Abstract
OBJECTIVES
Epidemiological evidence linking diet, one of the most important modifiable environmental factors, and risk of Alzheimer's disease (AD) is rapidly increasing. Several studies have shown that higher adherence to a Mediterranean diet (MeDi) is associated with reduced risk of AD. This study examines the associations between high vs. lower adherence to a MeDi and structural MRI-based brain atrophy in key regions for AD in cognitively normal (NL) individuals with and without risk factors for AD.
DESIGN
Cross-sectional study.
SETTING
Manhattan (broader area).
PARTICIPANTS
Fifty-two NL individuals (age 54+12 y, 70% women) with complete dietary information and cross-sectional, 3D T1-weighted MRI scans were examined.
MEASUREMENTS
Subjects were dichotomized into those showing higher vs. lower adherences to the MeDi using published protocols. Estimates of cortical thickness for entorhinal cortex (EC), inferior parietal lobe, middle temporal gyrus, orbitofrontal cortex (OFC) and posterior cingulate cortex (PCC) were obtained by use of automated segmentation tools (FreeSurfer). Multivariate general linear models and linear regressions assessed the associations of MeDi with MRI measures.
RESULTS
Of the 52 participants, 20 (39%) showed higher MeDi adherence (MeDi+) and 32 (61%) showed lower adherence (MeDi-). Groups were comparable for clinical, neuropsychological measures, presence of a family history of AD (FH), and frequency of Apolipoprotein E (APOE) ε4 genotype. With and without controlling for age and total intracranial volume, MeDi+ subjects showed greater thickness of AD-vulnerable ROIs as compared to MeDi- subjects (Wilk's Lambda p=0.026). Group differences were most pronounced in OFC (p=0.001), EC (p=0.03) and PCC (p=0.04) of the left hemisphere. Adjusting for gender, education, FH, APOE status, BMI, insulin resistance scores and presence of hypertension did not attenuate the relationship.
CONCLUSION
NL individuals showing lower adherence to the MeDi had cortical thinning in the same brain regions as clinical AD patients compared to those showing higher adherence. These data indicate that the MeDi may have a protective effect against tissue loss, and suggest that dietary interventions may play a role in the prevention of AD.
Keywords: Alzheimer's disease, diet, Mediterranean diet, magnetic resonance imaging (MRI), early detection, brain imaging
INTRODUCTION
Epidemiological evidence linking diet, one of the most important modifiable environmental factors, and risk of Alzheimer's disease (AD), the most common cause of dementia, is rapidly increasing. Given the current lack of disease-modifying treatments, as well as increasing awareness that symptoms develop over many years or even decades, there has been growing interest in identifying effective strategies for prevention (1, 2). Delaying symptoms onset by as little as one year could potentially lower AD prevalence by more than 9 million cases over the next 40 years (1).
Several studies have provided evidence for dietary patterns that are protective against AD (3-8). Among possible dietary patterns (DPs), there is consensus that higher adherence to a Mediterranean diet (MeDi) is associated with reduced risk of AD (3, 4, 8-12). While regional differences may subsist, the MeDi is characterized by high intake of plant foods (i.e., fruits, nuts, legumes, and cereals); moderate consumption of dairy products, fish, poultry; with olive oil as the primary source of monounsaturated fats; low to moderate intake of wine, low intake of red meat and poultry, and very low intake of processed foods (12). This diet is known to be one of the healthiest dietary patterns in the world, and it has been associated with reduced risk of cardiovascular disease, cancer, and overall mortality rates (10, 13-15).
While there is growing interest in implementing dietary recommendations prior to the onset of symptoms of AD, the overall picture remains equivocal as clinical trials failed to show consistent relationships between the hypothesized protective nutrients and clinical outcome (16). These studies would greatly benefit from biological markers of disease as surrogate endpoints of clinical change (16), especially during the recently re-conceptualized preclinical stages of AD (2). In vivo biomarkers are needed to clarify how diet promotes healthy brain aging, and can therefore be protective against AD.
Pathologically, AD is characterized by presence of amyloid-beta (Aβ) plaques, neurofibrillary tangles and neuronal loss in selectively vulnerable brain regions (17). Neuronal loss in AD originates in the medial temporal lobes during the normal stages of cognition and spreads to cortical regions, especially posterior cingulate and parieto-temporal cortices, along with clinical progression (18). These changes can be visualized in vivo by means of Magnetic Resonance Imaging (MRI). Several studies have shown that brain atrophy can be detected on MRI several years prior to dementia onset and correlates with AD progression (17, 19-21).
MRI studies have shown that higher adherence to the MeDi is associated with reduced cerebrovascular disease burden (i.e., white matter lesions) in the elderly (22, 23). However, to the best of our knowledge, there are no MRI studies that examined the MeDi in relation to brain atrophy in cognitively normal (NL) individuals with and without risk factors of AD. Here, we investigated whether structural MRI-based measures of cortical thickness (i.e., brain atrophy) in key AD-regions differ among young to late middle-aged NL individuals as a function of higher vs. lower adherence to the MeDi.
METHODS
Participants
Among a larger pool of clinically and cognitively normal (NL) individuals participating in longitudinal brain MRI imaging studies at NYU School of Medicine, this study included a sub-set of 52 NL participants who completed clinical, laboratory, MRI exams and dietary questionnaires within 4 months of each other between 2013-2014. Subjects were derived from multiple community sources, including individuals interested in research participation, family members and caregivers of impaired patients. Informed consent was obtained from all subjects for participation in this NYU institutional review board-approved study.
