Abstract
The Kruppel-like factor 2 (KLF2) and Kruppel-like factor 4 (KLF4) transcription factors have recently been shown to act as critical regulators of endothelial homeostasis. While several insights have been made into the signaling mechanisms orchestrating endothelial KLF2 expression, those governing the expression of KLF4 in the vascular endothelium remain largely unknown. Here, we show that diverse vasoprotective stimuli including an atheroprotective shear stress waveform, simvastatin, and resveratrol induce the expression of KLF4 in cultured human endothelial cells. We further demonstrate that the induction of KLF4 by resveratrol and atheroprotective shear stress occurs via a MEK5/MEF2-dependent signaling pathway. Since MEK5 activation is also critical for the expression of KLF2, we assessed the individual contribution of KLF4 and KLF2 to the global transcriptional activity triggered by MEK5 activation. Genome-wide transcriptional profiling of endothelial cells overexpressing KLF4, KLF2, or constitutively active MEK5 revealed that 59.2% of the genes regulated by the activation of MEK5 were similarly controlled by either KLF2 or KLF4. Collectively, our data identify a significant degree of mechanistic and functional conservation between KLF2 and KLF4, and importantly, provide further insights into the complex regulatory networks governing endothelial vasoprotection.
Keywords: Endothelial cells, KLF4, MEK5, KLF2, Shear stress, Resveratrol
Introduction
The vascular endothelium, which forms the inner most lining of blood vessels, plays a critical role in the transduction and regulation of biological responses to various types of physiological stimuli, including biomechanical and humoral signals [1-3]. The inability of the endothelium to modulate these responses and maintain a normal homeostatic state, a process known as endothelial dysfunction, is a hallmark in the development of several cardiovascular diseases including atherosclerosis [4].
The Kruppel-like family of transcription factors, in particular Kruppel-like factor 2 (KLF2) and Kruppel-like factor 4 (KLF4), are key regulators of endothelial function. Specifically, these transcription factors have been shown to coordinate transcriptional programs important for the establishment of an anti-inflammatory, vasodilatory, and anti-thrombotic vascular endothelial phenotype [5-9]. Recent work by our laboratory and others has identified KLF2 as a common critical mediator of the vascular protective effects conferred by distinct physiological and pharmacological stimuli, namely atheroprotective shear stress, statins, and resveratrol [5,10-12]. In the context of these three distinct stimuli, KLF2 is necessary for the induction of key anti-inflammatory, anti-thrombotic, and vasodilatory factors such as thrombomodulin (TM), endothelial nitric oxide synthase (eNOS), and c-type natriuretic peptide (CNP). Phylogenetic studies indicate that KLF4 is most closely related to KLF2 amongst the other members of the KLF family [13]. Indeed, these transcription factors have recently been shown to play functionally similar roles both in the regulation of self-renewal of embryonic stem cells [14] as well as the induction of key vasoprotective genes such as eNOS, TM, and CNP in the vascular endothelium [6].
While several insights have been made regarding the stimuli and molecular mechanisms governing the expression of KLF2 in endothelial cells, those mediating KLF4 expression remain largely unknown. Given the functional similarity between KLF2 and KLF4, we hypothesized that KLF4 is similarly induced by known physiological and pharmacological stimuli of KLF2, and furthermore, that some of the signaling pathways mediating the expression of these two transcription factors in endothelial cells may be conserved.
Materials and Methods
Endothelial Cell Culture, Dynamic Flow System, RNA Isolation, and Real-Time Taqman PCR
Primary human umbilical vein endothelial cells (HUVEC) were isolated and cultured as previously described [11]. For shear stress experiments, cells were exposed to an atheroprotective waveform using a dynamic flow system as described by Dai et al. [15]. Following the completion of each respective experiment, cells were lysed, RNA isolated, and real-time Taqman PCR performed as described previously by our laboratory [11].
