Abstract
As a neurotropic virus, human immunodeficiency virus type 1 (HIV-1) invades the brain and causes severe neuronal, astrocyte, and myelin damage in AIDS patients. To gain access to the brain, HIV-1 must migrate through brain microvascular endothelial cells (BMECs), which compose the blood-brain barrier (BBB). Given that BMECs lack the entry receptor CD4, HIV-1 must use receptors distinct from CD4 to enter these cells. We previously reported that cell surface proteoglycans serve as major HIV-1 receptors on primary human endothelial cells. In this study, we examined whether proteoglycans also impact cell-free HIV-1 invasion of the brain. Using an artificial BBB transmigration assay, we found that both heparan and chondroitin sulfate proteoglycans (HSPGs and CSPGs, respectively) are abundantly expressed on primary BMECs and promote HIV-1 attachment and entry. In contrast, the classical entry receptors, CXCR4 and CCR5, only moderately enhanced these processes. HSPGs and CSPGs captured HIV-1 in a gp120-dependent manner. However, no correlation between coreceptor usage and transmigration was identified. Furthermore, brain-derived viruses did not transmigrate more efficiently than lymphoid-derived viruses, suggesting that the ability of HIV-1 to replicate in the brain does not correlate with its capacity to migrate through the BBB as cell-free virus. Given that HIV-1-proteoglycan interactions are based on electrostatic contacts between basic residues in gp120 and sulfate groups in proteoglycans, HIV-1 may exploit these interactions to rapidly enter and migrate through the BBB to invade the brain.
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) is currently one of the most challenging aspects of HIV-induced disease (4, 6, 13, 64). HIV-1 causes neurologic abnormalities in infected individuals ranging from mild cognitive and motor disorders to frank dementia (termed neuroAIDS). More than 25% of infected individuals suffer some form of CNS disorder during the course of their infection. The neuropathology associated with HIV-1 infection in the brain is characterized by widespread axonal damage, astrocytosis, myelin loss, and infiltration by blood-derived monocyte/macrophages, resident microglia, and multinucleated giant cells. The main target cells for HIV replication in the brain are macrophages and microglial cells (69, 71, 91). HIV-infected macrophages/microglia overproduce viral proteins, chemokines, and cytokines that induce dysfunction or apoptosis of neurons and astrocytes (reviewed in references 3, 5, 16, 18, 41, 44, 58, 85, and 98). Since AIDS patients develop dementia or neurobehavioral changes despite highly active antiretroviral therapy (18, 68), the development of novel therapies that prevent HIV-1 entry into the CNS remains of critical importance.
To invade the CNS, HIV-1 must migrate through brain microvascular endothelial cells (BMECs), which compose the blood-brain barrier (BBB) (20). HIV-1 may utilize at least two potential routes to reach the brain: either HIV-1 itself crosses the BBB (cell-free invasion) or it first infects blood cells (T cells or monocytes) and uses them as Trojan horses to cross the BBB (cell-associated invasion). Several scenarios have been proposed for BBB transmigration of HIV-1 as cell-free virus. In one scenario, BMECs directly infected by HIV-1 release infectious particles into the brain (8, 54, 67, 84). In an alternative scenario, HIV-1 enters BMECs from the blood, migrates through the cells, and is released into the CNS from the brain side of BMECs (10, 11, 47). In addition to these two transcellular routes, cell-free HIV-1 may also use a paracellular route via tight junctions (25) or by perforating the BMEC monolayer by inducing apoptosis (7, 40, 83). Although it is likely that HIV-1 uses both cell-free and cell-associated routes to ensure successful entry into the brain, our study focuses exclusively on transcellular invasion of the brain by cell-free HIV-1.
Given that BMECs lack the entry receptor CD4 (23, 54), HIV-1 must use attachment and entry receptors distinct from CD4 to enter these cells. Several receptors have been reported to facilitate HIV-1 entry into CD4-negative cells. Specifically, galactosyl ceramide (34, 35, 95), adhesion molecules such as ICAM-1 and LFA-1 (27, 28, 72), C-type lectins such as DC-SIGN, DC-SIGNR, langerin, and the mannose receptor (12, 30, 66, 87), and proteoglycans containing chondroitin or heparan sulfate proteoglycan chains (CSPGs or HSPGs, respectively) (8, 15, 53, 75, 94) have all been shown to promote HIV-1 attachment and/or entry into cells that lack CD4. To date, there is no demonstration that these receptors are capable of mediating fusion between viral and cellular membranes. Thus, these receptors represent prime candidates for HIV-1 entry into BMECs, the major component of the BBB.
Proteoglycans bear covalently linked long unbranched anionic sulfated glycosaminoglycan chains (i.e., chondroitin sulfate, dermatan sulfate, heparan sulfate, and heparin) (14). These glycosaminoglycans consist of disaccharide units (40 to 100) of uronic acid (glucuronic acid/iduronic acid) and N-acetylgalactosamine in chondroitin sulfate and dermatan sulfate or N-acetylglucosamine in heparan sulfate and heparin. Sulfation of the chains varies as well. For example, chondroitin sulfate chains usually contain one sulfate per disaccharide unit attached to an N-acetylgalactosamine unit, whereas heparan sulfate chains contain sections that are intensely and sparsely sulfated (25). These sulfated regions act as binding sites for various growth factors, enzymes, and matrix proteins. A growing body of evidence suggests that one class of proteoglycans, the HSPGs, possesses the capacity to modulate HIV-1 pathogenesis. Patel and colleagues originally showed that HIV-1 exploits the long anionic heparan sulfate chains of HSPGs to attach to and enter into several T-cell lines (63). Enzymatic removal of cell surface heparan sulfate chains drastically impaired the capacity of HIV-1 to infect these cells (63, 73). We and others confirmed these findings by showing that HSPGs on the surface of specific cell types may greatly influence HIV infection (33, 38, 62, 76, 97). Primary human endothelial cells richly express HSPGs both in vitro and in vivo, and these HSPGs efficiently capture HIV-1 particles on the surfaces of the cells (15). Their abundance on the surface of primary endothelial cells and their high capacity to capture HIV-1 make HSPGs prime candidate receptors to facilitate the invasion of the brain by HIV-1. In the present study, we examined the contribution of HSPGs in the sequential steps of HIV-1 transmigration through the BBB.
MATERIALS AND METHODS
Cells.
TZM-bl cells (generously contributed by John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.) were obtained through the AIDS Research and Reference Reagent Program. TZM-bl cells express CD4, which renders them susceptible to infection, and contain an integrated Escherichia coli lacZ gene driven by the HIV long terminal repeat (90). Upon infection, Tat production from the integrated provirus leads to activation of the lacZ reporter, resulting in synthesis of β-galactosidase in these cells. Infected cells are identified by staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 48 h postinfection, allowing quantitation after a single round of infection (90). The University of Arizona Institutional Review Board for Research involving Human Subjects approved the isolation of BMECs from discarded temporal lobe tissues (5 to 15 mm3) obtained during temporal lobectomies. The tissue was fragmented in phosphate-buffered saline (PBS) with a 16-gauge needle. After centrifugation (300 × g for 5 min), the cell pellet was digested in 1 mg of collagenase-dispase (Roche Molecular Biochemicals)/ml containing 1 mg of DNase (Sigma)/ml and 0.15 mg of Nα-p-tosyl-l-lysine chloromethyl ketone (Sigma)/ml for 1 h at 37°C. The digest was spun down (1,000 × g for 20 min) and resuspended in 250 μg of PBS-bovine serum albumin (BSA)/ml. After additional centrifugation, the pellet containing capillaries was resuspended in 5 mg of PBS-BSA/ml and loaded onto a 50% Percoll gradient. The capillary fragments were removed and placed in a three-dimensional collagen type I gel and maintained in DMEM containing 10% fetal calf serum and endothelial cell growth supplement (50 μg/ml) (Sigma) as described previously (47). The microvessels were harvested, explanted into collagen type I-coated T-25 flasks, and propagated as monolayers. As a second source of BMECs, primary human BMECs were obtained from Cell System Corp. (ACBRI; Kirkland, Wash.) and cultured in human endothelial growth medium (Cell System Corp.) as described previously (8, 56, 57). Importantly, primary BMECs (mainly from brain cortex) obtained from Cell System Corp. were not passaged more than four times prior to use, to maintain the original features of primary BMECs.
Viruses.
All cloned viruses were produced from electroporated Jurkat-CCR5 (generous gift from M. Emerman) and normalized by p24 enzyme-linked immunosorbent assay (ELISA) (Perkin-Elmer Life Sciences). We generated a panel of pNL4.3-derived infectious molecular clones in which the envelope gene had been replaced with those encoded by a selection of isolates, including wild-type pNL4.3 (generously contributed by Malcolm Martin through the AIDS Research and Reference Reagent Program) (1), the gp120-deficient virus pNL4.3-E- (generously contributed by Nathaniel Landau through the AIDS Research and Reference Reagent Program) (19, 36), pNL-ADA, pNL-JRFL, pNL-YU2, pNL91US005.11, pNL-92MW965.26, pNL-RW020.5, and pNL-92BR020.4 as R5 viruses, pNL-HXB, pNL-92UG021.6, pNL-92UG024.2, and pNL-92HT599.24 as X4 viruses, and finally, pNL-89.6 as R5X4 virus (a generous gift from P. Bieniasz) (97). The primary viruses isolated from matched brain and lymphoid tissues were derived and amplified as described previously (32).
Fluorescence-activated cell sorter (FACS) analyses.
