were used to inoculate 105 target cells in 96-well flat-bottom tissue culture plates (Microtest III; Beckton Dickinson). The luciferase activity was monitored with a microplate luminometer (MLX; Dynex Technologies, Chantilly, Va.) and is expressed as relative light units. In some experiments, virus infection was performed with fully competent NL4-3 particles. In such instances, isogenic NL4-3 virus stocks (10 ng of p24) were used to inoculate 105 target cells in 96-well flat-bottom tissue culture plates, and HIV-1 production was assessed by monitoring the levels of p24 in the culture medium. Virus stocks were pretreated on some occasions with either L-307.4, BU-63, or an isotype-matched irrelevant control antibody (final concentration, 1 μg/ml) for 30 min at 37°C before infection. Target cells were either left untreated or were treated with the anti-CD28 (clone 9.3) or anti-CTLA-4 (clone 10A8) antibody (1 μg/ml) for 30 min at 4°C before being inoculated with virus preparations.
Attachment and entry assays.
The binding of HIV-1 to the cell surface was monitored as described previously (23). Briefly, virus stocks (25 ng of p24) were exposed to 2.5 × 105 Jurkat cells for 1 h at 37°C in a total volume of 250 μl of complete culture medium. Next, the cells were gently washed twice with 500 μl of PBS and resuspended in 250 μl of disruption buffer (0.5% Triton X-100 in PBS). Samples were stored at −20°C until they were assayed for their p24 content. For this set of experiments, virus stocks were pretreated or not treated with L307.4 or BU-63 (final concentration, 1 μg/ml) for 30 min at 37°C. In some experiments, Jurkat cells were pretreated or not treated with the 9.3 or 10A8 antibody (final concentration, 1 μg/ml) for 30 min at 4°C before being inoculated with the virus preparations. The internalization of HIV-1 was estimated by incubating the virus preparations (50 ng of p24) with target cells (5 × 106 cells/ml, 250 μl/well) for 2 h at 37°C. The cells were next washed twice with 250 μl of ice-cold PBS and were incubated for 5 min at 4°C with 250 μl of cold RPMI 1640 (without FBS) supplemented with pronase (Boehringer Mannheim, Laval, Quebec, Canada) at 0.1 mg/ml. The cells were washed immediately with 2 ml of ice-cold RPMI 1640 containing 10% FBS and three times with ice-cold PBS to eliminate the pronase. Cells were resuspended in 1 ml of disruption buffer (0.5% Triton X-100 in PBS). Samples were stored at −20°C until they were assayed for their p24 content.
Transfection and stimulation tests.
Transient transfections were done by the DEAE-dextran method. In brief, Jurkat cells (5 × 106) were first washed once in transfection solution (TS) (25 mM Tris-HCl [pH 7.4], 5 mM KCl, 0.6 mM Na2HPO4, 0.5 mM MgCl2, and 0.7 mM CaCl2) and then were resuspended in 0.5 ml of TS containing 15 μg of used plasmid and 500 μg of DEAE-dextran/ml (final concentration). The cell-TS-plasmid-DEAE-dextran mix was incubated for 25 min at room temperature. Thereafter, the cells were diluted to a concentration of 106 cells/ml in complete culture medium supplemented with 100 μM chloroquine (Sigma). After 45 min of incubation at 37°C, the cells were centrifuged, resuspended in complete culture medium with or without doxycycline (100 ng/ml), and incubated at 37°C for 24 h. Transiently transfected Jurkat cells (100 μl at 106 cells/ml) were stimulated with 100 μl of the following stimuli: an anti-CD3 antibody (clone OKT3) at 1 μg/ml, DT30 cells (ratio 1:1), or a combination of the anti-CD3 antibody and DT30 cells. Jurkat cells were equally incubated with HIV-1 preparations (wild type [WT], CD80, or CD86; 30 ng of p24) with or without the anti-CD3 antibody. After an incubation period of 8 h at 37°C, 100 μl of cell-free supernatant was drawn from each well and 25 μl of cell culture lysis buffer (25 mM Tris phosphate [pH 7.8], 2 mM dithiothreitol, 1% Triton X-100, and 10% glycerol [final concentrations]) was added before an incubation at room temperature for 30 min. The extracts (20 μl) were analyzed for luciferase activity in 96-well plates by use of a Dynex MLX luminometer. Each well was injected with 100 μl of luciferase assay buffer [20 mM Tricine, 1.07 mM (MgCO3)4 · Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 270 mM coenzyme A, 470 μM luciferin, 530 μM ATP, and 33.3 mM dithiothreitol). The light output was measured for 20 s, with a 2-s delay. Values are expressed in relative light units, as measured by the apparatus. The results are expressed as fold induction levels relative to the basal luciferase activity in untreated control cells.
RESULTS
A positive correlation is seen between the surface expression level and the degree of virus-associated host CD80 and CD86.