Adenoviral-mediated Infection
Endothelial cells at 85-90% confluency were infected with either Ad-MEK5-DN or Ad-GFP (MOI=20) for 12h, washed with media, and incubated for an additional 12h after media exchange. Cells were then treated for 8h with either 100μM resveratrol (Sigma) or ethanol vehicle. For Ad-MEF2ASA and Ad-GFP (MOI=50) experiments, endothelial cells at 85-90% confluency were infected for 24h, then washed with media and incubated for an additional 16h after media exchange. Cells were then treated for 8h with either 100μM resveratrol or ethanol vehicle. For Ad-MEK5-CA and Ad-GFP (MOI=20) experiments, cells were infected for 18h, washed with media, and incubated an additional 24h. For Ad-hKLF4-V5 and Ad-NC-V5 control (MOI=10) experiments, cells were infected and lysed 24 hours later. Samples were then processed for microarray analysis as previously described [5].
siRNA Experiments
Transfections were conducted as previously described [11] with minor modifications. Briefly, endothelial cells were transfected with siERK5 (Invitrogen Stealth siRNA HSS183373; 100nM) or siControl (Invitrogen Stealth siRNA LO GC negative control; 100nM) at a confluency of 30-40% using Oligofectamine (Invitrogen). At 24h post-transfection, cells were replated at 90-100% confluency. 45h post-transfection, cells were incubated for an additional 8h with either 100μM resveratrol or ethanol vehicle. ERK5 siRNA results were validated using an additional siRNA (Ambion; s11149) targeting a different region of the transcript.
Transcriptional Profiling
Total genome oligonucleotide microarrays from Applied Biosystems containing approximately 30,096 features representing 28,790 human genes were used. Labeling, hybridization, spot normalization, and analyses were performed as previously described [5]. Three independent experiments were run for each condition. Ad-hKLF4-V5 vs. Ad-NC-V5 control and Ad-MEK5-CA vs. Ad-GFP microarray excel data are located in the supplemental material section. Microarray data for Ad-KLF2 vs. Ad-GFP used for the multiple comparisons presented here were previously reported [5].
Western Blotting
HUVEC at 100% confluency were treated for 8 h with 100 μM resveratrol, 1.0 μM simvastatin, or ethanol vehicle. Following lysis, SDS-PAGE and immunoblotting were performed as previously described [5]. ERK5 polyclonal antibody (Cell Signaling; #3372) and alpha-tubulin monoclonal antibody (Santa Cruz Biotechnology Inc.; sc-14262) were both used at a dilution of 1:1000.
Statistics
Statistical significance was determined using Student's t-test or one-way ANOVA followed by Tukey's HSD Post-hoc test when appropriate. Differences were considered significant at P<0.05. For microarray data, gene regulation differences of p<0.001 were considered significant as determined using Z-pool statistical methodology as described previously [16].
Results
KLF4 expression in endothelial cells is induced by distinct vasoprotective stimuli
To assess our hypothesis that KLF4 expression is similarly increased by known physiological and pharmacological inducers of KLF2, human endothelial cells (EC) were exposed to an atheroprotective shear stress waveform, simvastatin, and resveratrol. As shown in Figure 1A, EC cultured under an atheroprotective shear stress waveform for 24 h displayed a significant upregulation of KLF4 mRNA expression. Likewise, EC incubated with increasing concentrations of simvastatin or resveratrol exhibited a significant induction in the expression of KLF4 (Figures 1B and 1C). Since the endothelial vasoprotective effects conferred by treatment with 1.0 μM simvastatin or 100 μM resveratrol are well characterized [10-12,17-20], we next conducted a time course analysis of KLF4 upregulation using these selected concentrations. As seen in Figure 1D and 1E, simvastatin and resveratrol displayed differing kinetics in their induction of KLF4. While the resveratrol-mediated upregulation in KLF4 expression seen at 8 h was steadily maintained through later time points, the expression of KLF4 in simvastatin-treated cells followed a statistically significant reduction after 12 h.
Fig. 1.

Distinct physiological and pharmacological vasoprotective stimuli induce the expression of endothelial KLF4. (A) KLF4 mRNA expression in HUVEC exposed to either static (no flow) or an atheroprotective shear stress waveform for 24h. (B) KLF4 mRNA expression in HUVEC following 12h treatment with 0.1, 0.25, 0.5, and 1.0 μM simvastatin or DMSO control. (C) mRNA expression of KLF4 in HUVEC incubated for 8 h with 10, 50, 100, and 250 μM resveratrol or ethanol control. (D) KLF4 mRNA expression following incubation with 1.0 μM simvastatin or DMSO control for the indicated periods of time. (E) mRNA levels of KLF4 in HUVEC cultured with 100 μM resveratrol or ethanol control for the indicated periods of time. All data are expressed as the mean +/- S.E.M. from three or four independent experiments (*p<0.05 vs. respective control; #p<0.05).
