INTRODUCTION
Urolithiasis has been a documented medical affliction since at least ancient Egyptian civilization,1 and continues to be responsible for an increasing number of practitioner visits worldwide.2,3 Furthermore, the recurrence rates of symptomatic stones are high at more than 50% within 5 years of a first episode,4 suggesting that identifiable high-risk cohorts may experience common pathways in the pathogenesis of stone formation that can be targeted for prevention efforts. This exciting field of research continues to grow. The goal of this article is to discuss new frontiers of understanding regarding the pathophysiology of urinary stone disease.
PHYSIOCHEMICAL ASPECTS OF URINARY STONE FORMATION
At the root of the pathophysiology of urolithiasis is the physiochemical formation of urinary stones. As the glomerular filtrate passes through the nephron, the urine becomes concentrated with stone-forming salts which, when supersaturated, can precipitate out of solution into crystals that can either be expulsed with voided urine or grow and aggregate under the relative influences of various stone-promoting or stone-inhibiting agents, resulting in stone formation.5 Given an estimated transit time through the nephron of 5 to 7 minutes, traditional thought was that this did not allow sufficient time for free particles to aggregate enough to increase in size to occlude a tubular lumen and serve as a site of stone formation.6 This theory suggested that some adhesion to tubular epithelial cells as fixed particles would be required to allow for crystal growth and subsequent stone formation. Although recalculation of nephron dimensions in the context of crystal conglomeration during acute increases in supersaturation have concluded that a free-particle theory of stone formation is a potential mechanism of disease,7 research into the fixed particle mechanism has gained some favorable results. In particular, the theory has been evaluated that intraluminal deposits, mostly within the distal nephron, could serve as sites of stone formation.
Histopathologic evidence of plugs of mineral deposits has been noted in several stone-forming groups of patients, such as those with brushite stones, hyperparathyroidism, cystinuria, and distal renal tubular acidosis, and those with a history of intestinal surgery, including bypass surgery for obesity, small bowel resection, and ileostomy creation. This theory is supported by observations of stone material growing from the ends of these plugs; however, whether these intraluminal deposits lead to stone formation remains unknown. Much research must be performed to elucidate this question, and most likely this is one of multiple pathways in the pathogenesis of urinary stone disease.8,9
RANDALL PLAQUES
Associations with Urinary Stone Disease
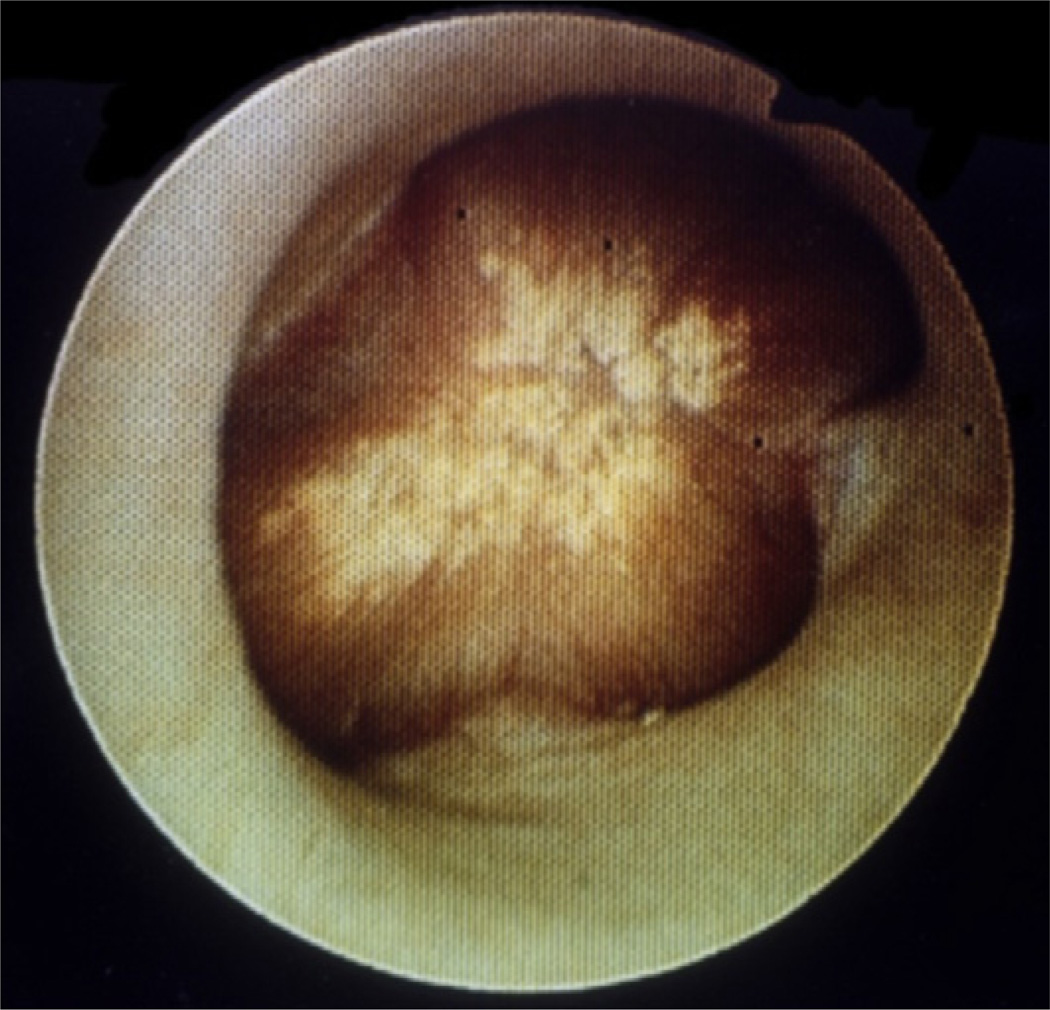
In contrast to the free and fixed particle theories of stone pathogenesis is the Randall plaque hypothesis. The theory evolved during a search for an initiating lesion for renal stones, which Alexander Randall thought originated in the renal papilla. Strong evidence was gained for this hypothesis during human autopsy studies in which calcium deposits were found in nearly 20% of renal papillae, with nearly one-third of these patients having a primary renal stone at the site. These deposits were coined “Randall plaques” and histologic analysis showed that the lesions were in the interstitial tissues of the papilla near segments of the nephron, rather than within the nephron lumen itself. Randall hypothesized the exposed plaque material served as a nidus for stone formation.10,11 This hypothesis has regained popularity over the past decade, particularly in regard to the pathogenesis of idiopathic calcium oxalate stones, the most commonly encountered stone in clinical practice. Published observations by Miller and colleagues12 during endoscopy confirmed that most stones in their study population of patients with idiopathic calcium oxalate stones, which were visually noted to be attached to and primarily originating from these Randall plaques (Fig. 1). Further evaluation from this group lent more credence to this observation. The group decided to also analyze free-floating stones encountered during ureteroscopy. More than half of these stones had mucus-covered, concavely cupped regions on one side of the stone that were found to contain apatite on micro-CT analysis of internal structure. This evidence supported the idea that these stones had also grown from a papillary plaque and then subsequently fallen off. Internal structure analysis of the remainder of the stones showed similar evidence of previous attachment to a Randall plaque at one end indicated by the presence of apatite. This finding also provided strong evidence that calcium oxalate stones arise from Randall plaques.13
Fig. 1.
