Abstract
Long peptide immunization is a promising strategy to clear established tumors. In the current study, we investigated the therapeutic effect of a naturally existing long peptide that contained two HLA-A2.1 restricted epitopes (CRPVE1/149-157 and CRPVE1/161-169) from cottontail rabbit papillomavirus (CRPV) E1 using our CRPV/HLA-A2.1 transgenic rabbit model. A universal Tetanus Toxin helper motif (TT helper) was tagged at either the N-terminus or the carboxyl-terminus of this long peptide and designated as TT-E1 peptide and E1 peptide-TT respectively. Four groups of HLA-A2.1 transgenic rabbits were infected with wild type CRPV DNA. Three weeks post-infection, the rabbits were immunized four times with TT-E1 peptide, E1peptide only, E1 peptide -TT or TT-control peptide with two-week intervals between immunizations. Tumor outgrowth was monitored and recorded weekly. After the third booster immunization, tumors on two of the four E1 peptide-TT immunized rabbits began to shrink. One animal from this group was free of tumors at the termination of the study. The mean papilloma size of E1 peptide-TT immunized rabbits was significantly smaller when compared with that of the three other groups (P<0.05, one way ANOVA analysis). It is interesting that E1 peptide-TT vaccination not only stimulated stronger T cell mediate immune responses but also stronger antibody generations. We conclude that the location of a TT helper motif tagged at the long peptide vaccine is critical for the outcome of therapeutic responses to persistent tumors in our HLA-A2.1 transgenic rabbit model.
Keywords: Long peptide, therapeutic vaccine, CRPV, HLA-A2.1, transgenic rabbit, viral infection, animal model, T-cell mediated immune response, TT helper, CD8+ T cells, CD4+ T cells
1. Introduction
Human papillomaviruses (HPVs) are associated with anogenital infections and cervical cancer in women [1]. HPV persistent infections are also detected in other related diseases including vaginal condylomas, recurrent respiratory papillomas (RRP), common plantar warts, and anal cancer [2-4]. HPV infections can be cleared in the majority of healthy people within one year of detection. Host immunity including innate and adaptive immune responses plays important roles in eliminating the infected cells because higher incidences of HPV related diseases and cancers are found in immunocompromised people such as transplant and HIVinfected patients [5-7].
Two prophylactic vaccines (Gardasil and Cervarix) developed by Merck and GSK respectively have been implemented in young adolescents [8,9]. These two vaccines, however, have shown no therapeutic effects against pre-existing infections [10]. People who have persistent HPV infections are at risk of developing cancers two decades later. Therapeutic vaccines that elicit anti-tumor immune responses in these infected individuals and subsequently eradicate the infected cells are highly desired [11].
T-cell mediated immune responses are the key players for tumor regression in patients. Increasing evidence indicates that both CD4+ T helper and functional CD8+ T cells are required for effective tumor control [12,13]. Therefore, stimulating both CD4+ helper T cells and functional CD8+T cells are the key to designing successful T cell targeted vaccines for persistent infections [13]. Previous studies have demonstrated that control of several chronic CMV, EBV, and HCV infections require both virus-specific CD4+ Th1-cells and CD8+ T cells [14,15]. The lack of functional CD4+ T cell immunity against several HPV early genes such as E6 and E7 in patients with chronic HPV infections or cervical cancer is considered one of the major reasons for persistence of the disease [5,16,17]. Therefore, combinations of CD4+ Th1 cells and specific strong CD8 T cell responses are essential for successful therapeutic responses in HPV infected patients [18,19].
The Cottontail rabbit papillomavirus (CRPV) rabbit model has played a pivotal role in the development of the two existing prophylactic vaccines [20,21]. HPV was not linked to cervical cancer until definitive evidence was found by ground breaking studies from Dr. ZurHausen's group in early 1980s [22]. HPV does not infect any laboratory animals because of the strict species-specific restrictions of these viruses. Therefore, it is not possible to directly test HPV vaccines against HPV infections experimentally [23,24]. CRPV infection shares many HPV disease properties including persistent infections that can progress to cancer. Previous studies have shown that vaccination with the non-structural early antigens E1, E2, E6, E7 and E8 prevent progression of CRPV-induced epithelial lesions and may even induce regression of CRPV-induced carcinomas [25-27]. Clinical trials based on the findings from animal PV models have been conducted in humans [28]. At the same time, novel strategies to stimulate strong therapeutic immune responses are being continuously explored. One such strategy includes long-peptide vaccines consisting of peptides that overlap and span the entire sequence of papillomavirus E6 and E7 proteins. These peptide vaccines have shown promising results in both preclinical animal models and clinical trials [29,30]. The long peptide vaccines induced strong CD4+ and CD8+ T-cell immunity in immunized hosts [31,32].
In this study, we tested long-peptide vaccines in our recently established CRPV/HLA-A2.1 transgenic rabbit model system [33]. The CRPV/HLA-A2.1 transgenic rabbit model has been used to test DNA and short peptide vaccines against HLA-A2.1 restricted epitopes from CRPV, HPV 16 and HSV antigens [34] (and unpublished observations). We have verified HLA-A2.1 restricted targets from CRPV E1 for both prophylactic and therapeutic T-cell bases vaccines [35]. Two of these CRPVE1 epitopes (E1/149-157 and E1/161-169) are adjacent to each other in E1 and we designed a long peptide vaccine containing a TT helper motif and both these two epitopes to test therapeutic potential in persistently infected transgenic rabbits.
