Abstract
We demonstrate the feasibility of microscale molecular imaging using hyperpolarized proton and carbon-13 MRI contrast media and low-field (47.5 mT) preclinical scale (38 mm i.d.) 2D magnetic resonance imaging (MRI). Hyperpolarized proton images with 94 × 94 μm2 spatial resolution and hyperpolarized carbon-13 images with 250 × 250 μm2 in-plane spatial resolution were recorded in 4–8 s (largely limited by the electronics response), surpassing the in-plane spatial resolution (i.e., pixel size) achievable with micro-positron emission tomography (PET). These hyperpolarized proton and 13C images were recorded using large imaging matrices of up to 256 × 256 pixels and relatively large fields of view of up to 6.4 × 6.4 cm2. 13C images were recorded using hyperpolarized 1-13C-succinate-d2 (30 mM in water, %P13C = 25.8 ± 5.1% (when produced) and %P13C = 14.2 ± 0.7% (when imaged), T1 = 74 ± 3 s), and proton images were recorded using 1H hyperpolarized pyridine (100 mM in methanol-d4, %PH = 0.1 ± 0.02% (when imaged), T1 = 11 ± 0.1 s). Both contrast agents were hyperpolarized using parahydrogen (>90% para-fraction) in an automated 5.75 mT parahydrogen induced polarization (PHIP) hyperpolarizer. A magnetized path was demonstrated for successful transportation of a 13C hyperpolarized contrast agent (1-13C-succinate-d2, sensitive to fast depolarization when at the Earth’s magnetic field) from the PHIP polarizer to the 47.5 mT low-field MRI. While future polarizing and low-field MRI hardware and imaging sequence developments can further improve the low-field detection sensitivity, the current results demonstrate that microscale molecular imaging in vivo is already feasible at low (<50 mT) fields and potentially at low (∼1 mM) metabolite concentrations.
Molecular imaging1 differs from traditional medical imaging modalities by utilizing probes known as biomarkers to image particular biochemical targets or metabolic pathways. These molecular imaging probes typically require use of radioactive nuclei, e.g., 18F-fluorodeoxyglucose for positron emission tomography (PET),2 or chemical modification of the tracer molecules, e.g., fluorescent contrast media.3 In comparison, hyperpolarized magnetic resonance imaging (HP MRI) contrast media are uniquely free from the above shortcomings because nonradioactive nuclei such as 13C in naturally occurring metabolites are being traced. Moreover, the signatures of injected HP MRI contrast agents and their metabolites can be spectrally differentiated to yield additional molecular information,4 an advantage that has already been exploited in clinical trials for prostate cancer.5 Hyperpolarization4,6,7 is a key step in the preparation of these agents; generally, conventional magnetic resonance (MR) methods are otherwise not sensitive enough to track and image such molecules in vivo because of low concentrations and as a consequence of the miniscule equilibrium nuclear spin polarization (P ≤ 10–5). Although fundamentally transient in nature, HP spin states can be prepared by increasing the nuclear spin polarization to nearly 100% in some cases, directly translating to orders-of-magnitude enhancement of MR sensitivity. To date, near-unity levels of polarization have been achieved for several nuclei. For example, spin exchange optical pumping (SEOP) has produced P129Xe values as high as ∼0.9 for Xe gas.8 Similarly, values as high as PH = 0.9 for protons and P13C = 0.7 for carbon9 have been achieved by dissolution dynamic nuclear polarization (DNP). Alternatively, the chemically based hyperpolarization techniques such as parahydrogen induced polarization (PHIP)10 and signal amplification by reversible exchange (SABRE)11 utilize the pure spin order of the singlet state of protons in parahydrogen as the polarization source.12
When slowly relaxing nuclear spins with spin–lattice relaxation time (T1) of tens of seconds are hyperpolarized in biologically relevant molecules (e.g., 13C-pyruvate), they can be successfully used to image cellular metabolism in living organisms4,5 to act as HP contrast agents (HCAs) enabling true molecular imaging.13 HP agents have already served as useful probes for reporting on metabolic changes in several deadly diseases14 including cancer,15 where the agents fulfill the role of imaging biomarkers.4 Moreover, these agents can noninvasively report on early response to treatment16 and enable disease grading.17
While HCA technology provides clear benefits of rich molecular information content, this emerging advanced imaging modality engenders additional complexity and costs. Normally a relatively expensive “hyperpolarizer” for on-site HCA production18 is required, which must be situated in close proximity to a high-field MRI scanner equipped with multinuclear capability. For example, the leading HCA technology, dissolution-DNP, is being tested on a 3 T MRI platform.5 Combined with the relatively low throughput rate of patient examination of high-field MRI (≥1.5 T), HCA technology in its present state is likely to carry a high molecular imaging examination cost similar to that of PET imaging. In addition to the expected financial burden to first-world economies, such a trend would likely render this technology unavailable to patient populations in poorer countries comprising most of the world’s population.