A Randall plaque forming at a renal papilla, as visualized on endoscopy. No stone is currently attached.
Formation Theories
The origin of Randall plaques themselves remains an issue of debate. On histopathologic examination of the lesions, Evan and colleagues14 suggested that these plaques arise from the basement membrane of the thin loops of Henle and subsequently protrude into the epithelium of the renal papillae after expanding through the interstitium. This theory has been based on examination of renal papillae from patients with idiopathic calcium oxalate stone formation. Using light microscopic analysis, the group first confirmed that Randall’s plaques were limited only to the papillary interstitium and did not reside within the renal tubule, and then examined regions with limited versus heavy plaque burden to identify patterns of progression.14 In separate studies, they have also noted small deposits within the basement membrane of the thin loops of Henle containing varying numbers of ring-like layers of proteins, suggesting the origin of stones to be within the basement membrane itself.15,16
Other analyses of Randall plaques from cadaveric samples with radiographic and immunohistochemical analysis, however, have noted the plaques to extend deep into the papilla, into the basement membrane of the collecting tubules and the vasa recta.17 These observations have led to a vascular theory of Randall plaque formation and subsequent calcium oxalate stone development, which suggests that repair of injured papillary vasculature in an atherosclerotic-like fashion results in calcification near vessel walls that eventually erodes a calculus into the papilla through the renal papillary interstitium.
The vascular theory of Randall plaque formation is supported by 3 properties of renal physiology. The first is based on the idea that areas of turbulent flow are predisposed to inflammation and atherosclerosis. In the case of arterial plaques these locations include the bifurcation of the aorta, iliac arteries, and carotid arteries.18 Laminar blood flow changes to turbulent flow at the tip of the renal papilla because of a 180° transition, likely predisposing the area to atherosclerotic-like reactions and subsequent plaque formation. Secondly, a 10-fold or higher increase in osmolality occurs between the renal cortex and the tip of the papilla.19 In this hyperosmolar microenvironment, resident inflammatory cytokines and proteins can accumulate and promote plaque aggregation in response to vascular injury. Lastly, a decreasing gradient of oxygen-carrying capacity occurs from the renal cortex to the tip of the papilla.20 In severe cases, as with diabetes mellitus, this can translate to events such as papillary necrosis and sloughed papillae that may obstruct the ureter and create a microenvironment of inflammation. These 3 factors can promote an atherosclerotic-like response to inflammation with perivascular calcification, which may lead to Randall plaque formation.
Given a known association of esterified cholesterol with atherosclerotic processes, this vascular theory was investigated with cholesterol extraction studies on calcium oxalate stones. Analysis noted high esterified-to-free cholesterol ratios in stones with high calcium oxalate composition, providing some support for this hypothesis.21 Indirect evidence of the interaction between the vascular system and urinary stone formation has also been noted after the interesting finding that urinary stones tend to be largely unilateral and on the dependent sleeping side of patients.22 These observations prompted renal perfusion studies of patients in various sleep positions, with results noting that renal perfusion is also position-dependent. Increased renal blood flow on the dependent sleeping side of patients may lead to increased turbulence and accumulation of inflammatory elements contributing to a vascular event leading to urinary stone formation.23 This association may implicate increased renal blood flow as a contributory cause of urinary stone disease. This mechanism may work in concert with hyperfiltration, leading to increased solute deposition and subsequent accumulation of stone-forming elements. These observations open the doorway to explore the associations between vascular disease and urolithiasis.
