Abstract
Background
Activation of the RAS-RAF-MEK-ERK signaling pathway is thought to be the key driver of pediatric low-grade astrocytoma (PLGA) growth. Sorafenib is a multikinase inhibitor targeting BRAF, VEGFR, PDGFR, and c-kit. This multicenter phase II study was conducted to determine the response rate to sorafenib in patients with recurrent or progressive PLGA.
Methods
Key eligibility criteria included age ≥2 years, progressive PLGA evaluable on MRI, and at least one prior chemotherapy treatment. Sorafenib was administered twice daily at 200 mg/m2/dose (maximum of 400 mg/dose) in continuous 28-day cycles. MRI, including 3-dimensional volumetric tumor analysis, was performed every 12 weeks. BRAF molecular testing was performed on tumor tissue when available.
Results
Eleven patients, including 3 with neurofibromatosis type 1 (NF1), were evaluable for response; 5 tested positive for BRAF duplication. Nine patients (82%) came off trial due to radiological tumor progression after 2 or 3 cycles, including 3 patients with confirmed BRAF duplication. Median time to progression was 2.8 months (95% CI, 2.1–31.0 months). Enrollment was terminated early due to this rapid and unexpectedly high progression rate. Tumor tissue obtained from 4 patients after termination of the study showed viable pilocytic or pilomyxoid astrocytoma.
Conclusions
Sorafenib produced unexpected and unprecedented acceleration of tumor growth in children with PLGA, irrespective of NF1 or tumor BRAF status. In vitro studies with sorafenib indicate that this effect is likely related to paradoxical ERK activation. Close monitoring for early tumor progression should be included in trials of novel agents that modulate signal transduction.
Keywords: low-grade glioma, pediatric low-grade astrocytoma, phase II trial, sorafenib
Recurrent pediatric low-grade astrocytoma (PLGA) represents a major clinical problem in neuro-oncology, and novel, less toxic and more effective therapies are needed.1 Recently, our increased understanding of the key molecular pathways driving PLGA growth and the increasing availability of targeted therapies for those pathways have led to great interest in exploring molecular targeted therapies for PLGA.
Pilocytic astrocytoma (PA) is the most common histological subtype of PLGA. Patients with neurofibromatosis type 1 (NF1) are predisposed to developing PAs, predominantly in the optic tract (ie, optic pathway gliomas [OPGs]).2 NF1 is characterized by the loss of the NF1 gene product neurofibromin, resulting in activation of the RAS/RAF/MEK/ERK signaling pathway.3 The majority of sporadic PAs harbor a unique tandem duplication at chromosome 7q34, which produces a fusion gene between KIAA1549 and the kinase domain of BRAF that result in constitutive BRAF and ultimately MAPK activation. In recent genomic studies, almost all PAs that do not harbor KIAA-BRAF have been shown to harbor other genetic lesions that also result in constitutive MAPK pathway activation, such as activating genetic hits in FGFR1, NTRK2, and RAF1.4,5
PLGAs express pro-angiogenic factors (vascular endothelial growth factor [VEGF], platelet derived growth factor [PDGF]), and their receptors (VEGFR and PDGFR).6–8 Bevacizumab, a VEGF inhibitor, has recently shown very encouraging activity in PLGA.9,10
Sorafenib is an oral, small-molecule multikinase inhibitor with potent in vitro activity against both wild-type and mutant (V600E) BRAF.11 Recent preclinical data showed that overexpression of activated BRAF led to increased proliferation of primary mouse astrocytes that could be inhibited by treatment with sorafenib.12 Sorafenib also exerts anti-angiogenic activity via inhibition of VEGFR-1/2/3, PDGFRβ, Flt-3, and c-kit, which has been studied in a variety of preclinical models13,14 as well as in clinical studies using dynamic, contrast-enhanced MRI.15
Because sorafenib is a potent inhibitor of several key molecular pathways that are relevant in PLGAs and encouraging preclinical data,12 we conducted this prospective phase II clinical trial to assess the objective response rate to sorafenib in patients with PLGA.
Materials and Methods
Patient Eligibility and Enrollment
Adult and pediatric patients (aged ≥2 years) with recurrent or progressive PLGA or OPG after at least one prior chemotherapy regimen were eligible. Histological confirmation of a WHO grade I or II low-grade glioma was required, except for OPG, as were measurable disease on MRI, a performance score of ≥60% (Karnofsky or Lansky), and adequate bone marrow, renal, hepatic, and cardiopulmonary function. An interval of at least 6 months from prior craniospinal radiotherapy (3 months for focal radiotherapy) and 4 weeks from prior chemotherapy (6 weeks for nitrosoureas) was required. Any neurological deficits had to be stable for ≥1 week; corticosteroids were allowed for control of progressive symptoms as long as the patient was on a stable or decreasing dose for ≥1 week prior to enrollment. Key exclusion criteria included baseline uncontrolled hypertension, concurrent treatment with other investigational drugs or anticancer agents or therapies, concurrent therapy with cytochrome P450 inducers or inhibitors, or any prior central nervous system bleeding.