2. Materials and methods
2.1. Rabbits, viral DNA infection and tumor measurement
HLA-A2.1 transgenic outbred rabbits were maintained in our animal facility [33]. Sixteen HLA-A2.1 transgenic rabbits (average weight 4kg, N=4/per group) were used for this study. The experiment was conducted following IACUC approval from Pennsylvania State University College of Medicine. The rabbits were challenged with wild type CRPV at four back skin sites as described previously [36]. 5μg DNA was used for each challenge site. The papillomas became evident approximately 3-4 weeks after challenge and were measured in three dimensions (length ×width ×height) and recorded weekly. Tumor size was calculated based on the geometric volume of each papilloma. The same investigator performed the measurements throughout the study.
2.2. HLA-A2.1 expression levels on different cell surfaces
HLA-A2.1 expression on both skin tissues (ear) and spleen cells were tested by either immunohistochemistry or flow cytometry as described previously [33]. Positivity of skin cells were graded as minimum of “+” to maximum of “++++” according to microscopic visual evaluation. The percentage (%) of A2 positive cells were calculated automatically based on cells staining stronger than M1 from flow cytometry profiles from peripheral blood mononuclear cells.
2.3. Peptides and vaccination
Peptides
Three of the four peptides were synthesized by ChinaPeptides Co., Ltd. (Shanghai, China). The purity of peptides was over 85%. Peptide 1 (TT-E1 peptide): QYIKANSKFIGITEL ILNANTARVKHLLLFRQAHSVSFS; peptide 2 (E1 peptide): ILNANTARVKHLLLFRQAHSVSFS (CRPVE1/149-172); peptide 3 (E1 peptide-TT): ILNANTARVKHLLLFRQAHSVSFS QYIKANSKFIGITEL and peptide 4 (TT-control peptide): QYIKANSKFIGITELLLMGTLGIV (Synthesized in the core facility of Hershey Medical Center). The underlined amino acid residues represent the Tetanus Toxin helper motif. Peptides 1-3 contained CRPVE1/141-172 with two HLA-A2.1 restricted epitopes (E1/149-157 and E1/161-169) that were demonstrated to be protective and /or therapeutic in previous studies using DNA-based vaccines [37]. Peptide 4 contained a HLA-A2.1 restricted eptiope (HPV16E7/82-90) and was used as control for peptide 1 to 3.
Vaccination
Sixteen HLA-A2.1 outbred transgenic rabbits were divided into four groups (N=4/per group). For each vaccination, one hundred micrograms of each peptide was mixed with phosphate buffered saline (PBS) in 0.1% DMSO to a final volume of approximately 300 μL for each rabbit. The concentration of peptide used was based on the initial studies demonstrating efficacy [29]. An emulsion was made with peptide (100μg) and Montanide ISA 51 (SEPPIC, 1:1, Vol/Vol) and 600ul was injected intramuscularly in the hind leg for a total of four times at a two-week interval.
2.3. Detection of peptide-specific antibodies in sera
Sera from each rabbit were collected at the termination of the experiment and tested by ELISA for peptide specific antibodies. 1 microgram peptide/per well was used to coat a 96 well plate (50μM sodium carbonate, pH9.6) at 37°C overnight. Triplicate wells were tested for each serum sample. Multiple negative controls were used including second antibody alone, PBScoated wells and pre-immune sera for all rabbits. The plate was blocked with PBS, 2% BSA at room temperature for 1 h, incubated with 1:100 dilution of sera from each rabbit followed by incubation with goat anti-rabbit IgG-AP (1:2000, SouthernBiotech) for 1 hour at room temperature. After washing, the plate was incubated with 100μl/each well 1% 4-nitrophenyl phosphate disodium salt hexahydrate tablet (Sigma) in alkaline phosphate substrate buffer (pH9.5). Color development was measured at 450 nm using a microplate reader (Thermolabsystems). The mean and standard error of the OD values of triplicates from each sample were calculated automatically and graphs were generated with SigmaPlot 11.2 software.
2.4.Tetramer binding assay
Spleen cells from all rabbits were harvested at the termination of the experiment. 0.5·106 suspending spleen cells from each rabbit were washed with RPMI1640 medium and cultured in RPMI1640 with 5% autologous serum together with human recombinant IL-2 (5-10U/ml) as described previously [38]. After two in vitro stimulations with autologous cells pulsed with corresponding peptide, the bulk CTLs were analyzed with corresponding tetramers (generously prepared by the NIH tetramer facility). The tetramer positive CD8 T cells were gated and calculated automatically with cytometry analysis software in the core facility of Pennsylvania State University College of Medicine.
2.6. Histology of tumors
Two of the four tumors from each animal from all four groups were harvested at the termination of the experiment. The tumor tissues were fixed in 10% formalin and processed for hematoxylin and eosin staining as described previously [39]. The slides were examined under the microscope and the digital images of each sample were taken for further analysis. The approximate infiltrated lymphocytes were counted and recorded. The cell number less than 10 was considered negative (-), more than 10 was considered positive (+).
2.7. Immunohistochemistry
Frozen tumor tissues harvested at the termination of the experiment were examined for CD4 and CD8+T cell infiltrates. Anti-rabbit CD8 (Antigenix American, Cat#MRB8020) and CD4 (Spring Valley laboratory, MD) monoclonal antibody was diluted to 1:20 for immunostaining as described previously [33]. The positive CD4 and CD8 T cells were counted and recorded accordingly.