All subjects underwent a thorough physical examination and a detailed medical history was recorded. Individuals with medical conditions or history of conditions that may affect brain structure or function, i.e. stroke, diabetes, head trauma, any neurodegenerative diseases, depression, hydrocephalus, intracranial mass, and infarcts on MRI, and those taking psychoactive medications were excluded. Subjects were 25-72 y of age, with education>12 y, Clinical Dementia Rating (CDR)=0, Global Deterioration Scale (GDS)<2, Mini Mental State Examination (MMSE)>28, Hamilton depression scale<16, Modified Hachinski Ischemia Scale<4 and normal cognitive test performance for age and education (24). None of the participants were diabetics, smokers, or met criteria for obesity as defined by a Body-Mass index (BMI)>30 kg/m2. While all subjects were normoglycemic young adults, the Homeostasis Model Assessment (HOMA) (25) for insulin sensitivity was calculated, as there is evidence for an association between increased insulin resistance (IR) and reduced brain volumes in AD-regions (26). Presence of hypertension (HTN) was determined based on current antihypertensive treatment or blood pressure assessments performed in a sitting position after 5 min rest (systolic blood pressure≥ 140 mmHg or diastolic blood pressure≥ 90 mmHg) (27, 28). Subjects were divided into 2 groups based on presence (HTN+) or absence of HTN (HTN-). A family history (FH) of late-onset AD that included at least one 1st degree relative whose AD onset was after age 60 was elicited using standardized questionnaires (24). DNA was obtained from venous blood samples to determine APOE genotypes using standard polymerase chain reaction (PCR) techniques (29, 30).
Dietary intake of nutrients
Dietary data regarding average food consumption over the prior year were obtained using the 61-item version of Harvard/Willett's semi-quantitative food frequency questionnaire (SFFQ) (31). The SFFQ has been used and validated for the determination of nutrient intake in the elderly as well as in young adults, yielding high reliability (31-35). The 61 food items were categorized into 30 food groups based on similarities in food and nutrient composition, and intake (grams per day) of each food group was then calculated by summing the intakes of member food items. The daily intake of nutrients was computed by multiplying the consumption frequency of each portion of every food by the nutrient content of the specified portion( 31).
Published methods were followed for the construction of the MeDi (3, 4, 8-12). Briefly, we first regressed caloric intake (in kilocalories) and calculated the derived residuals of daily gram intake for each of the following seven food categories: dairy, meat, fruits, vegetables, legumes, cereals, and fish. The median value was determined for each caloric intake-residual food category. Categories were divided into beneficial (fruits, vegetables, legumes, cereals and fish) or detrimental (meat and dairy products). A value of 0 or 1 was assigned to each subject based on their scores on each of the seven above categories, using sex-specific medians as cut-offs, following standardized scoring procedures. Specifically, (a) subjects whose consumption of beneficial components was below the median were assigned a value of 0, while those whose consumption was at or above the median were assigned a value of 1, for each of the 5 categories.
(b) Subjects whose consumption of detrimental components was at or above the median were assigned a value of 0, while those whose consumption was below the median were assigned a value of 1, for each of the 2 categories. (c) For fat intake (8th food category), we used the ratio of daily consumption of monounsaturated to saturated fats (in grams) (12) using sex-specific median cutoffs for assignment of values of 0 for low monounsaturated/saturated fats ratio (reflecting higher intake of saturated vs. monounsaturated fats) and 1 for high monounsaturated/saturated fats ratio (reflecting higher intake of monounsaturated vs. saturated fats). (d) For alcohol intake (9th food category), alcohol consumption was dichotomized into mild to moderate alcohol consumption (>0 drinks per week but <2 drinks per day in the previous year) and no (0 g/day) or more than moderate (>2 drinks per day) consumption (3, 4, 8-12). Subjects showing mild to moderate consumption were assigned a value of 1, other subjects a value of 0, as a moderate amount of alcohol consumption with meals is a characteristic component of the MeDi. The MeDi score was generated for each participant as the sum of the scores in the food categories (range 0-9), with a greater score indicating higher adherence to the MeDi. The MeDi score was analyzed as a dichotomous variable (<5: low vs. >5: high adherence) and as a continuous variable to facilitate comparison with other studies of the MeDi score.
Data Acquisition and Preparation
All subjects received a diagnostic and a research MRI study on a 1.5 T GE Signa imager (General Electric, Milwaukee, USA). The diagnostic study was performed using contiguous 3 mm axial T2-weighted images. The research scan was a 124 slice T1-weighted Fast-Gradient-Echo acquired in a sagittal orientation as 1.2 mm thick sections (field of view=25 cm, NEX=1, matrix=256x128, repetition time= 35 ms, echo time= 9 ms and flip angle=60 0, no interslice gaps). Clinical scans were used to rule out MRI evidence of hydrocephalus, intracranial mass, strokes, subcortical gray matter lacunes, non-specific white matter disease, and to identify focal white matter hyperintensities.