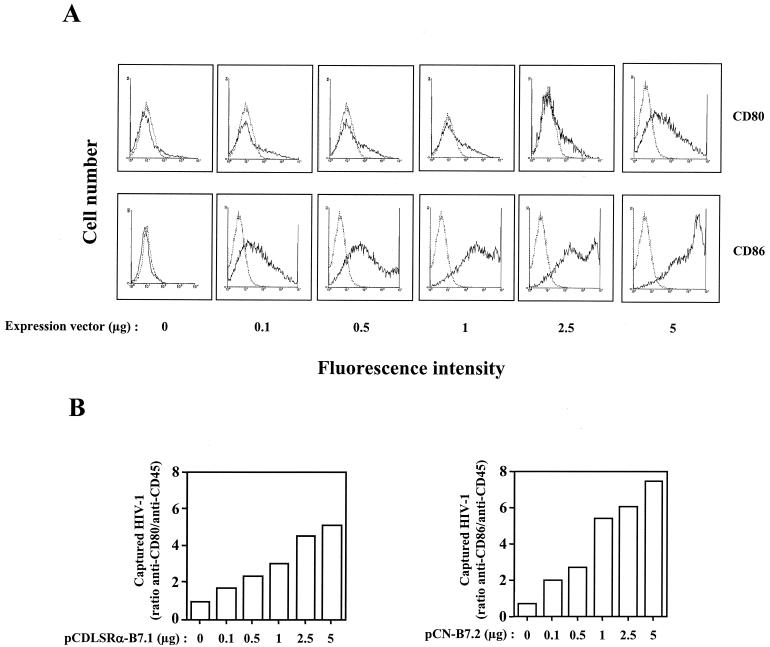
The costimulatory molecules CD80 and CD86 have been previously reported to be present within HIV-1 (16). The level of ICAM-1 surface expression on cells producing HIV-1 modulates the amount of virion-bound host ICAM-1 (41), and the expression of both CD80 and CD86 can fluctuate in response to a variety of stimuli on cells that are known to harbor HIV-1, i.e., T helper cells and macrophages (reviewed in reference 28). Thus, our first series of investigations was aimed at defining whether the incorporation process of CD80 and CD86 into mature HIV-1 progeny viruses was quantitatively affected by the amounts of these costimulatory proteins on the surfaces of virus producer cells. This objective was reached by transiently cotransfecting 293T cells with a fully infectious molecular clone of HIV-1 (pNL4-3) in combination with increasing doses of a mammalian expression vector coding for human CD80 (pCDLSRα-CD80) or CD86 (pCN-CD86). Data from flow cytometric analyses demonstrated that there is a positive correlation between the introduced CD80 or CD86 expression plasmid and the level of surface expression on transfected 293T cells (Fig. 1A). Next, the physical presence in HIV-1 of both cell surface constituents was semiquantitatively assessed by a virus capture assay (11). As illustrated in Fig. 1B, both host-derived molecules were acquired by mature HIV-1 particles and the virus recovery rate was influenced by the levels of CD80 and CD86 expressed on the surfaces of 293T cells. For the following experiments, virus stocks were produced on 293T cells expressing comparable surface levels of CD80 and CD86 (i.e., 5 μg of a CD80-encoding plasmid and between 0.1 and 0.5 μg of a CD86-encoding vector).
FIG. 1.
Positive correlation between levels of cell surface expression of CD80 and CD86 on virus producer cells and the degree of incorporation into HIV-1. (A) 293T cells were cotransfected with pNL4-3 and increasing amounts of an expression vector coding for human CD80 (pCDLSRα-B7.1) or CD86 (pCN-B7.2). Forty-eight hours later, virus-containing supernatants were harvested and cells were subjected to fluorescence-activated cell sorting analysis to monitor the surface levels of CD80 or CD86. An irrelevant isotype-matched antibody was used as a control. (B) Similar amounts of each virus stock (2.5 ng of p24) were incubated with magnetic beads coated with antibodies specific for CD80, CD86, or CD45. Magnetic beads coated with the anti-CD45 antibody served as controls to define the background levels of captured viruses. The data shown represent ratios of captured virions that were reactive with the human CD80, CD86, or CD45 antibody, respectively. The results shown are from triplicate samples and are representative of two separate experiments.
Virus-bound host CD80 and CD86 are functional and augment HIV-1 infectivity in several T-lymphoid cell lines and primary human mononuclear cells.
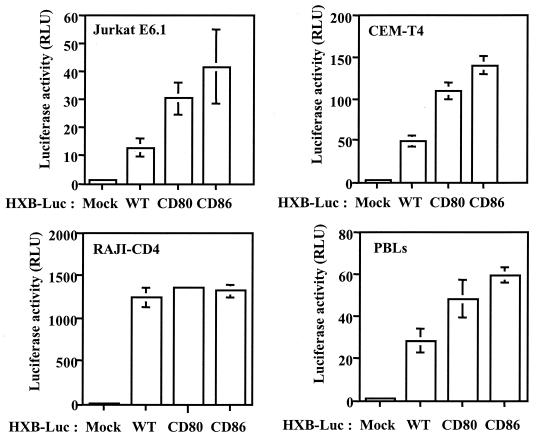
The effect of virion-anchored host CD80 and CD86 on the biology of HIV-1 was tested by infecting susceptible target cells that express CD28, one of the natural ligands of these two costimulatory molecules. Two CD28-expressing lymphoid cell lines (Jurkat and CEM-T4) were initially used as targets for reporter viruses either lacking or bearing the host-encoded cell membrane components. A CD4-expressing B-cell line that is negative for CD28, i.e., RAJI-CD4, was also used as a control. As depicted in Fig. 2, HIV-1 infectivity was enhanced by the insertion of CD80 and CD86 into the virus envelope when the infection was allowed to proceed in CD28-positive T-cell lines, but it was not enhanced in RAJI-CD4 cells. Interestingly, a comparable upregulatory effect on virus infectivity was obtained when a more natural reservoir of HIV-1, i.e., mitogen-stimulated PBLs, was used.
FIG. 2.
Incorporation of host CD80 and CD86 increases HIV-1 infectivity in established cell lines and primary human cells. Similar amounts of isogenic reporter viruses (HXB-Luc) either lacking (WT) or bearing host-derived CD80 or CD86 were used to inoculate Jurkat, CEM-T4, and RAJI-CD4 cells and PBLs. Cells were incubated at 37°C for 48 h. Finally, the cells were lysed, and luciferase activities were monitored as described in Materials and Methods. The data shown represent the means ± standard deviations (SD) from triplicate samples and are representative of three separate experiments. RLU, relative light units.