Resveratrol induces endothelial KLF4 expression via a MEK5/MEF2-dependent, ERK5-independent signaling pathway
Given the similar response of KLF4 to well-characterized KLF2 stimuli, in addition to their known overlapping functional roles in endothelial cells, we sought to assess if the mechanisms governing KLF4 and KLF2 upregulation were conserved. Since MEK5 has been shown to be critical for the resveratrol-mediated induction of KLF2 [12], we first investigated the involvement of this kinase in the induction of KLF4 by resveratrol. To this end, EC were infected with either a dominant negative MEK5 adenovirus (Ad-MEK5-DN) or control GFP virus (Ad-GFP) and subsequently treated with resveratrol. As shown in Figure 2A, MEK5-DN significantly blocked the upregulation of KLF4 by resveratrol. To determine whether MEK5 was sufficient for KLF4 induction, EC were infected with either a constitutively active form of MEK5 (Ad-MEK5-CA) or control GFP virus (Ad-GFP). As seen in Figure 2B, MEK5-CA led to a significant increase in KLF4 mRNA expression as compared to control infected cells. Together, these data establish MEK5 as a critical component for the regulation of endothelial KLF4 expression in EC.
Fig. 2.
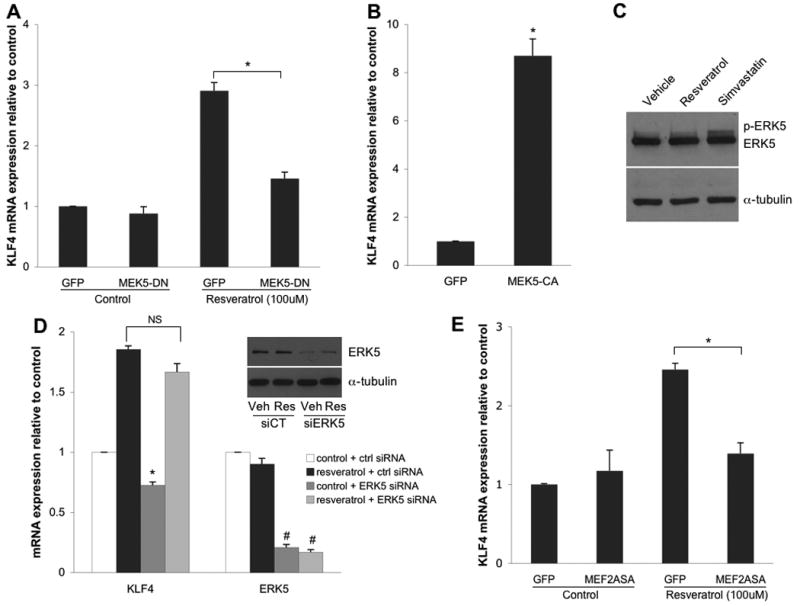
Resveratrol induces endothelial KLF4 expression via a MEK5/MEF2-dependent, ERK5-independent pathway. (A) KLF4 mRNA expression in HUVEC infected with either control GFP or MEK5-DN adenovirus followed by incubation with 100 μM resveratrol or its vehicle. (B) KLF4 mRNA expression from HUVEC infected with either control GFP or MEK5-CA adenovirus. (C) Representative western blot showing the effect on ERK5 phosphorylation from HUVEC stimulated for 8 h with either vehicle, resveratrol (100 μM), or simvastatin (1.0 μM). (D) mRNA expression of KLF4 and ERK5 from HUVEC transfected with either ERK5 siRNA or control siRNA followed by incubation with 100 μM resveratrol or its vehicle (*p<0.01 vs. control + ctrl siRNA; #p<0.001 vs. respective ctrl siRNA group). Insert shows representative western blot demonstrating the silencing efficiency of ERK5. (E) Effect of MEF2ASA or GFP adenovirus on the induction of KLF4 mRNA expression by 100 μM resveratrol or its vehicle. All data are expressed as the mean +/- S.E.M. from three independent experiments (*p<0.01).