One million cells were incubated with antibodies (1 μg) in 500 μl of PBS containing 0.25% human serum. Anti-CD4 monoclonal antibody (MAb) SIM.4 (generously contributed by James Hildreth), anti-DC-SIGN MAb DC28 (generously contributed by F. Baribaud, S. Pohlmann, J. A. Hoxie, and R. W. Doms), anti-CXCR4 MAb 12G5 (generously contributed by James Hoxie), and anti-CCR5 MAb 2D7 (generously contributed by Millennium Pharmaceuticals, Inc., and PharMingen) were obtained through the AIDS Research and Reference Reagent Program. Anti-HSPG 10E4 and 3G10 MAb were obtained from Seikagaku; anti-chondroitin sulfate CS-56 immunoglobulin G's were obtained from Sigma; anti-galactosyl ceramide (anti-GalCer) MAB342 MAb was obtained from Chemicon; anti-CD147 HIM6, anti-ZO-1 C-19 MAb was obtained from Zymed; anti-CD4 RP4-T4 MAb was obtained from BD Pharmingen; anti-syndecan-1 (CD138) ID4 MAb was obtained from Biogenesis; and anti-syndecan-2 10H4, anti-syndecan-3 1C7, anti-syndecan-4 8G3, and anti-glypican-1 S1 MAb were provided by G. David. Note that the antibody staining was performed on adherent BMECs prior to cell stripper (CellGro; Mediatech Inc.) detachment. Cell surface removal of heparan and chondroitin sulfates by using heparinase (heparitinases I and III [30 and 6 mIU/ml] from Seikagaku) or chondroitinase ABC (10 U from Sigma) were performed as described previously (53), whereas glycosylphosphatidyl inositol (GPI)-linked protein removal by phospholipase C (25 U from Sigma) was performed as described previously (76).
Immunostainings.
Heparan sulfate was localized in paraffin sections of paraformaldehyde-fixed material (4% in 0.1 M phosphate buffer), with heparitinase digestion (10 mIU; Seikagaku) for 3 h at 37°C followed by the anti-ΔHS MAb 3G10 (5 μg/ml). Sections without digestion were used as controls. Endogenous peroxidase was blocked with H2O2 (0.3%) in methanol. Syndecan-2 and syndecan-4 were localized in cryosections, with antibodies 10H4 (50 μg/ml) and 8G3 (50 μg/ml), respectively. After a blocking step with PBS-1% BSA-10% goat serum, primary antibody binding was detected with biotin-conjugated goat anti-mouse antibody (1/300 dilution; Dako) in PBS/BSA/goat serum and the Vectastain Elite kit (Vector), with diaminobenzidine tetrahydrochloride (Sigma) as the substrate.
Infections.
TZM-bl cells (100,000 cells/ml) were exposed to increasing volumes of medium present in the basal chamber (which contains transcytosed viruses) for 2 h. Cells were washed to remove unbound virus, and viral infection was measured at 48 h postinfection by β-galactosidase (X-Gal staining).
Transmigration assay.
BMECs were placed on Biocoat cell culture inserts (Collaborative Biomedical Products), with 40,000 BMECs on the upper surface of a polyethylene teraphthalate membrane (3-μm pore size) coated with fibronectin and collagen I (Sigma), as described previously (47). BMECs seeded onto the upper face of collagen I- and fibronectin-coated 12-mm-diameter transwells were cultured until formation of tight junctions was achieved. The inserts were fed every 2 days. The monolayer on the filter effectively divides the well into an apical compartment and a basolateral compartment. The integrity of each cell monolayer was measured by using an endothelial volt/ohm meter (Millipore). To ensure the integrity of the BMEC barrier, we monitored the elevated transendothelial electrical resistance of each cell monolayer and measured the paracellular passage of the extracellular marker inulin by the permeability coefficient, as measured by diffusion assay with 25,000 to 30,000 cpm of 14C-carboxylated inulin (molecular weight, 5,000; Sigma) in the upper chamber as described previously (47). After verifying the integrity of the monolayer on the transwell filters, BMECs were exposed to HIV-1 (added to the upper chamber) and attachment, internalization, and release of HIV-1 into the basal chamber was monitored as indicated in Results. The small-molecule antagonist TAK779 (against CCR5) was generously contributed by Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and distributed with the permission of Takeda Chemical Industries, Ltd. (9), whereas AMD3100 (against CXCR4) was generously contributed by Division of AIDS, National Institute of Allergy and Infectious Diseases, and distributed with the permission of AnorMED (22, 77).
RESULTS
Cell surface expression of proteoglycans on primary human BMECs.
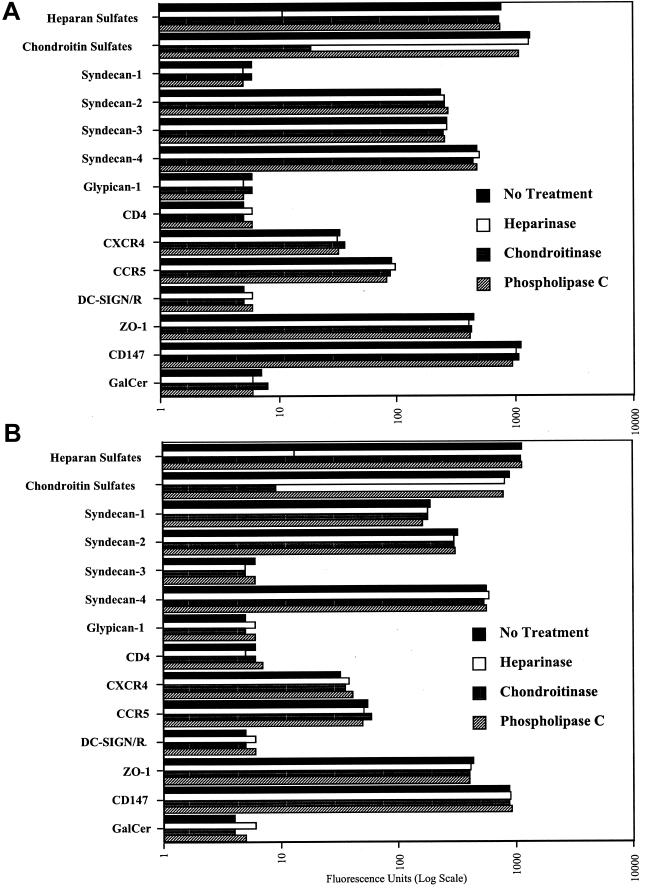
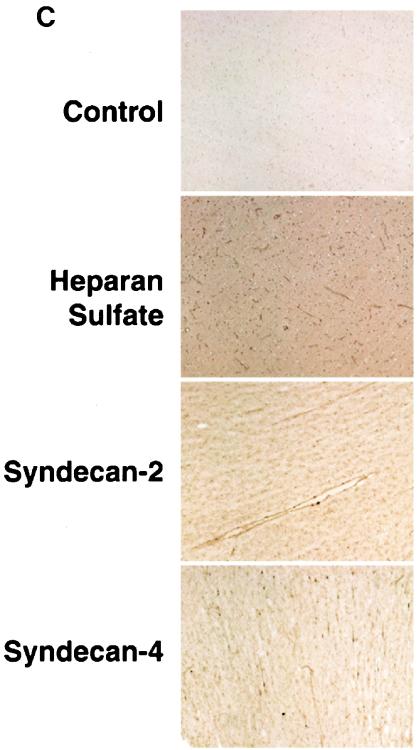
Given the demonstrated efficacy of HSPGs to function as HIV-1 receptors on CD4-negative primary human umbilical vein endothelial cells (15), we examined whether they play a central role in the transmigration of HIV-1 through BMECs. To date, there are two major classes of cell surface HSPGs, the syndecans and the glypicans. The four members of the syndecan family (syndecan-1 to -4) are transmembrane proteins consisting of a short cytoplasmic domain and an extended extracellular domain, which bears three heparan sulfate chains (far from the plasma membrane) and one to two chondroitin sulfate chains (close to the plasma membrane). In contrast to syndecans, glypicans (glypican-1 to -6) are GPI-linked proteins consisting of a globular extracellular domain, which bears two to three heparan sulfate chains and no chondroitin sulfate chains (14, 24). To determine whether HSPGs affect HIV-1 transmigration through the BBB, we first analyzed the expression of syndecans and glypicans on BMECs both in vitro and in vivo. To address this issue, we used two different sources of primary human BMECs, including primary BMECs isolated from discarded temporal lobe tissues obtained from temporal lobectomies from adults with epilepsy surgery (47) (Fig. 1A) and fetal or adult primary BMECs obtained from Cell Systems Corp. (Fig. 1B). We found that BMECs derived from all origins express high levels of heparan and chondroitin sulfates (Fig. 1A and B), supporting the notion that proteoglycans are richly expressed on the surface of primary human endothelial cells derived from different tissues, including the brain (15, 26, 39, 45, 51). We found that BMECs derived from all origins express high levels of syndecan-2 and -4 (Fig. 1A and B). In contrast, syndecan-1 and -3 expression seems to depend on the BMEC's origin. For example, syndecan-3 is highly expressed on BMECs isolated from discarded temporal lobe tissues (Fig. 1A) but absent on the commercially available BMECs (Fig. 1B), whereas syndecan-1 is expressed in an opposite pattern (Fig. 1A and B). Furthermore, we found that BMECs in general lack or weakly express glypicans. Indeed, glypican-1 is not detectable on BMECs by using an anti-glypican-1 antibody. Since anti-glypican-2 to -6 antibodies are not yet available, we used phospholipase C to detach all GPI-linked proteins from the cell surface and measured the loss of heparan sulfate from the cells. Treating BMECs with phospholipase C did not decrease heparan sulfate levels, suggesting that glypicans are absent or weakly expressed on BMECs. These results agree with those of recent studies, which show that brain endothelial cells richly express syndecan-2 and -4 but do not express glypican-1 (26, 70). As previously reported, we found that BMECs lack the entry receptor CD4 as well as GalCer (Fig. 1A and B) (23, 54). We also found that BMECs express low levels of both coreceptors CCR5 and CXCR4 (Fig. 1A and B). In contrast to a previous study (57), we were unable to detect DC-SIGN/R on BMECs (Fig. 1A and B). This may be related to the donor (i.e., difference in expression between individuals), the isolation procedure, or the number of passages of BMEC cultures. The detection of the tight junction marker Zonula occludens (ZO-1) (even in the absence of astrocytes) (Fig. 1A and B), by staining directly adherent BMEC monolayers with a specific antibody, suggests that the isolated BMECs used in this study represent appropriate targets for HIV-1 transmigration analysis (42, 74). Consistent with the in vitro immunostaining data, we found that human brain capillaries also express high levels of heparan sulfates in vivo (Fig. 1C, top panels), likely contributed by the presence of syndecan-2 and -4 (Fig. 1C, bottom panels). Syndecan-4 was consistently present on the surface of brain capillaries (data not shown). In contrast, we observed some variation of expression for syndecan-1, -2, and -3 among individuals (data not shown). Brain vessels lack or weakly express CD4, GalCer, and DC-SIGN/R but express significant CXCR4 and CCR5 levels (data not shown). Together, these in vitro and in vivo immunostaining data demonstrate that CSPGs and HSPGs, such as syndecans, are expressed on the surface of an important route of entry into the brain for HIV. Given that syndecans are capable of mediating attachment of HIV-1 particles onto the surface of CD4-negative cells (15, 76), their presence on BMECs may profoundly influence HIV neuroinvasion.