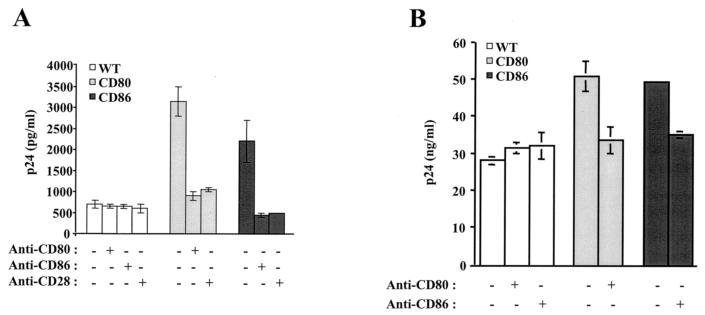
Infectivity studies were next performed with fully competent HIV-1, and virus production was monitored by measuring the amount of p24 in culture supernatants. Moreover, the role played by interactions between CD80/CD86 and CD28 in the noticeable enhancement of HIV-1 infectivity was studied by using blocking antibodies. In agreement with the experiments conducted with a reporter progeny virus, the infectivity of the fully infectious virus was still augmented by the acquisition of host CD80 or CD86 when Jurkat cells (Fig. 3A) and PBLs (Fig. 3B) were used as targets. The increase in HIV-1 infectivity conferred by the insertion of the tested costimulatory molecules into the virus envelope was abolished by the pretreatment of CD80- and CD86-bearing virions with blocking anti-CD80 and anti-CD86 antibodies, respectively. A similar observation was made when cells were pretreated with a blocking anti-CD28 antibody. Virus replication in Jurkat cells and PBLs was unaffected by the use of isotype-matched irrelevant antibodies (data not shown).
FIG. 3.
The infectivity of CD80- and CD86-bearing HIV-1 virions is diminished by antibodies blocking interactions with CD28. Similar amounts of isogenic fully infectious viruses (NL4-3) either lacking (WT) or bearing host-derived CD80 or CD86 were used to inoculate Jurkat cells (A) and PBLs (B). Virus stocks were either left untreated or were treated with blocking antibodies against CD80 or CD86 before virus infections. In some instances, the cells were left untreated or were treated with a blocking anti-CD28 antibody before HIV-1 infection. Cell-free supernatants were harvested at 24 (Jurkat) or 96 (PBLs) h postinfection to evaluate the p24 content. The data shown represent the means ± SD from triplicate samples and are representative of three separate experiments.
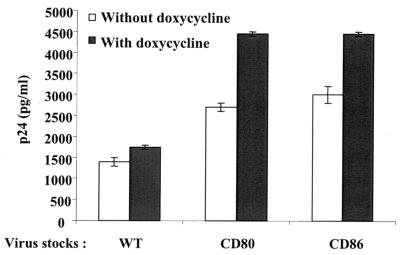
In addition to CD28, CTLA-4 also acts as a ligand for both CD80 and CD86. However, CTLA-4 is not constitutively expressed on resting lymphocytes but appears at the cell surface after T-cell activation. Thus, we also evaluated the contribution of the interaction between virus-anchored CD80/CD86 and cell surface CTLA-4 to HIV-1 replication. A derivative Jurkat cell line that expresses CTLA-4 in an inducible manner upon treatment with doxycycline was used in this set of experiments. It should be specified that this Jurkat cellular clone also carries on its surface a level of CD28 molecules comparable to that seen on parental Jurkat cells and that the CD28 surface level is not modulated upon treatment with doxycycline (data not shown). The replication of fully infectious NL4-3 virions was augmented in Jurkat cells expressing both CD28 and CTLA-4 compared to cells expressing CD28 only (Fig. 4). These data demonstrate the importance of this second type of interaction for the biology of CD80- and CD86-bearing progeny viruses.
FIG. 4.
The infectivity of CD80- and CD86-bearing HIV-1 particles is positively affected by interaction with CTLA-4. CTLA-4-inducible Jurkat cells were either left untreated or were treated overnight with doxycycline before infections with similar amounts of isogenic fully infectious viruses (NL4-3) either lacking (WT) or bearing host-derived CD80 or CD86. Cell-free supernatants were harvested at 24 h postinfection to evaluate the p24 content. The data shown represent the means ± SD from triplicate samples and are representative of three separate experiments.
HIV-1 adsorption and uptake are facilitated by the incorporation of CD80 and CD86 into mature viruses.
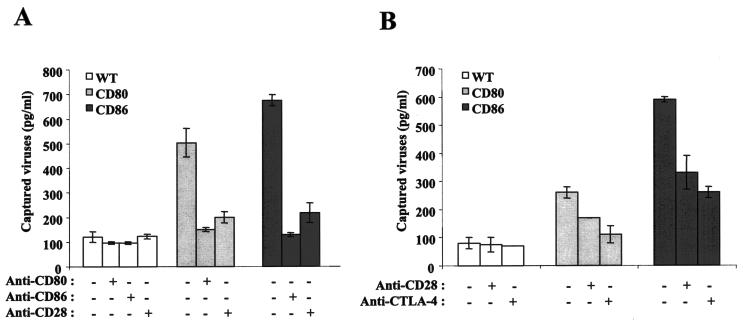
We compared the capacities of isogenic HIV-1 particles either lacking or bearing host-derived CD80 and CD86 to attach and bind to Jurkat cells expressing CD28 only or both CD28 and CTLA-4. As shown in Fig. 5A, the insertion of CD80 and CD86 into HIV-1 led to a significant increase in the attachment of viruses to the cell surface, a process which was eliminated by a blocking antibody specific for CD80, CD86, or CD28. These antibodies did not affect the attachment of progeny viruses lacking host CD80 or CD86 to the cell surface. Moreover, a treatment with isotype-matched irrelevant control antibodies had no effect on HIV-1 attachment to the studied target cells (data not shown). Studies performed with CD28+ CTLA-4+ Jurkat cells in combination with a blocking anti-CD28 or anti-CTLA-4 antibody confirmed that both cell surface molecules are involved in the binding of CD80- or CD86-bearing virions to such target cells (Fig. 5B).