Volumetric segmentation, cortical surface reconstruction and parcellation of the research scans were performed using a data analysis pipeline based on the FreeSurfer software package (20, 36-38). The automated whole-brain segmentation procedure for volumetric measures of the different brain structures uses a probabilistic atlas and applies a Bayesian classification rule to assign a neuroanatomic label to each voxel (38). A label is automatically assigned to each voxel in the MRI volume based on probabilistic information automatically estimated from a manually labeled atlas. The atlas consists of a manually derived training set created by the Center for Morphometric Analysis (Massachusetts General Hospital, Harvard Medical School) from 40 individuals across the adult age range. The classification technique uses a registration procedure that is robust to anatomical variability, including the ventricular enlargement typically associated with aging, and which has been shown to be comparable in accuracy to manual labeling (38). Automated volumetric segmentation required only qualitative review to ensure that there was no technical failure of the application. The cortical surface was then reconstructed to measure thickness at each surface location, or vertex, to allow visualization of group differences at each vertex. Cortical thickness was obtained by reconstructing representations of the gray/white matter boundary and the cortical surface, and the distance between these surfaces at each point across the cortical mantle was calculated (20, 36-38). This method uses both intensity and continuity information from the entire 3D MR volume in segmentation and deformation procedures to construct representations of cortical thickness. The maps were created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps are not restricted to the voxel resolution of the original data and are thus capable of detecting sub-millimeter differences between groups (37). Maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a full width at half maximum of 15 mm and averaged across participants using a non-rigid high-dimensional spherical averaging method to align cortical folding patterns (39). This procedure provides accurate matching of morphologically homologous cortical locations among participants on the basis of each individual's anatomy while minimizing metric distortion, resulting in a measure of cortical thickness for each person at each point on the reconstructed surface. The surface was parceled into distinct regions of interest (ROIs). The cortical-surface model was manually reviewed and edited for accuracy. Minimal editing was performed according to standard, objective rules, including correction of errors in removal of non-brain areas and inclusion of white-matter areas of hypointensity adjacent to the cortical ribbon. Qualitative review and editing were performed, with blinding to the diagnostic status, by an expert neuroanatomist with more than 10 years of experience (Y.L.).
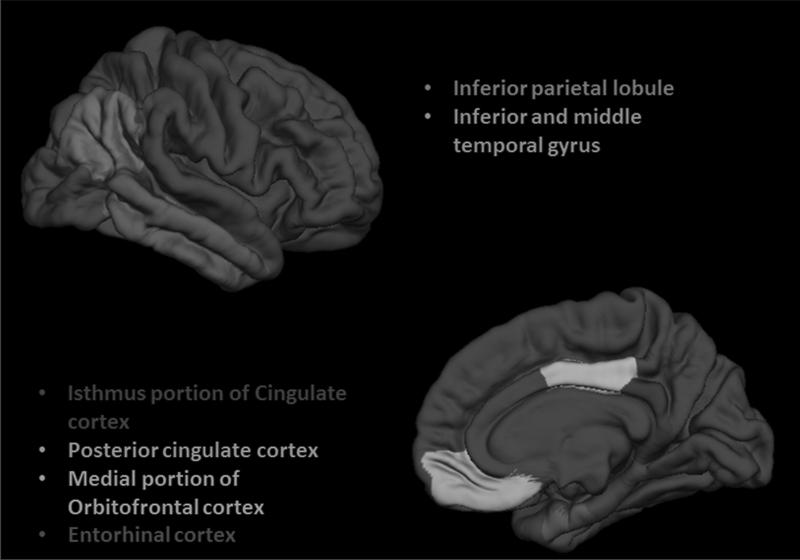
Thickness measures were calculated for 5 a priori selected ROIs which are known to show early atrophic changes in AD, including: entorhinal cortex, orbito-frontal cortex, inferior parietal lobule, inferior and middle temporal cortex and posterior cingulate cortex (17, 19-21). These ROIs were sampled separately for each hemisphere (Figure 1). The total intracranial volume (TIV) was used as the reference to account for possible differences in brain size.
Figure 1.
Three-dimensional representations of the 5 ROIs examined in the current study (only right hemisphere is shown). All of the ROIs are visible in the lateral (top) and medial (bottom) views of the gray matter surface
Statistics
SPSS v.21 (SPPS Inc., 2013) was used for data analysis. Differences in clinical and demographical measures between groups were examined with χ2 tests and the general linear model (GLM), as appropriate. All regression models were tested for violations of the model assumptions. All dependent variables were normally distributed.
Multivariate GLMs with follow-up univariate post-hoc comparisons performed using F statistics were used to test for differences in ROI thickness between groups. Multivariate GLMs were used to test for main effects of MedDi group, with brain structure (5x2 levels) as the within-subjects factor (i.e., dependent variables), and MedDi group as the between-subjects factor (i.e., independent variable). The multivariate GLM is a statistical test procedure for comparing multivariate (population) means of two or more dependent variables of several groups. Unlike univariate analysis, it uses the variance-covariance between variables in testing the statistical significance of the mean differences, and in testing for interactions among the dependent variables. Age, gender, education, TIV, FH status (positive vs. negative FH), APOE genotype (APOE ε4 carriers, APOE4+ vs. non carriers, APOE4-) [Model 1], BMI, HOMA-IR and HTN group [Model 2] were examined as confounders using two regression models so as to avoid over-fitting. Only covariates showing significant effects were retained in the models. Confounding variables which showed significant effects on the association between MRI measures and MeDi group were examined for interaction effects in adjusted models that included a 2-way interaction term. For example, to test main effects of MeDi group and MeDi × APOE interactions, GLMs were used, with brain structure (10 levels) as the within-subjects factor, and MeDi and APOE as between-subjects factors. Only significant interaction terms were retained in the model.