3. Results
3.1 HLA-A2.1 expression levels on ear tissues and spleen cells
We tested HLA-A2.1 expression levels on both rabbit ear skin and spleen cells by either immunohistochemistry or flow cytometry (Table 1). The expression levels on ear skin was evaluated and graded following microscopic examination (Table 1, supplemental Figure 1A). The animals were assigned into different groups based by balancing gender, birth date as well as A2 expression levels at their ear skins. No significant difference in A2 expression levels on spleen cells was found among the animals from the four groups in the experiment (supplemental Figure 1B, P>0.05, one way ANOVA analysis).
Table 1. HLA-A2.1 expression levels on spleen cells and ear skin tissues by flow cytometry and immunohistochemistry respectively.
| Group (Vaccine) |
Rabbit ID | HLA-A 2.1 | |
|---|---|---|---|
| Positive cells on spleen cells (M1%) | Positivity on ear tissue * | ||
| Group 1 (TT-E1peptide) |
3206 | 56.40 | ++/+++ |
| 3219 | 30.02 | +/++ | |
| 2914 | 38.49 | ++ | |
| 3214 | 37.52 | ++ | |
| Group 2 (E1 peptide) |
3207 | 32.51 | ++/+++ |
| 3222 | 56.70 | +++ | |
| 2916 | 28.15 | ++ | |
| 3216 | 31.82 | ++ | |
| Group 3 (E1 peptide-TT) |
3210 | 54.19 | ++ |
| 3223 | 57.14 | +/++ | |
| 2917 | 52.28 | ++/+++ | |
| 3217 | 36.48 | +/++ | |
| Group 4 (TT-control) |
3211 | 46.06 | +/++ |
| 3224 | 73.40 | ++ | |
| 2918 | 52.70 | +/++ | |
| 3218 | 44.91 | +/++ | |
grading of A2 positivity by microscope based on visual inspection. “+” to “ +++” represents from weak to strong A2 staining (as shown in supplemental Figure 1).
3.2 Antibody generation in long-peptide immunized animals
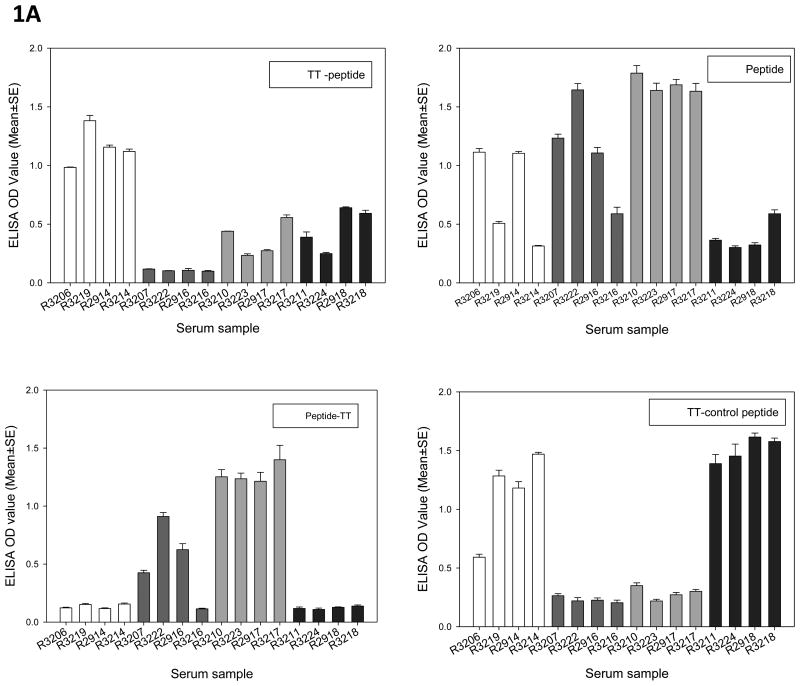
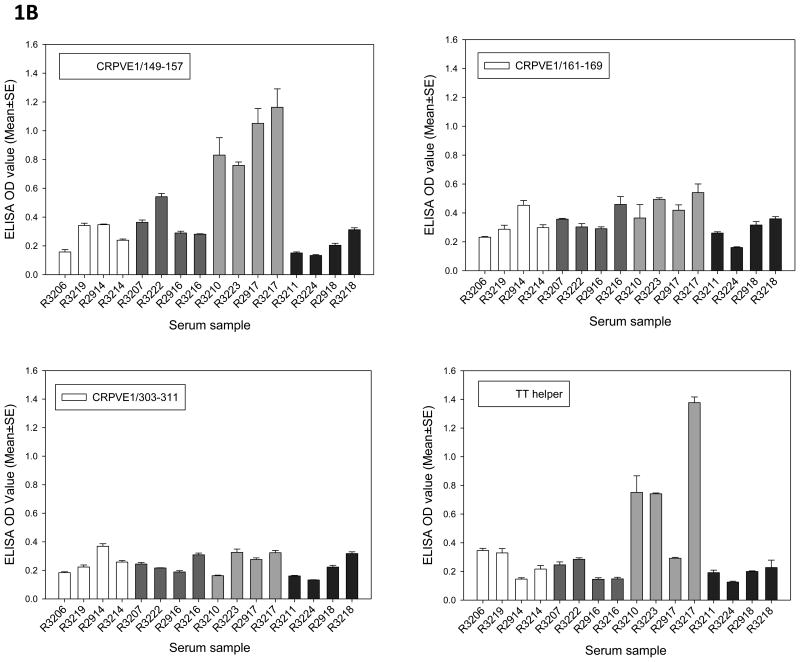
The long peptides contain two HLA-A2.1 restricted epitopes (CRPVE1/149-157 and CRPVE1/161-169) and/or a TT helper motif. Serum samples collected at the termination of the experiment were tested for antibodies against each long peptide as well as to the CD8 epitopes and the TT helper peptide. All rabbits generated strong antibody responses against their immunizing long peptide (Figure 1A). Cross-reactive antibodies against other peptides were also detected in some animals. For example, serum from TT-E1 peptide immunized rabbits also generated antibodies against TT-control peptide while serum from peptide and E1 peptide-TT immunized animals generated antibodies to the E1 epitope but not against the TT-E1 peptide. Sera from TT-control peptide immunized animals did not show any cross-reactivity to other E1 peptides (Figure 1A).