The trial enrolled participants at 3 centers: NYU Langone Medical Center, Children's Hospital of Philadelphia, and Johns Hopkins Hospital. NYU Langone Medical Center served as the primary study site, and patient eligibility, controlled patient registration and enrollment, and tracked toxicity and response were reviewed at that location. The study investigators at the primary site, as well as those at the sub-sites, were primarily responsible for determining disease progression according to study criteria but were encouraged to consult with investigators at the coordinating center for equivocal cases. All MRI was centrally reviewed by an experienced, board-certified pediatric neuro-radiologist (S.S.M.). The study was conducted under a protocol approved by the institutional review boards of all participating institutions, and the protocol was registered at ClinicalTrials.gov (NCT01338857). Informed consent was obtained from the patients and guardians in accordance with institutional policies. All consecutive patients who met study entry criteria and who consented to participate were enrolled.
Study Design
This study was a multicenter, prospective, open label, 2-stage phase II clinical trial. The primary endpoint was the objective response rate to sorafenib in children and adults with low-grade astrocytomas, including OPGs. Key secondary objectives included further characterization of the pharmacokinetics and toxicity of sorafenib in children (aged <18 years) treated at the currently defined, maximally tolerated dose,16 exploration of possible associations with response rates and participants′ NF1 status, and presence or absence of constitutive BRAF activation in the tumor. The study was originally designed to accrue participants in 4 distinct strata: non-NF1 patients with a tumor (A) positive for constitutive BRAF activation (KIAA-BRAF fusion or BRAF-activating mutation including BRAFV600E); (B) negative for constitutive BRAF activation; (C) no available tumor tissue; and (D) NF1 patients. Patients with prior surgery but without BRAF results available at the time of enrollment were allowed to enroll on stratum C and subsequently moved to a different stratum for analysis, depending on the subsequent availability of molecular testing results.
To test the null hypothesis that the response rate was ≤5% versus the alternative that the response rate was ≥25%, a 2-stage Simon design was used for each stratum.17 Nine participants were to be enrolled in the first stage for each of the 4 strata. If at least one of these 9 participants had at least a partial response (PR) within 48 weeks from the beginning of therapy, an additional 8 participants were to be enrolled in this stratum in the second stage. If there were no responses, sorafenib was to be considered inactive and of no interest for further evaluation, and the trial in that stratum was to be terminated. The overall alpha level for this design within each stratum was 0.05 with a power of 80%. Sorafenib was to be considered effective and of interest for further study in any given stratum if, after successful completion of both stages, there were at least 3 partial responses in the combined stages of that same stratum.
Adverse events were graded using version 4.0 of the National Cancer Institute Common Toxicity Criteria.
Treatment
Sorafenib was supplied by Bayer HealthCare Pharmaceuticals and administered orally twice daily in continuous 4-week cycles for up to a total of 12 cycles. For non-NF1 participants aged ≥6 and <18 years, starting dose was 200 mg/m2/dose (rounded to the nearest 50 mg increment) up to a maximum of 400 mg/dose.16 Non-NF1 participants aged ≥18 years received the standard recommended adult dose of 400 mg. For NF1 participants with plexiform neurofibromas, the dose-limiting toxicity was grade ≥3 pain.18 Therefore, the starting dose for NF1 participants aged ≥6 and <18 years was 80 mg/m2 (maximum 150 mg), with planned dose escalation until the maximum dose for non-NF1 participants was reached. Children aged ≥2 and <6 years, irrespective of NF1 status, received a starting dose of 100 mg/m2 with dose escalation after 2 weeks to a maximum of 150 mg/dose. For treatment-related rash, topical therapy for symptomatic relief was prescribed as needed.19
Clinical evaluations, including complete physical and neurological exam, complete blood count with differential, comprehensive metabolic panel, and serum pregnancy test (for females of child-bearing potential) were performed weekly during cycle 1 and every 4 weeks thereafter. To monitor for potential enamel changes or lesions, dental examinations were obtained at baseline and every 3 cycles thereafter in participants aged <18 years. Because effects on the bone structure (eg, thickening of the femoral growth plate) were observed in juvenile animals with sorafenib, participants aged <18 years were monitored with plain radiographs of tibial growth plates at baseline and every third cycle. Participants were allowed to remain on study for up to 12 cycles unless clinical or radiological progression or unacceptable toxicity occurred. Dose interruptions and modifications due to toxicity were allowed according to predefined protocol rules.
