Abstract
Background
Childhood cancer survivors (CCS) are at increased risk for the metabolic syndrome (MetSyn), which may be reduced with lifestyle modifications. The purpose of this investigation was to characterize lifestyle habits and associations with the MetSyn among CCS.
Methods
CCS ≥10 years from diagnosis, older than 18 years of age, and participating in the St. Jude Lifetime Cohort Study completed medical and laboratory tests and a food frequency questionnaire (FFQ). The Third National Cholesterol Education Program Adult Treatment Panel (NCEP-ATPIII) criteria were used to classify participants with MetSyn. Anthropometric, FFQ and self-reported physical activity data were used to characterize lifestyle habits according to World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations. Those who met ≥4 of 7 recommendations were classified as following guidelines. Sex stratified log-binomial regression models were used to evaluate associations between dietary/lifestyle habits and MetSyn, adjusted for age, age at cancer diagnosis, cranial radiation, education, and household income.
Results
Among 1598 CCS (49.2% male, median age 32.7 years, range, 18.9–60.0 years), 31.8% met criteria for MetSyn and 27.0 % followed WCRF/AICR guidelines. Females who did not follow WCRF/AICR guidelines were 2.4 (95% CI 1.7–3.3) and males were 2.2 (95% CI 1.6–3.0) times more likely to have MetSyn than those who followed WCRF/AICR guidelines.
Conclusion
Adherance to a heart healthy lifestyle is associated with lower risk of MetSyn among CCS. There is a need to determine if lifestyle interventions prevent or remediate MetSyn in CCS.
Keywords: childhood cancer survivor, metabolic syndrome, dietary intake
BACKGROUND AND RATIONALE
There are over 360,000 childhood cancer survivors (CCS) in the United States,1 70% of whom will experience at least one chronic health condition.2 Therefore, the long term health of survivors has become a focus of both observational and interventional research.3 One of the most significant findings among adult CCS is increased risk for developing cardiovascular disease including coronary artery disease.4–6 Anthracycline chemotherapy and chest radiation are known risk factors for cardiomyopathy,7, 8 likely worsened by modifiable risk factors including obesity, dyslipidemia, and insulin resistance,9 which are common in this population.2, 4 Metabolic syndrome (MetSyn) is a constellation of physical and laboratory abnormalities associated with risk of cardiovascular disease.10 The reported prevalence of MetSyn among CCS ranges from 7 to 60%. Those with a history of cranial radiation therapy are at highest risk.11, 12
Poor nutritional habits, specifically diets high in fat and simple sugars, are associated with development and progression of MetSyn in the general population. Because CCS report consuming less than recommended amounts of fruits and vegetables and high amounts of fat,13, 14 an evaluation of the contribution of poor dietary habits to MetSyn is important in this population. Other than small studies of adult survivors of childhood acute lymphoblastic leukemia (ALL),15, 16 few have investigated the association between dietary/lifestyle habits and the presence of MetSyn among CCS.
The purpose of this investigation was to evaluate the association between the World Cancer Research Fund/American Institute for Cancer Research (WCRF/ACIR) guidelines and MetSyn among adult CCS of varying diagnoses.
METHODS
Study population
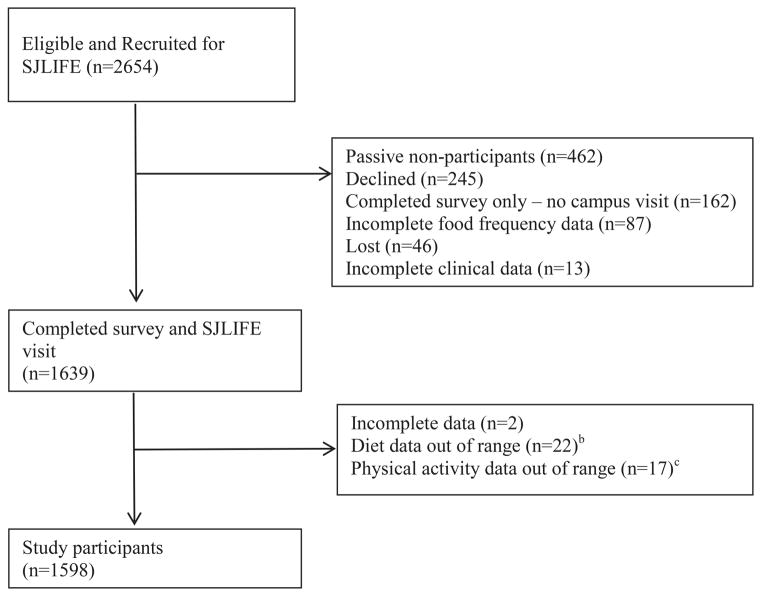
Participants were members of the St. Jude Lifetime cohort (SJLIFE), a study of adult CCS treated at St. Jude Children’s Research Hospital (SJCRH) using previously described recruitment strategies.17, 18 The primary aim of SJLIFE is to prospectively evaluate health outcomes among CCS as they age. Participants must be at least 18 years of age, ten years from cancer diagnosis, and willing to return to SJCRH for evaluation. Potentially eligible study participants were recruited and attended their on campus evaluation between October 2007 and October 2012 (Figure 1). All participants provided informed consent for participation in this Institutional Review Board approved study.