However, HCA technology offers one more significant technological advantage that has largely remained untapped: the induced nuclear spin hyperpolarization is independent of the MRI scanner’s magnetic field. We have recently provided a theoretical basis with experimental evidence that low-field MRI can be more sensitive than high-field MRI for HP detection. This was achieved through the introduction of resonance frequency-optimized MRI radio-frequency (rf) coils that mitigate unfavorable scaling of sensitivity with frequency.19 Low-field MRI (≤0.05 T) enjoys several advantages over high field. It is significantly less expensive due to reduced magnet costs. It is also a significantly higher examination throughput technique, because no time for patient-tailored scanner preparation (i.e., B0 and B1 field shimming) is needed. Furthermore, it has also been recently shown that patient rf power deposition is negligible at such low operating frequencies20 making it a significantly safer imaging modality.
Here, the feasibility of low-field molecular imaging with a very high spatial resolution (94 × 94 μm2) is demonstrated at 47.5 mT for 1H and 13C detection of HCA with 1H HP pyridine and 13C HP 1-13C-succinate-d2. The HCAs were prepared using the parahydrogen-based hyperpolarization techniques of SABRE and PHIP, respectively. These techniques are not instrumentation-demanding and offer high-throughput production of HP contrast media and thus have the potential to enable low-cost production of HCAs for clinical imaging. When combined with low-field MRI, such HCA production methods should ultimately enable a high-throughput molecular-imaging platform for clinical use at a relatively low cost.
Results
Hyperpolarization of Contrast Agents in PHIP Polarizer
Pyridine (Py) protons were hyperpolarized to %PH = 0.1 ± 0.02% (average polarization per each proton (five in total) corresponding to an enhancement factor ε ≈ 5000 at 47.5 mT), which was confirmed spectroscopically at 47.5 mT using a reference NMR signal from thermally polarized water in accordance with the referencing scheme previously reported.8,21 Using the experimentally determined T1 of 11.1 ± 0.1 s at 47.5 mT (average T1 for all HP protons of Py) and an estimated 12 s delivery time from the PHIP polarizer to the low-field MRI scanner, it was concluded that the initial Py hyperpolarization achieved at 5.75 mT was ∼0.3 ± 0.06% (per each proton). 1-13C-Succinate-d2 was hyperpolarized to %P13C = 25.8 ± 5.1% (30 mM substrate injected into 7 atm of para-H2 during 5 s reaction time at 73 °C) as measured in situ by the 5.75 mT PHIP polarizer using a 30° excitation rf pulse. This in situ13C detection allowed for quality assurance of the HCA prior to delivery to the low-field MRI system. The 30° rf pulse consumed ∼13% of the 13C magnetization while leaving the remaining 87% (i.e., %P13C = 22.4 ± 4.4%) available for further use.
“HyperBridge” and “HyperGate” Use for Hyperpolarized 13C-Succinate-d2
While transfer of HP agents has been recently demonstrated22,23 using a magnetic pathway, such work was conducted at 0.8 T using a significantly more sophisticated rigid structure. In this work, a two-piece magnetic pathway with mT fields was developed. The first device used here, dubbed the “HyperBridge”, consisted of a series of one-sided flexible magnetized strips (∼0.5 in. wide, Figure 1) assembled into a single flexible line. The 1/8 in. o.d. (1/16 in. i.d.) tubing attached to this flexible line is used for transferring the HP solution while a static magnetic field of ∼14–50 mT is maintained. A second device, dubbed the “HyperGate”, was used to generate a 4–6 mT magnetic field over ∼1 ft3 at the entrance of the low-field MRI magnet. While some HCAs such as HP 2-hydroxyethyl 1-13C-propionate (HEP) can be exposed to nearly zero magnetic field without substantial loss of polarization, other HCAs clearly require hyperpolarization protection by a sufficient static magnetic field during the transfer from hyperpolarizer to the MRI scanner.22,23 Thus, the HyperBridge and HyperGate were not used with HP Py but were required for HP 1-13C-succinate-d2.
Figure 1.