Response Evaluation
MRIs were required at baseline within 31 days prior to starting sorafenib and at the end of every third 4-week cycle. Target lesions were required to be measurable, and both enhancing and nonenhancing tumors were measured.
Three-dimensional measurements (using standard, commercially available analysis software [Vital Images Vitrea 2]) were used to assess response; when not available, the product of the 2 largest diameters was employed. Determination of tumor response (complete response [CR], partial response [PR], or stable disease [SD]) was defined based on the comparison of the baseline MRI performed at study entry or to the subsequent MRI that demonstrated best response. Progressive disease (PD) was based on the comparison to the study baseline MRI. PR and PD were defined by a >15% decrease or increase in tumor volume, respectively, as measured by 3D volumetric analysis. Overall response assessment was based on responses both in target and nontarget lesions as well as the appearance of any new lesions, as outlined in Table 1.
Table 1.
Definition of overall response based on assessment of target and nontarget lesions
| Target Lesions | Nontarget Lesions | New Lesions | Overall Response |
|---|---|---|---|
| CR | CR | No | CR |
| CR | SD | No | PR |
| PR | CR or PR or SD | No | PR |
| CR | CR or PR or SD | No | PR |
| SD | CR or PR or SD | No | SD |
| PD | Any | Yes or No | PD |
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
According to the study protocol, any participant who remained on therapy at least until the first on-study MRI was considered evaluable for response.
Molecular Tumor Analysis
Molecular analysis for BRAF alterations was performed based on tissue availability. Fluorescence in situ hybridization (FISH) was used to detect the presence or absence of KIAA1549-BRAF tandem duplications on formalin-fixed, paraffin embedded specimens, as well as staining for BRAFV600E with a mutant-specific antibody, as previously described.20,21 In addition, direct sequencing of BRAF was performed in participants with sufficient tissue available, as previously described.22 In addition, we performed additional targeted sequencing for KRAS and immunohistochemistry for phospho-PDGFR.5
Pharmacokinetics
Blood samples for measurement of sorafenib plasma concentration were collected from consenting participants <18 years of age after the patient had been on a stable uninterrupted dose for at least 14 consecutive days. Blood samples were collected immediately prior to a scheduled morning sorafenib dose, as well as at 2 and 4 hours after (optional). Plasma samples for Sorafenib and M2 (N-oxide) metabolite were analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay. Sample processing involved protein precipitation followed by a reversed phase high-performance liquid chromatography (HPLC) separation and positive mode MS/MS detection. The calibration range for Sorafenib was from 10 ng/mL to 10 600 ng/mL. The calibration range for M2 was from 10 ng/mL to 2,600 ng/mL. Assay precision for both analytes during analysis was within 15%, and accuracy was within 85%–115%, in compliance with FDA guidance on method validation.
Sorafenib in Vitro Studies
In order to explore the efficacy of sorafenib in treating cells harboring KIAA1549-BRAF, we used NIH-3T3 cell lines stably overexpressing BRAF fusion proteins by retroviral transduction, as previously described.23 Cells were incubated in increasing concentrations of (0, 0.1, 1 μM) of BAY-54-9085 (sorafenib tosylate) in serum-free conditions prior to lysing. Western blots were developed with chemiluminescence or with fluorescent secondary antibody. The fluorescent output was measured using the Odyssey Imaging systems. Constructs used in this study included “Fusion-1” (long-form of KIAA1549-BRAF fusion protein found in human tumors), “Fusion-2” (short-form of KIAA1549-BRAF fusion protein also found in human tumors), and “Fusion-3” and “Fusion-4”, truncated forms of the KIAA1549-BRAF fusion protein that truncate KIAA1549 N terminus just beyond 2 putative transmembrane domains, and full length wild-type BRAF.
Statistical Analysis
To evaluate efficacy, imaging response was treated as a binary variable, with participants who showed at least a partial response (PR) considered to be responders and all others nonresponders. Response rates were estimated with exact 95% confidence intervals.
Progression-free survival (PFS) was measured from date of first medication dose to date of volumetric progression using the Kaplan-Meier method. Point estimates for PFS with 95% confidence intervals were calculated.
Results
Patients
Twelve eligible participants with evaluable disease were enrolled between April, 2011, and January, 2012, including 3 with NF1. All participants except one were enrolled in 2011. Clinical characteristics, prior treatments, and tumor BRAF status of all enrolled participants are summarized in Table 2. The majority of participants (8/12, 66.6%) had received ≥2 prior chemotherapy regimens (range 2–5). There were 3 NF1 participants in stratum D (patients 2, 8, and 10), 5 participants with constitutive BRAF activation in stratum A (patients 1, 2, 5, 7 and 9), 2 participants negative for constitutive BRAF activation in stratum B (patients 3 and 4), and 2 non-NF1 participants with unknown BRAF status in stratum C (patients 6 and 11).