Figure 1.
Consort Diagram as of October 31st, 2012.
Metabolic Syndrome
MetSyn was defined using the Third Report of the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP III).19 Individuals having or being treated for three or more of the following were classified as having MetSyn: 1) abdominal obesity (waist circumference > 102 cm in males and > 88 cm in females); 2) triglycerides ≥ 150 mg/dL; 3) high density lipoprotein (HDL) cholesterol < 40 mg/dL in males and <50 mg/dL in females; 4) hypertension (systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg) and 5) fasting plasma glucose ≥ 100 mg/dL.
Abdominal circumference at the narrowest point between the xiphoid process and the navel was determined with a Gullick tape measure.20 The measure was repeated twice and recorded to nearest tenth centimeter. The highest circumference was used for analysis. Resting blood pressure was taken with the participant seated with both feet on the floor following a five minute rest period. The lowest of three measurements was used for analysis.
Blood samples were collected following an overnight fast. Glucose, triglycerides and HDL were measured using an enzymatic spectrophotometric assay (Roche Diagnostics, Indianapolis, IN).
Adherence to WCRF/ACIR guidelines
Anthropometrics, dietary intake data and self-reported physical activity information were used to calculate a score based on the WCRF/ACIR guidelines.21 The seven components that make up this scale are shown in Table 1. Participants received one point for each recommendation met. Based on a previous report,14 participants who followed a majority of recommendations (four or more of seven) were considered adherent.
Table 1.
WCRF/AICR Scoring
| Item description | Value | Score |
|---|---|---|
| Body mass index | ≤ 25 kg/m2 | 1 |
| > 25 kg/m2 | 0 | |
| Physical activity | Meets guidelinesa | 1 |
| Does not meet guidelinesa | 0 | |
| Daily fruit and vegetable consumption | ≥ 5 servings/day | 1 |
| < 5 servings/day | 0 | |
| Daily intake of complex carbohydrates | ≥ 400 g/day | 1 |
| < 400 g/day | 0 | |
| Daily alcohol intake | < 14 g/day(Females) < 28 g/day(Males) | 1 |
| ≥ 14 g/day(Females) ≥ 28 g/day(Males) | 0 | |
| Daily red meat intake | < 80 g/day | 1 |
| ≥ 80 g/day | 0 | |
| Daily sodium consumption | < 2400 mg | 1 |
| ≥ 2400 mg | 0 |
Center for Disease Control guidelines are 150 minute per week moderate physical activity.
Height and weight were measured using a wall mounted stadiometer and an electronic scale, respectively. Body mass index (BMI) was calculated by dividing weight in kilograms (kg) by height in meters squared (m2). BMI was defined as underweight (< 18.5 kg/m2), normal weight (18.5 to 24.9 kg/m2), overweight (25.0 to 29.9 kg/m2), and obese (≥ 30 kg/m2).