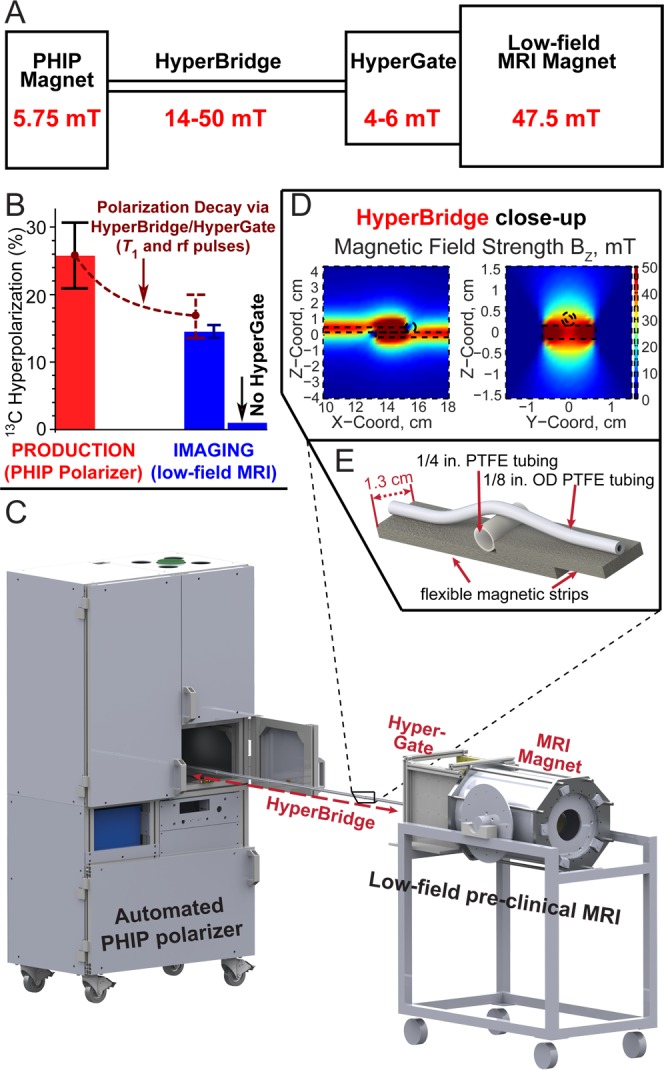
(A) Diagram of the experimental setup interfacing an automated parahydrogen induced polarization (PHIP) hyperpolarizer operating at 5.75 mT with a low-field (47.5 mT) preclinical MRI scanner (NMR console not shown) by using the HyperBridge and HyperGate to maintain the PHIP hyperpolarization of 1-13C-succinate-d2 during transfer. The inset shows additional details of the HyperBridge; the HyperBridge and HyperGate are fully described in Figures S1–S3 in the Supporting Information. (B) Hyperpolarization chart demonstrating the efficacy of transportation of hyperpolarized 1-13C-succinate-d2. 13C hyperpolarization was measured in situ of the PHIP polarizer (with a 30° rf pulse) before the transfer and in the MRI scanner (with a 15° rf pulse) after the transfer. (C) 3D-rendering of the experimental setup. (D, E) Close-up of the HyperBridge.
The combined efficacy of the HyperBridge and HyperGate was tested by 13C hyperpolarization detection before and after HCA transfer between the PHIP polarizer and the MRI scanner. The 13C T1 of 1-13C-succinate-d2 in aqueous solution was 74 ± 3 s and 75 ± 3 s at 5.75 mT and 47.5 mT, respectively. Although the HCA passes through variable magnetic fields in the range of ∼4–50 mT, a single value of T1 = 75 s was used to estimate T1 hyperpolarization losses based on the above results at 5.75 mT and 47.5 mT. In one experiment, %P13C = 25.8 ± 5.1% was produced in the PHIP polarizer. This sample was delivered via HyperBridge and HyperGate into the MRI scanner over a 22 ± 2 s period, wherein the total losses (from T1 decay and application of the sampling rf pulse in the PHIP polarizer) should diminish the 13C hyperpolarization by a factor of 1.54 to yield an estimated %P13C value of 16.7 ± 3.3% (in the MRI scanner), Figure 1A. Indeed, %P13C = 14.2 ± 0.7% was measured experimentally, corresponding to an enhancement factor ε ≈ 3 500 000 at 47.5 mT, which indicated that primary losses were largely due to T1 relaxation and rf excitation. A control experiment performed with a similar 13C hyperpolarization level but without the HyperGate yielded a 13C hyperpolarization of <1% at 47.5 mT (Figure 1B), demonstrating a significant loss of hyperpolarization and the efficacy of the HyperBridge and HyperGate.