Table 2.
Summary of general patient characteristics at enrollment
| Patient | Age at Diagnosis [y] | Age at Enrollment [y] | Sex | NF1 | Tumor Location | Pathology | Prior Treatments | BRAF Status | Baseline Tumor Volume [ml] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.4 | 15.1 | M | No | Lumbothoracic | Ganglioglioma | S, CBP/VCR | BRAF-KIAA (by FISH) | 32.49 |
| 2 | 3.9 | 9.2 | F | Yes | Optic pathway | Pilocytic astrocytoma | CBP/VCR, BEV | BRAF-KIAA (by FISH) | 14.18 |
| 3 | 2.4 | 6.6 | M | No | Cerebellar/disseminated | Pilocytic astrocytoma | S, CBP/VCR, TPCV | BRAF WT (by FISH and seq.) | 35.40 |
| 4 | 2.0 | 9.2 | F | No | Brainstem | Pilomyxoid astrocytoma | S, CBP/VCR | BRAF WT (by FISH) | 2.11 |
| 5 | 0.5 | 6.0 | M | No | Hypothalamic/disseminated | Fibrillary astrocytoma | S, CP/VCR, VBL/TMZ, TPCV, BEV/CPT11 | BRAF duplication (by SNP array) | 330.30* |
| 6 | 0.5 | 3.5 | M | No | Hypothalamic/disseminated | Pilocytic astrocytoma | S, CBP/VCR, TMZ, VBL, BEV | NA | 128.90* |
| 7 | 10.0 | 10.5 | M | No | Thalamic | Pilocytic astrocytoma | S, CBP/VCR | BRAF-KIAA (by FISH) | 9.02 |
| 8 | 1.2 | 8.8 | F | Yes | Optic pathway | Pilocytic astrocytoma | S, CBP/VCR, DACT/VCR, S, VBL, DACT/VCR | NA | 21.54 |
| 9 | 0.8 | 3.0 | M | No | Hypothalamic/disseminated | Pilomyxoid astrocytoma | S, CBP/VCR, VBL | BRAF-KIAA (by FISH) | 33.32 |
| 10 | 6.1 | 13.4 | F | Yes | Hypothalamic | Low-grade glioma NOS | CBP/VCR, TMZ, TPCV, S | NA | 36.00* |
| 11 | 0.7 | 13.8 | F | No | Optic pathway | Pilocytic astrocytoma | CBP/VCR, TPCV, VBL | NA | 25.96 |
| NE1 | 0.3 | 4.1 | F | No | Hypothalamic/disseminated | Pilomyxoid astrocytoma | CBP/VCR, S, BEV/CPT11, R | BRAF-KIAA (by FISH) | N/A |
Abbreviations: BEV, bevacizumab; CBP, carboplatin; CPT11, irinotecan; DACT, actinoymcin D; F, female; FISH, fluorescence in situ hybridization; M, male; N/A, not applicable; NE, not evaluable; NOS, not otherwise specified; RT, radiotherapy; S, surgery; seq., DNA sequencing; SNP, single nucleotide polymorphisms; TPCV, thioguanine/procarbazine/CCNU (lomustine)/vincristine; VBL, vinblastine; VCR, vincristine; WT, wild type.
*These patients did not have 3D MRI volumetrics available, and tumors were measured by the product of the 3 largest diameters.
One participant (patient NE1) developed a transaminase elevation after 1 week on treatment. Treatment was held as per protocol requirement. She was admitted 2 days later for urosepsis requiring ventilator support. She was definitively taken off study 5 weeks later after a repeat brain MRI disclosed progressive tumor growth. Response could not be assessed in this patient, with a remaining eleven participants evaluable for the primary outcome.
Due to an unexpectedly high rate of early radiological disease progression, an interim Safety Committee review was performed after an overall number of 12 participants (11 evaluable) were enrolled, and the decision was made to close the study for further accrual. None of the initially planned 4 strata on the first stage completed planned accrual of nine participants, and participants were therefore combined in a single group for study analysis.
Responses
Baseline patient characteristics and treatment outcome in eleven evaluable participants are summarized in Tables 2 and 3, respectively. The median number of cycles received by the eleven evaluable participants was three 4-week cycles (range 2–9). After 3 cycles of treatment, all but 2 participants had progressed, including 3 patients with BRAF tandem duplication. Patient 1 decided to discontinue sorafenib after 7 cycles, with stable disease. Patient 4, a non-NF1 9-year-old female, had a partial response after 3 cycles, with tumor shrinkage of -43% on volumetric measurement. Despite multiple dose interruptions and reductions, as low as 25% of her initial dose, sorafenib was discontinued after 9 cycles for recurrent hand-foot skin syndrome up to grade 3.