Participants completed the Block 2005 Food Frequency Questionnaire (FFQ)14 to estimate customary intake of nutrients and food groups over the past year. The data were processed using the Block Dietary Data Systems which utilizes a food list from the National Health and Nutrition Examination Survey (NHANES), and a nutrient database from the USDA Food and Nutrient Database for Dietary Studies.22–24
Self-reported physical activity was obtained by having participants complete the NHANES Physical Activity Questionnaire which asks questions about physical activity over the past seven days.25 Minutes per week of moderate physical activity were assigning one minute for each reported minute of moderate activity and 1.67 minutes for each minute of reported vigorous activity.26 Participants who met or exceeded the Center for Disease Control (CDC) recommendation for physical activity (150 minutes per week) were classified as physically active.26
Demographic and treatment information
Demographic and cancer treatment data were obtained from participant questionnaires and from medical records by trained abstractors.17
Statistics
Associations between adherence to the WCRF/AICR guidelines and MetSyn or components were analyzed in log-binomial regression models, with MetSyn or individual components treated as dependent variables in sex stratified models. Adherence to the WCRF/AICR guidelines was the primary independent variable, with current age, race, CRT, education, smoking status and age at diagnosis included as potential confounders. Final models were determined using AIC criterion to select the best subset of covariates among all possible models. Adherence to the WCRF/AICR guidelines was retained in all final models. The interaction between cranial radiation exposure and adherence to the WCRF/AICR guidelines was evaluated but not significant. Results are presented as relative risks (RR) with 95% Confidence Intervals (95% CI). Descriptive analyses were completed in SAS v9.2 (Cary, N.C.). Model selection was conducted in R (Vienna, Austria).
RESULTS
Study population and characteristics
Among 2654 potentially eligible survivors, 1639 (61.8%) agreed to participate. Non-participants included 46 (1.7%) lost to follow up, 707 (26.6%) who actively (n=245) or passively (n=462) chose not to participate, and 162 (6.1%) who completed the surveys but did not complete a campus visit. An additional 41 (1.5%) had incomplete or inaccurate dietary or MetSyn status data leaving 1598 participants for this analysis (Figure 1). Participants were more likely than the source population to be female (Table 2). Half of the survivors were female (50.8%) and just less than half were leukemia survivors (49.4%). Mean age at diagnosis was 7.9 ± 5.5 years and mean time since diagnosis was 25.6 ±7.6 years. Approximately 20% of the survivors were current smokers; less than 40% were college graduates. Over half of survivors were overweight (28.2%) or obese (38.1%). Over one third had been exposed to cranial radiation therapy (CRT).
Table 2.
Population Characteristics
| Study Participants (n=1639) | Non Participants (n=1015) | P Value | |||
|---|---|---|---|---|---|
|
| |||||
| Total n (%) | Females n (%) | Males n (%) | N (%) | ||