One of the well-characterized targets of MEK5 is the extracellular signal related kinase 5 (ERK5) [21,22]. ERK5 has been shown to be activated by and required for the upregulation of KLF2 by atheroprotective shear stress [5,23,24]. Previously, our laboratory demonstrated that MEK5 is both necessary and sufficient for the ERK5 phosphorylation by atheroprotective shear stress in EC [5]. To determine the importance of ERK5 for the resveratrol-mediated induction of KLF4, we first assessed its activation by resveratrol. As shown in Figure 2C, resveratrol did not induce ERK5 phosphorylation. In contrast, treatment with simvastatin, a known ERK5 activator [12], led to the phosphorylation of ERK5. These data suggest that ERK5 activation is not necessary for the upregulation of KLF4 by resveratrol. To further define the role of ERK5 in the induction of KLF4, we muted its expression using a specific siRNA (Figure 2D). Using this siRNA, we demonstrate that while ERK5 silencing modestly reduced KLF4 expression under basal conditions, its silencing had no significant effect on the KLF4 upregulation by resveratrol (Figure 2D). Thus, these observations demonstrate that ERK5 is not necessary for the resveratrol-mediated induction of KLF4 in endothelial cells.
Previous studies have identified MEK5 as a critical activator of the myocyte enhancer factor-2 (MEF2) family of transcription factors [22,25,26]. Indeed, recent work by our group and others has shown that the MEF2 family functions as a critical regulator of endothelial KLF2 expression [5,27]. Additionally, analysis of the human KLF4 promoter revealed several putative MEF2 binding sites. Therefore, we next elucidated the role of MEF2 in the resveratrol-mediated upregulation of KLF4 by infecting EC with either a dominant negative MEF2 mutant adenovirus (Ad-MEF2ASA) or control GFP virus (Ad-GFP). As seen in Figure 2E, the upregulation of KLF4 by resveratrol was abolished in MEF2ASA infected EC, demonstrating that the MEF2 family is necessary for the induction of KLF4 by resveratrol. Importantly, we also documented that the MEK5/MEF2 pathway plays a critical role in the upregulation of endothelial KLF4 by the atheroprotective shear stress waveform (Supplemental Figure 1), suggesting that MEK5/MEF2 may function as a common pathway in the activation of KLF4 by different vasoprotective stimuli.
KLF4 expression controls multiple endothelial vasoprotective transcriptional programs
To begin to comprehensively assess the role of KLF4 in global endothelial cell gene expression, genome-wide transcriptional profiling was performed in EC overexpressing either KLF4 or a control adenoviral vector. Statistical analysis of these transcriptional profiling data identified 1,899 genes differentially regulated by the expression of KLF4 in EC. Among these genes, several were found to mediate important endothelial functions including inflammation, thrombosis, vasomotor tone, blood vessel development, and oxidative stress (Table 1). While some of these genes such as endothelial nitric oxide synthase (eNOS), thrombomodulin (THBD), interleukin 6 (IL-6), and monocyte chemoattractant protein-1 (CCL2) are established targets of both KLF4 [6] and KLF2 [5], potentially novel KLF4-specific targets including forkhead box O1 (FOXO1), vascular endothelial growth factor (VEGF), and kelch-like ECH-associated protein 1 (KEAP1) were identified. These genes, which play important roles in angiogenesis and oxidative stress, displayed no overlap with previously published endothelial KLF2 targets derived from overexpression and silencing microarray datasets obtained by our laboratory and others [5,7]. Importantly, collective analysis of these gene expression data strongly suggests that KLF4 confers an anti-inflammatory, anti-thrombotic, and vasodilatory endothelial phenotype similar to that previously defined for KLF2.
Table 1.