Table 3.
Summary of evaluable patient treatment outcomes on study
| Patient | Treatment Duration [cycles] | Best Tumor Response | Tumor Volume Change | Reason for Treatment Discontinuation |
|---|---|---|---|---|
| 1 | 7 | SD | −4% | Patient preference |
| 2 | 3 | PD | +122% | Progression |
| 3 | 2 | PD | N/A* | Progression |
| 4 | 9 | PR | −43% | Toxicity (hand-foot skin syndrome) |
| 5 | 3 | PD | +21% | Progression |
| 6 | 2 | PD | +326% | Progression |
| 7 | 2 | PD | +26% | Progression |
| 8 | 3 | PD | +59% | Progression |
| 9 | 2 | PD | +53% | Progression |
| 10 | 3 | PD | +61% | Progression |
| 11 | 3 | PD | +24% | Progression |
Abbreviations: N/A, not applicable; PD, progressive disease; PR, partial response; SD, stable disease.
*Patient developed new lesion.
Surgery was performed in 3 participants (patients 2, 9, and 11) after removal from study for progressive disease at 3, 3 and 10 months off treatment, respectively. Neuropathological evaluation revealed viable PA or pilomyxoid astrocytoma in all specimens.
Progression-free Survival and Median Time to Progression
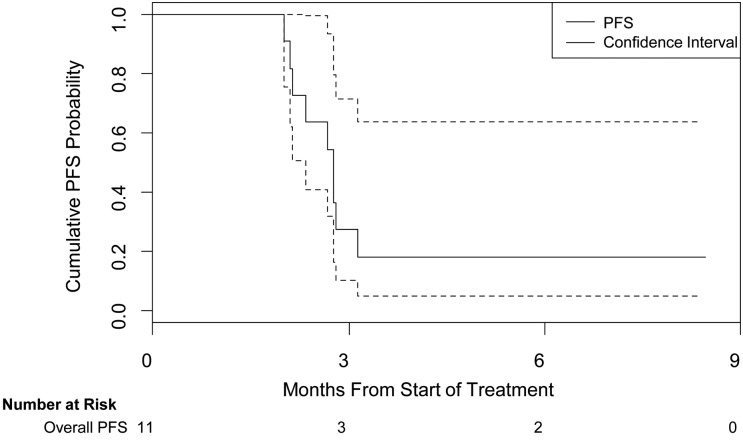
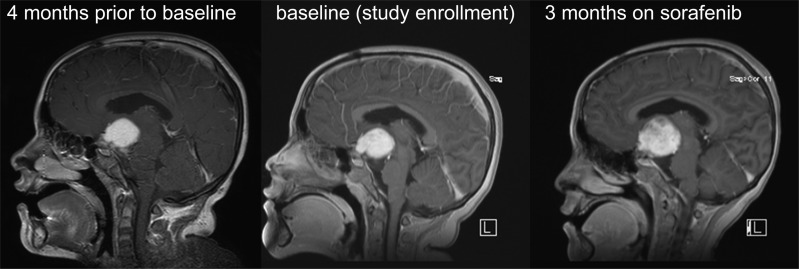
Nine participants experienced radiological tumor progression at a median time of 2.8 months (95% CI, 2.1–31.0 months), as illustrated in Figure 1. Rapid, accelerated tumor growth of an OPG on sorafenib (patient 8) is illustrated in Figure 2.
Fig. 1.
Kaplan-Meier estimate of cumulative progression-free survival (PFS) probability. This figure illustrates the PFS probability as measured from first dose of study drug to date of progression for all evaluable participants. Dotted lines indicate 95% confidence intervals.
Fig. 2.
Sequential gadolinium contrast-enhanced MRI studies showing rapid, accelerated tumor growth of an optic pathway glioma on sorafenib (patient 8). Imaging was obtained 4 months prior to sorafenib, at study enrollment (baseline), and after 3 months on study (sorafenib treatment week 12) showing a large contrast-enhancing suprasellar mass, stable in size prior to sorafenib and significant progression upon treatment.