| Gender | |||||
| Female | 832 (50.8) | 459 (45.2) | 0.006 | ||
| Male | 807 (49.2) | 556 (54.8) | |||
| Current age (years) | |||||
| 18–29 | 604 (36.9) | 304 (36.5) | 300 (37.2) | 351 (34.6) | 0.58 |
| 30–39 | 675 (41.2) | 355 (42.7) | 320 (39.7) | 423 (41.7) | |
| 40–49 | 311 (19.0) | 148 (17.8) | 163 (20.2) | 211 (20.8) | |
| 50–59 | 49 (3.0) | 25 (3.0) | 24 (3.0) | 30 (3.0) | |
| Age at diagnosis (years) | |||||
| 0–4 | 665 (40.6) | 333 (40.0) | 332 (41.1) | 411 (40.5) | 0.62 |
| 5–9 | 391 (23.9) | 199 (23.9) | 192 (23.8) | 263 (25.9) | |
| 10–14 | 346 (21.1) | 173 (20.8) | 173 (21.4) | 203 (20.0) | |
| 15–22 | 237 (14.5) | 127 (15.3) | 110 (13.6) | 138 (13.6) | |
| Survival (years) | |||||
| 10–19 | 407 (24.8) | 203 (24.4) | 204 (25.3) | 245 (24.1) | 0.084 |
| 20–29 | 768 (46.9) | 396 (47.6) | 372 (46.1) | 452 (44.5) | |
| 30–39 | 408 (24.9) | 205 (24.6) | 203 (25.2) | 264 (26.0) | |
| 40–48 | 56 (3.4) | 28 (3.4) | 28 (3.5) | 54 (5.3) | |
| Primary Diagnosis | |||||
| Leukemia | 809 (49.4) | 412 (49.5) | 397 (49.2) | 465 (45.8) | 0.32 |
| Lymphoma | 264 (16.1) | 142 (17.1) | 122 (15.1) | 154 (15.2) | |
| Sarcoma | 180 (11.0) | 77 (9.3) | 103 (12.8) | 121 (11.9) | |
| Neuroblastoma | 70 (4.3) | 39 (4.7) | 31 (3.8) | 55 (5.4) | |
| Wilms Tumor | 74 (4.5) | 44 (5.3) | 30 (3.7) | 57 (5.6) | |
| CNSa | 135 (8.2) | 60 (7.2) | 75 (9.3) | 97 (9.6) | |
| Other | 107 (6.5) | 58 (7.0) | 49 (6.1) | 66 (6.5) | |
| Race | |||||
| White | 1435 (87.6) | 722 (86.8) | 713 (88.4) | 873 (86.0) | 0.25b |
| Black | 188 (11.5) | 102 (12.3) | 86 (10.7) | 134 (13.2) | |
| Other | 16 (1.0) | 8 (1.0) | 8 (1.0) | 8 (0.8) | |
| Cranial Radiation | |||||
| Yes | 621 (37.9) | 303 (36.4) | 318 (39.4) | 361 (35.6) | 0.37 |
| No | 1018 (62.1) | 529 (63.6) | 489 (60.6) | 654 (64.4) | |
| Stem Cell Transplant | |||||
| Yes | 45(2.7) | 19(2.3) | 26(3.2) | 31(3.1) | 0.64 |
| No | 1594(97.3) | 813(97.7) | 781(96.8) | 984(96.9) | |
| Educational Attainment | |||||
| < College graduate | 1002 (61.1) | 468 (56.3) | 534 (66.2) | ||
| College graduate | 597 (36.4) | 349 (41.9) | 248 (30.7) | ||
| Not reported | 40 (2.4) | 15 (1.8) | 25 (3.1) | ||
| Smoking Status | |||||
| Current smoker | 332 (20.3) | 155 (18.6) | 177 (21.9) | ||
| Non smoker | 1307 (79.7) | 677 (81.4) | 630 (78.1) | ||
| Body Mass Index (BMI) | |||||
| < 18.5 kg/m2 | 55 (3.4) | 37 (4.4) | 18 (2.2) | ||
| 18.5–24.9 kg/m2 | 497 (30.3) | 281 (33.8) | 216 (26.8) | ||
| 25.0–29.9 kg/m2 | 462 (28.2) | 193 (23.2) | 269 (33.3) | ||
| >= 30 kg/m2 | 625 (38.1) | 321 (38.6) | 304 (37.7) | ||
Central nervous system;
comparison for race (white vs. black and other); P-values were from Chi-square test.
Metabolic Syndrome
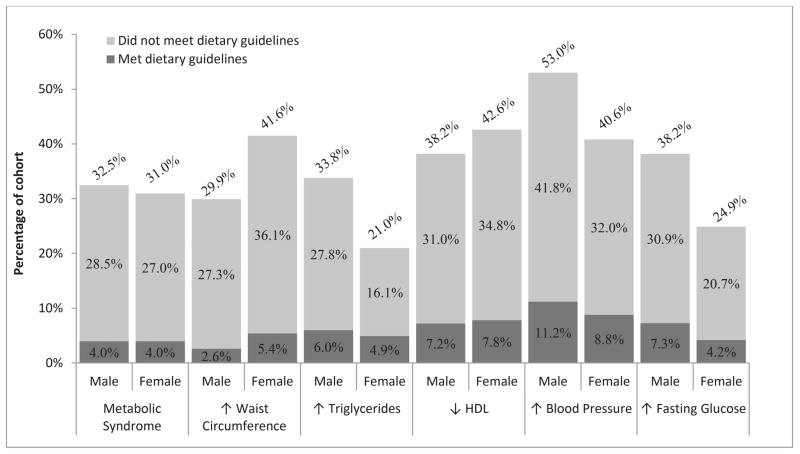
MetSyn was present in 32.5 % of males and 31.0% of females. Among males, the most prevalent MetSyn component was high blood pressure (53.0%) followed by elevated fasting glucose (38.2%) and low HDL (38.2%). Among females, low HDL was the most prevalent component (42.6%) followed by increased waist circumference (42.6 %) and high blood pressure (40.6%; Figure 2). Supplemental Table 1 includes detailed information by sex and age group for each MetSyn component.