FIG. 5.
Attachment to CD28- and CD28/CTLA-4-expressing target cells is enhanced by the presence of host CD80 and CD86 in HIV-1. (A) Similar amounts of isogenic fully infectious viruses (NL4-3) either lacking (WT) or bearing host-derived CD80 or CD86 were incubated with CD28-expressing Jurkat cells. In some instances, virus preparations were left untreated or were treated with blocking anti-CD80 or anti-CD86 antibodies prior to incubation with target cells. Additionally, Jurkat cells were either left untreated or were pretreated with a blocking anti-CD28 antibody before incubation with HIV-1 particles. (B) Similar studies were performed with CD28/CTLA-4-expressing Jurkat cells which were either left untreated or were pretreated with blocking anti-CD28 or anti-CTLA-4 antibodies before inoculation with virus stocks. After incubation, the cells were washed twice with PBS to remove unattached virus particles and the levels of cell-associated p24 were estimated by an enzymatic assay. The data shown represent the means ± SD from triplicate samples and are representative of three separate experiments.
By facilitating the initial step in the HIV-1 life cycle, additional interactions between virus-anchored host CD80 or CD86 and CD28/CTLA-4 can be proposed to increase the probability for viral gp120 to encounter its two natural ligands, i.e., CD4 and the appropriate chemokine coreceptor, thus favoring the fusion of viral and cellular membranes and virus entry. This scenario was investigated by assessing the internalization of isogenic virions either lacking or bearing host CD80 or CD86 by an HIV-1 entry assay (23). The data shown in Table 1 indicate that HIV-1 entry is more efficient for virions bearing CD80 and CD86 than for isogenic progeny viruses lacking these two host cell membrane constituents. Altogether, our observations reveal that the acquisition of CD80 or CD86 by mature virus particles represents a process that will ultimately favor the most proximal steps in the HIV-1 replicative cycle.
TABLE 1.
Internalization of isogenic HIV-1 particles either lacking (WT) or bearing host-derived CD80 or CD86 glycoprotein in Jurkat cells
| Virus stock | Total p24 (pg/ml)a | Intracellular p24 (pg/ml)a | % Virus entry (intracellular p24/ total p24 level) |
|---|---|---|---|
| WT | 1,194 ± 69 | 576 ± 14 | 48.2 |
| CD80 | 1.582 ± 18 | 1,407 ± 15 | 88.9 |
| CD86 | 1,761 ± 28 | 1.654 ± 46 | 93.9 |
The data shown represent the means ± SD from triplicate samples and are representative of three separate experiments.
CD80 and CD86 incorporation confers costimulatory properties on HIV-1 particles.
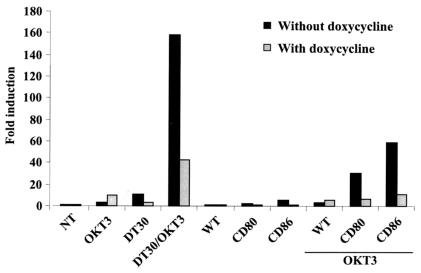
It was previously reported that CD86-bearing HIV-1 virions can provide a cosignal that forms a synergy with TCR/CD3-mediated biochemical events to fully activate some important transcriptional factors, such as NF-κB and NFAT (7). It should be emphasized that this study was performed with the CTLA-4-negative parental Jurkat cell line. Thus, we evaluated the possible activation of NF-κB by CD80- and CD86-bearing progeny viruses by using cells expressing both CD28 and CTLA-4. To this end, the CTLA-4-inducible Jurkat derivative was transfected with a molecular construct made of the luciferase reporter gene under the control of a basic promoter element (TATA box) joined to five tandem repeats of NF-κB binding elements (pNF-κB-Luc). Cells were next either left untreated (CD28+) or treated with doxycycline (CD28+ CTLA-4+) before being exposed to CD80- and CD86-bearing HIV-1 virions in the absence or presence of the anti-CD3 monoclonal antibody OKT3. The CD80-expressing DT30 cell line was preincubated with the anti-CD3 antibody and was used as a positive control. We found that both CD80- and CD86-bearing virions can efficiently provide the costimulatory signal necessary to achieve NF-κB induction (Fig. 6). Moreover, the presence of CTLA-4 at the cell surface resulted in a significant decline in NF-κB activation that was seen upon incubation with CD80- or CD86-bearing HIV-1 and TCR/CD3 engagement.
FIG. 6.
CD80- and CD86-bearing HIV-1 particles provide costimulatory signal leading to activation of NF-κB when combined with engagement of the TCR/CD3 complex. The CTLA-4-inducible Jurkat cell line was transfected with an NF-κB-driven reporter gene construct before treatment with an anti-CD3 antibody (clone OKT3), DT30 cells, a combination of anti-CD3 and DT30 cells, or viruses either lacking or bearing host CD80 or CD86 in the absence or presence of the anti-CD3 antibody. After 8 h of incubation, the cells were lysed and assayed for luciferase activity. The results shown are the means (± SD) from triplicate samples and are expressed as the fold induction relative to the basal luciferase activity in the untreated control (NT). These data are representative of two separate experiments.
Efficient incorporation of CD80 and CD86 in clinical isolates of HIV-1 produced by cytokine-treated macrophages.