FIG. 1.
Human primary adult BMECs derived from temporal lobe tissues (A) and human primary fetal BMECs obtained from Cell System Corp. (B) were analyzed for cell surface expression by FACS. BMECs (106 cells) were incubated with anti-CD4 SIM.4, anti-DC-SIGN DC28, anti-CXCR4 12G5, anti-CCR5 2D7, anti-HSPG 10E4, anti-CSPG CS-56, anti-GalCer MAB342, anti-CD147 HIM6, anti-ZO-1 C-19, anti-CD4 RP4-T4, anti-syndecan-1 ID4, anti-syndecan-2 10H4, anti-syndecan-3 1C7, anti-syndecan-4 8G3, and anti-glypican-1 S1 antibodies (1 μg) in 500 μl of PBS containing 0.25% human serum. Levels of cell surface attachment and entry receptors were determined by FACS analysis. Values are the geometric means expressed in fluorescence units (log scale). Results are representative of those from two independent experiments. Cell surface removal of heparan and chondroitin sulfates with heparinase, chondroitinase ABC, and phospholipase C were performed as described previously (53, 76). (C) Immunostained cryosections of human brain tissue, revealing the expression of HSPGs (3G10 MAb), syndecan-2 (10H4 MAb), and syndecan-4 (8G3 MAb).
HIV-1 transmigration assay.
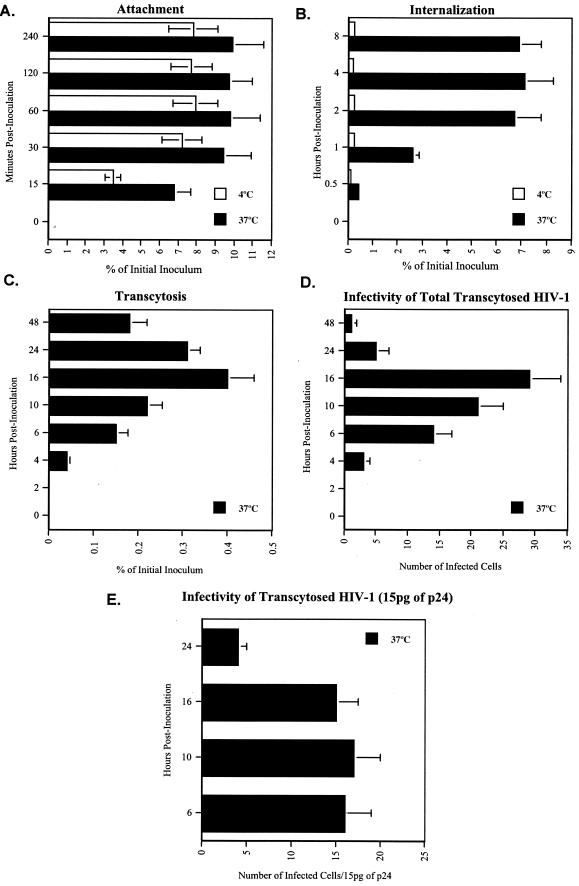
After analyzing the cell surface expression of candidate attachment and entry HIV receptors in BMECs, we developed assays to discriminate between initial viral attachment, subsequent viral internalization, and viral release from BMECs. The transendothelial transport assay was performed in Biocoat cell culture inserts (Collaborative Biomedical Products) with 40,000 BMECs plated on the upper surface of a polyethylene teraphthalate membrane coated with fibronectin and collagen I, as described previously (47). The cells were cultured until formation of tight junctions occurred (around 4 days). The integrity of the monolayer was verified by measuring the transendothelial electrical resistance of the monolayer and the permeability coefficient of 14C-inulin as described previously (47). Using this transwell filter assay, we first examined how long it takes for HIV-1 to attach to the surface of BMECs. HIV-1 was added to the apical surface of the BMEC monolayer. After different time intervals, BMECs were extensively washed to remove unbound virus, detached (Cell Stripper), and lysed. Amounts of attached virus were determined by p24 ELISA in BMEC lysates. Experiments were conducted at both 4 and 37°C (Fig. 2A). We found that HIV-1 rapidly attached to BMECs, with a plateau occurring after 30 min (Fig. 2A). During the first hour, we did not observe a significant difference in the efficiency of attachment between 4 and 37°C, suggesting that the initial attachment of HIV-1 to BMECs is not temperature dependent. Secondly, we examined how long it takes for HIV-1 to enter BMECs. HIV-1 was added to BMECs, and at different time intervals, cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA in BMEC lysate. In contrast to viral attachment, HIV-1 internalization into BMECs was strictly dependent on temperature. Indeed, less than 1% of attached particles penetrated BMECs at 4°C, whereas more than 80% of attached viruses entered BMECs (Fig. 2B). Furthermore, we observed that viral internalization is a slower process than attachment, since it took 90 min to 2 h for HIV-1 to enter BMECs at 37°C (Fig. 2B). Third, we examined how long it takes for HIV-1 to transmigrate through the BMEC monolayer. To mimic physiological transmigration conditions, HIV-1 was added to the apical surface of BMECs and amounts of transcytosed viruses were quantified by measuring capsid levels in the lower chamber corresponding to the basal surface. We found that 6 h was sufficient for HIV-1 to transmigrate through the BMEC monolayer and observed a peak after 16 h (Fig. 2C). To determine whether viruses that have crossed BMECs represent infectious particles, the medium from infected BMECs was collected at different time intervals, filtered, and added to CD4+ CXCR4+ CCR5+ HeLa indicator cells (TZM-bl cells). Importantly, basal chamber medium collected between 6 and 16 h represented a source of infection for TZM-bl cells (Fig. 2D), indicating that viruses which crossed the BMEC monolayer represent authentic infectious particles. We observed a decrease in p24 levels in the basal chamber (Fig. 2D), suggesting that BMECs, at least under our culture conditions, do not support robust HIV-1 replication, as previously reported (47, 60). Furthermore, medium from the basal chamber of BMECs collected after 16 h was significantly less infectious than medium collected prior to 16 h even after p24 normalization (Fig. 2D). Similar results were obtained after p24 standardization (Fig. 2E). This finding suggests that if particles which crossed the BMEC monolayer do not rapidly encounter target cells, they lose their infectivity, probably due to particle degradation or gp120 shedding. Together, these results suggest that HIV-1 rapidly attaches to the apical surface of BMECs (15 to 30 min), enters the monolayer after 2 h, and is released from the basal BMEC membrane after 6 h. Although viruses that transmigrate represent only a small fraction of the original inoculum (0.5%), our findings indicate that even this tiny amount of infectious particles released into the basal side of BMECs can infect target cells that reside in the brain, such as macrophages and microglia.
FIG. 2.
(A) Attachment. BMECs (Cell System Corp.) were exposed to 10 ng of p24 of HIV-1 pNL-ADA at 4 or 37°C. After different intervals of time, cells were washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates. (B) Internalization. HIV-1 was added to BMECs as described above, and at different intervals of time, cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. (D) Infectivity of transcytosed viruses. Medium (100 μl) from the basal chamber was collected at different intervals of time, filtered, and added to TZM-bl indicator cells. Infection was measured 48 h postinfection by X-Gal staining by counting the number of blue foci. Results are representative of those from two independent experiments. (E) Same experiments as for panel D, except that virus was first standardized for p24 (15 pg) prior to infection.
gp120 is required for HIV-1 transcytosis through BMECs.
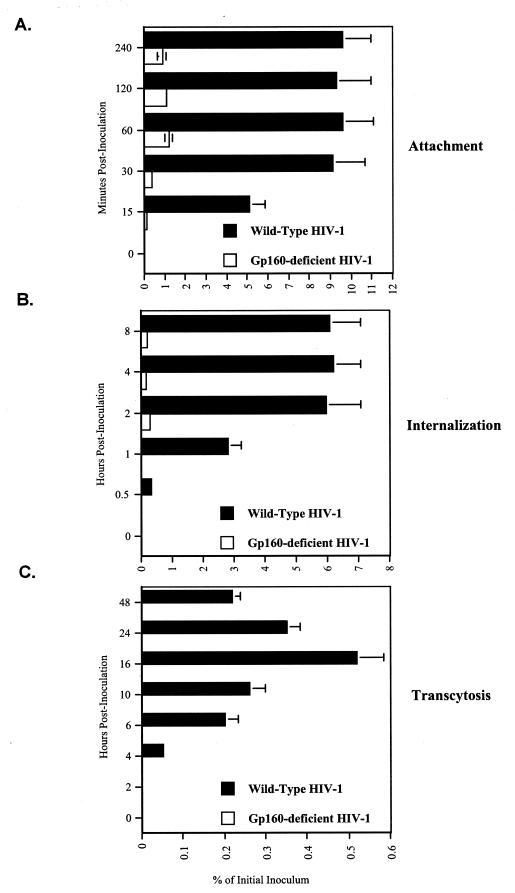
To address whether gp120 is necessary for the transcellular routing of HIV-1 through BMECs, we compared the transmigration capacity of a gp160-deficient virus (called pNL4.3-E-) (generous gift from N. Landau) with that of wild-type virus (called pNL4.3) (generous gift from M. Martin) by using our transmigration assay developed as described above. In contrast to wild-type HIV-1, the gp160-deficient mutant virus attached weakly to BMECs (Fig. 3A), suggesting that the viral envelope glycoprotein is the main ligand for HIV attachment to the brain microvascular endothelium. Consistent with the failure of attachment, the gp160-deficient virus weakly entered BMECs (Fig. 3B) and failed to transmigrate into the basal chamber (Fig. 3C). These data are in agreement with those of previous studies showing that envelope-deficient viruses exhibit an attenuated capacity to enter or transmigrate through BMECs (11, 47). Since the gp160-deficient virus does not appear to cross the BMEC barrier, this suggests that the integrity of tight junctions was maintained and that the BMEC monolayer does not allow paracellular transport.