Figure 2. Dietary status among participants with metabolic syndrome or metabolic syndrome components.
Metabolic Syndrome and Components by Dietary Status. The light gray portion of the bar indicates the percentage of those who have metabolic syndrome and who did not follow WCRF/ACIR guidelines. The dark gray portion of the bar indicates the percentage who have metabolic syndrome and who follow WCRF/ACIR guidelines.
Dietary intake
On average, males reported consuming 2419.9±1549.9 and females reported consuming 1905.3±1122.9 kilocalories (Kcal) per day (Table 3). Daily caloric intake from fat and sugars was similar among males and females. Mean fruit intake was nearly one serving per day and vegetable intake was just over 2.5 servings per day among males and females. Sodium intake was higher in males than females.
Table 3.
Dietary intake
| Females (n=832) | Males (n=807) | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SD | RDIa | Mean ± SD | RDIa | |
| Nutrient intake, Daily | ||||
| Energy (kcal) | 1905.3 ± 1122.9 | 1800–2400 | 2419.9 ± 1549.9 | 1800–2400 |
| Fat (g) | 76.6 ± 49.7 | 20–35 | 98.4 ± 69.3 | 20–35 |
| Energy fat (%) | 35.8 ± 6.0 | 25–35 | 36.2 ± 5.9 | 25–35 |
| Energy saturated fat (%) | 12.1 ± 3.0 | <10 | 11.4 ± 2.5 | <10 |
| Protein (g) | 72.6 ± 45.7 | 46 | 94.3 ± 70.1 | 56 |
| Carbohydrate (g) | 235.7 ± 137.2 | 130 | 287.5 ± 172.9 | 130 |
| Energy from sugar (%) | 4.6 ± 1.9 | <7 | 4.7 ± 2.1 | <7 |
| Total fiber (g) | 17.2 ±9.7 | 25 | 17.8 ± 11.4 | 31 |
| Sodium (mg) | 3151.3 ± 1916.5 | <2300 | 3979.2 ± 2672.9 | <2300 |
| Daily food group servings | ||||
| Fruit | 1.2 ± 0.9 | 4–5 | 1.0 ± 0.8 | 5–6 |
| Vegetables | 3.0 ± 2.2 | 4–5 | 2.8 ± 2.1 | 5–6 |
| Total grains | 2.6 ± 4.6 | 6–8 | 2.0 ± 5.2 | 8–11 |
| Whole grains | 0.6 ± 0.7 | 3–4 | 0.6 ± 0.8 | 4–5 |
| Dairy | 1.3 ± 1.0 | 2–3 | 1.5 ± 1.2 | 2–3 |
| Meat, poultry, fish (oz) | 3.9 ± 3.4 | <6 | 5.8 ± 5.5 | <6 |
RDI=Recommended daily intake based on: U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, December 2010. www.dietaryguielines.gov; SD=Standard Deviation
Adherence to dietary guidelines
Only 25.2 % of males and 28.8% of females met four or more of seven components of the WFCR/AICR guidelines (Table 4). Obesity, excessive red meat and sodium consumption, and inadequate servings of fruits and vegetables were common among both males and females. Over half of survivors reported activity levels below recommended guidelines. Less than half of females (39.9 %) consumed over 400 grams per day of complex carbohydrates.
Table 4.