Results were considered significant at p<0.05. The multivariate approach controls for the multiple chances to find group differences, and it does so without assuming independence of the dependent variables, yielding corrected p values. We first examined all ROIs together (left and right hemisphere, i.e. 10 levels), and then each hemisphere separately (i.e., 5 levels). For the latter analysis, multivariate results were considered significant at a Bonferroni corrected p=0.05/2=0.025.
Linear regressions were used to evaluate the associations between MRI measures, neuropsychological measures (dependent variables), MeDi scores (independent variable), and the same confounds as above. MRI measures were regressed by age and TIV to generate age- and TIV-adjusted residuals. Neuropsychological measures were regressed by age and education to generate age- and education-adjusted residuals. Results were considered significant at p<0.05.
RESULTS
Subjects
Subjects’ characteristics are found in Table 1. Of the 52 subjects, 20 (39%) showed higher adherence to a Mediterranean diet (MeDi+) and 32 (61%) showed lower adherence (MeDi-). There were no differences between MeDi groups for clinical, demographical and neuropsychological measures, frequency of APOE4 genotype and presence of a FH of AD. The MeDi- group showed a trend towards a higher frequency of HTN+ subjects than the MeDi+ group (p=0.06, Table 1).
Table 1.
Demographic and clinical characteristics by MeDi group
| MeDi- | MeDi+ | |
|---|---|---|
| N | 32 | 20 |
| Age, y, mean (SD) | 53(13) | 55(12) |
| Gender, % female | 63% | 85% |
| Education, y, mean (SD) | 16(2) | 16(2) |
| Family history of LOAD, % positive | 69% | 60% |
| APOE ε4 status, % positive | 39% | 56% |
| Ethnicity | ||
| White | 83% | 80% |
| Black | 6% | 3% |
| Hispanic | 3% | 6% |
| Other | 8% | 11% |
| MeDi score [unitless] | 3.6(1.1) range 0-5 | 6.9(1.2) range 6-9 |
| Hypertension, % positive | 31% | 10% |
| HOMA-IR score [unitless] | 1.5(1.9) | 1.7(2.0) |
| Hip to waist ratio [unitless] | 1.18(0.12) | 1.16(0.11) |
| Blood pressure (mm/Hg) | ||
| Systolic | 117(15) | 122(12) |
| Diastolic | 69(15) | 69(18) |
| Glucose (mg/dl) | 76(9) | 81(15) |
| Cholesterol (mg/dl) | 193(35) | 206(41) |
| HDL: LDL ratio (unitless) | 0.6(0.2) | 0.5(0.2) |
| Triglycerids (mg/dl) | 86(34) | 96(49) |
| Homocysteine (micromol/l) | 10(2) | 10(3) |
| Neuropsychological tests | ||
| Mini Mental State Exam | 29(1) | 29(1) |
| Digit symbol substitution | 65(10) | 63(11) |
| Paired associates delayed recall | 7(2) | 6(3) |
| Paragraph delayed recall | 10(2) | 10(3) |
| Designs | 8(2) | 8(2) |
| Object naming | 55(12) | 53(13) |
| WAIS-vocabulary | 67(10) | 63(18) |
Values are mean (SD) unless otherwise specified; Abbreviations: MeDi = Mediterranean diet, lower (MeDi-) vs. higher (MeDi+) adherence
MeDi group differences on MRI
All regions of interest. A multivariate GLM with 10 ROI measures (i.e., dependent variables), MeDi group (i.e., independent variable), age, gender, education, APOE, FH and TIV (i.e., covariates) showed significant effects of MeDi group on MRI measures (Wilk's Lambda p=0.015). In this fully corrected model, MeDi+ subjects had overall greater thickness of AD-vulnerable ROIs as compared to MeDi- subjects. Post-hoc analysis for each structure is presented in Table 2. On post-hoc examination, group differences were most pronounced in orbitofrontal cortex (OFC, 9%, p=0.004), entorhinal cortex (EC, 6%, p=0.028) and posterior cingulate cortex (PCC, 4%, p=0.05) of the left hemisphere, and there was a non-significant linear trend for PCC of the right hemisphere (3%, p=0.11).
Table 2.