FIG. 3.
(A) Attachment. BMECs (Cell System Corp.) were exposed to 10 ng of p24 of HIV-1 pNL4.3 (wild type) or pNL4.3-E (gp160 deficient) virus at 37°C. After different intervals of time, cells were washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates. (B) Internalization. HIV-1 was added to BMECs as described above, and at different intervals of time, cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. Results are representative of those from two independent experiments.
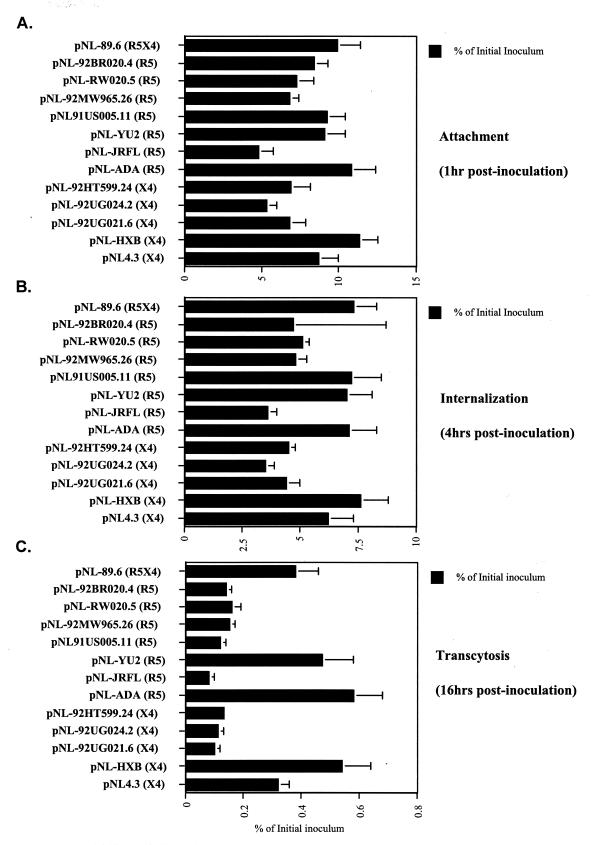
HIV-1 transcytosis does not depend on gp120 coreceptor usage.
Given that most HIV-1 strains isolated from the brain use CCR5 as a coreceptor (2, 17, 36, 46, 78, 82), we sought to determine whether coreceptor usage represents a critical parameter for HIV-1 transmigration through BMECs. To explore this issue, we tested a panel of viruses expressing various envelopes with different tropisms. Specifically, we used pNL4.3-derived infectious molecular clones in which the envelope gene was replaced with envelopes derived from a panel of isolates including pNL-ADA, pNL-JRFL, pNL-YU2, pNL91US005.11, pNL-92MW965.26, pNL-RW020.5, and pNL-92BR020.4 as R5 viruses, pNL4.3, pNL-HXB, pNL-92UG021.6, pNL-92UG024.2, and pNL-92HT599.24 as X4 viruses, and pNL-89.6 as an R5X4 virus (a generous gift from P. Bieniasz) (97). All viruses were grown in Jurkat-CCR5 cells, standardized for p24, and tested for transmigration through BMECs as described above. We did not observe a correlation between coreceptor usage and the capacity of the virus to cross BMECs. For example, both pNL-HXB (X4) and pNL-ADA (R5) transmigrated very efficiently, whereas pNL-UG024.2 and pNL-JRFL transmigrated very poorly (Fig. 4). However, we observed a correlation between the ability of the virus to attach to BMECs and to be released into the basal chamber. The unique exception to this rule is the R5 virus 91US005.11, which attaches and enters efficiently but is poorly released by infected BMECs (Fig. 4). Thus, the predominance of R5 viruses in the brains of AIDS patients cannot be explained by the possibility that cell-free R5 viruses cross the BBB more efficiently than X4 viruses.
FIG. 4.
(A) Attachment. BMECs (Cell System Corp.) were exposed to 10 ng of p24 of HIV-1 pNL4.3 derivatives for 1 h at 37°C. Cells were then washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA in cell lysates. (B) Internalization. Viruses were added to BMECs for 4 h at 37°C. Cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs for 16 h at 37°C, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. Results are representative of those from two independent experiments.
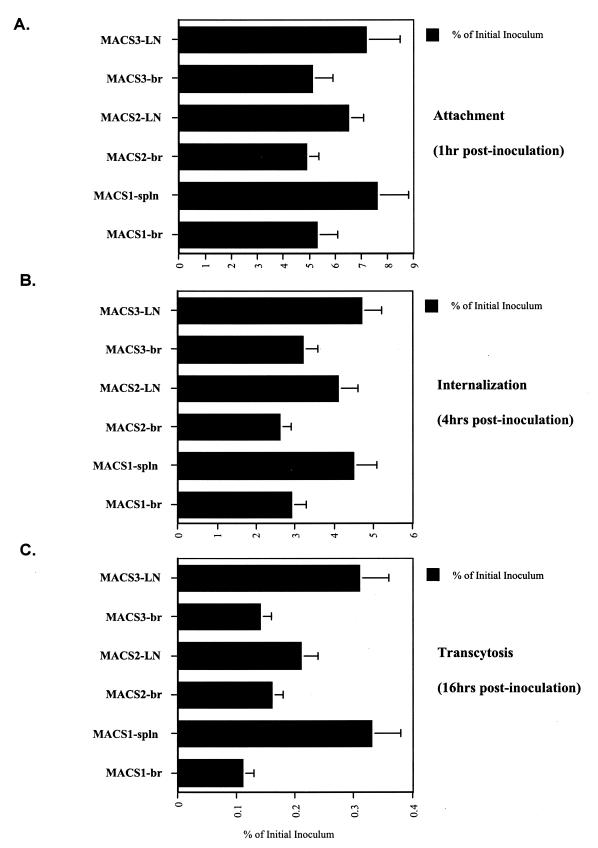
Brain-derived viruses do not transmigrate through BMECs more efficiently than lymphoid-derived viruses.
We next sought to determine whether viruses isolated from the brain exhibit a higher capacity to cross the BBB than virus isolated from lymphoid tissues. To address this issue, we compared the transmigration capacities of pairs of primary viruses isolated from the same AIDS patients with dementia, either from the brain (frontal lobe) or from lymphoid tissues (spleen or lymph node) (32). Isolated viruses were amplified in CD8-depleted peripheral blood mononuclear cells as described previously (32). The following three pairs of viruses isolated from the brain (br) and either the spleen (spln) or lymph nodes (LN) were tested for transmigration by using the assay described above: MACS1-br and MACS1-spln, MACS2-br and MACS2-LN, and MACS3-br and MACS3-LN (32). MACS1-br and MACS1-spln are R5X4 viruses, whereas MACS2-br, MACS2-LN, MACS3-br, and MACS3-LN are R5 viruses (32). The brain-derived viruses did not cross the BMEC monolayer more efficiently than lymphoid-derived viruses (Fig. 5). In fact, the lymphoid-derived viruses transmigrated more effectively than the brain-derived viruses (Fig. 5). Furthermore, we found that coreceptor usage (R5X4 versus R5) did not correlate with the capacity of the virus to cross the artificial BBB (Fig. 5), as observed above (Fig. 4). Thus, these data suggest that the property of HIV-1 to replicate in the brain of AIDS patients does not correlate with its capacity to migrate through the BBB as cell-free virus.
FIG. 5.
(A) Attachment. BMECs (Cell System Corp.) were exposed to 10 ng of p24 of pairs of viruses derived from brain (br) or from lymphoid tissues such as spleen (spln) or lymph nodes (LN) for 1 h at 37°C. Cells were then washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates. (B) Internalization. Viruses were added to BMECs for 4 h at 37°C. Cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs for 16 h at 37°C, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. Results are representative of those from two independent experiments.
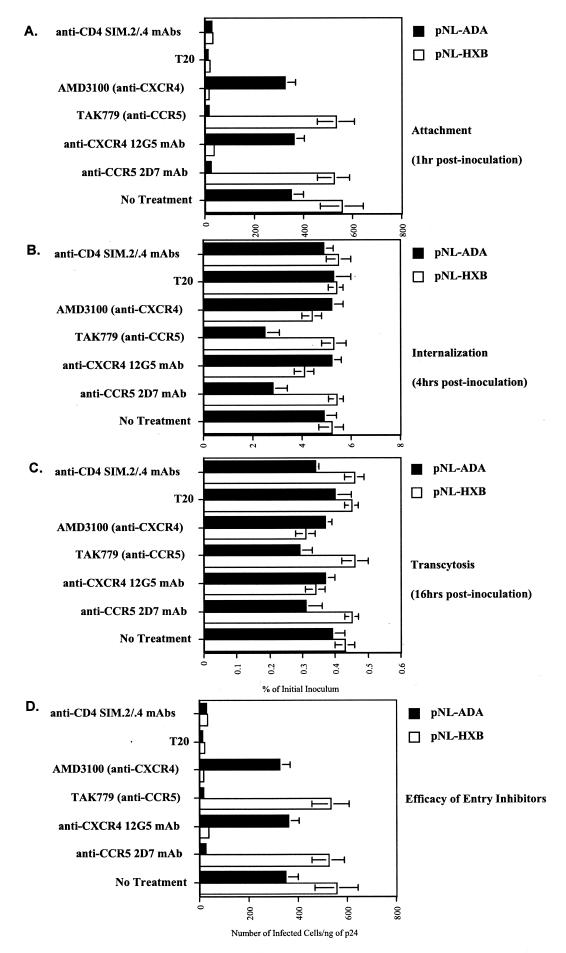
HIV-1 entry receptors do not facilitate HIV-1 transcytosis through BMECs.