VASCULAR DISEASE ASSOCIATIONS WITH UROLITHIASIS
To explore new frontiers in the pathogenesis of urinary stone disease, it is helpful to explore associations between urolithiasis and other phenomena, such as vascular disease. The link between urolithiasis and vascular disease is well documented in the literature. Nephrolithiasis has been associated with a 31% increased risk of myocardial infarction (MI), as documented by Rule and colleagues24 in a study of more than 4500 stone formers compared with nearly 11,000 control patients with 9 years of follow-up. The risk was noted to be independent of kidney disease or other common risk factors for MI. Data from a large cohort of nearly 10,000 women participating in the Study of Osteoporotic Fractures has similarly revealed that patients with a history of nephrolithiasis have an increased relative risk (RR) of MI (RR, 1.78) and angina (RR, 1.63).25
Although the precise mechanisms underlying these associations remain to be elucidated, one speculation is that the disease processes may have shared risk factors that have not been fully identified. One potential risk factor could be atherosclerosis, as supported by the vascular theory of Randall plaque formation. The association between nephrolithiasis and subclinical atherosclerosis was recently investigated within the Coronary Adult Risk Development in Young Adults (CARDIA) cohort, which identified a significant association between kidney stones and carotid artery atherosclerosis (odds ratio [OR], 1.6), even after adjusting for known major atherosclerotic risk factors. This study provided further support for possible common systemic pathophysiology that may be shared between vascular and urinary stone disease.26
Perhaps most well studied is the association between urinary stone disease and hypertension, which was recognized as early as the 1760s when Morgagni described a patient with clinical and anatomic findings suggestive of both diseases.27 More recent studies have confirmed these observations. In their prospective analysis of 503 men, Cappuccio and colleagues27 noted an RR of 1.96 for the development of kidney stones in hypertensive men compared with normotensive men at 8 years. Similarly, in another prospective analysis, Borghi and colleagues28 noted an OR of 5.5 linking a baseline history of hypertension to the formation of a kidney stone at 5 years of follow-up. This risk seemed particularly pronounced for individuals who were overweight. The link between hypertension and urinary stone disease seems to be potentially bidirectional, as supported by studies that have demonstrated stone formation to predate the onset of hypertension. In their prospective study of a cohort of more than 50,000 men, Madore and colleagues29 noted an association between nephrolithiasis and risk of hypertension (OR, 1.31), and reported that in men who had both disorders, 79.5% experienced the occurrence of nephrolithiasis before or concomitant to their diagnosis of hypertension. A similar association was seen in women, with an RR of 1.36 for developing a new diagnosis of hypertension in those with a history of nephrolithiasis, as demonstrated from data secured from the Nurses’ Heath study, a cohort with nearly 90,000 women.30
Although an association seems to exist between hypertension and urinary stone disease, the pathophysiology responsible for this link remains unclear. Multiple theories have been proposed, some highlighting the contribution of urinary composition to the mechanism of disease. Strazzullo and colleagues31 in a case-controlled study of 110 patients, evaluated calcium metabolism in cohorts with and without essential hypertension, noting higher urinary calcium excretion rates in hypertensive individuals despite similar total and ionized serum calcium levels. The response to intravenous calcium infusion was also investigated, showing that hypertensive patients excreted more calcium at all serum calcium concentrations, suggesting that a form of urinary leak of calcium could be occurring within hypertensive patients. Cappuccio and colleagues32 similarly recorded abnormalities of calcium metabolism in hypertensive patients, specifically highlighting increased parathyroid gland activity, urinary cyclic AMP, and intestinal calcium absorption. Increased levels of urinary uric acid33 and decreased levels of urinary citrate34 have also been seen in studies of hypertensive individuals. These risk factors for the development of urinary stones are well established.35,36 Differences in urinary composition of magnesium and oxalate may also contribute to the link between hypertension and urinary stone disease.28 Diet has also been implicated as a potential link between hypertension and a predisposition for urolithiasis. In particular, the known effects of increased dietary sodium, known to promote urolithiasis via hypercalciuria37 and also promote hypertension,38 has led to its consideration as a potential parsimonious factor.
Animal models have also demonstrated this association between hypertension and urinary stone disease. Although otherwise rare in animals, Wexler and McMurtry39 showed that strains of spontaneously hypertensive rats that were born normotensive and developed hypertension with maturation were prone to the development of urinary stone disease. The substrain most prone to urolithiasis also became obese with maturity and stereotypically formed microscopic stones within the kidney. These stones began in a subepithelial location before detaching and serving as a nidus for further stone growth, a mechanism reminiscent of current Randall plaque theories of stone formation. This finding also implicates other metabolic associations with urinary stone disease, such as obesity.
OBESITY, DIABETES, AND URINARY STONE DISEASE
Several studies have found significant associations between weight and body mass index (BMI) and urinary stones. Taylor and colleagues,40 in an analysis of 3 large prospective cohorts of nearly 250,000 individuals, showed that the RR of incident kidney stone formation for people weighing more than 100 kg, compared with those weighing less than 68.2 kg, was 1.44 in men, 1.89 in older women, and 1.92 in younger women. Using a BMI cutoff of 30, the RRs were 1.33, 1.90, and 2.09, respectively. Similarly, in a study of more than 800 renal stone formers, Del Valle and colleagues41 showed that most patients (nearly 60%) were either overweight or obese. In 2006, Taylor and Curhan42 investigated the relationship of BMI as a continuous variable to stone formation, and noted that even in nonobese patients (BMI <30), an increasing BMI lent itself to a higher risk of urolithiasis. The effect was most significant in women, wherein those with a BMI of 23 to 24.9 had a 25% increased incidence of stones compared with those with a BMI of 21 to 22.9. Those with a BMI of 27.5 to 29.9 had a 65% to 75% increased incidence. Similar results were seen in men, wherein those with a BMI of 25 to 29.9 had a 15% to 25% increase in stone incidence compared with those with a BMI of 21 to 22.9. These findings support the idea that increasing weight and BMI are directly correlated to susceptibility to urinary stone formation.
Multiple groups have investigated urine chemistries to better characterize the links between BMI and urinary stone disease. Ekeruo and colleagues,43 for example, noted that obese (BMI >30) urinary stone formers most commonly had evidence of hypocitraturia (54%) and hyperuricosuria (43%) compared with nonobese stone formers. Taylor and Curhan42 and Powell and colleagues44 similarly investigated urine chemistries, showing increased urinary excretion of oxalate, uric acid, phosphate, sodium, sulfate, and cysteine44 in obese versus nonobese patients. Urinary composition in the obese population seems to contain higher levels of substances known to precipitate urinary stones compared with the nonobese population.
The close association between obesity and diabetes, another known risk factor for urolithiasis, may compound the influence of obesity on the development of urinary stones. Obesity has been shown to carry with it a well-established increased risk for diabetes mellitus.45,46 In several large-scale studies, patients with diabetes have been closely linked to increased risk of formation of all types of urinary calculi47,48 and increased risk of uric acid stone formation in particular.49 Several pathophysiologic mechanisms have been suggested to explain these observations. One explanation offered by Canda and Isgoren50 stems from their observation of decreased function of interstitial cells and neural tissue within the urothelial tissue of diabetic rabbits. They suggested that these perturbations of function could affect ureteral peristalsis and promote urinary stone formation by virtue of urinary stasis. Other authors, however, suggest that the insulin resistance seen in diabetics is the underlying mechanism through which stones form. Insulin resistance has been noted to impair renal ammoniagenesis, resulting in acidic urine. It also promotes reabsorption of uric acid in the proximal tubule, resulting in hyper-uricemia. Both of these factors could contribute to an increased propensity for uric acid urolithiasis.51 Hyperglycemia has also been associated with increased urinary calcium52 and oxalate53 excretion. Taken together, these metabolic changes may explain the consistent association seen between diabetes and urinary stone disease.