Regional MRI thickness measures by MeDi group
| Uncorrected data | Age and TIV-adjusted data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MeDi- | MeDi+ | MeDi- | MeDi+ | ||||||
| ROI thickness (cm) | Side | mean | SEM | mean | SEM | mean | SEM | mean | SEM |
| EC | Left | *3.17 | 0.06 | 3.38 | 0.08 | *3.16 | 0.06 | 3.40 | 0.08 |
| Right | 3.35 | 0.07 | 3.38 | 0.08 | 3.33 | 0.07 | 3.41 | 0.08 | |
| IPL | Left | 2.52 | 0.03 | 2.54 | 0.04 | 2.52 | 0.03 | 2.55 | 0.04 |
| Right | 2.54 | 0.03 | 2.52 | 0.03 | 2.55 | 0.03 | 2.52 | 0.03 | |
| MTG | Left | 2.95 | 0.03 | 2.93 | 0.04 | 2.96 | 0.03 | 2.94 | 0.04 |
| Right | 3.01 | 0.03 | 3.05 | 0.03 | 3.02 | 0.03 | 3.04 | 0.03 | |
| OFC | Left | **2.44 | 0.04 | 2.67 | 0.05 | **2.45 | 0.04 | 2.67 | 0.05 |
| Right | 2.39 | 0.03 | 2.43 | 0.04 | 2.39 | 0.03 | 2.43 | 0.04 | |
| PCC | Left | *2.56 | 0.03 | 2.66 | 0.04 | *2.56 | 0.03 | 2.66 | 0.04 |
| Right | 2.50 | 0.03 | 2.58 | 0.04 | 2.50 | 0.03 | 2.59 | 0.04 | |
| TIV (cm3) | n.a. | 1514 | 0.286 | 1577 | 0.399 | n.a. | |||
Lower than MeDi+
p<0.05
p<0.01 on post-hoc univariate GLM analysis
Abbreviations: EC = entorhinal cortex, IPL = MeDi = inferior parietal lobule, Mediterranean diet, lower (MeDi-) vs. higher (MeDi+) adherence, MTG = middle temporal gyrus, n.a. = not applicable, OFC = orbitofrontal cortex, PCC = posterior cingulate cortex, TIV = total intracranial volume
Gender, education and FH were not significantly associated with MRI measures and did not show interactions with MeDi group. Removing these variables from the model left results unchanged (Wilk's Lambda p=0.026), with group differences being most pronounced in left OFC (8%, p=0.001), EC (7%, p=0.034) and PCC (4%, p=0.041), and with a trend for right PCC (4%, p=0.10).
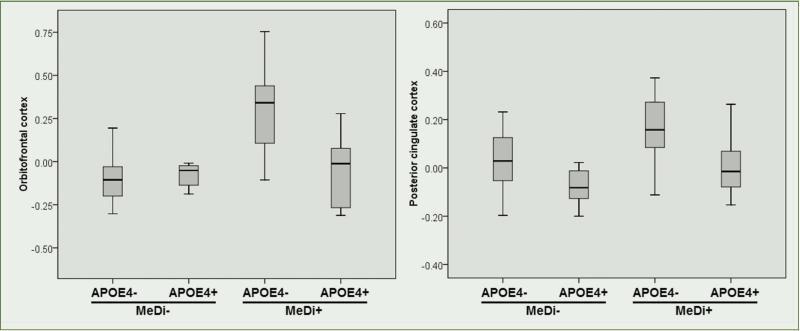
While APOE was borderline associated with MRI measures (p=0.12), there was a significant interaction between MeDi and APOE group (Wilk's Lambda p=0.013). Post-hoc examination showed that the interaction was driven by the fact that APOE4- subjects showing higher MeDi adherence had the greatest ROI thickness of all other subgroups (Figure 2).
Figure 2.
Mediterranean diet and APOE genotype interactions on regional MRI thickness
Abbreviations: MeDi = Mediterranean diet group (MeDi- = lower adherence vs MeDi+ = higher adherence), APOE4 = Apolipoprotein E ε4 allele (APOE4- = non carriers, APOE4+ = carriers). MRI measures are age and total intracranial volume-adjusted residuals
A multivariate GLM with 10 ROI measures (i.e., dependent variables), MeDi group (i.e., independent variable), age, TIV, BMI, HOMA-IR, and HTN group (i.e., covariates) left results substantially unchanged (Wilk's Lambda p=0.029), with MeDi+ subjects showing overall greater ROI thickness than MeDi- subjects, with most pronounced group differences in left OFC (p=0.004) and EC (p=0.028). BMI, HOMA-IR and HTN were not significantly associated with MRI measures and did not show interactions with MeDi group. Removing these variables resulted in the same results as with Model 1 (age and TIV-adjusted data).
Left hemisphere. A multivariate GLM with 5 ROI measures, MeDi group, and covariates confirmed results from the entire ROI data set (Wilk's Lambda p=0.003; Table 2). Gender, education, FH, BMI, HOMA-IR and HTN were not significantly associated with MRI measures of the left hemisphere. Removing these variables from the model left results unchanged (Wilk's Lambda p=0.002), with more pronounced group differences in OFC (p=0.002), EC (p=0.02) and PCC (p=0.04). The interaction between MeDi and APOE group remained significant (Wilk's Lambda p=0.02; Figure 2).
Right hemisphere. A multivariate GLM with 5 ROI measures, MeDi group, and covariates showed no significant effects of MeDi group on MRI measures (Wilk's Lambda p=0.56, n.s., Table 2). None of the covariates, except for TIV, were significantly associated with MRI measures of the right hemisphere. Results remained unchanged after removing non-significant confounds from the model (Wilk's Lambda p=0.55, n.s.).