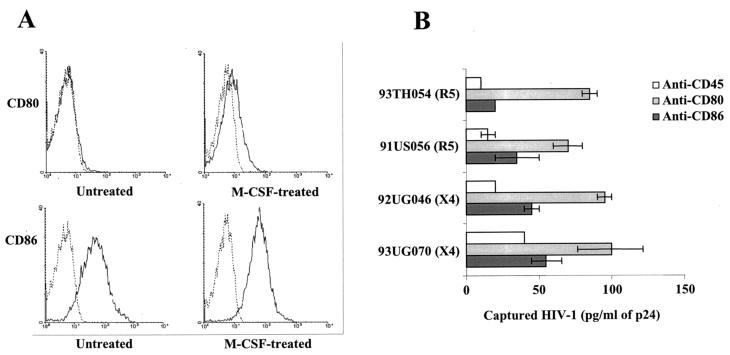
As specified above, the two most important cellular reservoirs of HIV-1, i.e., monocytes/macrophages and CD4+ T lymphocytes, can also express both CD80 and CD86 (3, 4, 14, 24, 25, 48, 56). Since CD80 and CD86 are upregulated after the treatment of cells of the monocytic lineage with cytokines (reviewed in reference 28), we finally studied the process of CD80 and CD86 incorporation in field isolates of HIV-1 bearing distinct coreceptor utilization profiles (i.e., R5 and X4). Treatment with M-CSF led to an important increase in CD80 and to a more modest augmentation of CD86 on the surfaces of primary human MDM (Fig. 7A). By using a sensitive antibody-based virus capture assay, we found that the four clinical isolates of HIV-1 tested incorporated both CD80 and CD86 when they were expanded in M-CSF-treated MDM (Fig. 7B).
FIG. 7.
R5- and X4-tropic field isolates of HIV-1 grown on M-CSF-treated MDM incorporate CD80 and CD86. (A) MDM were either left untreated or were treated with M-CSF before CD80 and CD86 surface expression was monitored by flow cytometric analysis. (B) Four clinical isolates of HIV-1 were produced in M-CSF-treated MDM and subjected to a virus capture assay as described in Materials and Methods. Similar amounts of each virus stock were incubated with magnetic beads coated with antibodies specific for CD80, CD86, or CD45. The data shown represent the means (± SD) from triplicate samples and are representative of two independent experiments.
DISCUSSION
The acquisition of host components by retroviruses, particularly HIV-1, has been extensively described during the last decade. Such host molecules are incorporated into newly formed viral entities during the budding process, but the precise mechanism(s) governing this phenomenon remains vague. However, it is becoming clear that the insertion of some defined host cell membrane components into mature virions has various impacts on the HIV-1 life cycle and/or virus susceptibility to immune defense. For example, CD55 and CD59 contribute to complement resistance (47); HLA-DR, CD28, and some adhesion molecules (e.g., ICAM-1, LFA-1, and VLA-4) increase virus infectivity by facilitating the binding and entry process when the target cell expresses the appropriate counterligand (10, 18, 23, 30); ICAM-1 renders HIV-1 virions less susceptible to antibody-mediated neutralization (17, 44); major histocompatibility complex class II (MHC II) molecules present superantigens, leading to a polyclonal activation of T cells (45); and CD86 results in intracellular signal transduction events which, when coupled with the engagement of the TCR/CD3 complex, culminate in the nuclear translocation of both NF-κB and NFAT (7).
Studies aimed at monitoring the presence of the costimulatory molecules CD80 and CD86 in HIV-1 have been limited despite the fact that the CD28/B7 receptor/ligand system is considered one of the dominant costimulatory pathways. Frank and colleagues performed a phenotypic characterization of host-derived molecules acquired by HIV-1 and reported the presence of CD86 in virus isolates produced by DCs, T lymphocytes, and DC-T-cell cocultures (20). CD86 has been reported to be efficiently incorporated by an X4-tropic laboratory isolate of HIV-1 (7). More recently, it was shown that clinical isolates of HIV-1 amplified in primary human cells were captured by anti-CD86 antibodies, and to a lesser extent, by anti-CD80 antibodies (16).
We demonstrate here that HIV-1 replication is improved by the acquisition of CD80 and CD86 through a mechanism facilitating the binding and entry process. Moreover, we demonstrate that this phenomenon results from the specific interaction between CD80 and CD86 and their two natural ligands, CD28 and CTLA-4. We also found that both CD80 and CD86 confer on the virus the capacity to provide the cosignal required for the activation of NF-κB, a phenomenon that is abolished upon expression of CTLA-4. Finally, the physiological relevance of the incorporation of CD80 and CD86 into HIV-1 was provided by the observation that both R5 and X4 field variants of HIV-1 produced by cytokine-treated MDM were efficiently captured by either anti-CD80 or anti-CD86 antibodies. In the present work, the involvement of the CD28/B7 system in favoring virus production was established by using two CD28-expressing T-lymphoid cell lines (Jurkat and CEM-T4), whereas the contribution of CTLA-4/B7 immunobiology to this phenomenon was illustrated by using a derivative Jurkat cell line expressing doxycycline-inducible WT human CTLA-4 molecules in addition to CD28. It is interesting that, despite the low level of cell surface expression of CTLA-4 compared with CD28 (data not shown), the interaction between CTLA-4 and virion-anchored CD80 or CD86 contributes significantly to the noted increase in HIV-1 infectivity. Such a finding can be explained by the fact that the avidity of CTLA-4 for CD80 and CD86 is 100-fold higher than that for CD28 (37, 53). This last observation might have some significance in vivo, since it is known that only a small percentage of CTLA-4 reaches the cell surface of activated CD4+ T lymphocytes (29, 31). The importance of interactions between virus-associated host CD80/CD86 and CD28/CTLA-4 in HIV-1 biology was confirmed in a natural cellular reservoir, PBLs. However, the impact of virus-anchored host CD80 and CD86 on HIV-1 infectivity in primary human cells was more modest than the effect on virus infectivity when lymphoid cell lines were used as targets. The explanation for such differences is unknown but could relate to differences in the surface expression levels of CD80 and CD86 ligands (CD28 and CTLA-4) on primary human cells versus established cell lines. It can also be proposed that interactions between virus-bound host molecules other than CD80 and CD86 and their normal receptors are different when primary human cells or lymphoid cells are used. Given that sugar groups located on gp120 have been shown to associate through CD4-independent mechanisms with sugars or lectin-like domains on some specific cell surface receptors (reviewed in reference 13), it is also possible that the different impact of virus-associated host CD80 and CD86 on HIV-1 infectivity in PBLs compared to that in established cell lines is linked to the nature of additional interactions between cell surface constituents that can interact with gp120. Further studies are needed to shed light on this matter.