DYSLIPIDEMIA AND URINARY STONE DISEASE
The links between dyslipidemia and urinary stone disease have also been investigated. Kadlec and colleagues,54 in their retrospective review of nearly 600 endourologic stone procedures for which stone composition data were available, noted that more than 30% of their cohort was characterized as dyslipidemic (defined by the use of a cholesterol-lowering medication). Of these patients with dyslipidemia, nearly 70% had calcium oxalate stones and 15% had uric acid stones. A recent study by Inci and colleagues55 similarly found that total cholesterol levels were significantly higher in stone formers compared with patients who do not form stones, with the association noted to be particularly prominent for calcium oxalate and uric acid stone formers.
To evaluate the potential pathophysiologic mechanisms linking dyslipidemia with urinary stone disease, related research on atorvastatin may be useful to consider. Atorvastatin is a commonly prescribed drug used to decrease serum cholesterol levels. Tsujihata and colleagues56,57 reported that the administration of atorvastatin to stone-forming rats significantly lowered crystalline deposits on quantitative light microscopy analysis of excised kidney specimens. They hypothesized that anti-inflammatory and antioxidative effects of the drug were responsible, through preventing renal tubular cell injury from oxalate and subsequently inhibiting renal crystal retention. In their experimental model, they found that urinary levels of biomarkers for renal tubular cell injury (N-acetyl glucosamidase) and oxidative stress (8-OHdG) were decreased significantly by atorvastatin treatment. Furthermore, atorvastatin treatment decreased the apoptosis of renal tubular cells. These results suggest that common pathophysiology shared between dyslipidemia and urinary stone formation may be related to inflammation and subsequent cellular injury of renal tubular cells.
THE METABOLIC SYNDROME AND UNIFICATION OF THE METABOLIC LINKS TO URINARY STONE DISEASE
Metabolic syndrome is the term given to a combination of risk factors that may include impaired fasting glucose, elevated blood pressure, central obesity, and dyslipidemia in the form of high serum triglycerides or low high-density lipoprotein cholesterol levels. The presence of at least 3 of these traits establishes a diagnosis.58 This syndrome has been strongly associated with various disease states, most notably diabetes and cardiovascular disease, with a documented relative risk of 3 for diabetes, and 1.78 for cardiovascular disease and death.59,60
More recently, metabolic syndrome has become the subject of increased urologic research because of continued observations that it is associated with an increased risk of urinary stone disease. West and colleagues61 examined the association between the number of metabolic syndrome traits and risk of nephrolithiasis using a national sample of patients in the United States. Prevalence of kidney stones increased with the number of traits, from 3% with 0 traits to 9.8% with 5 traits. The presence of 2 or more traits significantly increased the odds of stone disease, and the presence of 4 or more traits was associated with an approximate 2-fold increase. In a study of Italian adults, Rendina and colleagues62 similarly found an approximate 2-fold increase in the risk of stone disease for patients with metabolic syndrome. In an analysis of the individual components of the syndrome, they found that the only syndrome trait independently associated with increased stone risk on its own was hypertension. The risk of nephrolithiasis with hypertension was reported with an OR of 2.1 for men and 4.9 for women. The presence of hypertension with any other trait of metabolic syndrome further increased the risk of urolithiasis, with an OR of 2.2 compared with those individuals with hypertension alone. Jeong and colleagues63 confirmed a similar pattern in an American population, finding metabolic syndrome and the trait of hypertension as independent risk factors for the presence of urinary stones. The other components of metabolic syndrome did not independently carry a risk for kidney stone disease. Patients with metabolic syndrome had an OR of 1.25 for stone disease, and those with hypertension had an OR of 1.47. These studies suggest that synergistic effects of the components of the syndrome lead to an increased risk of urolithiasis. Therefore, the pathophysiology explaining increased urinary stone risk related to metabolic syndrome likely goes beyond simple cumulative effects on urine chemistry by the individual components of the syndrome. Underlying shared systemic influences are likely at play. The vascular theory of stone development is one hypothesis that attempts to link the components of the metabolic syndrome with urinary stone disease by considering a possible common systemic malfunction of inflammation and tissue damage as an underlying mechanism. However, further research is needed to investigate this hypothesis further, and to consider other possible unifying mechanisms of disease. This research will likely need to go beyond epidemiologic and urine composition studies to tease out the mechanisms behind the individual disease states themselves.
INTESTINAL CALCIUM ABSORPTION AND URINARY STONE DISEASE
The physiochemical understanding of stone formation has identified hypercalciuria as a clear risk factor for calcium-based stone formation, with increasing saturation of calcium within urine pushing crystallization and resultant stone formation. Absorption of calcium within the intestine has been associated with hypercalciuria,64,65 highlighting the importance of understanding the path-ophysiologic mechanisms behind this aspect of calcium metabolism. A recent study by Sorensen and colleagues66 evaluated a cohort of nearly 10,000 women followed for 20 years who were administered radioactive oral calcium assays. The impact of dietary and supplemental calcium on intestinal fractional calcium absorption and the development of urinary stone disease was determined within the cohort. Fractional calcium absorption was found to be associated with increased risk of stone formation; however, it decreased with increased dietary calcium intake. As a result, increased intake of calcium decreased the likelihood of nephrolithiasis. The effect was noted to be a decrease of at least 45% for all levels of dietary calcium intake compared with patients in the lowest quintile of intake. This observation was thought to be from active absorption of intestinal calcium at low calcium intakes compared with passive paracellular diffusion of calcium at higher intake levels, which tends to be more linear.67 With decreased intestinal calcium to bind to oxalate in the gut of these individuals, the oxalate is absorbed and ultimately excreted in greater concentration into the already hypercalciuric urine, increasing the likelihood for calcium oxalate stone formation.66
This understanding of the pathogenesis of urinary stones is not only important for this disease process but also has important implications in other disease processes. For example, several epidemiologic studies have noted an increased risk of osteoporotic fractures in patients with urinary stone disease.68–70 This association is thought to be related to multiple risk factors, including metabolic acidosis, mutual genetic factors, and abnormal bone remodeling in hypercalciuric stone formers thought to be from elevated vitamin D levels and aberrant local cytokine and growth factor signals seen in both of these patient populations.68 Sorensen and colleagues66 in their study noted that women with a history of nephrolithiasis were less likely to supplement calcium in their diet, and those who did, did so at low doses. Given that low dietary calcium is associated with osteoporotic fracture risk,71 this suggests that another simple and modifiable reason for the association between urolithiasis and osteoporotic fractures is low calcium intake by stone formers. Although the influence of calcium intake on urinary stone formation is still a subject of debate, based on these data, the authors do not recommend the restriction of dietary calcium supplementation, because no clear increased risk for urinary stones has been shown. However, calcium supplementation is important for reducing the risk of osteoporotic fracture and for maintaining bone health.72,73
HEAVY METALS AND URINARY STONE DISEASE
Traditionally, calcium hydroxyapatite is regarded as the predominant nidus for calcium-based urinary stone formation. A recent study has found that other heavy metal compounds may act comparably. Strontium is a heavy metal that is processed by the human body in much the same way as calcium, as demonstrated in intestinal absorption and renal filtration studies.74,75 This similarly divalent cation has been observed to substitute for calcium during the process of bio-mineralization in bone studies, incorporating into hydroxyapatite in bones through replacing a proportion of the calcium ions.76 These observations, and the finding that hypercalciuric stone formers were noted to have increased strontium absorption compared with normocalciuric patients,77 have recently led to investigations regarding strontium incorporation into uroliths. Using synchrotron radiation imaging techniques on human stone samples, a recent study showed that 80% of strontium in these stones appeared as strontium apatite and 20% as strontium carbonate.78 Although strontium research in urolithiasis is still in its infancy, this study suggests that strontium hydroxyapatite may serve as a nidus for calcium-based stone formation and could potentially serve as a valuable marker to study calcium-based stone pathogenesis.