The preceding experiments show that the presence of gp120, but not coreceptor usage, governs HIV-1 transmigration. We next sought to determine whether CCR5 or CXCR4 plays a role in this process. To address this issue, CCR5 or CXCR4 was blocked with either neutralizing MAbs (2D7 against CCR5 and 12G5 against CXCR4) or small antagonists (TAK779 against CCR5 and AMD3100 against CXCR4). BMEC monolayers were preincubated with 10 μg of neutralizing antibodies/ml or with 100 nM TAK779 (9) or 1.2 μM AMD3100 (22, 77) for 1 h at 37°C prior to the addition of R5 (pNL-ADA) or X4 (pNL-HXB) virus. CXCR4 and CCR5 inhibitors did not block pNL-ADA or pNL-HXB attachment to BMECs (Fig. 6A), in agreement with previous work by Liu et al. and Mukhtar et al., who showed that SDF-1α, RANTES, and MIP-1α and β do not prevent HIV-1 binding to BMECs (47, 57). Together, these results indicate that HIV-1 uses receptors other than CXCR4 and CCR5 to initially attach to the surface of BMECs. Furthermore, we found that CCR5 and CXCR4 inhibitors only partially diminished HIV-1 entry into and transmigration through BMECs, suggesting that CCR5 and CXCR4 play minor roles in the transcytosis process. Interestingly, viruses that transmigrated into the basal chamber in the presence of the coreceptor inhibitors were as infectious as those that transmigrated in the absence of inhibitors (data not shown). Surprisingly, we found that the T20 peptide fusion inhibitor (5 μg/ml) does not diminish HIV-1 entry and transmigration (Fig. 6B and C), suggesting that fusion between viral and BMEC membranes is not required for successful transcytosis. Lastly, anti-CD4 antibodies (SIM2 and SIM.4) (100 μl of culture supernatant per ml of each) failed to prevent HIV-1 attachment, entry, and transcytosis (Fig. 6), further suggesting that HIV-1 crosses the BBB in a CD4-independent manner as proposed previously (23, 54). Note that we verified the potency of these entry inhibitors (Fig. 6D). Given that classical entry receptors, CD4, CXCR4, and CCR5, only moderately facilitated HIV-1 transmigration, our data strongly suggest that HIV-1 uses unconventional receptors or mechanisms to cross the BMEC monolayer.
FIG. 6.
(A) Attachment. BMECs (Cell System Corp.) preincubated with a panel of inhibitors (anti-CD4 MAb, anti-CCR5 MAb, anti-CXCR4 MAb, AMD3100, TAK779, or T20) were exposed to 10 ng of p24 of pNL-ADA (R5) or pNL-HXB (X4) for 1 h at 37°C. Cells were then washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates. (B) Internalization. Viruses were added to BMECs for 4 h at 37°C. Cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs for 16 h at 37°C, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. Results are representative of those from two independent experiments. (D) Inhibitory efficacy. The above inhibitors were added to TZM-bl indicator cells followed by the addition of HIV-1 (1 ng of p24). Infection was measured at 48 h postinfection by X-Gal staining by counting the number of blue foci.
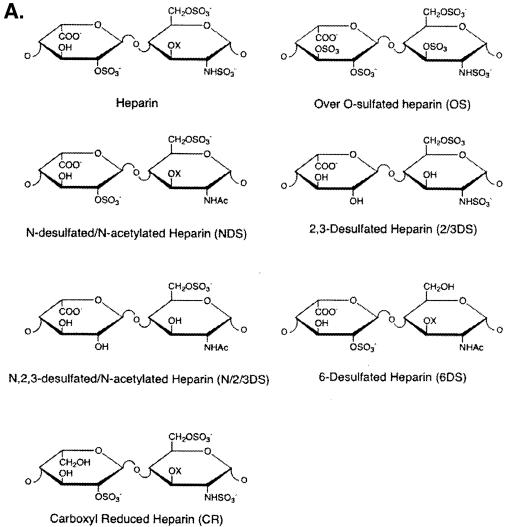
Soluble glycosaminoglycans prevent HIV-1 transcytosis.
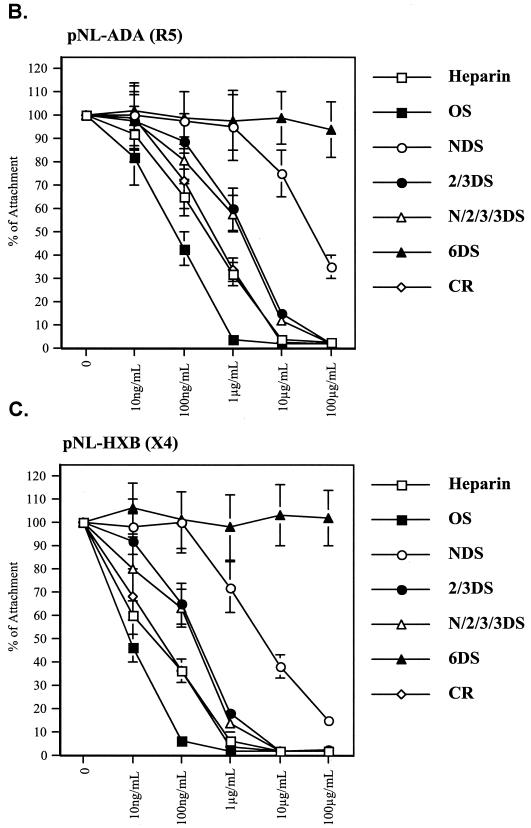
It was previously reported that proteoglycans on primary human endothelial cells efficiently capture HIV-1 (15), suggesting the possibility that proteoglycans participate in the HIV-1 transmigration process. To address this issue, we first sought to determine whether soluble glycosaminoglycans can prevent HIV-1 transcytosis. We tested a panel of soluble chemically modified heparin derivatives to block HIV-1 transcytosis, including heparin, N-desulfated/N-acelytated heparin (NDS-heparin), oversulfated heparin (OS-heparin), carboxyl-reduced heparin (CR-heparin), 2-O, 3-O-desulfated heparin (2/3DS-heparin), N-, 2-O, 3-O-desulfated heparin (N/2/3DS-heparin), and 6-O-desulfated heparin (6DS-heparin) (Fig. 7A) (89). Although the majority of these derivatives blocked the initial attachment of HIV-1 to BMECs (Fig. 7B and C), they differed significantly in their potency. Oversulfated heparin was more potent (50% inhibitory concentration [IC50] of 57 ng/ml) than native heparin (IC50 of 221 ng/ml), the 2-O, 3-O-desulfated heparin exhibited an intermediate inhibitory effect (IC50 of 1.7 μg/ml), whereas the completely 6-O-desulfated heparin was ineffective (Fig. 7B and C). These data suggest that the degree of sulfation and in particular the presence of 6-O-sulfate groups (6-O-sulfo GlcNAc and the GlcNSO3) represent important parameters for HIV-1 adsorption onto BMECs. Additionally, the negatively charged carboxyl group on the uronic acids plays an important role, since the carboxyl-reduced material was relatively inactive. Supporting the notion that R5 viruses are less sensitive to polyanion inhibition than X4 viruses (52, 55, 97), we found that more soluble proteoglycans (10-fold excess) are required to block the attachment of R5 viruses (pNL-ADA) (Fig. 7B) than that of X4 viruses (pNL-HXB) (Fig. 7C).
FIG. 7.
(A) Structure of soluble glycosaminoglycans. (B and C) Attachment. BMECs (Cell System Corp.) were exposed to 10 ng of p24 of pNL-ADA (R5) (B) or pNL-HXB (X4) (C) for 1 h at 37°C in the presence of increasing concentrations of soluble glycosaminoglycans. Cells were then washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates.
Cell surface proteoglycans facilitate HIV-1 transmigration.