Correlations between MeDi scores and MRI measures
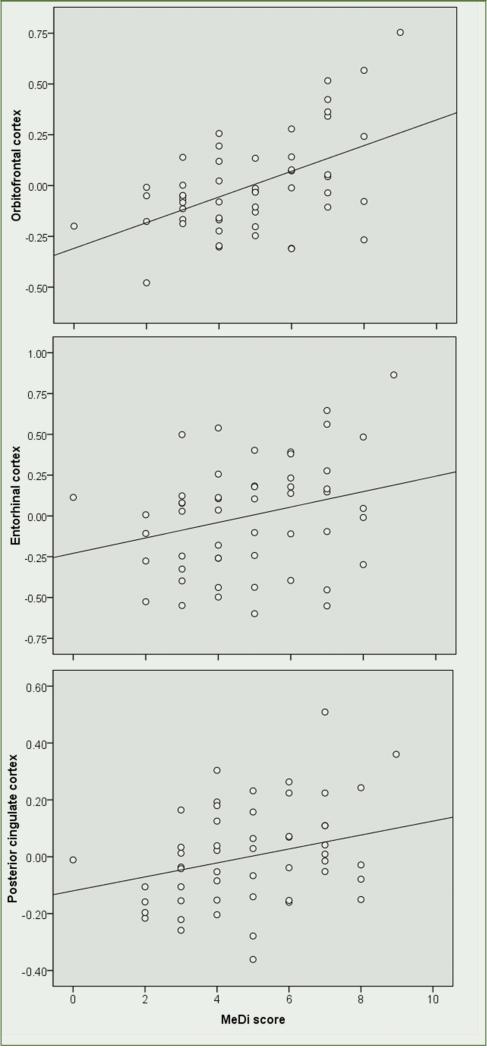
MeDi scores were significantly associated with OFC, EC and PCC of the left hemisphere, with and without correcting for covariates (Figure 3). For every unit increase in MeDi scores, thickness of OFC increased by β=0.51 units (R2=0.28, p<0.001), EC by β=0.25 units (R2=0.07, p=0.05), and PCC by β=0.28 units (R2=0.08, p=0.04; Figure 3). Given the semi-categorical nature of the MeDi scores, non-parametric tests were used to confirm these associations (Spearman's rho: OFC σ=0.47, p<0.001, EC σ=0.26, p=0.03, PCC σ=0.29, p=0.02).
Figure 3.
Associations between Mediterranean diet scores and regional MRI thickness
MRI measures are age and total intracranial volume-adjusted residuals
Correlations between MeDi scores, clinical and cognitive measures
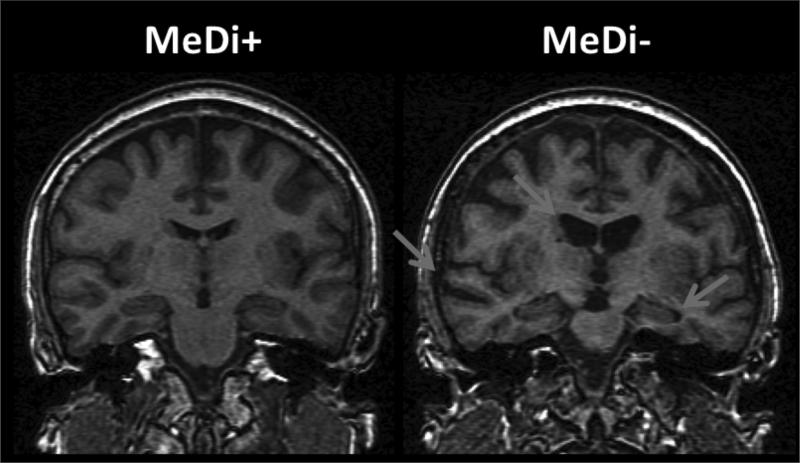
Controlling for age, higher MeDi scores were associated with a smaller hip-to-waist ratio (β=−0.25, p=0.03) and were borderline associated with lower plasma insulin and triglycerides levels (β=−0.18 and β=−0.13, p<0.09). There were no significant associations between MeDi score and neuropsychological measures, with or without controlling for covariates. The MRI scans of two representative cases showing higher vs. lower adherence to the MeDi are shown in Figure 4.
Figure 4.
MRI scans of two representative NL cases showing higher vs. lower adherence to the MeDi.
Participants were 52 and 50 year old, respectively, with MMSE>28, education>12 y, normal cognitive test performance by age and education. The MeDi+ subject shows no ventricular enlargement or hippocampal atrophy by age. The MeDi- subject shows mild ventricular enlargement, hippocampal and temporal cortex atrophy by age (arrows)
Discussion
Among young to late middle aged NL individuals, lower adherence to a MeDiet was associated with structural MRI-based cortical thinning (i.e., atrophy) in key AD-regions as compared to a higher adherence. These effects were restricted to brain areas of the left hemisphere, and were most pronounced in OFC, EC and PCC. These results were independent of possible risk factors for LOAD such as age, gender, education, APOE genotype, FH, as well as of BMI, insulin resistance and hypertension.
Prospective studies have provided evidence for a favorable relation of a MeDi-type diet with slower cognitive decline, reduced risk of progression from mild cognitive impairment (MCI) to AD, lower risk of AD, and reduced mortality in AD patients (3, 4, 8-12). These effects were independent of physical activity (40) and were not mediated by vascular comorbidity (10).
Various nutrients have been associated with the MeDi pattern, including B-complex vitamins, antioxidants, vitamin D, and polyunsaturated fatty acids (PUFA), which are all known to have neuro-protective effects ranging from anti-oxidant, anti-inflammatory and Aβ anti-oligomerization properties, to vasculo-protective and synaptic plasticity-enhancing effects, and to modulation of vascular endothelial factor expression, angiogenin, and advanced glycation end products (41-47). Conversely, higher intake of saturated fats is known to have negative effects on cardiovascular function (5, 7).