Figure 1.
Triplicates were set up for each serum sample as described in the Methods section. Serum samples from Rabbits immunized with TT-peptide (white), peptide(dark gray), peptide-TT(gray) or TT-control peptide(dark) were tested for antibody generation against each peptide. A) Animals from each group responded to their immunized peptide strongly. B) Animals from peptide-TT immunized group also generated detectable antibodies to one of the CD8 epitopes (CRPVE1/149-157) and the TT helper motif. No strong antibody response was found in other groups.
Antibodies against individual epitopes were also detected in the animals from vaccinated groups (Figure 1B). Interestingly, rabbits from E1 peptide-TT and peptide immunized groups not only generated stronger antibodies against one of the epitopes (CRPVE1/149-157) but also to the TT helper peptide. Animals from the three other groups failed to generate antibodies against any of the E1 peptides (Figure 1B).
3.3 More tetramer positive cells were found in E1 peptide-TT helper immunized rabbits
To examine whether epitope-specific T cells were generated in these long peptide immunized animals, we tested the rabbit spleen cells after two in vitro stimulations with tetramers specific for either CRVPE1/161-169 or CRPVE1/149-157(Figure 2A and 2B). Significantly more tetramer specific CD8+ T cells to both HLA-A2.1 restricted epitopes were found in E1 peptide-TT helper immunized rabbits when compared with the control group (P<0.05, one way ANOVA analysis). More tetramer specific CD8+ T cells were also found in the two tested groups but were not significantly different when compared with the control group (P<0.05, one way ANOVA analysis).
Figure 2.
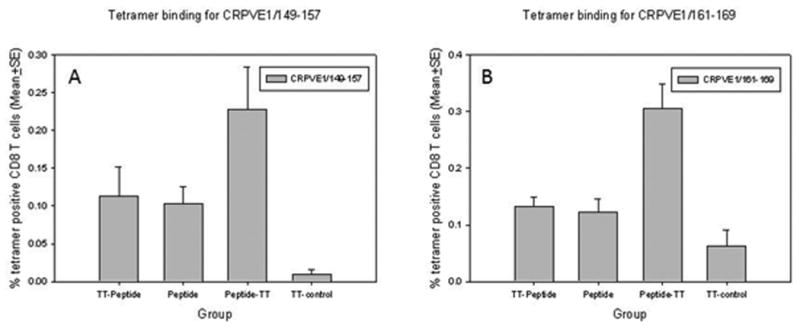
Tetramer positive CD8+ T cells after in vitro stimulation with two HLA-A2.1 restricted epitopes (A) CRPVE1/149-157 and (B) CRPVE1/161-169. Significantly more tetramer positive CD8+ T cells were found in E1 peptide-TT immunized rabbits when compared with those in the control group (P<0.05, one way ANOVA analysis). Increased numbers of tetramer binding cells were found in the other two long peptide vaccinated groups when compared to the control group but the difference was not significant (P>0.05, one way ANOVA analysis).
3.4 Tumor regression was found in rabbits immunized with E1 peptide-TT
Rabbits were immunized with long peptide vaccines at 3 weeks after viral DNA infection. Tumor outgrowth was recorded as shown in Figure 3. No significant difference in papilloma size was found among the four groups until week 9 (P>0.05, one way ANOVA analysis). At the fourth immunization time point (week 9), tumors from two out of four animals in the E1 peptide-TT immunized group regressed or significantly reduced in size. The average tumor size of E1 peptide-TT immunized rabbits was significantly smaller when compared with the other three groups (P<0.05, one way ANOVA analysis). No significant difference in papilloma size was found among the other three groups (P>0.05, one way ANOVA analysis).
Figure 3.
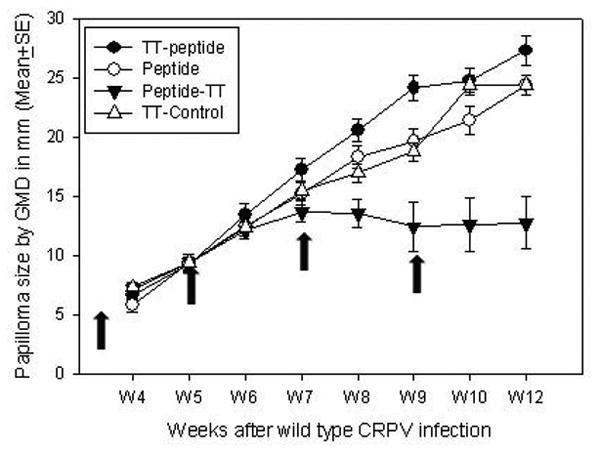
Tumor outgrowth after viral DNA infection and long peptide immunizations (arrows indicate the four immunization times: week 3, 5, 7 and 9 post infection). Significantly smaller papillomas induced by wild type CRPV infection were found in E1 peptide-TT immunized animals when compared with those from the other three groups after week 9 (P<0.05, one way ANOVA analysis). No significant difference was found among the other three groups at all the time points (P>0.05, one way ANOVA analysis).