Molecular Tumor Analysis
Results of testing for KIAA1549-BRAF by FISH and BRAFV600E by immunohistochemistry (IHC) and direct sequencing are summarized in Table 2 and detailed in Table 4. Patients 3, 4, 5, and 7 had tumor tissue available for molecular analysis prior to study entry. In patients 1 and 2, tissue became available from surgery after sorafenib. Patient 9 had tissue available for molecular studies from a biopsy at initial diagnosis and surgical resection post sorafenib. Tumors from 4 participants (patients 1, 2, 7, and 9) were positive for KIAA1549-BRAF tandem duplication by FISH, including both the initial biopsy and the subsequent resection specimen from patient 9. Another tumor (from patient 5), which had been tested prior to study entry, harbored a copy number gain at 7q34 as determined by single nucleotide polymorphism microarray, consistent with BRAF duplication and most likely representing a KIAA1549-BRAF tandem duplication. No BRAFV600E mutations were detected by direct sequencing or IHC in any of the tumors tested.
Table 4.
Results of molecular tumor analysis in available tumor specimen of evaluable patients
| Patient | KIAA-BRAF (FISH) | BRAF Duplication (SNP array) | BRAFV600E (IHC) | BRAF (direct sequencing) |
|---|---|---|---|---|
| 1 | Positive | |||
| 2* | Positive | Negative | ||
| 3 | Negative | Wild-type | ||
| 4 | Negative | Wild-type | ||
| 5 | Positive | Wild-type | ||
| 6 | ||||
| 7 | Positive | Negative | ||
| 8* | ||||
| 9 | Positive | Negative | ||
| 10* | ||||
| 11 |
*NF1 patients.
Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; SNP, single nucleotide olymorphism.
Toxicity
Toxicity was assessed in all 12 enrolled participants who received at least one dose of sorafenib. The only grade 4 toxicity possibly related to sorafenib was an increase of alanine aminotransferase in the context of developing urosepsis in patient NE1. Grade 3 toxicities included hand-foot skin syndrome (n = 1) at the end of cycle 9. Other grade 3 toxicities included diarrhea (n = 1), increased aspartate aminotransferase (n = 1), headache (n = 1), and mucositis (n = 1). Most participants tolerated the full protocol dose with only minor (grades 1–2) expected toxicities including rash (75.0%), dry skin (50.0%), hand-foot skin syndrome (33.3%), alanine aminotransferase (25.0%) or aspartate aminotransferase (50.0%) elevation, fatigue (41.7%), alopecia (41.7%), anorexia (25.0%), diarrhea (41.7%), hypophosphatemia (33.3%), and lymphopenia (25.0%). Only one NF1 patient (patient 10) did not tolerate escalation to the full protocol dose due to hand-foot skin reaction (maximum dose tolerated 80 mg/m2/dose twice daily). Regarding the non-NF participants, only one (patient 4) required permanent dose reductions due to toxicity, (ie, hand-foot skin reaction) and required 2 dose level reductions down to 100 mg/m2/dose once daily.
Pharmacokinetics
Informed consent/assent for PK blood sampling was provided by 7 evaluable participants. The median maximum plasma concentration observed was 5.0 μg/mL, similar to prior pharmacokinetic data from a pediatric phase I trial, which showed a median peak plasma concentration at steady-state of 5.4 ± 1.8 μg/mL at the same dose level used in our study (ie, 200 mg/m2 twice daily).16
Sorafenib in Vitro Studies
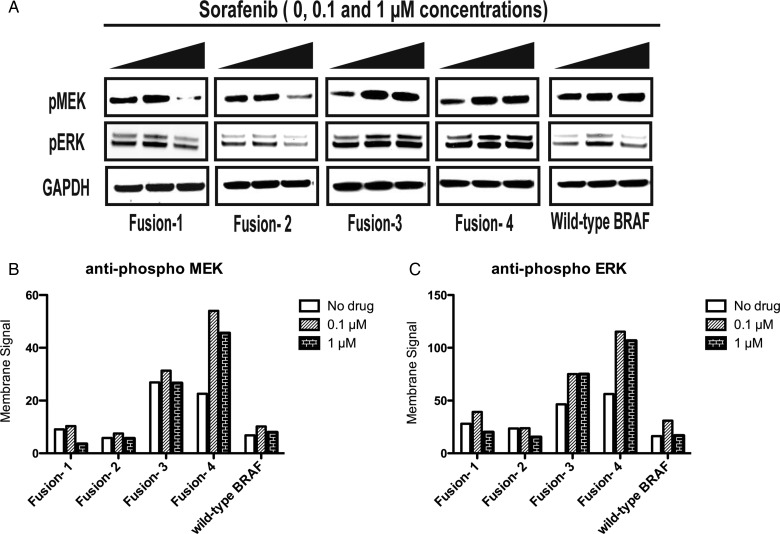
NIH/3T3 cell lines stably expressing KIAA-BRAF fusion and wild-type BRAF constructs exhibited dose-dependent resistance and enhanced paradoxical activation in the presence of sorafenib, as were recently described for PLX4720, a research analog to the targeted BRAF inhibitor vemurafenib.23 Figure 3 demonstrates MAPK pathway responsiveness in the presence of increasing concentrations (0, 0.1, 1 μM) of the drug. Membrane signals for both phospho-MEK 1/2, and especially phospho-ERK 1/2, were raised in both KIAA-BRAF fusion and full-length, wild-type BRAF-expressing cells.