Our findings of increased cortical thinning in NL showing lower adherence to the MeDi are consistent with epidemiological findings, and provide a possible pathophysiological substrate to the clinical data. Moreover, while all our subjects had lab values within normal limits and MeDi groups were comparable for clinical measures, lower MeDi scores were associated with larger hip-to-waist ratios and, to a lesser extent, with higher plasma insulin and triglycerides levels, which lends further support to prior observations of less favorable medical profiles.
MeDi effects on MRI biomarkers were significant in the left, but not in the right hemisphere, and were most pronounced in OFC, EC and PCC. Previous MRI studies have shown that atrophic changes in these AD-vulnerable regions, especially of the left hemisphere, are associated with increased risk for developing memory impairments and dementia (48-51). Given the known relationship between brain atrophy and onset of clinical symptoms in AD (17), our data suggests that the pathological AD process leading to neuronal loss may be influenced by modifiable lifestyle practices, such as a healthy diet, during the normal stages of cognition. Additionally, a novel association between MeDi and APOE status was observed, as APOE4- showing higher adherence to the MeDi diet had the largest ROI thickness of all other subgroups. The APOE ε4 genotype is a well-established risk factor for late-onset AD and has been associated with increased brain atrophy in NL elderly (52). To our knowledge, there are no prior investigations of interactive effects of APOE status and MeDi diet on MRI biomarkers in NL individuals. Within the MeDi+ group, APOE4- showed greater cortical thickness than APOE4+, whereas no APOE group differences were observed within the MeDi- group. These findings suggest that diet may have greater impact on APOE4-, as APOE4+ subjects seem to develop brain atrophy regardless of diet, while APOE4- may put themselves at greater risk for AD-related brain changes by not following a healthy diet. These findings are consistent with previous reports of more beneficial effects of physical activity for APOE4-than APOE4+ individuals (53), although results are not always consistent (54, 55). More studies with larger samples and longitudinal follow-ups are warranted to replicate our preliminary research studies and to specifically examine the effects of APOE status on dietary patterns in AD, and whether the relationship varies with age and disease.
The biological mechanisms for the observed associations between MeDi and cortical thickness remain to be clarified. In the adjusted models, the association between MeDi and MRI features was essentially unchanged by including age, gender, education, presence of family history, APOE status, BMI, insulin resistance scores and presence of hypertension as possible confounds. These data suggest that MeDi is a protective factor independent of traditional AD risk factors. Other studies are needed to assess whether the observed association would change depending on additional factors such as vascular structure/function or markers of inflammation (56).
Most, if not all participants reported stability of their dietary patterns over the past 2-5 years. Examination of our records showed that approximately 90% of the surveyed participants have been living the lifestyle reported in the surveys for 5 years or more, with a very conscientious focus on their diet and food choices. Approximately 8% of those surveyed reported their nutritional intake to be a lifestyle span of about 2-5 years. Only 1 participant in the MeDi- group reported their nutritional behavior starting within the last 1-2 years. Overall, our MeDi+ cohort included people for whom the MeDi was their normal dietary pattern, and most of the MeDi+ participants reported following the MeDi since childhood. Previous longitudinal studies of the MeDi with repeated dietary assessments over up to 13 years, demonstrated that adherence to the MeDi is remarkably stable over time, especially in healthy individuals (10, 57, 58). However, while we consider it more likely that the MeDi adherence reported reflects our population's longstanding dietary habits, because of the synchronous timing of dietary and MRI assessments and the cross-sectional nature of our study, we cannot exclude that adherence the MeDi may be a more recent lifestyle choice in our cohort. Since this is the first study demonstrating an association between the MeDi and MRI biomarkers of AD in a relatively young NL population, future studies are needed to replicate our preliminary research findings, to test whether cortical thickness changes only after long-term exposure to certain ingredients of MeDi (e.g., vitamin B, antioxidants, etc.) or whether short-term exposure is sufficient to preserve brain volumes, and whether adherence to the MeDi from a very young age would be particularly beneficial to healthy brain aging. These biomarker findings are valuable for future research studies as well as possible randomized clinical trials in which participants are assigned to a standard low-fat diet vs. MeDi, with change in ROI thickness being a primary endpoint or outcome measure.
MeDi scores were not associated with neuropsychological measures, most likely because our subjects were cognitively normal and all high-school graduates, which resulted in a “ceiling-effect”. As such, present cross-sectional findings do not offer information on risk of future AD in our NL cohort. Longitudinal studies with larger samples are warranted to determine whether reduced brain thickness in MeDi- vs. MeDi+ subjects is predictive of cognitive decline, and whether the relationship between MeDi, AD-biomarkers and cognitive performance varies with age and disease. Our preliminary results provide the rationale for performing a larger, longitudinal study to assess how diet, biomarkers and risk factors of AD modulate AD-risk years in advance of possible clinical symptoms. We caution that present results were found in small numbers of carefully screened subjects under controlled clinical conditions. Most participants belonged to middle-class; none were smokers, diabetics, met criteria for obesity or had significant cardiovascular disease. Replication of these preliminary findings in community-based populations with more diversified socio-economic and medical status, as well as with other biomarkers of AD, particularly of AD pathology (i.e., amyloid beta and neurofibrillary tangles), is warranted and clinical application is not justified.