Select endothelial genes identified by transcriptional profiling to be differentially regulated by Ad-hKLF4-V5 vs. Ad-NC-V5 Control infected cells
| RefSeq | Gene Name | Ratio | Function |
|---|---|---|---|
| Upregulated | |||
| NM_000050 | Argininosuccinate Synthetase 1 | 25.1 | Vessel Tone |
| NM_000641 | Interleukin 11 | 18.6 | Vasoprotection |
| NM_002514 | Nephroblastoma overexpressed gene | 13.9 | Cell Communication |
| NM_000501 | Elastin | 7.0 | ECM Structural Protein |
| NM_000903 | NAD(P)H dehydrogenase, quinone 1 | 5.8 | Oxidative Stress |
| NM_001025366 | Vascular endothelial growth factora | 4.1 | Angiogenesis |
| NM_000361 | Thrombomodulin | 3.8 | Thrombosis/Hemostasis |
| NM_002133 | Heme oxygenase 1 | 3.2 | Inflammation/Oxidative Stress |
| NM_000603 | Nitric oxide synthase 3 | 2.6 | Vessel Tone |
| NM_000459 | TEK tyrosine kinase | 1.6 | Vascular Development |
| Downregulated | |||
| NM_203500 | Kelch-like ECH-associated protein 1a | -1.4 | Oxidative Stress |
| NM_002015 | Forkhead box O1a | -1.7 | Cell Proliferation/Oxidative Stress |
| NM_001200 | Bone morphogenetic protein 2 | -1.8 | Inflammation |
| NM_016270 | Kruppel-like factor 2 | -2.7 | Vasoprotection |
| NM_001147 | Angiopoietin 2 | -2.9 | Angiogenesis |
| NM_000584 | Interleukin 8 | -2.9 | Inflammation |
| NM_001955 | Endothelin 1 | -3.0 | Vessel Tone |
| NM_000600 | Interleukin 6 | -3.1 | Inflammation |
| NM_003246 | Thrombospondin 1 | -3.5 | Angiogenesis/Platelet Aggregation |
| NM_002982 | Chemokine (C-C motif) ligand 2 | -7.3 | Inflammation |
Gene not a published transcriptional target of endothelial KLF2
MEK5 activation evokes endothelial gene expression programs similar to those triggered by the expression of KLF2 or KLF4 in endothelial cells
Since we have established MEK5 as a critical inducer of both KLF2 [5] and KLF4 expression in endothelial cells, we next sought to assess the global transcriptional activity evoked by MEK5 activation and compare it with that triggered by the expression of KLF2 or KLF4. To this end, we conducted a genome-wide transcriptional profiling screen of EC infected with either Ad-MEK5-CA or Ad-GFP as a control. Comparison of the downstream targets regulated by MEK5 and either KLF2 or KLF4 revealed a substantial overlap (Figure 3A). Specifically, 383 (59.2%) of the MEK5-regulated genes were similarly modulated (up and down) by either KLF2 or KLF4, suggesting that these Kruppel factors function as critical transcriptional regulators for the activation/repression of genes downstream of MEK5 activation. Among the genes identified to be commonly regulated by MEK5, KLF2, and KLF4 (Figure 3B) were TEK tyrosine kinase (Tie-2), argininosuccinate synthetase 1 (ASS1), nephroblastoma overexpressed gene (NOV), and interleukin 8 (IL-8), all of which mediate diverse endothelial functions including vasomotor tone, inflammation, and blood vessel development. Given the strong similarity between the previously published downstream transcriptional targets of KLF2, and those described here for KLF4, we next determined the degree of global overlap between KLF2 and KLF4-regulated genes. Analyses of these datasets demonstrated that 552 (42.4%) of the genes regulated by KLF2 were similarly controlled by KLF4 (Figure 3A), suggesting a significant degree of transcriptional redundancy between these two closely related KLF members.
Fig. 3.