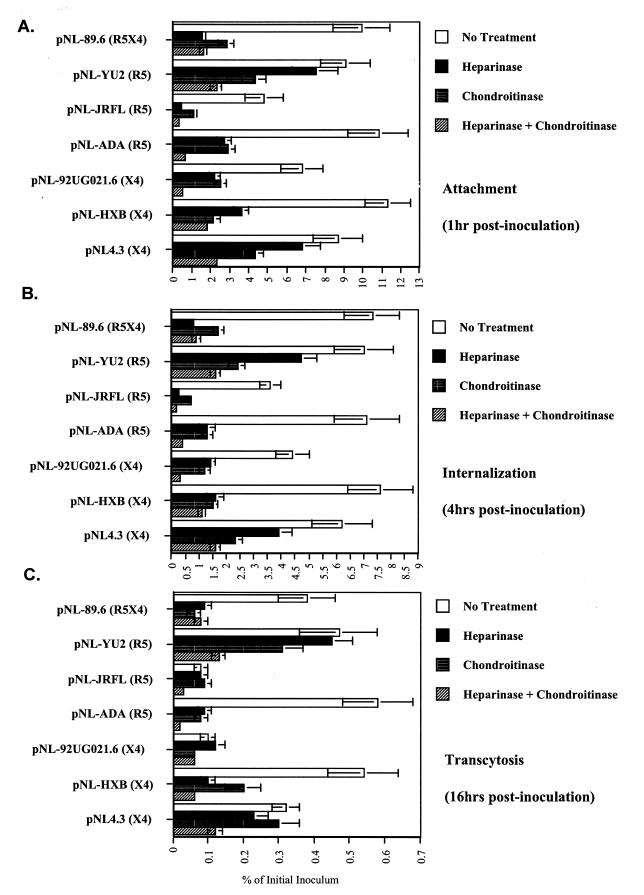
Our observation that soluble proteoglycans significantly inhibited the initial adsorption of HIV-1 to BMECs led us to postulate that cell surface proteoglycans richly expressed on BMECs (Fig. 1) directly participate in HIV-1 attachment to the BBB. To address this issue, BMEC monolayers were pretreated or not with heparinase or chondroitinase to remove cell surface heparan or chondroitin sulfate moieties, respectively. We verified by FACS that heparan and chondroitin sulfates were removed by using the 10E4 antibody for heparan sulfates and the CS-56 antibody for chondroitin sulfates (Fig. 1A). We observed that the enzymatic treatment conditions (enzyme concentration, incubation time, and temperature) as well as the source (or the lot number) of these enzymes was of critical importance in significantly reducing the levels of heparan and chondroitin sulfates on the surfaces of BMECs. Therefore, for each enzymatic treatment, we verified by FACS that these enzymes removed heparan or chondroitin sulfate moieties (at least 80% removal) prior to the addition of virus as shown above (Fig. 1A). Importantly, we found that heparinase treatment of BMECs reduced HIV-1 attachment (Fig. 8B), although this inhibitory effect differed greatly depending on the HIV-1 strains tested. Heparinase efficiently blocked pNL-89.6 and pNL-JRFL attachment to BMECs, whereas it only slightly blocked pNL4.3 and pNL-YU2 attachment (Fig. 8A). Similarly, we found that chondroitinase also diminished viral attachment in a strain-dependent manner (Fig. 8A). However, the combination of both enzymes prevented the adsorption of a majority of HIV-1 strains (Fig. 8A). Importantly, heparinase and chondroitinase treatments did not affect the expression of CXCR4, CCR5, or other cell surface antigens such as CD147 (Fig. 1A), suggesting that these enzymes specifically removed heparan and chondroitin sulfate moieties without altering other cell surface molecules. Furthermore, we found that the heparinase- and chondroitinase-mediated decrease in HIV-1 entry and transcytosis (Fig. 8B and C) reflects the initial decrease in adsorption, suggesting that HSPGs and CSPGs function mainly as attachment receptors for HIV-1 on the surfaces of BMECs. Together, these results suggest that HIV-1 exploits both HSPGs and CSPGs as attachment receptors on the surfaces of BMECs.
FIG. 8.
(A) Attachment. BMECs (Cell System Corp.), pretreated or not with heparinase, chondroitinase, or both enzymes, were exposed to 10 ng of p24 of pNL derivatives for 1 h at 37°C. Cells were then washed, detached, and lysed. Amounts of attached virus were determined by p24 ELISA of cell lysates. (B) Internalization. Viruses were added to BMECs for 4 h at 37°C. Cells were washed, trypsinized, washed again, and immediately lysed. Amounts of internalized HIV-1 were quantified by p24 ELISA of cell lysates. (C) Transcytosis. HIV-1 was added to the apical surface of BMECs for 16 h at 37°C, and amounts of transcytosed viruses were quantified by p24 ELISA in the lower chamber, corresponding to the basal surface. Results are representative of those from two independent experiments.
DISCUSSION
In the present study, we investigated the transcellular migration of HIV as a cell-free virus through an intact BMEC monolayer. The absence of CD4 on BMECs and the fact that SDF-1α, RANTES, and MIP-1α and β do not prevent HIV-1 entry into BMECs (8, 47) implicate an unusual entry pathway for HIV-1. Here, we sought to determine whether HSPGs contribute to HIV-1 transcytosis. We found that BMECs express high levels of HSPGs likely contributed by syndecan-2 and -4. As expected, we did not detect CD4 or GalCer and found that BMECs express low levels of CXCR4 and CCR5, as previously described (47, 57). Importantly, we found that like HSPGs, CSPGs are also abundantly expressed on the surface of BMECs. This is in agreement with a recent study by Argyris et al., who showed that low passaged BMECs express high levels of both HSPGs and CSPGs (8). In contrast to a previous study, we were unable to detect DC-SIGN/R on BMECs (57). Note that the addition of neutralizing anti-DC-SIGN antibodies or an excess of mannan did not influence HIV-1 attachment to BMECs (data not shown). This apparent discrepancy may arise from the source of BMECs, the conditions of culture, and the number of passages in cell culture. Importantly, the cell surface expression pattern of low passaged BMECs may greatly differ from that of high passaged BMECs. For example, we found that after 8 passages, BMECs derived from discarded temporal lobe tissues from one donor lost their expression of the tight junction marker ZO-1, expressed high syndecan-3 levels, and even gained CD4 expression (data not shown). This underscores the importance of the number of passages of BMECs for studies on BBB transmigration. We observed that low passaged BMECs (with 8 different donors) express a uniform pattern of HIV-1 entry and attachment receptors among donors, suggesting that freshly isolated as well as low passaged BMECs are suitable for transmigration studies. Thus, as attachment receptors, HSPGs (syndecans) expressed on the surface of an important route of entry into the brain may profoundly influence HIV neuroinvasion.
We next found that HIV-1 possesses the capacity to rapidly attach to BMECs in a temperature-independent, but HSPG- and CSPG-dependent manner. We found that soluble glycosaminoglycans or the removal of either HSPGs or CSPGs greatly diminishes the ability of HIV-1 to bind to BMECs, suggesting that these proteoglycans serve as major attachment receptors. The removal of both HSPGs and CSPGs amplified the failure of HIV-1 to attach to BMECs, suggesting that HIV-1 may exploit HSPGs and CSPGs in a cooperative manner to facilitate its initial adsorption. Furthermore, we demonstrated that gp120 is required for the initial attachment of HIV-1 to BMECs. Indeed, a gp120-deficient virus fails to bind to and transcytose through BMECs. As previously described, we found that CCR5 and CXCR4 do not participate in the attachment of HIV-1 to BMECs (47, 57). Furthermore, coreceptor antagonists do not significantly affect HIV-1 entry and transcytosis of HIV-1 through BMECs. The finding that the affinity of HIV-1 for CCR5 and CXCR4 in the absence of CD4 is extremely low (93) may explain why HIV-1 does not use these coreceptors to enter BMECs. Supporting this hypothesis, T-20 does not influence HIV-1 transcytosis. Moreover, our finding that the coreceptor usage of gp120 is not a major determinant of the ability of HIV-1 to cross BMECs further supports the notion that CCR5 and CXCR4 are not required for HIV-1 transmigration. Together, these results suggest that HIV-1 uses alternative receptors such as HSPGs and CSPGs to cross the BBB.
Our findings that HSPGs promote initial HIV-1 attachment to and entry into BMECs are in accordance with previous studies including ours, which showed that HSPGs (i.e., syndecans) greatly enhance HIV-1 adsorption onto cells that express CD4 such as macrophages (76), CD4+ HeLa cells (53, 75), CD4+-T-cell lines (38, 62, 63, 73, 97), or cells that lack CD4, such as primary human vein endothelial cells (15), human genital epithelial cells (94), or HeLa cells (53). This finding is also consistent with a recent study, which showed that the uptake of the basic fibroblast growth factor by the BBB is greatly enhanced by cell surface HSPGs (21). Interestingly, Argyris et al., by using a method similar to ours, also found that the removal of both HSPGs and CSPGs reduces HIV-1 entry into BMECs (8). However, they mapped the requirement for proteoglycans at the internalization step rather than the attachment step (8). They postulated that proteoglycans facilitate HIV-1 entry into BMECs, either by rescuing HIV-1 from abortive endocytosis or by directing HIV-1 particles into safe transcytosis routes (8). Given that we and others showed that gp120 serves as the main ligand for HSPGs (15, 54, 72, 96), it is difficult to imagine that HSPGs and CSPGs abundantly expressed on BMECs would not, at least in part, contribute to the initial attachment of HIV-1 to BMECs. Nevertheless, these apparent discrepancies may arise from the cell origin or cell culture conditions. For example, our BMECs were plated on transwell filters precoated with fibronectin and collagen, whereas those of Argyris et al. were plated on plastic flasks precoated with attachment factor (8). These subtle variations in plating conditions may lead to different degrees of expression of receptors such as HSPGs and CSPGs on the surfaces of BMECs and may thereby influence HIV-1 transmigration. This might also explain why Argyris et al. found a more important contribution of CSPGs than HSPGs in HIV-1 entry into BMECs (8).
Our present data together with those of Argyris et al. identified HSPGs and/or CSPGs as primary attachment/entry receptors on BMECs. Interestingly, previous studies showed that both HSPGs (especially syndecans) and CSPGs are associated with lipid rafts (29, 50, 81, 86, 96). Interestingly, Slimani et al. demonstrated that syndecan-1 and -4, but not syndecan-2, form complexes with CCR5 on the surfaces of HeLa cells (81). These data may suggest a close proximity between HSPGs (at least syndecans) and HIV-1 coreceptors on the surfaces of BMECs. Supporting the possibility that HSPGs and CSPGs associated with lipid rafts promote HIV-1 entry into BMECs, Liu et al. showed that cholesterol-extracting agents such as cyclodextrin and nystatin efficiently prevent HIV-1 entry into BMECs (47). However, this notion has been recently challenged by Argyris et al., who showed that cyclodextrin and 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A reductase inhibitor treatments do not affect the attachment and entry of HIV-1 in BMECs but blocked HIV-1 infection of T cells (8). As suggested by Argyris et al., the fact that they used primary fetal BMECs (8), while Liu et al. used adult BMECs isolated from discarded temporal lobe tissues (47), may explain this apparent discrepancy. If HSPGs and/or CSPGs do not facilitate HIV-1 entry into BMECs via lipid rafts, they could mediate viral entry via macropinosomes, as previously suggested (43, 48). Thus, we cannot exclude the possibility that HSPGs and/or CSPGs may capture HIV-1 particles on the surfaces of BMECs and subsequently direct them into these macropinosomes. Supporting this hypothesis, Liu et al. showed that the macropinocytosis inhibitor dimethylamiloride strongly diminishes HIV-1 entry into BMECs (47).