CD80 and CD86 were found to be acquired by field isolates of HIV-1 grown on M-CSF-treated MDM. Moreover, such progeny virions were more efficiently captured by an anti-CD80 antibody than by an anti-CD86 antibody. This was in contrast with a study conducted by Esser and coworkers, who reported that HIV-1 preferentially acquires CD86 compared to CD80 (16). Differences in experimental methodologies may account for the discrepant results. For example, there may have been differences with respect to the virus capture assay, the antibodies used to precipitate viruses, and/or the virus producer cells. Furthermore, the data from Esser's group were obtained with virus stocks prepared in untreated MDM while our virus preparations came from M-CSF-treated MDM. Recent evidence has suggested that HIV-1 assembles on late endocytic membranes in primary human macrophages and acquires antigens that are characteristic of this intracellular compartment (42). There is currently no information available on the presence of CD80 and CD86 in late endosomes and of course on a possible modulatory effect of M-CSF on CD80/CD86 expression in such cellular compartments. Based on this new observation, we postulate that the treatment of MDM with M-CSF modulates the process of incorporation of host-derived CD80 and CD86 into late endosomes.
The expression of CD80 and CD86 is almost exclusively restricted to lymphoid tissues, primarily on professional antigen-presenting cells (APCs), including DCs, monocytes, and B cells. Both CD80 and CD86 expression is induced upon cell activation, with different kinetics and in response to a variety of stimuli (reviewed in reference 12). Microbial constituents such as bacterial lipopolysaccharide or cytokines produced during inflammation will induce or upregulate CD80 and CD86 on APCs, rendering them fully competent to activate T lymphocytes. For example, IFN-γ and GM-CSF increase the expression of both CD80 and CD86 on monocytes/macrophages (5), although interleukin-10 blocks their expression (55). Signaling through membrane proteins (e.g., CD40/CD40L, MHC-II, BCR, etc.) is another mechanism by which CD80/CD86 expression is regulated (21, 24, 39, 43, 46, 54). For instance, T lymphocytes activated through CD3/CD28 will express both CD80 and CD86 (4, 14, 48, 56). Thus, it will be interesting to investigate the possible relationships between the types of producer cells in which HIV-1 is expanded (e.g., CD4+ T cells versus macrophages), the types of stimuli used to activate virus producer cells, and the degree of incorporation of CD80 and CD86 into mature HIV-1 particles.
Infected macrophages serve as long-lived reservoirs of HIV-1 under in vivo conditions. The regulation of HIV-1 expression in this cell type is complex, but it is generally associated with cell activation and differentiation (27, 36). During the normal immune response, macrophages serve as APCs to present foreign antigens to CD4-expressing T lymphocytes. Intimate contact between an APC and a T cell leads to the upregulation of costimulatory molecules (e.g., CD80/CD86 on macrophages and CTLA-4 and CD40L on T cells) as well as the activation of HIV-1 transcription and production (35, 38, 50, 51). Thus, it can be proposed that antigen presentation and/or the presence of some specific cytokines in the milieu surrounding HIV-1-infected macrophages will result in the expression of CD80 and CD86 and also in the production of HIV-1 particles bearing CD80 and CD86 in their envelopes. The additional interactions between virus-anchored CD80 and CD86 with their natural cognate ligands might accelerate the process of infection of CD28/CTLA-4-bearing T lymphocytes, an event favoring the spread of HIV-1.
In this study, we confirmed the ability of virally embedded CD86 to mediate signal transduction through its natural receptor, CD28, and extended this property to CD80-bearing virions. Moreover, we showed that the presence of CTLA-4 at the cell surface impedes this costimulatory capacity. It is still unknown if the negative effect of CTLA-4 with regard to NF-κB activation is due to a CTLA-4-mediated signal transduction event or a sequestration of CD80- and CD86-bearing particles by CTLA-4 due to its higher affinity for CD80 and CD86 than CD28 has. Additional experiments are needed to solve this issue. Although previous works have shown that the engagement of CD28 in the absence of TCR-mediated biochemical events generally has no physiological effect (12), it should be kept in mind that HIV-1 incorporates significant quantities of MHC II molecules (1). Assuming that HIV-1 bears on its surface CD80 and/or CD86 in combination with MHC II molecules loaded with a nominal antigen, a possible contact with a T cell bearing a TCR specific for this antigenic peptide might result in cell activation. The establishment of an activated state can reveal the physiological significance in the pathogenesis of HIV-1 infection. Indeed, this process can promote virus transcription if the responding T cell is latently infected with HIV-1. In addition, cellular activation can create a cellular microenvironment that is more favorable for a productive HIV-1 infection.