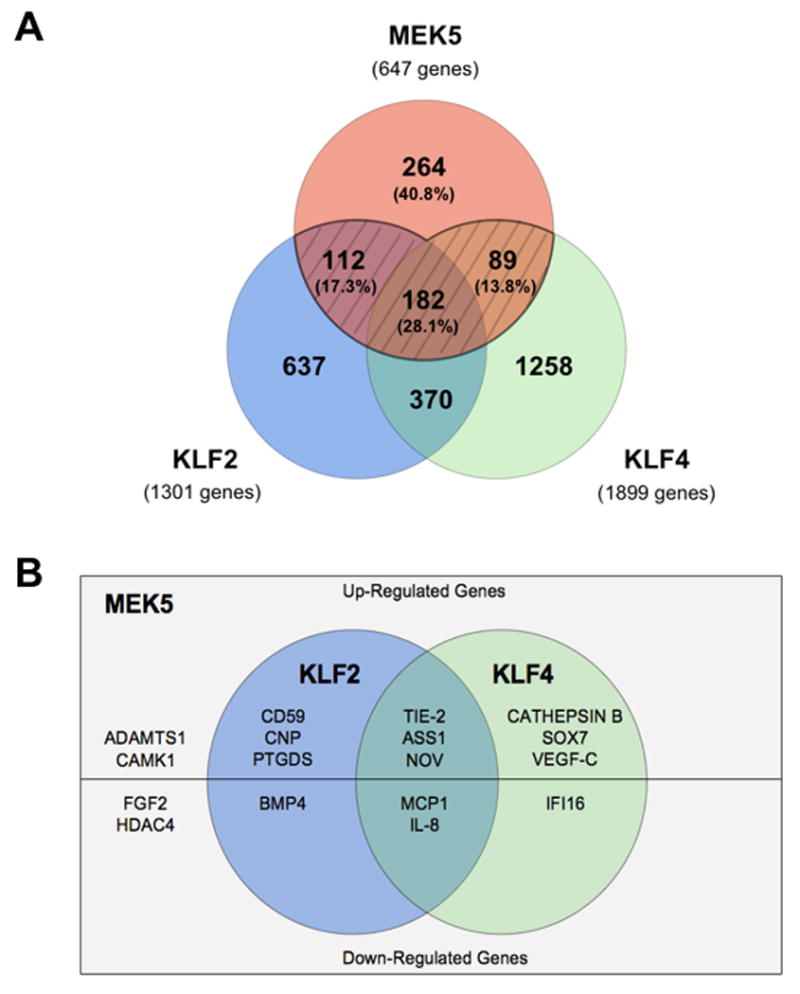
Comparative analysis of MEK5, KLF2, and KLF4 global gene regulation. (A) Venn diagram demonstrating the quantitative overlap between endothelial MEK5, KLF2, and KLF4-regulated genes. Percentages represent the proportion of all MEK5-controlled genes present in that particular sector. (B) MEK5-regulatory gene domain highlighting specific KLF2 and KLF4 targets contained within this domain.
Discussion
The vascular endothelium plays a fundamental role in the health and disease of the cardiovascular system [4]. The Kruppel-like transcription factors, KLF2 and KLF4, have previously been shown to control important aspects of endothelial function leading to the establishment of a vasoprotective phenotype [5-9]. Despite the advances that have been made in our understanding of the stimuli and molecular mechanisms governing KLF2 expression in endothelial cells, little is known regarding the regulation of KLF4 and the degree of functional convergence between these two transcription factors in the vascular endothelium.
In the present study, we demonstrate that distinct, well-characterized physiological and pharmacological stimuli of KLF2, including atheroprotective shear stress, simvastatin, and resveratrol, also induce the expression of endothelial KLF4. Using resveratrol stimulation as our specific experimental tool, we show that the signaling mechanisms governing KLF4 upregulation occur via a MEK5/MEF2-dependent, ERK5-independent pathway. These mechanisms are the same as those recently described by our group to mediate the induction of KLF2 by resveratrol in EC [12]. In addition, we demonstrate that MEK5/MEF2 are also important for KLF4 upregulation by atheroprotective shear stress, suggesting that this signaling pathway for KLF4 induction may be shared by multiple vasoprotective stimuli. Taken together, our data reveal that at least part of the regulatory machinery governing the induction of KLF2 and KLF4 by vasoprotective stimuli in endothelial cells is conserved. However, it remains to be defined if the previously documented dependencies of KLF2 induction on geranylgeranyl pyrophosphate (statins) and SIRT1 (resveratrol) are also important for KLF4 upregulation by these two stimuli.