WCRF/AICR guidelines
| Total (n=1639) | Females (n=832) | Males (n=807) | ||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | P-Valuec | |
| WCRF/AICR Guidelinesa | ||||
| < 4 | 1196 (73.0) | 592 (71.2) | 604 (74.8) | 0.093 |
| +4 | 443 (27.0) | 240 (28.8) | 203 (25.2) | |
| Physically Active | ||||
| Not reported | 17 (0.8) | 6 (0.7) | 11 (1.4) | |
| Yes | 786 (48.0) | 330 (39.7) | 456 (56.5) | <.0001b |
| No | 836 (51.0) | 496 (59.6) | 340 (42.1) | |
| Body mass index | ||||
| ≤ 25 kg/m2 | 560 (34.2) | 321 (38.6) | 239 (29.6) | 0.0001 |
| > 25 kg/m2 | 1079 (65.8) | 511 (61.4) | 568 (70.4) | |
| Fruit and vegetables | ||||
| ≥ 5 servings/day | 426 (26.0) | 245 (29.4) | 181 (22.4) | 0.0012 |
| < 5 servings/day | 1213 (74.0) | 587 (70.6) | 626 (77.6) | |
| Complex carbohydrates | ||||
| ≥ 400 g/day | 778 (47.5) | 332 (39.9) | 446 (55.3) | <0.0001 |
| < 400 g/day | 861 (52.5) | 500 (60.1) | 361 (44.7) | |
| Alcohol | ||||
| < 14 g/day (Female); < 28 g/day (Male) | 1543 (94.1) | 778 (93.5) | 765 (94.8) | 0.28 |
| ≥ 14 g/day (Female) ≥ 28 g/day (Male) | 96 (5.9) | 54 (6.5) | 42 (5.2) | |
| Red meat | ||||
| < 80 g/day | 164 (10.0) | 121 (14.5) | 43 (5.3) | <.0001 |
| ≥ 80 g/day | 1475 (90.0) | 711 (85.5) | 764 (94.7) | |
| Sodium | ||||
| < 2400 mg/day | 498 (30.4) | 314 (37.7) | 184 (22.8) | <.0001 |
| ≥ 2400 mg/day | 1141 (69.6) | 518 (62.3) | 623 (77.2) | |
World Cancer Research Fund/American Institute for Cancer Research;
Unknowns were not included in comparisons;
P-value from Chi-square tests.
Associations between meeting guidelines and metabolic syndrome
Among those with MetSyn, 87.8% of men and 87.2% of women did not follow the WFCR/AICR guidelines. Of the men with hypertension (53.0%), 78.9% did not follow WFCR/AICR guidelines. Of the nearly 40% of men with elevated fasting glucose, 80.8% were not adherent to the WFCR/AICR guidelines. Similarly, among the 42.6% of women with low HDL, 81.6% did not follow the WFCR/AICR guidelines. Over 40% of the women had elevated waist circumference and 87.0% of them were not adherent to the WFCR/AICR guidelines (Figure 2).
Survivors who did not follow the WFCR/AICR guidelines were more likely to have MetSyn than those who followed the guidelines, with relative risks 2.2 (95% CI 1.6–3.0) among males and 2.4 (95% CI 1.7–3.3) among females (Table 5). Among female survivors, advanced age, lower educational attainment, and cranial radiation were also associated with increased risk for MetSyn. Among male survivors, white race, advanced age, and lower educational attainment were also associated with increased risk for MetSyn.
Table 5.
Association between dietary and metabolic syndrome
| Variable | Metabolic Syndrome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | |||||||||
| Total N | n | Row % | RRb | 95% CI | Total N | n | Row % | RR | 95% CI | |
| WCRF/AICR | ||||||||||
| <4 | 580 | 222 | 38.3 | 2.4 | 1.7–3.3 | 583 | 223 | 38.3 | 2.2 | 1.6–3.0 |
| +4 | 236 | 33 | 14.0 | Refc | Ref | 198 | 31 | 15.7 | Ref | Ref |
| Race | ||||||||||
| White | 709 | 217 | 30.6 | 694 | 238 | 34.3 | 1.9 | 1.2–2.9 | ||
| Non-White | 107 | 38 | 35.5 | 87 | 16 | 18.4 | Ref | Ref | ||
| Age | ||||||||||
| 18–29 | 294 | 66 | 22.5 | Ref | Ref | 288 | 56 | 19.4 | Ref | Ref |
| 30–39 | 351 | 122 | 34.8 | 1.5 | 1.2–1.9 | 310 | 110 | 35.5 | 1.7 | 1.3–2.3 |
| 40–59 | 171 | 67 | 39.2 | 1.6 | 1.2–2.1 | 183 | 88 | 48.1 | 2.3 | 1.7–3.0 |
| Education | ||||||||||
| <College graduate | 467 | 177 | 37.9 | 1.6 | 1.2–1.9 | 533 | 181 | 34.0 | 1.2 | 1.0–1.5 |
| College graduate | 349 | 78 | 22.4 | Ref | Ref | 248 | 73 | 29.4 | Ref | Ref |
| Cranial Radiation | ||||||||||
| Yes | 299 | 129 | 43.1 | 1.4 | 1.2–1.8 | 309 | 110 | 35.6 | ||
| No | 517 | 126 | 24.4 | Ref | Ref | 472 | 144 | 30.5 | ||
World Cancer Research Fund/American Institute for Cancer Research;
Relative risks estimated using the log-binomial model. Final models based on AIC criteria;
Reference group.