Still elusive is a clear understanding of the initiating factors for the calcification process of urinary stone disease. For example, although Randall plaques are accepted as a nucleus for calcium oxalate stone formation,10–14 the process through which crystals enucleate to form the plaque remains unclear. To search for potentially responsible elements, a group in France led by Carpentier and Bazin79 performed x-ray diffraction and fluorescence studies on human Randall plaques and kidney stones to determine their chemical compositions and the nature and amount of trace elements in each. They demonstrated that zinc levels were dramatically increased in the carbapatite of Randall plaques compared with the carbapatite of kidney stones. This finding suggested a role for zinc in the formation of Randall plaques in the medullar interstitium.
The Role of Calcifying Nanoparticles
Calcifying nanoparticles (CNPs), also known as nanobacteria, were discovered more than 25 years ago as cell culture contaminants.80 They were originally described as novel microorganisms, and were isolated from human and bovine blood and blood products. They were characterized as fastidious and cytotoxic, and carbonate apatite–forming.81 The nature of these particles has since been debated, with contrasting theories—some describing them as a self-replicating form of life, and others describing them as a nonliving physicochemical phenomenon in the form of mineraloprotein complexes. Those who favor their existence as nanobacteria often cite characteristics such as morphologic similarities to bacteria; presence of DNA, RNA, and bacterial proteins; and their susceptibility to antimetabolic antimicrobials. In contrast, arguments favoring their existence as mineralo-protein complexes include their extremely small size, the absence of an accurately sequenced genome, morphologic similarities to other mineralo-protein complexes, resistance to DNase and RNase activity, and proposed chemical models of formation.82
Regardless of their origin, careful study has implicated these particles in the pathogenesis of multiple disease states, including polycystic kidney disease, cholelithiasis, prostatitis, HIV infection, atherosclerotic disease, and cardiovascular calcification.82 However, perhaps the most studied association is between these particles and urinary stone disease. Several investigators have isolated evidence of CNPs in 62% to 100% of urinary stone samples in various studies.81,83,84 Similarly, serum studies of patients with nephrolithiasis have also noted evidence of CNPs, with Chen and colleagues85 evaluating a 27-patient cohort and showing CNPs in the serum of 92% of patients with nephrolithiasis, compared with 0% of controls. The mechanism through which CNPs influence urinary stone disease has been suggested to be related to an etiologic role they may play in Randall plaques. This theory was supported by Ciftc¸ ioglu and colleagues,81 who detected CNPs in more than 70% of kidney papillae samples with Randall plaques, while conversely noting that more than 80% of papillae samples without Randall plaques were free of CNPs.
Although the precise mechanisms through which CNPs may be related to urinary stone disease remain elusive, evaluation of their involvement with atherosclerotic disease and cardiovascular calcification may provide some clues. The links between CNPs and these forms of cardiovascular disease have been evaluated in multiple studies. Puskás and colleagues86 serologically identified CNPs in most atherosclerotic plaques they examined, whereas their presence was lacking in control areas of the same vessels. Furthermore, CNPs were extracted and cultivated from most calcified sclerotic aortic and carotid samples, suggesting their involvement in atherosclerotic pathogenesis and subsequent blood vessel calcification. Similarly, Miller and colleagues87 and Bratos-Pérez and colleagues88 noted the presence of CNPs in calcified cardiac vessels and arterial plaques, and stenotic aortic valves, respectively. In an effort to investigate the nature of CNP arterial toxicity, Schwartz and colleagues89 exposed a rabbit model with unilaterally damaged carotid arteries to mineralized CNPs from kidney stones. Damaged arteries exposed to the CNPs became occluded and calcified, whereas the arteries with a healthy endothelium were resistant to exposure to the CNPs in this respect. These interesting findings note a connection between endothelial damage of blood vessels and calcification, with CNPs as a pathogenic factor. Although further studies are required to definitively establish association and theories of pathogenesis, this is one potential mechanism through which CNPs could be involved in the formation of urinary stones.