In conclusion, HIV-1 infections are highly dependent on the interaction between the viral protein gp120/gp41 and the cell surface receptor CD4. In addition to this interaction, the participation of one chemokine coreceptor (usually CXCR4 or CCR5) is required to give rise to fusion between the viral envelope and the cell membrane. However, recent data have indicated that the attachment of HIV-1 particles to cell surfaces is not only attributed to the interaction between the spike Env glycoproteins with appropriate receptors but is also influenced by a large number of molecules that may serve to concentrate the virus on the cell surface. For example, cell types such as macrophages and DCs express barely detectable levels of CD4, and their initial contact with HIV-1 is most likely due to CD4-independent interactions. In these situations, the attachment process is under the influence of interactions between sugar groups on the Env glycoproteins and other sugars or lectin-like domains on cell surface receptors, such as the mannose-specific macrophage endocytosis receptor (26). Additionally, virus binding to macrophages depends heavily on the glycosaminoglycan heparan sulfate since the treatment of target cells with heparinase abolishes HIV-1 attachment while blockers of the gp120-CD4 interaction have no effect on virus binding (49). Supplementary interactions between cell-derived molecules incorporated onto HIV-1 virions and their ligands will increase the frequency of Env-receptor interactions and enhance the overall efficiency of virus entry because fusion does not occur until a certain amount of CD4 and coreceptor molecules are recruited to elicit the formation of a fusion pore. In summary, this study further reinforces the concept that the biology of HIV-1 is affected by virion-associated host cell proteins. Our findings confirm the high degree of complexity of the interactions occurring between a pathogen such as HIV-1 and its cellular target.
Acknowledgments
We thank M. Dufour for technical assistance with flow cytometry studies and Sylvie St.-Amand for technical support.
This study was supported by a grant to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (grant HOP-14438). J.-F.G. is the recipient of a Ph.D. Fellowship from the Fonds de la Recherche en Santé du Québec and S.B. holds a Ph.D. Fellowship from the CIHR. M.J.T. is the recipient of the Canada Research Chair in Human Immuno-Retrovirology (senior level).
REFERENCES
- 1.Arthur, L. O., W. J. Bess, Jr., R. C. Sowder II, R. E. Benveniste, D. L. Mann, J.-C. Cherman, and L. E. Henderson. 1992. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science 258:1935-1938. [DOI] [PubMed] [Google Scholar]
- 2.Azuma, M., M. Cayabyab, D. Buck, J. H. Phillips, and L. L. Lanier. 1992. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J. Exp. Med. 175:353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma, M., D. Ito, H. Yagita, K. Okumura, J. H. Phillips, L. L. Lanier, and C. Somoza. 1993. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366:76-79. [DOI] [PubMed] [Google Scholar]
- 4.Azuma, M., H. Yssel, J. H. Phillips, H. Spits, and L. L. Lanier. 1993. Functional expression of B7/BB1 on activated T lymphocytes. J. Exp. Med. 177:845-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcy, S., M. Wettendorff, O. Leo, J. Urbain, M. Kruger, J. L. Ceuppens, and M. de Boer. 1995. FcR cross-linking on monocytes results in impaired T cell stimulatory capacity. Int. Immunol. 7:179-189. [DOI] [PubMed] [Google Scholar]
- 6.Baroja, M. L., D. Luxenberg, T. Chau, V. Ling, C. A. Strathdee, B. M. Carreno, and J. Madrenas. 2000. The inhibitory function of CTLA-4 does not require its tyrosine phosphorylation. J. Immunol. 164:49-55. [DOI] [PubMed] [Google Scholar]
- 7.Bounou, S., N. Dumais, and M. J. Tremblay. 2001. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kB- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J. Biol. Chem. 276:6359-6369. [DOI] [PubMed] [Google Scholar]
- 8.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood 90:1091-1100. [PubMed] [Google Scholar]
- 10.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 12.Chambers, C. A. 2001. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 22:217-223. [DOI] [PubMed] [Google Scholar]
- 13.Clapham, P. R., and A. McKnight. 2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83:1809-1829. [DOI] [PubMed] [Google Scholar]
- 14.Creery, W. D., F. Diaz-Mitoma, L. Filion, and A. Kumar. 1996. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur. J. Immunol. 26:1273-1277. [DOI] [PubMed] [Google Scholar]
- 15.Engel, P., J. G. Gribben, G. J. Freeman, L. J. Zhou, Y. Nozawa, M. Abe, L. M. Nadler, H. Wakasa, and T. F. Tedder. 1994. The B7-2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood 84:1402-1407. [PubMed] [Google Scholar]
- 16.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortin, J. F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257:228-238. [DOI] [PubMed] [Google Scholar]
- 18.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank, I., L. Kacani, H. Stoiber, H. Stossel, M. Spruth, F. Steindl, N. Romani, and M. P. Dierich. 1999. Human immunodeficiency virus type 1 derived from cocultures of immature dendritic cells with autologous T cells carries T-cell-specific molecules on its surface and is highly infectious. J. Virol. 73:3449-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman, G. J., A. S. Freedman, J. M. Segil, G. Lee, J. F. Whitman, and L. M. Nadler. 1989. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J. Immunol. 143:2714-2722. [PubMed] [Google Scholar]
- 22.Garoff, H., R. Hewson, and D. J. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giguère, J.-F., J. S. Paquette, S. Bounou, R. Cantin, and M. J. Tremblay. 2002. New insights into the functionality of a virion-anchored host cell membrane protein: CD28 versus HIV type 1. J. Immunol. 169:2762-2771. [DOI] [PubMed] [Google Scholar]