3.5 Infiltration of CD8+ and CD4+ T cells were found in the tumor sites under regression
The tumors were examined by H&E staining. A large number of lymphocytes was found in one of the tumor sites that was undergoing regression from the E1 peptide-TT immunized group (Figure 4A). For the persistent tumors, very limited numbers of infiltrates were found (Figure 4B). Several tumors from the control group showed a malignant histology again with limited lymphocyte infiltration (Figure 4C). Approximate number of lymphocyte infiltration at corresponding tumor sites from each group were summarized in Table 2. The number that was less than 10 was considered negative (-).
Figure 4.
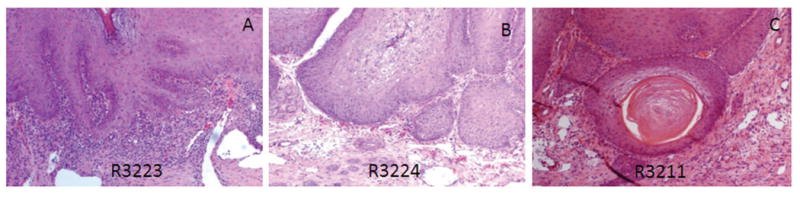
H&E staining of tumors harvested at the termination of the experiment. A) High lymphocytic infiltration was found in one (3223) of the E1 peptide-TT helper immunized rabbits; B) Low lymphocytic infiltration was found in persistent tumors as shown here from one (3224) of the control group (10×); C) A malignant tumor (early cancer) was found from one (3211) of the control group rabbits (10×).
Table 2. Summary of lymphocytic infiltration in tumor tissues (under 20×object lens).
| Group (vaccine) |
Rabbit ID | Approximate cell numbers | Outcome (histology) |
|
|---|---|---|---|---|
| Tumor site 1 | Tumor site 2 | |||
| Group 1 (TT-E1 peptide) |
3206 | -(9) | +(40) | Persistent |
| 3219 | - (10) | - (8) | Persistent | |
| 2914 | +(30) | +(30) | Persistent | |
| 3214 | - (4) | +(50) | Persistent | |
| Group 2 (E1 peptide) |
3207 | - (8) | - (8) | Persistent |
| 3222 | +(47) | - (5) | Persistent | |
| 2916 | - (9) | +(30) | Persistent | |
| 3216 | - (8) | +(35) | Persistent | |
| Group 3 (E1 peptide-TT) |
3210 | - (8) | +(30) | Persistent |
| 3223 | +(>100) | NA | Small tumor | |
| 2917 | NA | NA | Regressed | |
| 3217 | - | +(20) | Persistent | |
| Group 4 (TT-control) |
3211 | - (10) | - (5) | Early cancer* |
| 3224 | - (9) | - (6) | Early cancer | |
| 2918 | - (10) | - (4) | Persistent | |
| 3218 | +(20) | - (1) | Persistent | |
To characterize which cell populations were present in these infiltrates, the tumor tissues were further examined with anti-rabbit CD8 and CD4 monoclonal antibodies. Positive cells were counted under microscope (cell numbers/field). A population of CD8 T cells was found in the tumor sites containing large numbers of infiltrating lymphocytes (about 60 cells /field) (Figure 5B). Minimal CD8+ T cells were found in persistent tumors (about 14 cells/ field, Figure 5C). Interestingly, most of these CD8+ T cells were located along the basal layer of the tumor tissue. A large population of CD4+ T cells (>1000/field) was also identified in these infiltrates at the base of tumors (Figure 5E) and minimal numbers of CD4 + T cells (about 36 cells/field) were found in persistent tumors (Figure 5F).
Figure 5.
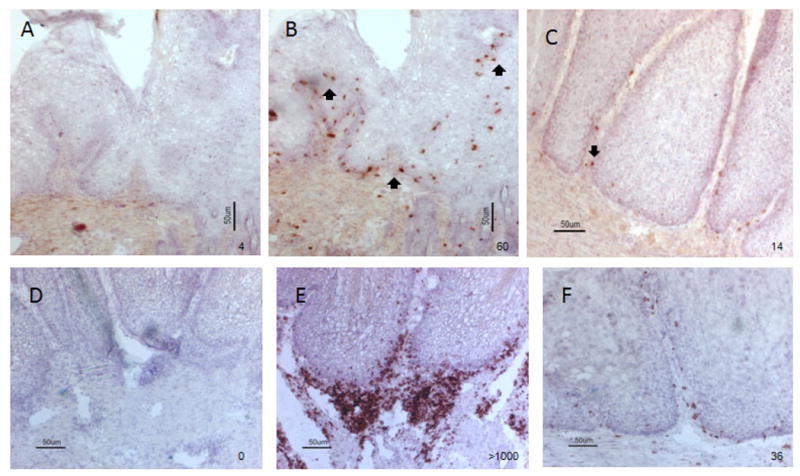
Immunohistological detection of CD8+ (A-C) and CD4+ T (D-F) cells at the sites of lymphocytic infiltration. A and D) Negative control omitting monoclonal antibody against rabbit CD8 and CD4 during the staining; B and E) A population of CD8+ and CD4+ T cells (red) were found around the basal layer of the tumor (shown by arrows) that showed high levels of lymphocytic infiltration as shown in figure 4A (10×); C and F) Low numbers of CD8+ and CD4+ T cells were found in the persistent tumors that had limited lymphocytic infiltration (10×) respectively.