2D Low-Field MRI of HCAs
HP proton images of Py in methanol-d4 in 10 mm NMR tubes collected in the transverse and sagittal imaging planes (corresponding to two views of the 10 mm NMR tube filled with ∼2 mL of HP Py solution and 1/16 in. o.d. PTFE tubing) are shown in Figures 2 and 3, respectively, at three different in-plane spatial resolutions. It should be noted that a 2D projection (i.e., without slice selection) gradient echo (GRE) sequence was used. This imaging sequence defined only pixel size (2D) with the most intense pixels lying in regions of greatest sample thickness but did not define the voxel size (3D). However, investigation of the resolution and sensitivity limits of potential in vivo HP low-field MRI motivated this study. Therefore, the voxel volume, Vvox, of the most intense pixels used to ascertain these limits was defined as the product of in-plane pixel resolution (as defined by the imaging sequence parameters) and the sample thickness at the location of the most intense pixel. This measure yielded the volumetric spatial resolution (in units of μm3 or alternatively in mm3), and in conjunction with the defined voxel’s associated measured signal-to-noise-ratio (SNR), SNRMAX, was used for detection sensitivity analysis.
Figure 2.
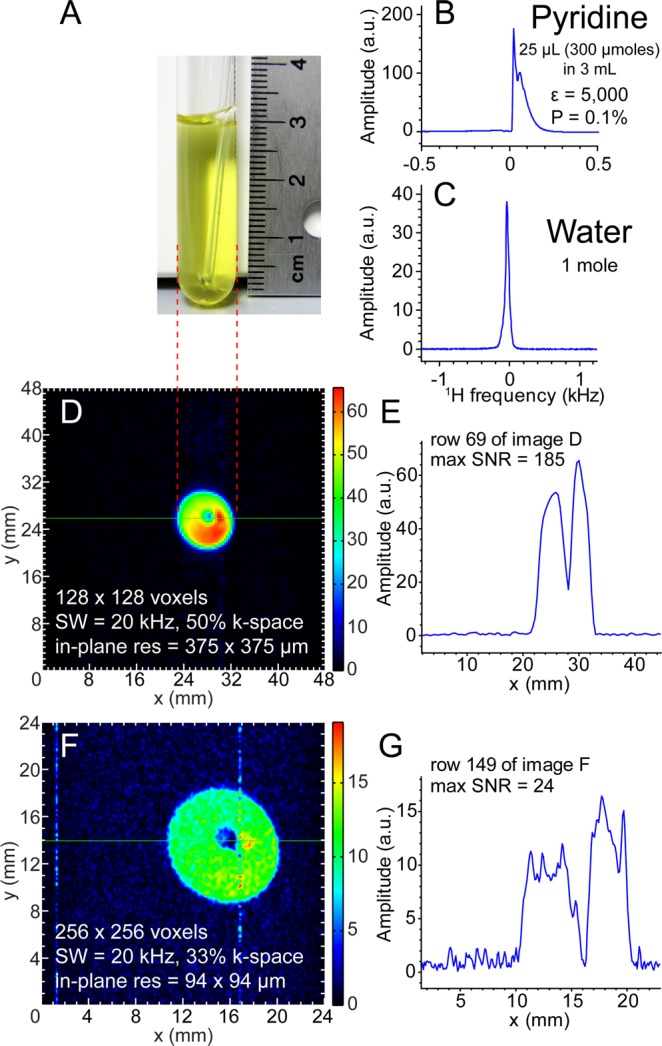
47.5 mT proton NMR spectroscopy and transverse-plane proton imaging of SABRE-hyperpolarized Py. (A) A photograph of ∼2 mL solution of 100 mM Py with 7 mM Ir catalyst in a 10 mm NMR tube with 1/16 in. o.d. PTFE tubing for parahydrogen bubbling at 1 atm. (B,C) Single scan proton NMR spectra of HP Py (B) and that from a reference sample of water (∼1 mol, part C). (D,E) Proton HP Py imaging with 375 × 375 μm2 in-plane pixel resolution using GRE imaging and spatial NMR signal (“slice”) from the selected row. (F,G) Proton HP Py imaging with 94 × 94 μm2 in-plane pixel resolution GRE imaging and spatial NMR signal from the selected row. Total imaging times were ∼3.9 s (D) and ∼5.1 s (F), respectively. The imaging data was under-sampled using only a fraction of k-space encodings (50% and 33%, respectively).
Figure 3.