GENETIC LINKS TO URINARY STONE DISEASE
Genetic links to urolithiasis have been long established in certain heritable disorders, such as primary hyperoxaluria and the AGXT gene90; cystinuria and the SLC3A1 and SLC7A9 genes91; and xanthinuria and the XDH gene.92 Familial and twin studies have suggested that calcium-based urolithiasis may also be genetically linked, with the latter studies implicating a 50% heritability for calcium nephrolithiasis.93 This suspected heritability has prompted genome-wide association studies to determine candidate genes that may underlie stone formation. These studies have implicated genes encoding the calcium-sensing receptor (CASR), osteopontin (OPN), vitamin D receptor (VDR), and the claudin family of genes (particularly CLDN14) in calcium urolithiasis.93,94 CASR protein inhibits calcium absorption in the ascending limb in response to increased interstitial calcium. Mutations in this gene have been found to be associated with idiopathic calcium stone formation and primary hyperparathyroidism.93,94 Polymorphisms of the OPN gene, which encodes a urinary crystallization inhibitor, have been implicated in calcium urolithaisis.93 The VDR gene has also been linked to nephrolithiasis. Polymorphisms resulting in less active versions of the gene have been hypothesized to result in increased citrate re-absorption and therefore less inhibition of stone formation.94,95 The CLDN14 gene was identified in a population of subjects from Iceland and The Netherlands. Polymorphisms in this gene were associated with patients showing higher urinary calcium exretion.95
These genome-wide association studies rely on large population-based cohorts with carefully sequenced genomic data to identify subtle variations in genetic expressions. They imply that calcium-based urinary stone disease may not simply be affected by a few major genes, but rather that many genetic polymorphisms may have a sum effect resulting in increased individual susceptibility to stone formation.93 The identification of a limited set of common genetic defects that contribute to a large proportion of stone disease remains elusive, most likely because of the contributions of diet, obesity, and other environmental factors in the pathogenesis of urinary stone disease. The search for genetic links to urolithiasis is currently in its infancy but certainly holds great promise for future research into origins of urinary stone formation.
ANIMAL MODELS OF URINARY STONE DISEASE
Animal models have long been used to dissect complex disease processes into simpler components to allow for study and testing of scientific hypotheses. The known presence of various types of urinary stones and the complex, likely multifactorial causes of pathogenesis within stone types makes the use of appropriate models particularly important in urolithiasis research. As an era of whole genome sequencing is ushered in and more candidate genetic changes leading to the development of stone disease are identified, more animal models will surely need to be developed to better study the pathogenic mechanisms of stone disease in an in vivo fashion. A variety of animal models have historically been used in the investigation of urinary stone disease, including mice, rabbits, rats, and pigs. However, most studies in the literature to date have preferentially used rat models, likely because of the similarities between experimentally induced nephrolithiasis in rats with human kidney stones and the ease of inducing urolithiasis under experimental conditions. Rats have a nearly identical oxalate metabolism, can be induced to have calcium oxalate nephrolithiasis with hyperoxaluria, and produce kidney stones located on renal papillary surfaces with a similar organic and crystal matrix to humans. All of these characteristics make the rat a reasonable animal model for urolithiasis.96–99 Disadvantages of a rat model, however, include the high costs of breeding, care, and performance of gene knockout experiments. Many of these models also rely on the feeding of ethylene glycol to induce urinary stones, which may not be representative of a physiologic mechanism through which stones normally form. Some have also noted the potential existence of uncharacterized promoters or inhibitors of stone formation in rat metabolic pathways as downsides.97
A novel model of stone disease using the common fruit fly, Drosophila melanogaster, was recently developed. The feasibility of this model was seeded in the observation that the Drosophila Malpighian tubule, as the site of solute transport and excretion of calcium, uric acid, and phosphorus, is the functional equivalent of the human kidney convoluted tubule.100 The use of a Drosophila model was first published by Chen and colleagues.97 This team dissected and analyzed Drosophila Malpighian tubules with electron microscopy and x-ray spectroscopy after feeding the flies prolithogenic agents. The investigators subsequently confirmed the presence of deposited calcium oxalate crystals within the tubules. Furthermore, they were able to demonstrate appropriate changes in crystal deposition with antilithogenic agents, such as potassium citrate. Additional studies to support the translational utility of this model are currently underway by other research groups.
SUMMARY
The pathophysiology of the various forms of urinary stone disease is a complex topic. Epidemiologic research to identify high-risk cohorts and the study of urine and serum chemistries have been important in raising hypothesis-generating questions. However, many of the answers are still outstanding. Multiple, varied mechanisms have been proposed to explain the observations. Although this is valuable, the development and study of unifying theories to couple these proposed mechanisms remains the next great frontier of discovery. Genetic studies and the use of animal models will likely be important as the next steps are taken in understanding this intriguing disease and its diverse origins.
KEY POINTS.
Epidemiologic research and the study of urine and serum chemistries have created an abundance of data to help drive the formulation of pathophysiologic theories of stone formation.
The abundance of associations between nephrolithiasis and metabolic disease states forces us to reconsider existing hypotheses of stone formation, including the etiology of Randall’s plaques.
Future steps in understanding the pathophysiology of urinary stone disease will likely include genetic studies and the use of animal models.
REFERENCES
- 1.Eknoyan G. History of urolithiasis. Clin Rev Bone Miner Metabol. 2004;2:177–185. [Google Scholar]
- 2.Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13(Suppl 3):S45–S50. [PubMed] [Google Scholar]
- 3.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 4.Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med. 1989;111:1006–1009. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 5.Stoller ML, Meng MV. Urinary stone disease. Totowa (NJ): Humana Press; 2007. [Google Scholar]
- 6.Finlayson B, Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978;15:442–448. [PubMed] [Google Scholar]