Conclusions
Our biomarker findings provide biological evidence in support of epidemiological studies showing that the MeDi diet may be protective against AD. In our study, lower adherence to the MeDi was associated with increased atrophy of key brain regions for AD among NL individuals, which provides support for further exploration of dietary behavior as a possible AD prevention strategy.
Acknowledgments
This study was supported by NIH/NIA grants AG035137, AG13616 and P30AG008051.
Footnotes
Contributions: Dr. Mosconi - study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Mr. Murray – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Tsui – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Li – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Ms. Davies – acquisition of data, critical revision of the manuscript for important intellectual content. Ms. Williams – acquisition of data, analysis and interpretation, study supervision. Dr. Pirraglia – analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Osorio – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Glodzik – study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. McHugh – study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content. Dr. de Leon – study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content
Statistical Analyses were done by Lisa Mosconi and Elizabeth Pirraglia
Competing interests: Disclosures: Dr. Mosconi has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company. Dr. Mosconi has received compensation for consulting services from Abiant Inc. Mr. Murray reports no disclosures. Dr. Tsui has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company. Dr. Li has received compensation for consulting services from Abiant Inc. Ms. Davies reports no disclosures. Ms. Williams reports no disclosures. Dr. Pirraglia reports no disclosures. Dr. Osorio reports no disclosures. Dr. Glodzik has received honoraria from the French Alzheimer Foundation and was PI on an investigator initiated clinical trial supported by Forrest Labs. Dr. McHugh was PI on an investigator initiated clinical trial supported by Bayer Healthcare Pharmaceuticals. Dr. de Leon has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company. Dr. de Leon has received compensation for consulting services from Abiant Inc., has received honoraria from the French Alzheimer Foundation, and was PI on an investigator initiated clinical trial supported by Neuroptix.
REFERENCES
- 1.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol. 2013;9:54–58. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Y, Scarmeas N. Dietary patterns in Alzheimer's disease and cognitive aging. Curr Alzheimer Res. 2011;8:510–519. doi: 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 6.Kesse-Guyot E, Andreeva VA, Ducros V, Jeandel C, Julia C, Hercberg S, Galan P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr. 2013:1–9. doi: 10.1017/S0007114513003188. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97:369–376. doi: 10.3945/ajcn.112.047993. [DOI] [PubMed] [Google Scholar]
- 10.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 13.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69:1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estruch R, Ros E, Martinez-Gonzalez MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med. 2013;369:676–677. doi: 10.1056/NEJMc1306659. [DOI] [PubMed] [Google Scholar]
- 16.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 19.Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119(Pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 20.Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Dale AM. Subregional neuroanatomical change as a biomarker for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr., Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Lowe V, Kantarci K, Bernstein MA, Senjem ML, Gunter JL, Boeve BF, Trojanowski JQ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Knopman DS. Shapes of the Trajectories of 5 Major Biomarkers of Alzheimer Disease. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, Brown T, Brickman AM. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol. 2011;69:257–268. doi: 10.1002/ana.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardener H, Scarmeas N, Gu Y, Boden-Albala B, Elkind MS, Sacco RL, DeCarli C, Wright CB. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch Neurol. 2012;69:251–256. doi: 10.1001/archneurol.2011.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91:1063–1077, viii. doi: 10.1016/j.mcna.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glodzik L, Mosconi L, Tsui W, de Santi S, Zinkowski R, Pirraglia E, Rich KE, McHugh P, Li Y, Williams S, Ali F, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Alzheimer's disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC. Accuracy of food-frequency questionnaires. Am J Clin Nutr. 2000;72:1234–1236. doi: 10.1093/ajcn/72.5.1234. [DOI] [PubMed] [Google Scholar]
- 33.Willett WC, Hu FB. The food frequency questionnaire. Cancer Epidemiol Biomarkers Prev. 2007;16:182–183. doi: 10.1158/1055-9965.EPI-06-0843. [DOI] [PubMed] [Google Scholar]
- 34.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87:43–47. [PubMed] [Google Scholar]
- 35.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–189. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 36.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 37.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 39.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 40.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira BF, Veloso CA, Nogueira-Machado JA, de Moraes EN, Santos RR, Cintra MT, Chaves MM. Ascorbic acid, alpha-tocopherol, and beta-carotene reduce oxidative stress and proinflammatory cytokines in mononuclear cells of Alzheimer's disease patients. Nutr Neurosci. 2012 doi: 10.1179/1476830512Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 42.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 43.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 44.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007;64:86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 45.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 46.Takasaki J, Ono K, Yoshiike Y, Hirohata M, Ikeda T, Morinaga A, Takashima A, Yamada M. Vitamin A has anti-oligomerization effects on amyloid-beta in vitro. J Alzheimers Dis. 2011;27:271–280. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 47.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 7:934–946. doi: 10.1016/j.neuroimage.2005.05.015. 22005. [DOI] [PubMed] [Google Scholar]
- 49.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;3(123 Pt):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- 50.Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr., Weiner MW, Saykin AJ. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, Puel M, Berry I, Fort JC, Celsis P. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 53.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 54.Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimers Dis. 2010;22:483–492. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 58.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]