A critical aspect of KLF4 biology that remains unexplored is the extent to which KLF4 orchestrates endothelial transcriptional programs. To begin addressing this issue, we conducted a genome-wide transcriptional profiling of cells overexpressing KLF4. These experiments revealed that 1,899 genes are differentially regulated by KLF4 expression. Among the KLF4-regulated transcripts found were a number of vasoprotective genes commonly induced by atheroprotective shear stress, simvastatin, and resveratrol. These genes included eNOS, thrombomodulin, and CNP, key mediators of the endothelial functional phenotype. Since several of the genes identified to be regulated by KLF4 in this study are also regulated by KLF2 [5], we compared the genome-wide transcriptional profiles of endothelial cells overexpressing either KLF2 or KLF4. This analysis revealed that 42.4% of the genes regulated by KLF2 were similarly controlled by KLF4, suggesting a significant degree of shared downstream transcriptional targets. Because KLF2 and KLF4 share a common canonical CACC transcriptional binding site, at the present time we cannot rule out the possibility of non-physiologically relevant gene activation/suppression resulting from this type of in vitro overexpression experiments. Importantly, Jiang et al. have demonstrated that 89% of KLF2 in vivo binding sites are shared by KLF4 in embryonic stem cells [14]. In this same study only 26% of KLF4 in vivo binding sites were shared by KLF2. This result is similar to our analysis in endothelial cells whereby 29.1% of KLF4 targets were shared by KLF2, suggesting that KLF4 may play a more extensive transcriptional regulatory role than KLF2 in endothelial cells. Notably however, KLF4 null mice do not display any reported vascular abnormalities [28], while mice lacking KLF2 show defects in vessel wall maturation, stability, and hemodynamic adaptation, and are embryonic lethal at approximately day 12.5-14.5 [29-31]. These data, together with in vitro KLF2 silencing experiments [5,11,12], suggest that KLF2 and KLF4 have important non-compensatory transcriptional regulatory functions. The specific roles of endothelial KLF2 and KLF4, and their functional overlap in the context of adult pathophysiological states remains to be defined.
In this study, we also demonstrate that MEK5 activation, which has been shown to be necessary for the establishment of an endothelial vasoprotective phenotype [5,12], functions as a critical upstream regulator of endothelial KLF4 expression. Comparisons of genome-wide expression profiles from cells overexpressing KLF2, KLF4, or a constitutively active MEK5 showed that 59.2% of the MEK5-regulated genes are controlled in a similar manner by either KLF2 or KLF4. These data indicate that these two KLF members serve as important transcriptional integrators of MEK5 activation, and importantly, identify MEK5 as a critical regulatory node for endothelial vasoprotective stimuli.
The studies presented here provide novel insights into the regulatory mechanisms regulating KLF4 expression in vascular endothelial cells. Furthermore, they identify a significant degree of mechanistic and functional conservation between KLF2 and KLF4, and indicate that this two transcription factors are critical for the downstream transcriptional programs triggered by MEK5 activation. Collectively, these observations reveal important molecular interactions involved in the complex regulatory processes mediating endothelial vasoprotection.
Supplementary Material
Supplemental Fig. 1. MEK5/MEF2 are necessary for the induction of endothelial KLF4 by atheroprotective shear stress. (A) KLF4 mRNA expression in HUVEC infected for 24 h with either control GFP or MEK5-DN adenovirus followed by 24 h exposure to either static (no flow) or atheroprotective shear stress. (B) mRNA expression of KLF4 in HUVEC infected for 24 h with either control GFP or MEF2ASA adenovirus followed by 24 h exposure to either static or atheroprotective shear stress. All data are expressed as the mean +/- S.E.M. from three independent experiments (*p<0.01).