- 7.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Unwin RJ, Williams JC., Jr. Renal stone disease: a commentary on the nature and significance of Randall’s plaque. Nephron Physiol. 2010;119:49–53. doi: 10.1159/000330255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coe FL, Evan AP, Lingeman JE, et al. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randall A. Papillary pathology as a precursor of primary renal calculus. J Urol. 1940;44:580. [Google Scholar]
- 12.Miller NL, Gillen DL, Williams JC, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int. 2009;103:966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller NL, Williams JC, Jr., Evan AP, et al. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall’s plaque. BJU Int. 2010;105:242–245. doi: 10.1111/j.1464-410X.2009.08637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evan AP, Coe FL, Rittling SR, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int. 2005;68:145–154. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 16.Evan AP, Bledsoe S, Worcester EM, et al. Renal inter-α-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007;72(12):1503–1511. doi: 10.1038/sj.ki.5002569. [DOI] [PubMed] [Google Scholar]
- 17.Stoller ML, Low RK, Shami GS, et al. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J Urol. 1996;156:1263–1266. doi: 10.1016/s0022-5347(01)65565-4. [DOI] [PubMed] [Google Scholar]
- 18.Olgun A, Akman S, Erbil MK. The role of RBC destruction in vascular regions with high turbulence on atherosclerosis. Med Hypotheses. 2004;63:283–284. doi: 10.1016/j.mehy.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Kwon MS, Lim SW, Kwon HM. Hypertonic stress in the kidney: a necessary evil. Physiology (Bethesda) 2009;24:186–191. doi: 10.1152/physiol.00005.2009. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol. 2006;33:961–967. doi: 10.1111/j.1440-1681.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 21.Stoller ML, Meng MV, Abrahams HM, et al. The primary stone event: a new hypothesis involving a vascular etiology. J Urol. 2004;171:1920–1924. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 22.Shekarriz B, Lu HF, Stoller ML. Correlation of unilateral urolithiasis with sleep posture. J Urol. 2001;165:1085–1087. [PubMed] [Google Scholar]
- 23.Rubenstein JN, Stackhouse GB, Stoller ML. Effect of body position on renal parenchyma perfusion as measured by nuclear scintigraphy. Urology. 2007;70:227–229. doi: 10.1016/j.urology.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 24.Rule AD, Roger VL, Melton LJ, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisner BH, Cooperberg MR, Kahn AJ, et al. Nephrolithiasis and the risk of heart disease in older women. J Urol. 2009;181:517–518. [Google Scholar]
- 26.Reiner AP, Kahn A, Eisner BH, et al. Kidney stones and subclinical atherosclerosis in young adults: the CARDIA study. J Urol. 2011;185:920–925. doi: 10.1016/j.juro.2010.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappuccio FP, Siani A, Barba G, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens. 1999;17:1017–1022. doi: 10.1097/00004872-199917070-00019. [DOI] [PubMed] [Google Scholar]
- 28.Borghi L, Meschi T, Guerra A, et al. Essential arterial hypertension and stone disease. Kidney Int. 1999;55:2397–2406. doi: 10.1046/j.1523-1755.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 29.Madore F, Stampfer MJ, Rimm EB, et al. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 30.Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 31.Strazzullo P, Nunziata V, Cirillo M. Abnormalities of calcium metabolism in essential hypertension. Clin Sci (Lond) 1983;65(2):137–141. doi: 10.1042/cs0650137. [DOI] [PubMed] [Google Scholar]
- 32.Cappuccio FP, Kalaitzidis R, Duneclift S, et al. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169–177. [PubMed] [Google Scholar]
- 33.Losito A, Nunzi EG, Covarelli C, et al. Increased acid excretion in kidney stone formers with essential hypertension. Nephrol Dial Transplant. 2008;24:137–141. doi: 10.1093/ndt/gfn468. [DOI] [PubMed] [Google Scholar]
- 34.Taylor EN, Mount DB, Forman JP, et al. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327(16):1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 36.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367(9507):333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 37.Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982;22:292–296. doi: 10.1038/ki.1982.168. [DOI] [PubMed] [Google Scholar]
- 38.Luft FC. Sodium intake and essential hypertension. Hypertension. 1982;4(5 Pt 2):14–19. doi: 10.1161/01.hyp.4.5_pt_2.iii14. III. [DOI] [PubMed] [Google Scholar]
- 39.Wexler BC, McMurtry JP. Kidney and bladder calculi in spontaneously hypertensive rats. Br J Exp Pathol. 1981;62:369. [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 41.Del Valle EE, Negri AL, Spivacow FR, et al. Metabolic diagnosis in stone formers in relation to body mass index. Urol Res. 2012;40:47–52. doi: 10.1007/s00240-011-0392-8. [DOI] [PubMed] [Google Scholar]
- 42.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159–163. doi: 10.1097/01.ju.0000128574.50588.97. [DOI] [PubMed] [Google Scholar]
- 44.Powell CR, Stoller ML, Schwartz BF, et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825–830. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 45.Thamer C, Machann J, Stefan N, et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007;15:531–538. doi: 10.1038/oby.2007.568. [DOI] [PubMed] [Google Scholar]
- 46.Blüher S, Markert J, Herget S, et al. Who should we target for diabetes prevention and diabetes risk reduction? Curr Diab Rep. 2012;12:147–156. doi: 10.1007/s11892-012-0255-x. [DOI] [PubMed] [Google Scholar]
- 47.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 48.Chung SD, Chen YK, Lin HC. Increased risk of diabetes in patients with urinary calculi: a 5-year followup study. J Urol. 2011;186:1888–1893. doi: 10.1016/j.juro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 50.Canda AE. Re: Increased risk of diabetes in patients with urinary calculi: a 5-year followup study: S.-D. Chung, Y.-K. Chen and H.-C. Lin J Urol 2011; 186: 1888–1893. J Urol. 2012;187:2279–2280. doi: 10.1016/j.juro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Abate N, Chandalia M, Cabo-Chan AV. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65(2):386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 52.Lemann J, Piering WF, Lennon EJ. Possible role of carbohydrate-induced calciuria in calcium oxalate kidney-stone formation. N Engl J Med. 1969;280:232–237. doi: 10.1056/NEJM196901302800502. [DOI] [PubMed] [Google Scholar]
- 53.Eisner BH, Porten SP, Bechis SK, et al. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010;183:2244–2248. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Kadlec AO, Greco K, Fridirici ZC, et al. Metabolic syndrome and urinary stone composition: what factors matter most? Urology. 2012;80(4):805–810. doi: 10.1016/j.urology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Inci M, Demirtas A, Sarli B, et al. Association between body mass index, lipid profiles, and types of urinary stones. Ren Fail. 2012;34(9):1140–1143. doi: 10.3109/0886022X.2012.713298. [DOI] [PubMed] [Google Scholar]