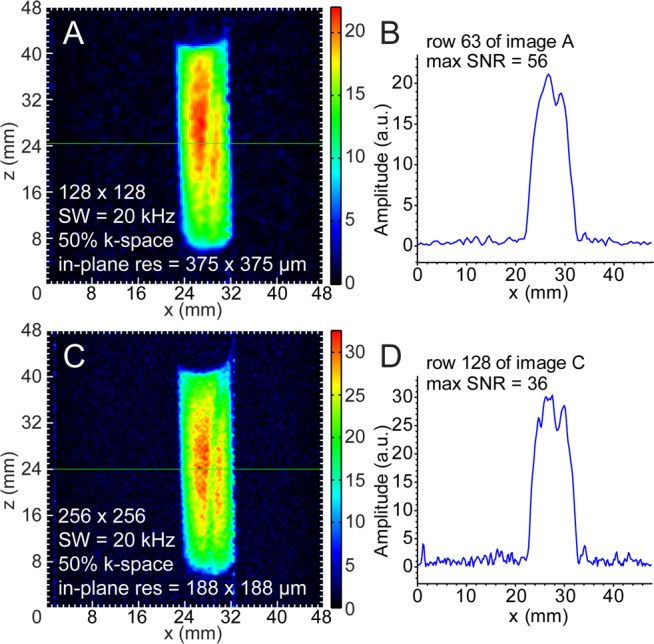
Sagittal-plane proton imaging of SABRE-polarized Py. (A,B) Proton HP Py with 375 × 375 μm2 in-plane pixel resolution GRE imaging and spatial NMR signal (“slice”) from the selected row. (C,D) Proton HP Py 188 × 188 μm2 in-plane pixel resolution GRE imaging and spatial NMR signal from the selected row. Total imaging times were ∼3.9 s (A) and ∼7.7 s (C), respectively.
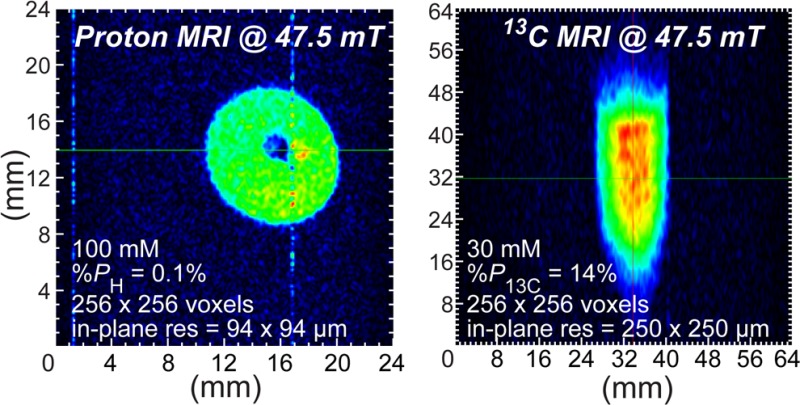
Four sets of 1H images (Figures 2 and 3) were acquired from individual hyperpolarization preparations and yielded the following voxel sizes and SNRs for sensitivity analysis: 375 × 375 × 32 000 μm3 (Vvox = 4.5 mm3, SNRMAX = 187, Figure 2D), 94 × 94 × 32 000 μm3 (Vvox = 0.28 mm3, SNRMAX = 24, Figure 2F), 375 × 375 × 8750 μm3 (Vvox = 1.2 mm3, SNRMAX = 56, Figure 3A), and 188 × 188 × 8750 μm3 (Vvox = 0.31 mm3, SNRMAX = 36, Figure 3C). The 1/16 in. PTFE tubing used for bubbling parahydrogen is clearly resolved in all four MR images. The 10 mm NMR tube was not placed perfectly coaxial with the imaging system’s z-axis, resulting in the observed tilt in the images.
Low-field HP 13C images of 1-13C-succinate-d2 in water are shown in Figure 4. HP solutions were loaded into tilted 15 mL Falcon tubes and imaged in the coronal and sagittal planes (corresponding to two orientations of the Falcon tubes filled with ∼1.5 mL of HP 1-13C-succinate-d2 solution and foam) with an in-plane spatial resolution of 250 × 250 μm2. These two images were acquired from individual hyperpolarization preparations and corresponded to the following voxel sizes of ∼250 × 250 × 3000 μm3 (Vvox = 0.19 mm3, SNRMAX = 58, Figure 4C) and ∼250 × 250 × 8000 μm3 (Vvox = 0.50 mm3, SNRMAX = 72, Figure 4D). The brown arrows shown in Figure 4 indicate the presence of foamed contrast agent, a consequence of high-pressure ejection from the polarizer and HCA surface tension. Despite the magnetic susceptibility artifacts that typically accompany bubbles at high field, the foamed agent was amenable to imaging at the low field of 47.5 mT used here. The images in Figures 2F and 3C show differential-mode gradient amplifier noise, which a low-pass gradient filter would reduce or eliminate.