Our study demonstrates that gp120 is necessary for HIV-1 transmigration through BMECs. However, we did not observe a correlation between coreceptor usage and the capacity of the virus to transcytose. This is surprising, given that the majority of viruses isolated from the brains of AIDS patients are R5 viruses (2, 17, 36, 46, 78, 82). CCR5 is the major coreceptor for HIV-1 infection of macrophages and microglia (2, 31, 36, 78) and the principal coreceptor used by HIV-1 viruses isolated from the brain (2, 36, 46, 49, 78, 82). Nevertheless, we found that viruses (both X4 and R5) which attach efficiently to the surface of BMECs usually transcytose better than those which did not attach efficiently. This lack of specificity, in terms of coreceptor usage, is in accordance with our observation that neither coreceptors nor fusion are required for efficient viral transmigration though the BBB. Further supporting the hypothesis that HIV-1 transmigration through the BBB does not correlate with coreceptor usage, we found that pairs of viruses isolated from the same AIDS patient and having the same coreceptor usage differ nonetheless in their abilities to cross BMEC monolayers. This also suggests that the properties conferring virus replication in the brain are distinct from those conferring virus entry into the brain. Only viruses that successfully crossed the BBB and subsequently infected target cells in the brain will productively replicate and persist in this body compartment. Factors other than CCR5 usage also play an important role in brain infection. Although macrophage tropism seems to predict HIV-1 neurotropism independent of coreceptor specificity (32), CCR5 usage by primary brain-derived HIV-1 isolates is neither necessary nor sufficient for neurotropism (defined as the ability of viruses to replicate in microglia) (32). Specifically, macrophages and microglia can support efficient replication by a subset of primary X4 viruses isolated from blood (32, 37, 61, 79, 80, 88) or brain tissue (32). Thus, it is likely that brain invasion and neurotropism arise from distinct HIV-1 properties.
We found that the efficiency of cell-free HIV-1 transmigration is extremely low (less than 0.5% of the inoculum). Given that the peak of viremia is high during primary infection, this high concentration of virus in plasma may result in the accumulation of viruses captured by HSPGs and CSPGs that cover the BBB. We cannot exclude the possibility that a low level of cell-free HIV-1 transcytosis occurs in vivo. However, we did not observe a correlation between coreceptor usage and transmigration, suggesting that the establishment of brain infection via cell-free HIV-1 transmigration is a rare event. A large body of evidence suggests that HIV-1 gains entry into the brain within perivascular macrophages (59, 60, 65, 92). This cell-associated transport of HIV into the brain would explain the predominance of macrophage-tropic strains isolated from the brain of AIDS patients. Further work is required to compare the respective contribution of cell-free and cell-associated transmigration to HIV brain invasion.
Acknowledgments
We thank J. Kuhns for secretarial assistance and A. C. S. Saphire for critically reading the manuscript. We thank H. Fox and E. Stephens for helpful discussions. We deeply thank M. Witte and M. Weinand for providing primary BMECs from discarded temporal lobe tissues. We thank M. Emerman for providing us with the Jurkat-CCR5 cells and P. Bieniasz for the pNL-envelope series (pNL-JRFL, pNL-YU2, pNL91US005.11, pNL-92MW965.26, pNL-RW020.5, pNL-92BR020.4, pNL-HXB, pNL-92UG021.6, pNL-92UG024.2, pNL-92HT599.24, and pNL-89.6).
This work was supported by U.S. Public Health Service grant no. AI 054196 (to P.A.G.), MH62261 (TSRI NeuroAIDS Preclinical Studies Developmental Award), and M01 RR00833 awarded to the General Clinical Research Center (GCRC) at The Scripps Research Institute. D.G. was supported by NS 37277 and NS 35734, and J.D.E. was supported by HL57345.
Footnotes
This is publication no. 16230-IMM from the Department of Immunology, The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright, A. V., J. T. C. Shieh, T. Itoh, B. Lee, D. Pleasure, M. J. O'Connor, R. W. Doms, and F. Gonzalez-Scarano. 1999. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J. Virol. 73:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright, A. V., S. S. Soldan, and F. Gonzalez-Scarano. 2003. Pathogenesis of human immunodeficiency virus-induced neurological disease. J. Neurovirol. 9:222-227. [DOI] [PubMed] [Google Scholar]
- 4.An, S. F., M. Groves, F. Gray, and F. Scaravilli. 1999. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 58:1156-1162. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, E., W. Zink, H. Xiong, and H. E. Gendelman. 2002. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J. Acquir. Immune Defic. Syndr. 31:S43-S54. [DOI] [PubMed] [Google Scholar]
- 6.Annunziata, P. 2003. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J. Neurol. 250:901-906. [DOI] [PubMed] [Google Scholar]
- 7.Annunziata, P., C. Cioni, S. Toneatto, and E. Paccagnini. 1998. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS 12:2377-2385. [DOI] [PubMed] [Google Scholar]
- 8.Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, J. K. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks, W. A., V. Akerstrom, and A. J. Kastin. 1998. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J. Cell Sci. 111:533-540. [DOI] [PubMed] [Google Scholar]
- 11.Banks, W. A., E. O. Freed, K. M. Wolf, S. M. Robinson, M. Franko, and V. B. Kumar. 2001. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. J. Virol. 75:4681-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell, J. E., A. Busuttil, J. W. Ironside, S. Rebus, Y. K. Donaldson, P. Simmonds, and J. F. Peutherer. 1993. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J. Infect. Dis. 168:818-824. [DOI] [PubMed] [Google Scholar]
- 14.Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 15.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 16.Brew, B. J., M. Rosenblum, and R. W. Price. 1998. AIDS dementia complex and primary HIV brain infection. J. Neuroimmunol. 20:133-140. [DOI] [PubMed] [Google Scholar]
- 17.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 18.Clifford, D. B. 2002. AIDS dementia. Med. Clin. N. Am. 86:537-550. [DOI] [PubMed] [Google Scholar]
- 19.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 20.Crone, C., and S. P. Olesen. 1982. Electrical resistance of brain microvascular endothelium. Brain Res. 241:49-55. [DOI] [PubMed] [Google Scholar]
- 21.Deguchi, Y., H. Okutsu, T. Okura, S. Yamada, R. Kimura, T. Yuge, A. Furukawa, K. Morimoto, M. Tachikawa, S. Ohtsuki, K. Hosoya, and T. Terasaki. 2002. Internalization of basic fibroblast growth factor at the mouse blood-brain barrier involves perlecan, a heparan sulfate proteoglycan. J. Neurochem. 83:381-389. [DOI] [PubMed] [Google Scholar]
- 22.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 23.Edinger, A. L., J. L. Mankowski, B. J. Doranz, B. J. Margulies, B. Lee, J. Rucker, M. Sharron, T. L. Hoffman, J. F. Berson, M. C. Zink, V. M. Hirsch, J. E. Clements, and R. W. Doms. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc. Natl. Acad. Sci. USA 94:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esko, J. D., and U. Lindahl. 2001. Molecular diversity of heparan sulfate. J. Clin. Investig. 108:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 26.Floris, S., J. van den Born, S. M. van der Pol, C. D. Dijkstra, and H. E. De Vries. 2003. Heparan sulfate proteoglycans modulate monocyte migration across cerebral endothelium. J. Neuropathol. Exp. Neurol. 62:780-790. [DOI] [PubMed] [Google Scholar]
- 27.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuki, I. V., M. E. Meyer, and K. J. Williams. 2000. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem. J. 351:607-612. [PMC free article] [PubMed] [Google Scholar]
- 30.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 31.Ghorpade, A., M. E. Xia, B. T. Hyman, Y. Persidsky, A. Nukuna, P. Bock, M. Che, J. Limoges, H. E. Gendelman, and C. R. Mackay. 1998. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J. Virol. 72:3351-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorry, P. R., G. Bristol, J. A. Zack, K. Ritola, R. Swanstrom, C. J. Birch, J. E. Bell, N. Bannert, K. Crawford, H. Wang, D. Schols, E. De Clercq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 75:10073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guibinga, G. H., A. Miyanohara, J. D. Esko, and T. Friedmann. 2002. Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells. Mol. Ther. 5:538-546. [DOI] [PubMed] [Google Scholar]
- 34.Harouse, J. M., S. Bhat, S. L. Spitalnik, M. Laughlin, K. Stefano, D. H. Silberberg, and F. Gonzalez-Scarano. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320-323. [DOI] [PubMed] [Google Scholar]
- 35.Harouse, J. M., R. G. Collman, and F. Gonzalez-Scarano. 1995. Human immunodeficiency virus type 1 infection of SK-N-MC cells: domains of gp120 involved in entry into a CD4-negative, galactosyl ceramide/3′ sulfo-galactosyl ceramide-positive cell line. J. Virol. 69:7383-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Buscigilo, X. Yang, W. Hofmann, W. Newman, C. R. MacKay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 37.Hibbitts, S., J. D. Reeves, G. Simmons, P. W. Gray, L. G. Epstein, D. Schols, E. De Clercq, T. N. C. Wells, A. E. I. Proudfoot, and P. R. Clapham. 1999. Coreceptor ligand inhibition of fetal brain cell infection by HIV type 1. AIDS Res. Hum. Retrovir. 15:989-1000. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim, J., P. Griffin, D. R. Coombe, C. C. Rider, and W. James. 1999. Cell-surface heparan sulfate facilitates human immunodeficiency virus type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 60:159-169. [DOI] [PubMed] [Google Scholar]