4. Discussion
This study reported therapeutic immunization by long peptides that contained two HLA-A2.1 restricted epitopes and a universal TT helper motif. Our results demonstrated unexpectedly, that the location of the TT helper motif played a critical role in the outcome of the therapeutic vaccination. Specifically, TT-helper motif at the N-terminus did not induce any therapeutic response and may in fact have worsened the therapeutic effect in our transgenic rabbits. In contrast, the TT helper motif at the carboxyl-terminus provided a strong therapeutic effect by reducing tumor size and triggering regression in some rabbits.
Long peptide vaccines have been reported to successfully induce tumor regression in the CRPV/rabbit model as well as in clinical trials [18]. The investigators used high doses of pooled overlapping long peptides that covered E6 and E7 of CRPV for the preclinical therapeutic vaccination and then the same strategy for HPV therapeutic vaccine clinical trials [40]. Although this vaccine strategy has promise, high doses of long peptides in such vaccines may trigger high levels of CD4 T cell-mediated cytokine levels that may increase the risk for potential mortality [41]. Therefore, improved design and preclinical testing of long peptide vaccines would be beneficial.
Our HLA-A2.1 transgenic rabbit model has been established to test therapeutic strategies against HPV related antigens [38]. However, most of the proof-of-concept studies were conducted with CRPV which induces a natural infection and tumor growth in rabbits [35,37,42]. We recently showed that a multivalent DNA vaccine containing five HLA-A2.1 restricted epitopes from CRPV E1 gene provided not only complete protective immunity but also strong therapeutic immunity in the HLA-A2.1 transgenic rabbits [35]. We further confirmed that all five epitopes could provide protective immunity while two of these five epitopes could also be potentially therapeutic [37]. Making use of the location of two HLA-A2.1 restricted epitopes from CRPVE1 (149-157 and 161-169), we designed a long peptide vaccine that overlapped these two naturally existing CD8 epitopes. We have included a TT helper motif at the N-terminus of the epitopes to augment immune responses in our previous DNA vaccination studies and demonstrated that this motif improved immune responses when mixed with short peptides [42]. Therefore, we decided to include the TT helper motif in the present study to preclude the possibility that the long peptide lacks CD4 epitopes. Importantly, we did not know whether the location of the TT-helper motif could impact the outcome of the therapy, so we designed two long peptides with the TT helper at either the N-terminus or the carboxyl-terminus together with a long peptide with no TT-helper motif. Surprisingly, the long peptide with the TT helper motif at the carboxyl-terminus rather than at the N-terminus showed much stronger therapeutic effects in our HLA-A2.1 transgenic rabbits. The long peptide without the TT-helper motif generated only marginal therapeutic responses in rabbits. These findings indicate that the TT-helper motif is required for an effective therapy for long peptide vaccine and that the location of TT-helper motif greatly impacts therapeutic outcomes.
We analyzed both humoral and cellular immune responses stimulated by these long peptide immunizations in order to determine why the E1peptide-TT induced a stronger therapeutic effect. One explanation might be that this latter peptide is more stable than the other two. A previous study demonstrated that the stability of antigen played an important role in DNA vaccination [43]. If the E1peptide-TT was a more stable antigen, then we would expect a much stronger immune response and this was what we observed.
Long peptides are proven effective antigens for antibody generation [29]. We found that all the long peptides successfully produced strong antibody responses to the corresponding immunogens. The TT-E1 peptide also generated a cross-reactive antibody against the control vaccine which contains a TT helper motif at the N-terminus. Interestingly, all the rabbits in E1 peptide-TT group also generated antibodies against one of the CD8 T cell epitopes (CRPVE1/149-157) as well as the TT helper motif. This may indicate this long peptide was properly processed in vivo as well. Our previous study found that CRPVE1/149-157 epitope vaccine was both protective and therapeutic [37]. Overall, animals vaccinated with the E1 peptide-TT showed the highest level of antibody generation when compared with the other two long peptides. Although the antibody responses have not been found to correlate with the therapeutic outcome because humoral immunity was reported to play a minimal role in tumor clearance, the anti-peptide antibodies may have played a role in improved antigen presentation pathways for cell-mediated immune responses as antigen-antibody complexes in the later vaccinations.
We further examined the role of T-cell mediated immune responses in the outcome of therapy in these rabbits. Because the in vitro culture system is not optimal for rabbit T cells, we only detected small populations of epitope specific T cells. Nevertheless, we were able to detect statistically significantly more tetramer binding CD8 T cells for both epitopes from E1 peptide-TT immunized animals, and this finding correlated with best therapeutic outcome. In vitro T cell stimulation assay was designed to test the systematic immune responses. For our model, the local immune responses might play a more important role in eliminating the tumor cells and therefore could be more appropriate indicators. Therefore, we analyzed the local immunity by both H&E staining and immunohistochemistry assay. Increased lymphocytic infiltration was detected in the small tumor tissues from E1 peptide-TT immunized animals by H&E staining. A portion of these infiltrating lymphocytes were further confirmed as CD8+ T cells. Interestingly, these CD8+T cells were located at the basal layer of the papillomas which make them available to attack the virally infected cells at the base of the papilloma. In control persistent tumors, very few CD8+T cells were found at the same location demonstrating their likely role in eliminating infected virally infected cells. A similar pattern was found for CD4+ T cells in these tumor tissues. Therefore, stronger T cell-mediated immune responses elicited in E1 peptide-TT helper immunized animals contributed to the strong therapeutic effect we observed.