Figure 4.
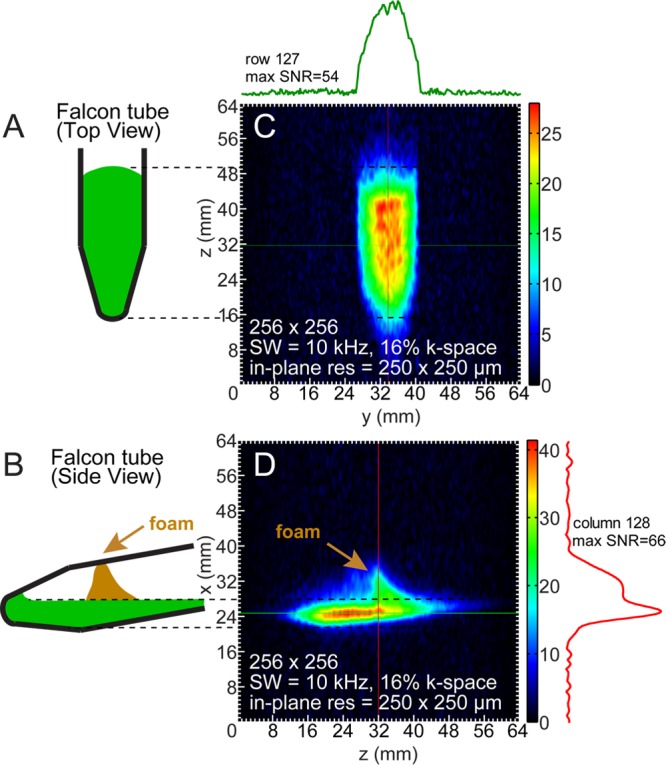
GRE imaging of 13C PHIP-polarized 1-13C-succinate-d2 in a partially filled Falcon tube. (A,C) Coronal-plane cartoon and the corresponding image of 13C-succinate 250 × 250 μm2 in-plane pixel resolution GRE imaging with corresponding spatial NMR signal (“slice”) of the selected row. Total imaging time was ∼4.5 s. (B,D) Sagittal-plane cartoon and the corresponding image of 13C-succinate 250 × 250 μm2 in-plane pixel resolution GRE imaging and spatial NMR signal from the selected column. Brown arrows mark the location of foamed HCA during in parts B and D.
Under-sampling (i.e., data recording with 16–50% of the imaging matrix’s corresponding frequency-domain k-space points) did not cause any significant imaging artifacts (albeit some blurring is visible as in Figure 4) resulting from loss of information. The coherent k-space under-sampling scheme for each MRI acquisition was conducted using the built-in algorithm and routine of the Prospa software environment (v3.12, Magritek, Wellington, New Zealand). Given the nonequilibrium nature of hyperpolarization, under-sampling k-space importantly decreases the total imaging acquisition time. For example, collecting only 50% of k-space projections accelerates acquisition by a factor of 2. The % of k-space sampled is therefore reported in Figures 2–4. Additional details about Prospa’s under-sampling scheme can be found in the Supporting Information. Fast scan speeds are especially desirable for contrast agents with rapidly decaying polarization due to low T1, as exemplified by the agents used here. The MR images presented in Figures 2–4 and the Supporting Information were automatically reconstructed from the under-sampled data by Prospa as supplied by the manufacturer. No additional data or image manipulations were performed to improve image quality, such as zero-filling or smoothing, beyond that as acquired and shown here and in the Supporting Information.
Discussion
To the best of our knowledge (e.g., refs (24−30)), the results shown here compare favorably with other low-field images of hyperpolarized or nonhyperpolarized media from the perspectives of both the achieved in-plane resolution as well as the small effective voxel sizes. Low-field, frequency-optimized rf coils comparable in SNR performance to commercial preclinical coils at 4.7 T for hyperpolarized contrast agents19 were used to achieve the high sensitivity necessary for microscale spatial resolution of 1H and 13C HP MRI at low magnetic field. Figures 2–4 show that the required sensitivity is indeed attainable at a magnetic field strength of 47.5 mT. Not only the presented HP 1H images but also the relatively large FOV 13C HP images are high-resolution owing to a large imaging matrix size (256 × 256 points) allowing small pixel sizes of only 250 × 250 μm2 or less.