Acknowledgments
This work was supported by the National Institutes of Health [HL-076686 and HL-090856 to G.G.-C.]; the Howard Hughes Medical Institute Research Training Fellowship for Medical Students [G.V.]; the American Federation for Aging Research/National Institute on Aging [T35 AG026781 to G.V.]; the Department of Innovation, Universities and Enterprise, Government of Catalonia, Spain, and the Spanish Association for the Study of the Liver [J.G.-S.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Cardena G, Gimbrone MA., Jr Biomechanical modulation of endothelial phenotype: implications for health and disease. Handb Exp Pharmacol. 2006:79–95. doi: 10.1007/3-540-36028-x_3. [DOI] [PubMed] [Google Scholar]
- 3.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 5.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 7.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 8.Methe H, Balcells M, Alegret Mdel C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol. 2007;292:H2167–2175. doi: 10.1152/ajpheart.00403.2006. [DOI] [PubMed] [Google Scholar]
- 9.Hiroi T, Deming CB, Zhao H, Hansen BS, Arkenbout EK, Myers TJ, McDevitt MA, Rade JJ. Proteasome Inhibitors Enhance Endothelial Thrombomodulin Expression via Induction of Kruppel-Like Transcription Factors. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 11.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 12.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of Sirt1 by Resveratrol Induces Klf2 Expression Conferring an Endothelial Vasoprotective Phenotype. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 15.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comander J, Natarajan S, Gimbrone MA, Jr, Garcia-Cardena G. Improving the statistical detection of regulated genes from microarray data using intensity-based variance estimation. BMC Genomics. 2004;5:17. doi: 10.1186/1471-2164-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles KA, Sowden H, Porter KE, Parkin SM, Homer-Vanniasinkam S, Graham AM. Simvastatin alters human endothelial cell adhesion molecule expression and inhibits leukocyte adhesion under flow. Atherosclerosis. 2008;200:69–79. doi: 10.1016/j.atherosclerosis.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Gonzalez J, Raposo B, Rodriguez C, Badimon L. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition prevents endothelial NO synthase downregulation by atherogenic levels of native LDLs: balance between transcriptional and posttranscriptional regulation. Arterioscler Thromb Vasc Biol. 2001;21:804–809. doi: 10.1161/01.atv.21.5.804. [DOI] [PubMed] [Google Scholar]
- 19.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Hao X, Yang Q, Si L. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Biochem Biophys Res Commun. 2009;388:389–394. doi: 10.1016/j.bbrc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Merritt AJ, Seyfried J, Guo C, Papadakis ES, Finegan KG, Kayahara M, Dixon J, Boot-Handford RP, Cartwright EJ, Mayer U, Tournier C. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinderlerer AR, Ali F, Johns M, Lidington EA, Leung V, Boyle JJ, Hamdulay SS, Evans PC, Haskard DO, Mason JC. KLF2-dependent, shear stress-induced expression of CD59: a novel cytoprotective mechanism against complement-mediated injury in the vasculature. J Biol Chem. 2008;283:14636–14644. doi: 10.1074/jbc.M800362200. [DOI] [PubMed] [Google Scholar]
- 24.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 25.Seyfried J, Wang X, Kharebava G, Tournier C. A novel mitogen-activated protein kinase docking site in the N terminus of MEK5alpha organizes the components of the extracellular signal-regulated kinase 5 signaling pathway. Mol Cell Biol. 2005;25:9820–9828. doi: 10.1128/MCB.25.22.9820-9828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron SJ, Abe J, Malik S, Che W, Yang J. Differential role of MEK5alpha and MEK5beta in BMK1/ERK5 activation. J Biol Chem. 2004;279:1506–1512. doi: 10.1074/jbc.M308755200. [DOI] [PubMed] [Google Scholar]
- 27.Sako K, Fukuhara S, Minami T, Hamakubo T, Song H, Kodama T, Fukamizu A, Gutkind JS, Koh GY, Mochizuki N. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 28.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Bohanan CS, Neumann JC, Lingrel JB. KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem. 2008;283:3942–3950. doi: 10.1074/jbc.M707882200. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. MEK5/MEF2 are necessary for the induction of endothelial KLF4 by atheroprotective shear stress. (A) KLF4 mRNA expression in HUVEC infected for 24 h with either control GFP or MEK5-DN adenovirus followed by 24 h exposure to either static (no flow) or atheroprotective shear stress. (B) mRNA expression of KLF4 in HUVEC infected for 24 h with either control GFP or MEF2ASA adenovirus followed by 24 h exposure to either static or atheroprotective shear stress. All data are expressed as the mean +/- S.E.M. from three independent experiments (*p<0.01).