One of the animals in the E1 peptide-TT group, however, failed to respond to the treatment although this animal showed similar in vitro humoral and cellular immune responses. No lymphocytic infiltration was identified in the tumor tissues. This result resonates with findings in human studies in which populations are heterogenic and patient responses stratify into responders and non-responders. Given the fact that no human papillomavirus infection model is available, this model system will shed light on a better understanding of human papillomavirus infection in heterogenic human populations.
It is challenging to treat existing large tumors through immunization [44]. Improved preventive and therapeutic endpoints have been found in previous studies using combinations of topical treatments and DNA vaccination [45], peptide and DNA vaccines [42], DNA vaccines based on recombinant VSV [44,46] and combinations of multiple antigens [26,35,47]. Adjuvants such as Toll-like receptor agonists and CpG have been included in tumor therapeutic studies to enhance the specific adaptive immunity in hosts [48,49]. Recently, we tested siRNA targeted anti-viral therapy in our rabbit model system and showed promising preliminary findings (unpublished observations). We believe that combination therapeutic strategies that include immunotherapeutic approaches will be the optimal way to cure papillomavirus induced tumors.
Supplementary Material
Supplemental Figure 1. HLA-A2.1 expression on cell surface of rabbit ear tissues and spleen cells. A) Biopsies of rabbit ear were taken and frozen for immunohistochemistry as described previously [33]. A monoclonal antibody against HLA-A2.1 (BB7.2) was used for immunostaining. A representative sample from each group is shown. B) Spleens of rabbits were harvested and cell suspensions were tested for HLA-A2.1 expression with BB7.2 by flow cytometry. A2 positive cell populations (>M1%) were calculated automatically and recorded. No significant difference in A2 expression on spleen cells was found among the animals from the four groups (P>0.05, one way ANOVA analysis).
Acknowledgments
This work was supported by the National Cancer Institute grant R01 CA47622 from the National Institutes of Health and the Jake Gittlen Memorial Golf Tournament.
Footnotes
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Palefsky J. Human papillomavirus and anal neoplasia. Curr HIV /AIDS Rep. 2008 May;5(2):78–85. doi: 10.1007/s11904-008-0013-5. [DOI] [PubMed] [Google Scholar]
- 2.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009 Apr 1;124(7):1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi S, De Vuyst H. Human papillomavirus vaccines and anal carcinoma. Curr Opin HIV AIDS. 2009 Jan;4(1):57–63. doi: 10.1097/COH.0b013e32831b9c81. [DOI] [PubMed] [Google Scholar]
- 4.Campisi G, Giovannelli L. Controversies surrounding human papilloma virus infection, head & neck vs oral cancer, implications for prophylaxis and treatment. Head Neck Oncol. 2009;1:8. doi: 10.1186/1758-3284-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009 Feb;21(1):54–9. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 6.Van der Burg SH, Palefsky JM. Human Immunodeficiency Virus and Human Papilloma Virus - why HPV-induced lesions do not spontaneously resolve and why therapeutic vaccination can be successful. J Transl Med. 2009;7:108. doi: 10.1186/1479-5876-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theiler RN, Farr SL, Karon JM, et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women: risk factors for cervical viral shedding. Obstet Gynecol. 2010 Jun;115(6):1150–8. doi: 10.1097/AOG.0b013e3181e00927. [DOI] [PubMed] [Google Scholar]
- 8.Weaver B, Shew M, Qadadri B, et al. Natural History of Multiple Human Papillomavirus Infections in Female Adolescents With Prolonged Follow-up. J Adolesc Health. 2011 May;48(5):473–80. doi: 10.1016/j.jadohealth.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Romanowski B. Long term protection against cervical infection with the human papillomavirus: Review of currently available vaccines. Hum Vaccin. 2011 Feb 1;7(2) doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 10.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006 May;116(5):1167–73. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers AE, Kaufmann AM. Therapeutic human papillomavirus vaccination. Public Health Genomics. 2009;12(5-6):331–42. doi: 10.1159/000214923. [DOI] [PubMed] [Google Scholar]
- 12.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002 May;106(1):113–21. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubert RD, Kamphorst AO, Sarkar S, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21182–7. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson SA, Sherritt MA, Medveczky J, et al. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998 Feb 15;160(4):1717–23. [PubMed] [Google Scholar]
- 15.Renard N, Boucreux D, Lemonnier F, Inchauspe G. HLA-A2 transgenic mouse model: potential utility for development of an HCV vaccine. J Hepatol. 2000 Feb;32(2):363–4. doi: 10.1016/s0168-8278(00)80087-2. [DOI] [PubMed] [Google Scholar]
- 16.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008 Nov;69(11):771–5. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Todd RW, Roberts S, Mann CH, Luesley DM, Gallimore PH, Steele JC. Human papillomavirus (HPV) type 16-specific CD8+ T cell responses in women with high grade vulvar intraepithelial neoplasia. Int J Cancer. 2004 Mar 1;108(6):857–62. doi: 10.1002/ijc.11645. [DOI] [PubMed] [Google Scholar]
- 18.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009 Nov 5;361(19):1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 19.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010 Jun;118(6-7):455–70. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen ND, Han R, Kreider JW. Cottontail Rabbit Papillomavirus (CRPV) In: Ahmed R, Chen I, editors. Persistent Viral Infections. Sussex, England: John Wiley & Sons Ltd.; 2000. pp. 485–502. [Google Scholar]