HyperBridge and HyperGate
These devices were essential for efficient preservation of nuclear spin hyperpolarization of 1-13C-succinate-d2 (susceptible to depolarization in a near zero magnetic field) during transportation from the PHIP hyperpolarizer to the low-field MRI scanner. Overall, our experience is similar to others working with similar contrast agents.22,23 The range of magnetic field variation along the transfer path was almost entirely within the magnetic field bounds defined by the T1 measurements at 5.75 mT and 47.5 mT. The HyperBridge and HyperGate may also prove useful for other similar HCAs such as 1-13C-phospholactate.31
Detection Sensitivity and Other Limits of Detection
The presented imaging results allow estimation of the corresponding detection sensitivity. Since HCA media can utilize variable % polarization and concentration or may involve detection of nuclei other than spin-1/2 nuclei (i.e., 1H, 13C, 129Xe, etc.), we were prompted to implement a new quantity of measure incorporating these variables as described below.
SNR in MRI19,32,33 is proportional to the gyromagnetic ratio, γ, concentration, C, and nuclear spin polarization, P, in a voxel volume, VVOX, or
| 1 |
where A is a numerical constant accounting for rf coil sensitivity, the imaging pulse sequence efficiency, and other experimental parameters. The value of this constant A yields a useful quantification of the detection sensitivity of the molecular imaging method because it can be used for direct comparison of imaging approaches and protocols. For example,
| 2 |
Here, we introduce the concept of fully (P = 1 = 100%) polarized proton spins (pps), wherein AHγH in eq 2 is a useful quantity derived from the SNR from a hypothetical 1 mol of fully polarized proton spins (pps) in the imaging voxel of interest. When using the experimental results from Figure 3C (CH = 0.5 M (5 Py protons at 0.1 M concentration) in 0.31 mm3 voxel volume with 0.1% polarization), AHγH = 2.3 × 1011 units of SNR per 1 mol of pps. The utility of this experimental rf-coil parameter lies in its enabling prediction of the SNR of biomedical imaging experiments with known metabolic fluxes, HCA concentrations, and anticipated % polarization on target hyperpolarized molecule.
For example, when using the low-field imaging setup (Figure 1) demonstrated in Figure 3C, if 1 mM concentration of proton spins with PH = 0.01 (1%) is achieved in a tissue with an imaging voxel size of 10 mm3, one should expect a SNR of 23. A SNR value >20 is sufficient for MRI image reconstruction as well as for quantitative in vivo imaging analysis of metabolite concentrations.34 Moreover, this expected fine spatial resolution (i.e., voxel size of ∼10 mm3) is similar to or better than that of high-field spectroscopic HP MRI performed with HCA with a significantly (factor of 10+) greater payload of net magnetization (i.e., CVVOXP, eq 1)16 and represents nearly an order of magnitude improvement over the 50–100 mm3 voxel sizes of conventional high-field preclinical MR spectroscopy enabled by signal averaging of hundreds of scans.35 A voxel size of ∼10 mm3 already enables molecular imaging of deadly diseases such as cancer with isotropic spatial resolution of 2–3 mm, which can be further improved (to 1–2 mm range) through the use of more polarized media (e.g., P = 0.2 vs P = 0.01) or by exploiting metabolic pathways with higher metabolic fluxes and in vivo concentration (e.g., C = 10 mM vs 1 mM). Thus, the imaging sensitivity of HP low-field MRI certainly paves the way to molecular imaging of many metabolic pathways such as glycolysis13,16 or glutamine36 and choline37,38 metabolism, which are often up-regulated in cancer and other diseases, with spatial resolution (defined as the pixel size) exceeding that of micro-PET (>2 mm in each dimension).39
It should also be stressed that the effective spectroscopic line-width corresponding to B0 field homogeneity is typically limited only by the homogeneity of the magnet at low magnetic fields (our study) rather than by subject-induced perturbations to B0 (i.e., inhomogeneity due to tissue magnetic susceptibility) at high magnetic fields. In addition, subject-associated rf (also referred to as B1) losses are negligible at low resonance frequencies.20 Therefore, the SNR factor analysis performed above is fully applicable to in vivo conditions.