DISCUSSION
Among a large, well characterized cohort of adult CCS, nearly a third had evidence of MetSyn which was associated with not following dietary and lifestyle guidelines recommended by the WFCR/AICR. This association persisted even after adjusting for race, age, smoking status, educational attainment, and CRT in multivariable models. Importantly, the association between an unhealthy lifestyle and MetSyn was even stronger than the association between CRT and MetSyn in females, suggesting that individuals predisposed to adverse cardiovascular outcomes following treatment for childhood cancer may be able to modify this risk through behavioral change.
The prevalence of MetSyn and its components in our cohort are higher than that documented by previous investigators in younger cohorts of CCS, but similar to those documented in the general population among much older adults. In a cohort of 75 childhood ALL survivors (mean age 30.2±7.1 years), the authors reported that 16.6% had MetSyn, 8.0% had abnormal fasting glucose and 20.0% had hypertension,11 compared to the 31.5, 31.9 and 46.9%, respectively, in our cohort. Similarly, Van-Waas et al,12 described a MetSyn prevalence of 13% among of 500 adult CCS with a younger age distribution (5% over 40 years old) than our cohort. The overall prevalence (31.5%) of MetSyn in our cohort, 22% of whom were older than age 40 years, was similar to that (34%) reported in the general population, 68% of whom were older than age 40 years. Elevated blood pressure (53.0 vs. 43.4% males; 40.6 vs. 35.2% females) and low HDL cholesterol (38.2% vs. 21.6% males; 42.6% vs. 27.8% females) were more, whereas increased waist circumference (29.9 vs. 44.8% males; 41.6 vs. 60.2% females) and impaired fasting glucose (38.2 vs. 45.8% males; 24.9 vs. 31.3% females) were less prevalent among CCS when compared to the general population.27
Significant findings in this study were that the association between poor lifestyle choices and MetSyn persisted even after taking into account cancer survivor specific (e.g. CRT) and other known risk factors (e.g. smoking, age, etc.), and that the influence of lifestyle on each MetSyn component was apparent for both male and female survivors. These results are concordant with those of Tonorezos et al who reported that among 117 adult survivors of childhood ALL, each unit increase in adherence to the Mediterranean diet, increased the odds of MetSyn 31%, 28 but expand their findings by accounting for CRT exposure and including survivors of a variety of diagnoses. While reports from large cohort studies such as the Atherosclerosis Risk in Community study and the NHANES indicate that the association between poor dietary habits and the incidence of MetSyn is a general population phenomenon,29, 30 results in our younger CCS population are particularly troubling. Recent data indicate that CCS who have both a cardiotoxic treatment exposure and a known population based cardiovascular risk factor (e.g. hypertension) have a more than additive risk for an adverse cardiac outcome, including death.9
Our findings that the majority (over 70%) of adult CCS cancer do not follow diets that promote heart health are in concordance with the few studies documenting dietary habits among CCS.13, 14 Robein et al evaluated dietary intake among 72 adult survivors of childhood ALL and noted that few met WFCR/AICR guidelines.14 Similar to our study participants, these authors reported that their cohort consumed excessive red meat, sodium, and dietary fat.14 Another study reported that nearly 80% of 209 CCS did not consume recommended servings of fruits and vegetables per day.13 The body of literature regarding dietary habits among CCS is not yet robust. However, there is a pattern of evidence that suggests that survivors’ dietary habits are not optimal for heart health.
Our study has limitations that should be considered when interpreting the results. Dietary intake data may be under reported.31, 32 To address this we applied a critical evaluation of energy intake33 to allowing us to exclude from analysis those (1.3%) with unrealistic values based on physiological energy demands. Additionally, not all eligible individuals participated. Although Ojha et al previously assessed selective non-participation in this cohort and reported no substantial differences between participants and non-participants,18 it is possible that those who were unable to participate differed from study participants. Rates of obesity (38.1 vs. 33.3%) and smoking (20.3 vs. 24.1%) among survivors were fairly similar to those of the population in the Southeastern U.S.34 However, generalizability to other regions may be limited. In addition, while we used the WRCF/AICR guidelines as our model for a heart healthy lifestyle, an optimal dietary strategy to reduce disease risk has not been identified.35, 36 It is possible that other diet or lifestyle guidelines may have a greater impact on MetSyn.