- 24.Hathcock, K. S., G. Laszlo, C. Pucillo, P. Linsley, and R. J. Hodes. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeannin, P., N. Herbault, Y. Delneste, G. Magistrelli, S. Lecoanet-Henchoz, G. Caron, J. P. Aubry, and J. Y. Bonnefoy. 1999. Human effector memory T cells express CD86: a functional role in naive T cell priming. J. Immunol. 162:2044-2048. [PubMed] [Google Scholar]
- 26.Larkin, M., R. A. Childs, T. J. Matthews, S. Thiel, T. Mizuochi, A. M. Lawson, J. S. Savill, C. Haslett, R. Diaz, and T. Feizi. 1989. Oligosaccharide-mediated interactions of the envelope glycoprotein gp120 of HIV-1 that are independent of CD4 recognition. AIDS 3:793-798. [DOI] [PubMed] [Google Scholar]
- 27.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 29.Leung, H. T., J. Bradshaw, J. S. Cleaveland, and P. S. Linsley. 1995. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J. Biol. Chem. 270:25107-25114. [DOI] [PubMed] [Google Scholar]
- 30.Liao, Z., J. W. Roos, and J. E. Hildreth. 2000. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retrovir. 16:355-366. [DOI] [PubMed] [Google Scholar]
- 31.Linsley, P. S., J. Bradshaw, J. Greene, R. Peach, K. L. Bennett, and R. S. Mittler. 1996. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4:535-543. [DOI] [PubMed] [Google Scholar]
- 32.Linsley, P. S., W. Brady, L. Grosmaire, A. Aruffo, N. K. Damle, and J. A. Ledbetter. 1991. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linsley, P. S., E. A. Clark, and J. A. Ledbetter. 1990. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl. Acad. Sci. USA 87:5031-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linsley, P. S., J. L. Greene, P. Tan, J. Bradshaw, J. A. Ledbetter, C. Anasetti, and N. K. Damle. 1992. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 176:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancino, G., R. Placido, S. Bach, F. Mariani, C. Montesano, L. Ercoli, M. Zembala, and V. Colizzi. 1997. Infection of human monocytes with Mycobacterium tuberculosis enhances human immunodeficiency virus type 1 replication and transmission to T cells. J. Infect. Dis. 175:1531-1535. [DOI] [PubMed] [Google Scholar]
- 36.Martin, J. C., and J. C. Bandres. 1999. Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 22:413-429. [DOI] [PubMed] [Google Scholar]
- 37.Metzler, W. J., J. Bajorath, W. Fenderson, S. Y. Shaw, K. L. Constantine, J. Naemura, G. Leytze, R. J. Peach, T. B. Lavoie, L. Mueller, and P. S. Linsley. 1997. Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat. Struct. Biol. 4:527-531. [DOI] [PubMed] [Google Scholar]
- 38.Mikovits, J. A., N. C. Lohrey, R. Schulof, J. Courtless, and F. W. Ruscetti. 1992. Activation of infectious virus from latent human immunodeficiency virus infection of monocytes in vivo. J. Clin. Investig. 90:1486-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nabavi, N., G. J. Freeman, A. Gault, D. Godfrey, L. M. Nadler, and L. H. Glimcher. 1992. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature 360:266-268. [DOI] [PubMed] [Google Scholar]
- 40.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 41.Paquette, J. S., J. F. Fortin, L. Blanchard, and M. J. Tremblay. 1998. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J. Virol. 72:9329-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelchen-Matthews, A., B. Kramer, and M. Marsh. 2003. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 162:443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranheim, E. A., and T. J. Kipps. 1993. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 177:925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossio, J. L., J. Bess, Jr., L. E. Henderson, P. Cresswell, and L. O. Arthur. 1995. HLA class II on HIV particles is functional in superantigen presentation to human T cells: implications for HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1433-1439. [DOI] [PubMed] [Google Scholar]
- 46.Roy, M., A. Aruffo, J. Ledbetter, P. Linsley, M. Kehry, and R. Noelle. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596-603. [DOI] [PubMed] [Google Scholar]
- 47.Saifuddin, M., C. J. Parker, M. E. Peeples, M. K. Gorny, S. Zolla-Pazner, M. Ghassemi, I. A. Rooney, J. P. Atkinson, and G. T. Spear. 1995. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 182:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sansom, D. M., and N. D. Hall. 1993. B7/BB1, the ligand for CD28, is expressed on repeatedly activated human T cells in vitro. Eur. J. Immunol. 23:295-298. [DOI] [PubMed] [Google Scholar]
- 49.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shattock, R. J., D. Burger, J. M. Dayer, and G. E. Griffin. 1996. Enhanced HIV replication in monocytic cells following engagement of adhesion molecules and contact with stimulated T cells. Res. Virol. 147:171-179. [DOI] [PubMed] [Google Scholar]
- 51.Shattock, R. J., G. P. Rizzardi, P. Hayes, and G. E. Griffin. 1996. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44, and CD58) enhances human immunodeficiency virus type 1 replication in monocytic cells through a tumor necrosis factor-modulated pathway. J. Infect. Dis. 174:54-62. [DOI] [PubMed] [Google Scholar]
- 52.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 53.van der Merwe, P. A., D. L. Bodian, S. Daenke, P. Linsley, and S. J. Davis. 1997. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 185:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts, T. H., N. Alaverdi, W. F. Wade, and P. S. Linsley. 1993. Induction of costimulatory molecule B7 in M12 B lymphomas by cAMP or MHC-restricted T cell interaction. J. Immunol. 150:2192-2202. [PubMed] [Google Scholar]
- 55.Willems, F., A. Marchant, J. P. Delville, C. Gerard, A. Delvaux, T. Velu, M. de Boer, and M. Goldman. 1994. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 24:1007-1009. [DOI] [PubMed] [Google Scholar]
- 56.Wyss-Coray, T., D. Mauri-Hellweg, K. Baumann, F. Bettens, R. Grunow, and W. J. Pichler. 1993. The B7 adhesion molecule is expressed on activated human T cells: functional involvement in T-T cell interactions. Eur. J. Immunol. 23:2175-2180. [DOI] [PubMed] [Google Scholar]