Fig. 3.
Western blots demonstrating the resistance and enhanced paradoxical activation of MEK/ERK in NIH/3T3 cell lines generated by retroviral transduction and stably expressing KIAA1549-BRAF fusion and full-length, wild-type BRAF constructs in the presence of increasing concentrations of BAY-54-9085 (sorafenib tosylate). Cells were washed and incubated with drug for 1 hour in the absence of serum or growth factors. (A) Increase in phospho-MEK and phospho-ERK from baseline after treatment with BAY-54-9085 at 0.1 and/or 1 µM. (B) Graphical quantification of phospho-MEK membrane signal. (C) Graphical quantification of phospho-ERK membrane signal. Constructs include “Fusion-1” (long-form of KIAA1549-BRAF fusion protein found in human tumors) and “Fusion-2” (short-form of protein also found in human tumors) as well as “Fusion-3” and “Fusion-4” (truncated forms of the KIAA1549-BRAF fusion protein that truncate KIAA1549 N terminus just beyond 2 putative transmembrane domains), and wild-type BRAF.
Discussion
Although sorafenib was tolerated at the full protocol dose by most participants with only minor (grades 1 and 2) expected toxicities, the most striking observation was the unexpectedly high rate of early and rapid progression in the majority of PLGA patients (ie, 9 of 11 [81.8%]) within 3 treatment cycles. The observed PFS was extremely short in these typically slow-growing tumors, with a median time to progression of 2.8 months (95% CI, 2.1–3.1 months). We also calculated median time to progression on the study patients′ prior chemotherapy regimen and found it to be considerably higher (16 months), which was consistent with our observation of accelerated tumor growth in at least a subset of participants on sorafenib.
First-line chemotherapy in PLGA achieves objective response rates in the range of 50%–60%,24,25 and regimens for recurrent/progressive disease have reported an objective response rate of 36% and PFS at 5 years of 42% with vinblastine26 or stable disease in at least 41% of patients with temozolomide.27 In comparison, our results with sorafenib were far inferior and in striking contrast to the observed 86% objective response rate in recurrent PLGAs treated with bevacizumab.10
Because our only responder, patient 4, was negative for KIAA-BRAF duplication and BRAFV600E, we performed additional molecular testing to identify alternative oncogenic drivers. Targeted sequencing of KRAS revealed wild-type, and IHC for phospho-PDGFR was negative, consistent with absence of an FGFR1 activating mutation.5 Further molecular analysis was not possible due to exhaustion of the available tissue.
Since the time of conception of this study, our knowledge regarding the oncogenic drivers in PLGA, as well as our understanding of RAS-RAF-MEK-ERK signaling pathway inhibition with molecular targeted agents and resistance mechanisms, have increased vastly. First, recent comprehensive genomic studies of PLGA have uncovered alternative oncogenic mutations leading to constitutive MAPK pathway activation in almost all PAs that do not harbor KIAA-BRAF fusions, such as activating mutations in FGFR1, NTRK2 and RAF1.4,5 Although PA represents a prototypic “single pathway” tumor, these data indicate that pathway activation occurs through a number of different mechanisms and at different levels along the pathway, with important implications for rational drug selection. Second, our understanding of the mechanisms of molecular targeted drug sensitivity and resistance in tumors with oncogenic RAS-RAF-MEK-ERK pathway activation has evolved. We observed in vitro activation of MEK and ERK signaling in the presence of sorafenib, providing a mechanistic explanation for the observed rapid tumor progression in the majority of PLGA patients treated with sorafenib on this study. A comparable in vitro response at 1 μM was recently reported in a melanoma cell line harboring a similar BRAF fusion.28 Correspondingly, we have recently described similar paradoxical activation of MEK and ERK in KIAA-BRAF-expressing cells when treated with a first-generation BRAF-specific kinase inhibitor, PLX4720.23
Other studies have also shown that RAF inhibitors can paradoxically activate the MAPK signaling pathway and lead to treatment resistance and/or tumor growth.29–32 In the presence of oncogenic RAS, selective BRAF inhibitors promote RAS-dependent BRAF binding and activation of CRAF, which in turn enhance MEK-ERK signaling.32 Furthermore, although RAF inhibitors suppress ERK signaling in cells with mutant BRAF, signaling appears to be amplified in cells with wild-type BRAF.30,31 Of note, paradoxical activation of ERK by sorafenib in the context of NF1 loss has also been demonstrated in vitro,33 providing a possible explanation for the rapid disease progression in the NF1 participants included in our study.