CONCLUSIONS
MetSyn was prevalent in over 30% of the adult CCS in our cohort, and only 28% reported a lifestyle consistent with WFCR/AICR guidelines. Even after adjusting for known treatment and demographic risk factors, failure to follow a heart healthy lifestyle was associated with more than a two-fold increased risk of having MetSyn. These data suggest that a heart healthy lifestyle is associated with improved metabolic control in CCS, even in individuals treated with CRT. Additional work is needed to evaluate the impact of lifestyle interventions on risk for MetSyn among CCS especially in individuals predisposed to adverse cardiovascular outcomes following treatment for childhood cancer.
Supplementary Material
Acknowledgments
Supported provided by Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
The authors have no financial disclosures.
References
- 1.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012. [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-Term Survivors of Childhood Cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Buchanan GR, Eshelman DA, et al. Cardiovascular Risk Factors in Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2001;23(7):424–430. doi: 10.1097/00043426-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45(1):55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 8.Shankar SM, Marina N, Hudson MM, et al. Monitoring for Cardiovascular Disease in Survivors of Childhood Cancer: Report From the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 11.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107(6):1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 12.van Waas M, Neggers SJCMM, Pieters R, van den Heuvel-Eibrink MM. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol. 2010;21(5):1121–1126. doi: 10.1093/annonc/mdp414. [DOI] [PubMed] [Google Scholar]
- 13.Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians. Cancer. 2005;103(10):2171–2180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- 14.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(11):815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djousse L, Padilla H, Nelson TL, Gaziano JM, Mukamal KJ. Diet and metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2010;10(2):124–137. doi: 10.2174/187153010791213056. [DOI] [PubMed] [Google Scholar]
- 16.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56(5):825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude lifetime cohort study. Pediatr Blood Cancer. 2012;60(5):856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM’s Guidelines for Exercise Prescription. 7. Baltimore, MD: Lippincott, Williams, & Wilkins; 2006. [Google Scholar]
- 21.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: WCRF/AICR; 2007. [Google Scholar]
- 22.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 23.Johnson BA, Herring AH, Ibrahim JG, Siega-Riz AM. Structured measurement error in nutritional epidemiology: applications in the Pregnancy, Infection, and Nutrition (PIN) Study. J Am Stat Assoc. 2007;102(479):856–866. doi: 10.1198/016214506000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeckner LS, Pullen CH, Walker SN, Abbott GW, Block T. Use and reliability of the World Wide Web version of the Block Health Habits and History Questionnaire with older rural women. J Nutr Educ Behav. 2002:34. doi: 10.1016/s1499-4046(06)60307-2. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) 2003–2004 Available from URL: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/paqiaf_c.pdf.
- 26.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 27.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- 28.Tonorezos E, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24(2):313–321. doi: 10.1007/s10552-012-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutsey PL, Steffen LM, Stevens J. Dietary Intake and the Development of the Metabolic Syndrome: The Atherosclerosis Risk in Communities Study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 30.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 31.Schoeller DA. How Accurate Is Self-Reported Dietary Energy Intake? Nutr Rev. 1990;48(10):373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 32.Schoeller DA. Limitations in the assessment of dietary energy intake by self-report. Metabolism. 1995;44(Supplement 2):18–22. doi: 10.1016/0026-0495(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen SJ, Adair L. An Alternative to Dietary Data Exclusions. J Am Diet Assoc. 2007;107(5):792–799. doi: 10.1016/j.jada.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Behavioral Risk Factor Surveillance System. [accessed December 24, 2013];Prevelance and Trends Data. Available from URL: http://apps.nccd.cdc.gov/brfss/
- 35.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Nori Janosz KE, Miller WM, Odom J, Lillystone M, McCullough PA. Optimal diabetes management during medical weight loss for cardiovascular risk reduction. Expert Rev Cardiovasc Ther. 2005;3(4):761–775. doi: 10.1586/14779072.3.4.761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.