- 56.Tsujihata M, Momohara C, Yoshioka I, et al. Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol. 2008;180:2212–2217. doi: 10.1016/j.juro.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Tsujihata M, Yoshioka I, Tsujimura A, et al. Why does atorvastatin inhibit renal crystal retention? Urol Res. 2011;39:379–383. doi: 10.1007/s00240-011-0370-1. [DOI] [PubMed] [Google Scholar]
- 58.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 59.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 60.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 61.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. 2009;24:900–906. doi: 10.1093/ndt/gfn548. [DOI] [PubMed] [Google Scholar]
- 63.Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58:383–388. doi: 10.1053/j.ajkd.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Pak CY, East DA, Sanzenbacher LJ, et al. Gastrointestinal calcium absorption in nephrolithiasis. J Clin Endocrinol Metab. 1972;35:261–270. doi: 10.1210/jcem-35-2-261. [DOI] [PubMed] [Google Scholar]
- 65.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorensen MD, Eisner BH, Stone KL, et al. Impact of calcium intake and intestinal calcium absorption on kidney stones in older women: the study of osteoporotic fractures. J Urol. 2012;187:1287–1292. doi: 10.1016/j.juro.2011.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ireland P, Fordtran JS. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest. 1973;52:2672–2681. doi: 10.1172/JCI107461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maalouf NM, Kumar R, Pasch A, et al. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int. 2011;79(4):393–403. doi: 10.1038/ki.2010.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauderdale DS, Thisted RA, Wen M, et al. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res. 2001;16:1893–1898. doi: 10.1359/jbmr.2001.16.10.1893. [DOI] [PubMed] [Google Scholar]
- 70.Melton LJ, Crowson CS, Khosla S, et al. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int. 1998;53:459–464. doi: 10.1046/j.1523-1755.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 71.Garriguet D. Bone health: osteoporosis, calcium and vitamin D. Health Rep. 2011;22:7–14. [PubMed] [Google Scholar]
- 72.Dawson-Hughes B, Harris SS, Krall EA. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 73.Tang BM, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 74.Samachson J, Scheck J, Spencer H. Radiocalcium absorption at different times of day. Am J Clin Nutr. 1966;18:449–451. doi: 10.1093/ajcn/18.6.449. [DOI] [PubMed] [Google Scholar]
- 75.Vezzoli G, Baragetti I, Zerbi S, et al. Strontium absorption and excretion in normocalciuric subjects: relation to calcium metabolism. Clin Chem. 1998;44(3):586–590. [PubMed] [Google Scholar]
- 76.Li C, Paris O, Siegel S, et al. Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res. 2010;25:968–975. doi: 10.1359/jbmr.091038. [DOI] [PubMed] [Google Scholar]
- 77.Vezzoli G, Rubinacci A, Bianchin C. Intestinal calcium absorption is associated with bone mass in stone-forming women with idiopathic hypercalciuria. Am J Kidney Dis. 2003;42(6):1177–1183. doi: 10.1053/j.ajkd.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Blaschko SD, Miller J, Chi T, et al. Micro-composition of human urinary calculi using advanced imaging techniques. J Urol. 2012 doi: 10.1016/j.juro.2012.09.098. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carpentier X, Bazin D, Combes C, et al. High Zn content of Randall’s plaque: a m-X-ray fluorescence investigation. J Trace Elem Med Biol. 2011;25:160–165. doi: 10.1016/j.jtemb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Kajander EO, Kuronen I, Akerman KK, et al. Nano-bacteria from blood: the smallest culturable autonomously replicating agent on Earth. Proceedings of SPIE. 1997;3111:420–428. [Google Scholar]
- 81.Ciftçioglu N, Björklund M, Kuorikoski K, et al. Nano-bacteria: an infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–1898. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 82.Kutikhin A, Brusina, Yuzhalin A. The role of calcifying nanoparticles in biology and medicine. Int J Nanomedicine. 2012;7:339–350. doi: 10.2147/IJN.S28069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kajander EO, Ciftçioglu N. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci U S A. 1998;95:8274–8279. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khullar M, Sharma SK, Singh SK, et al. Morphological and immunological characteristics of nanobacteria from human renal stones of a north Indian population. Urol Res. 2004;32:190–195. doi: 10.1007/s00240-004-0400-3. [DOI] [PubMed] [Google Scholar]
- 85.Chen L, Huang XB, Xu QQ, et al. Cultivation and morphology of nanobacteria in sera of patients with kidney calculi. Beijing Da Xue Xue Bao. 2010;42(4):443–446. [in Chinese] [PubMed] [Google Scholar]
- 86.Puskás LG, Tiszlavicz L, Rázga Z, et al. Detection of nanobacteria-like particles in human atherosclerotic plaques. Acta Biol Hung. 2005;56:233–245. doi: 10.1556/ABiol.56.2005.3-4.7. [DOI] [PubMed] [Google Scholar]
- 87.Miller VM, Rodgers G, Charlesworth JA, et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:H1115–H11124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 88.Bratos-Pérez MA, Sánchez PL, García de Cruz S, et al. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008;29:371–376. doi: 10.1093/eurheartj/ehm592. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz MA, Lieske JC, Kumar V, et al. Human-derived nanoparticles and vascular response to injury in rabbit carotid arteries: proof of principle. Int J Nanomedicine. 2008;3:243–248. doi: 10.2147/ijn.s2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cellini B, Oppici E, Paiardini A, et al. Molecular insights into primary hyperoxaluria type 1 pathogenesis. Front Biosci. 2012;17:621–634. doi: 10.2741/3948. [DOI] [PubMed] [Google Scholar]
- 91.Eggermann T, Venghaus A, Zerres K. Cystinuria: an inborn cause of urolithiasis. Orphanet J Rare Dis. 2012;7:19. doi: 10.1186/1750-1172-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arikyants N, Sarkissian A, Hesse A, et al. Xanthinuria type I: a rare cause of urolithiasis. Pediatr Nephrol. 2007;22:310–314. doi: 10.1007/s00467-006-0267-3. [DOI] [PubMed] [Google Scholar]
- 93.Vezzoli G, Terranegra A, Arcidiacono T, et al. Genetics and calcium nephrolithiasis. Kidney Int. 2010;80:587–593. doi: 10.1038/ki.2010.430. [DOI] [PubMed] [Google Scholar]
- 94.Sayer JA. Renal stone disease. Nephron Physiol. 2011;118:p35–p44. doi: 10.1159/000320902. [DOI] [PubMed] [Google Scholar]
- 95.Thorleifsson G, Holm H, Edvardsson V, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 96.Evan AP, Bledsoe SB, Smith SB, et al. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int. 2004;65:154–161. doi: 10.1111/j.1523-1755.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 97.Chen YH, Liu HP, Chen HY, et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney Int. 2011;80:369–377. doi: 10.1038/ki.2011.80. [DOI] [PubMed] [Google Scholar]
- 98.Khan SR. Animal models of kidney stone formation: an analysis. World J Urol. 1997;15:236–243. doi: 10.1007/BF01367661. [DOI] [PubMed] [Google Scholar]
- 99.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res. 2010;38:429–438. doi: 10.1007/s00240-010-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dow JA, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol. 2010;299:F1237–F1244. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]