In summary, our study shows that sorafenib is generally well tolerated in PLGA patients but may promote accelerated tumor growth. Results of in vitro testing indicate that sorafenib may lead to paradoxical ERK activation in both BRAF wild-type and KIAA-BRAF tumor cells as well as in NF1-deficient cells. Further preclinical data are required for a better understanding of the effects of molecular targeted agents on RAS-RAF-MEK-ERK signaling and growth in PLGA. Molecular targeting downstream of BRAF (ie, at the level of MEK) should be explored in future studies, and a MEK inhibitor is currently being tested in a phase I study for PLGA (ClinicalTrials.gov identifier NCT01089101). Importantly, recent data indicate that differential activation of MEK in RAS versus RAF-mutant tumors requires selection of specific subtypes of MEK inhibitors based on the upstream oncogenic driver,34 which is highly relevant to designing future MEK-inhibitor studies for PLGA. Based on our experience, close clinical monitoring with implementation of predetermined stopping rules for early tumor progression should be standard in exploratory studies with novel, molecular targeted agents, especially if tumor tissue is unavailable for molecular genetic testing.
Funding
This study was supported by the Pediatric Low-Grade Astrocytoma Foundation, Making Headway Foundation and Bayer HealthCare Pharmaceuticals; NYU Cancer Center support grant 5P30CA016087 from the NCI and NYU CTSA grant UL1TR000038 from the National Center for Advancing Translational Sciences (NCATS), NIH to the Laura and Isaac Perlmutter Cancer Center Biostatistics Shared Resource, BioRepository and Immunohistochemistry Core Laboratory; and the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through Grant UL1TR000003 to the University of Pennsylvania Institute for Translational Medicine and Therapeutics. The project content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgments
This study was presented in part at the 2nd Biennial 2013 Pediatric Neuro-Oncology Basic and Translational Research Conference, Fort Lauderdale, Florida, May 2013, and the Children′s Tumor Foundation 2013 NF conference, Monterey, California, June 2013.
Conflict of interest statement. None declared.
References
- 1.Qaddoumi I, Sultan I, Broniscer A. Pediatric low-grade gliomas and the need for new options for therapy: Why and how? Cancer Biol Ther. 2009;8(1):1–7. doi: 10.4161/cbt.8.1.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albers AC, Gutmann DH. Gliomas in patients with neurofibromatosis type 1. Expert Rev Neurother. 2009;9(4):535–539. doi: 10.1586/ern.09.4. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neuro Oncol. 2007;9(2):113–123. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DT, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatva E, Kaipainen A, Mentula P, et al. Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumors. Am J Pathol. 1995;146(2):368–378. [PMC free article] [PubMed] [Google Scholar]
- 7.Bartels U, Hawkins C, Jing M, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg. 2006;104(5 Suppl):314–320. doi: 10.3171/ped.2006.104.5.314. [DOI] [PubMed] [Google Scholar]
- 8.Guha A, Dashner K, Black PM, et al. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60(2):168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 9.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 10.Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2013;60(5):776–782. doi: 10.1002/pbc.24297. [DOI] [PubMed] [Google Scholar]
- 11.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 12.Gronych J, Korshunov A, Bageritz J, et al. An activated mutant BRAF kinase domain is sufficient to induce pilocytic astrocytoma in mice. J Clin Invest. 2011;121(4):1344–1348. doi: 10.1172/JCI44656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 14.Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008;7(4):496–501. doi: 10.4161/cbt.7.4.5624. [DOI] [PubMed] [Google Scholar]
- 16.Widemann BC, Kim A, Fox E, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18(21):6011–6022. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim A, Dombi E, Tepas K, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401. doi: 10.1002/pbc.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, Mateus C, Spatz A, et al. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60(2):299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Korshunov A, Meyer J, Capper D, et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 2009;118(3):401–405. doi: 10.1007/s00401-009-0550-z. [DOI] [PubMed] [Google Scholar]
- 21.Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122(1):11–19. doi: 10.1007/s00401-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 22.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71(1):66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110(15):5957–5962. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 25.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110(7):1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 28.Botton T, Yeh I, Nelson T, et al. Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell Melanoma Res. 2013;26(6):845–851. doi: 10.1111/pcmr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spirli C, Morell CM, Locatelli L, et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56(6):2363–2374. doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 30.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 32.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Dombi E, Jousma E, et al. Preclincial testing of sorafenib and RAD001 in the Nf(flox/flox) ;DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2012;58(2):173–180. doi: 10.1002/pbc.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatzivassiliou G, Haling JR, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501(7466):232–236. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]