- 21.Brandsma JL. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods Mol Med. 2005;119:217–35. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- 22.Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80:3812–5. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010 Feb;84(3):1214–20. doi: 10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fausch SC, Da Silva DM, Eiben GL, Le Poole IC, Kast WM. HPV protein/peptide vaccines: from animal models to clinical trials. Front Biosci. 2003 Jan 1;8:s81–s91. doi: 10.2741/1009. [DOI] [PubMed] [Google Scholar]
- 25.Han R, Reed CA, Cladel NM, Christensen ND. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gunmediated intracutaneous delivery but not by intramuscular injection. Vaccine. 2000 Jul 1;18(26):2937–44. doi: 10.1016/s0264-410x(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 26.Brandsma JL, Shlyankevich M, Zelterman D, Su Y. Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine. 2007 Aug 14;25(33):6158–63. doi: 10.1016/j.vaccine.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J, Cladel NM, Christensen ND. Increased immunity to cottontail rabbit papillomavirus infection in EIII/JC inbred rabbits after vaccination with a mutant E6 that correlates with spontaneous regression. Viral Immunol. 2007;20(2):320–5. doi: 10.1089/vim.2006.0104. [DOI] [PubMed] [Google Scholar]
- 28.Trimble CL, Frazer IH. Development of therapeutic HPV vaccines. Lancet Oncol. 2009 Oct;10(10):975–80. doi: 10.1016/S1470-2045(09)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005 Nov 1;23(45):5271–80. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008 Jan 1;14(1):178–87. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 31.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in endstage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008 Jan 1;14(1):169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 32.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008 Apr;38(4):1033–42. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Peng X, Budgeon LR, Cladel NM, Balogh KK, Christensen ND. Establishment of a Cottontail Rabbit Papillomavirus/HLA-A2.1 Transgenic Rabbit Model. J Virol. 2007 Jul;81(13):7171–7. doi: 10.1128/JVI.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chentoufi AA, Dasgupta G, Christensen ND, et al. A Novel HLA (HLA-A*0201) Transgenic Rabbit Model for Preclinical Evaluation of Human CD8+ T Cell Epitope-Based Vaccines against Ocular Herpes. J Immunol. 2010 Mar 1;184(5):2561–71. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, Cladel N, Peng X, Balogh K, Christensen ND. Protective immunity with an E1 multivalent epitope DNA vaccine against cottontail rabbit papillomavirus (CRPV) infection in an HLA-A2.1 transgenic rabbit model. Vaccine. 2008 Feb 6;26(6):809–16. doi: 10.1016/j.vaccine.2007.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J Virol Methods. 2008 Mar;148(1-2):34–9. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Schell TD, Peng X, Cladel NM, Balogh KK, Christensen ND. Using HLA-A2.1 Transgenic Rabbit Model to Screen and Characterize New HLA-A2.1 Restricted Epitope DNA Vaccines. J Vaccines Vaccin. 2010 Aug 20;1(1) doi: 10.4172/2157-7560.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-Transgenic Rabbit Model to Study Immunity to Papillomavirus Infection. J Immunol. 2006 Dec 1;177(11):8037–45. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, Cladel NM, Budgeon L, Balogh KK, Christensen ND. Papillomavirus DNA complementation in vivo. Virus Res. 2009 Apr 18; doi: 10.1016/j.virusres.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melief CJ, Van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008 May;8(5):351–60. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura H, Sedlik C, Jacquet A, et al. Long peptide vaccination can lead to lethality through CD4+ T cell-mediated cytokine storm. J Immunol. 2010 Jul 15;185(2):892–901. doi: 10.4049/jimmunol.1000933. [DOI] [PubMed] [Google Scholar]
- 42.Hu J, Cladel N, Balogh K, Christensen N. Mucosally delivered peptides prime strong immunity in HLA-A2.1 transgenic rabbits. Vaccine. 2010 Mar 20; doi: 10.1016/j.vaccine.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bins AD, Wolkers MC, van dB, Haanen JB, Schumacher TN. In vivo antigen stability affects DNA vaccine immunogenicity. J Immunol. 2007 Aug 15;179(4):2126–33. doi: 10.4049/jimmunol.179.4.2126. [DOI] [PubMed] [Google Scholar]
- 44.Brandsma JL, Shlyankevich M, Su Y, Zelterman D, Rose JK, Buonocore L. Reversal of papilloma growth in rabbits therapeutically vaccinated against E6 with naked DNA and/or vesicular stomatitis virus vectors. Vaccine. 2009 Jul 14; doi: 10.1016/j.vaccine.2009.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen ND, Han R, Cladel NM, Pickel MD. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob Agents Chemother. 2001 Apr;45(4):1201–9. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandsma JL, Shylankevich M, Su Y, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J Virol. 2007 Jun;81(11):5749–58. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han R, Cladel NM, Reed CA, Peng X, Christensen ND. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J Virol. 1999 Aug;73(8):7039–43. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007 Feb 1;67(3):1326–34. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta K, Cooper C. A review of the role of CpG oligodeoxynucleotides as toll-like receptor 9 agonists in prophylactic and therapeutic vaccine development in infectious diseases. Drugs R D. 2008;9(3):137–45. doi: 10.2165/00126839-200809030-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. HLA-A2.1 expression on cell surface of rabbit ear tissues and spleen cells. A) Biopsies of rabbit ear were taken and frozen for immunohistochemistry as described previously [33]. A monoclonal antibody against HLA-A2.1 (BB7.2) was used for immunostaining. A representative sample from each group is shown. B) Spleens of rabbits were harvested and cell suspensions were tested for HLA-A2.1 expression with BB7.2 by flow cytometry. A2 positive cell populations (>M1%) were calculated automatically and recorded. No significant difference in A2 expression on spleen cells was found among the animals from the four groups (P>0.05, one way ANOVA analysis).