- 39.Kim, C. W., O. A. Goldberger, R. L. Gallo, and M. Bernfield. 1994. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim, T. A., H. K. Avraham, Y. H. Koh, S. Jiang, I. W. Park, and S. Avraham. 2003. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J. Immunol. 170:2629-2637. [DOI] [PubMed] [Google Scholar]
- 41.Kolson, D. L., and R. J. Pomerantz. 1996. AIDS dementia and HIV-1-induced neurotoxicity: possible pathogenic associations and mechanisms. J. Biomed. Sci. 3:389-414. [DOI] [PubMed] [Google Scholar]
- 42.Krause, D., U. Mischeck, H. J. Galla, and R. Dermietzel. 1991. Correlation of zonula occludens ZO-1 antigen expression and transendothelial resistance in porcine and rat cultured cerebral endothelial cells. Neurosci. Lett. 128:301-304. [DOI] [PubMed] [Google Scholar]
- 43.Lanzavecchia, A. 1996. Mechanisms of antigen uptake for presentation. Curr. Opin. Immunol. 8:348-354. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence, D. M., and E. O. Major. 2002. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 4:301-308. [DOI] [PubMed] [Google Scholar]
- 45.Leong, J. M., H. Wang, L. Magoun, J. A. Field, P. E. Morrissey, D. Robbins, J. B. Tatro, J. Coburn, and N. Parveen. 1998. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect. Immun. 66:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H. M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGavin, C. H., S. A. Land, K. L. Sebire, D. J. Hooker, A. D. Gurusinghe, and C. J. Birch. 1996. Syncytium-inducing phenotype and zidovudine susceptibility of HIV-1 isolated from post-mortem tissue. AIDS 10:47-53. [DOI] [PubMed] [Google Scholar]
- 50.McQuade, K. J., and A. C. Rapraeger. 2003. Syndecan-1 transmembrane and extracellular domains have unique and distinct roles in cell spreading. J. Biol. Chem. 278:46607-46615. [DOI] [PubMed] [Google Scholar]
- 51.Mertens, G., J. J. Cassiman, H. Van den Berghe, J. Vermylen, and G. David. 1992. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 267:20435-20443. [PubMed] [Google Scholar]
- 52.Meylan, P. R., R. S. Kornbluth, I. Zbinden, and D. D. Richman. 1994. Influence of host cell type and V3 loop of the surface glycoprotein on susceptibility of human immunodeficiency virus type 1 to polyanion compounds. Antimicrob. Agents Chemother. 38:2910-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moses, A. V., F. E. Bloom, C. D. Pauza, and J. A. Nelson. 1993. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc. Natl. Acad. Sci. USA 90:10474-10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhtar, M., and R. J. Pomerantz. 2000. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. J. Hum. Virol. 3:324-334. [PubMed] [Google Scholar]
- 57.Mukhtar, M., S. Harley, P. Chen, M. BouHamdan, C. Patel, E. Acheampong, and R. J. Pomerantz. 2002. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology 297:78-88. [DOI] [PubMed] [Google Scholar]
- 58.Nath, A. 2002. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 186:S193-198. [DOI] [PubMed] [Google Scholar]
- 59.Nottet, H. S., Y. Persidsky, V. G. Sasseville, A. N. Nukuna, P. Bock, Q. H. Zhai, L. R. Sharer, R. D. McComb, S. Swindells, C. Soderland, and H. E. Gendelman. 1996. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 156:1284-1295. [PubMed] [Google Scholar]
- 60.Nottet, H. S. 1999. Interactions between macrophages and brain microvascular endothelial cells: role in pathogenesis of HIV-1 infection and blood-brain barrier function. J. Neurovirol. 5:659-669. [DOI] [PubMed] [Google Scholar]
- 61.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohshiro, Y., T. Murakami, K. Matsuda, K. Nishioka, K. Yoshida, and N. Yamamoto. 1996. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol. Immunol. 40:827-835. [DOI] [PubMed] [Google Scholar]
- 63.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 64.Persidsky, Y., H. S. Nottet, V. G. Sasseville, L. G. Epstein, and H. E. Gendelman. 1995. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J. Neurovirol. 1:229-243. [DOI] [PubMed] [Google Scholar]
- 65.Persidsky, Y., and H. E. Gendelman. 2003. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 74:691-701. [DOI] [PubMed] [Google Scholar]
- 66.Pohlmann, S., E. J. Soilleux, F. Baribaud, G. J. Leslie, L. S. Morris, J. Trowsdale, B. Lee, N. Coleman, and R. W. Doms. 2001. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. USA 98:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poland, S. D., G. P. Rice, and G. A. Dekaban. 1995. HIV-1 infection of human brain-derived microvascular endothelial cells in vitro. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:437-445. [DOI] [PubMed] [Google Scholar]
- 68.Pomerantz, R. J. 2001. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. Biomed. Pharmacother. 55:7-15. [DOI] [PubMed] [Google Scholar]
- 69.Pumarola-Sune, T., B. A. Navia, C. Cordon-Cardo, E. S. Cho, and R. W. Price. 1987. HIV antigen in the brains of patients with the AIDS dementia complex. Ann. Neurol. 21:490-496. [DOI] [PubMed] [Google Scholar]
- 70.Qiao, D., K. Meyer, C. Mundhenke, S. A. Drew, and A. Friedl. 2003. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J. Biol. Chem. 278:16045-16053. [DOI] [PubMed] [Google Scholar]
- 71.Resnick, L., J. R. Berger, P. Shapshak, and W. W. Tourtellotte. 1988. Early penetration of the blood-brain-barrier by HIV. Neurology 38:9-14. [DOI] [PubMed] [Google Scholar]
- 72.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roderiquez, G., T. Oravecz, M. Yanagishita, D. C. Bou-Habib, H. Mostowski, and M. A. Norcross. 1995. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J. Virol. 69:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rutten, M. J., R. L. Hoover, and M. J. Karnovsky. 1987. Electrical resistance and macromolecular permeability of brain endothelial monolayer cultures. Brain Res. 425:301-310. [DOI] [PubMed] [Google Scholar]
- 75.Saphire, A. C., M. D. Bobardt, and P. A. Gallay. 1999. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 18:6771-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shieh, J. T. C., A. V. Albright, M. Sharron, S. Gartner, J. Strizki, R. W. Doms, and F. Gonzalez-Scarano. 1998. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J. Virol. 72:4243-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simmons, G., D. Wilkinson, J. D. Reeves, M. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmons, G., J. D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. I. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slimani, H., N. Charnaux, E. Mbemba, L. Saffar, R. Vassy, C. Vita, and L. Gattegno. 2003. Binding of the CC-chemokine RANTES to syndecan-1 and syndecan-4 expressed on HeLa cells. Glycobiology 13:623-634. [DOI] [PubMed] [Google Scholar]
- 82.Smit, T. K., B. Wang, T. Ng, R. Osborne, B. Brew, and N. K. Saksena. 2001. Varied tropism of HIV-1 isolates derived from different regions of adult brain cortex discriminate between patients with and without AIDS dementia complex (ADC): evidence for neurotropic HIV variants. Virology 279:509-526. [DOI] [PubMed] [Google Scholar]
- 83.Stephens, E. B., D. K. Singh, M. E. Kohler, M. Jackson, E. Pacyniak, and N. E. Berman. 2003. The primary phase of infection by pathogenic simian-human immunodeficiency virus results in disruption of the blood-brain barrier. AIDS Res. Hum. Retrovir. 19:837-846. [DOI] [PubMed] [Google Scholar]
- 84.Strelow, L. I., D. D. Watry, H. S. Fox, and J. A. Nelson. 1998. Efficient infection of brain microvascular endothelial cells by an in vivo-selected neuroinvasive SIVmac variant. J. Neurovirol. 4:269-280. [DOI] [PubMed] [Google Scholar]
- 85.Strelow, L. I., D. Janigro, and J. A. Nelson. 2001. The blood-brain barrier and AIDS. Adv. Virus Res. 56:355-388. [DOI] [PubMed] [Google Scholar]
- 86.Tkachenko, E., and M. Simons. 2002. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J. Biol. Chem. 277:19946-19951. [DOI] [PubMed] [Google Scholar]
- 87.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 88.Verani, A., E. Pesenti, S. Polo, E. Tresoldi, G. Scarlatti, P. Lusso, A. G. Siccardi, and D. Vercelli. 1998. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J. Immunol. 161:2084-2088. [PubMed] [Google Scholar]
- 89.Wang, L., J. R. Brown, A. Varki, and J. D. Esko. 2002. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J. Clin. Investig. 110:127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiley, C. A., R. D. Schrier, J. A. Nelson, P. W. Lampert, and M. B. Oldstone. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83:7089-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams, K. C., S. Corey, S. V. Westmoreland, D. Pauley, H. Knight, C. deBakker, X. Alvarez, and A. A. Lackner. 2001. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 94.Wu, Z., Z. Chen, and D. M. Phillips. 2003. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ cells: implications for mechanisms of sexual transmission. J. Infect. Dis. 188:1473-1482. [DOI] [PubMed] [Google Scholar]
- 95.Yahi, N., S. Baghdiguian, H. Moreau, and J. Fantini. 1992. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J. Virol. 66:4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamauchi, S., Y. Tokita, S. Aono, F. Matsui, T. Shuo, H. Ito, K. Kato, K. Kasahara, and A. Oohira. 2002. Phosphorylation of neuroglycan C, a brain-specific transmembrane chondroitin sulfate proteoglycan, and its localization in the lipid rafts. J. Biol. Chem. 277:20583-20590. [DOI] [PubMed] [Google Scholar]
- 97.Zhang, Y. J., T. Hatziioannou, T. Zang, D. Braaten, J. Luban, S. P. Goff, and P. D. Bieniasz. 2002. Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection. J. Virol. 76:6332-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zink, W. E., J. Zheng, Y. Persidsky, L. Poluektova, and H. E. Gendelman. 1999. The neuropathogenesis of HIV-1 infection. FEMS Immunol. Med. Microbiol. 26:233-241. [DOI] [PubMed] [Google Scholar]