A corresponding analysis applies to 13C imaging. When using the experimental parameters in Figure 4C (C = 0.030 M, %P = 14.2%, VVOX = 0.19 mm3, SNR = 54), A13Cγ13C = 6.7 × 1010 units of SNR per 1 mol of 13C polarized spins (cps, i.e., 100% polarized 13C nuclei). It should be noted that γ13C = γH/3.98, and consequently it is unsurprising that A13Cγ13C in units of cps is significantly lower than AHγH. It reflects the inherently greater NMR sensitivity of protons with their greater magnetic moment under otherwise identical conditions compared to those of 13C spins. However, A13Cγ13C of the 13C rf coil can be expressed in units of pps (corresponding to HP proton imaging in the 13C rf coil at 3.98 times lower B0) yielding A13CγH = 2.7 × 1011 units of SNR per 1 mol of pps. This value of A13CγH is very similar to AHγH despite an ∼4-fold difference in the resonance frequency (0.508 MHz vs 2.02 MHz). This agreement is consistent with our recent work demonstrating a very weak MR SNR dependence on the resonance frequency (SNR ∝ ω00.25) for this type of MRI rf coil.19 Moreover, this result indicates that high-resolution and high-sensitivity (i.e., large Aγ values) MRI of HCA should be readily achieved at even lower magnetic fields. Although future hardware and imaging-sequence improvements can certainly improve Aγ, low-field MRI still largely remains unexplored territory, the values already reported here demonstrate the feasibility of in vivo preclinical imaging using HCAs.
Feasibility of Direct Proton HCA Imaging in Vivo
While protons are the most sensitive MRI nuclei, the direct proton imaging of HP MRI contrast media is challenging at high magnetic fields because of background signal from ∼102 M protons.40 A potential mitigation is indirect proton imaging of 13C HCA by initial water suppression, followed by intramolecular polarization transfer from HP 13C to 1H, with final imaging of proton spins.41,42 While this approach has been demonstrated at high field, it poses two challenges: (i) efficient water background suppression and (ii) the requirement of 13C isotopic labeling. Fundamentally, for direct proton HCA imaging, the condition CHCA × PHCA ≫ CB × PB (or CHCA ≫ CB × PB/PHCA) must be met (where CHCA, CB, PHCA, and PB correspond to the concentrations and polarizations of HCA and the background proton species, respectively). The low B0 magnetic field used here reduces proton background by orders of magnitude, reduction of B0 linearly decreases PB. Reasonably assuming that %PHCA = 10%, corresponding to an enhancement factor of 6 × 105 (i.e., PHCA/PB) at 47.5 mT and water proton concentration of ∼102 M, CHCA during image acquisition must be significantly greater than 0.2 mM, which makes direct proton imaging of many HP metabolites above the 1 mM level feasible. Moreover, further minimization of the ratio CB × PB/PHCA simply entails additional reduction of B0.
Low-Field MRI Hardware Challenges and Opportunities
A key metric of MRI rf coil performance is the quality factor Q (ω0/Δω0) of the rf receiver network, where Δω0 corresponds to the −3 dB bandwidth of the matched rf circuit resonance at the detection frequency ω0. The use of high-sensitivity coils implies a high Q, which limits the maximum spectroscopic imaging bandwidth (SW). High SW is required for high-resolution MRI with large imaging matrices, because Δω0 must be greater than SW to avoid imaging artifacts.43 The rf circuits used here had Q values of 62 at 2.02 MHz and 28 at 0.508 MHz corresponding to Δω0 of 32 kHz and 18 kHz, respectively. Therefore, nearly the maximum SWs of 20 kHz and 10 kHz were utilized. However, use of high-Q yet low-SW rf coils would advantageously enable greater values of the SNR and Aγ constant. A potential solution to these contradictory requirements (high-Q coils versus high-SW MRI) without loss of SNR as noted by Baudin et al.43 involves rf probe Q-spoiling by active feedback to increase the imaging bandwidth at low resonance frequencies. Such Q-spoiling is most desirable as it simultaneously enables MRI using high-Q rf probes for high-SW imaging at very low resonance frequencies.
Conclusion
High-resolution (94 × 94 μm2) MRI of proton and 13C HP contrast agents has been demonstrated at a low magnetic field of 47.5 mT. The achieved spatial resolution of the presented molecular imaging rivals that of micro-PET.39 The HyperBridge and HyperGate magnetic devices were successfully demonstrated for transportation of hyperpolarized contrast agents from the hyperpolarizer to the preclinical low-field MRI scanner. The presented technological advances of low-field MRI already can enable preclinical molecular imaging of proton and 13C hyperpolarized contrast agents, and there are no fundamental barriers for future clinical translation. For example, DNP hyperpolarized 1-13C-pyruvate is already being used in clinical trials.5 With respect to parahydrogen-based methods for hyperpolarization, hyperpolarized succinates and hyperpolarized phospholactate are particularly well-suited for molecular imaging using the technologies described here. 13C-succinates potentially report on abnormal citric acid cycle metabolism in cancer.4,44,45 1-13C-phospholactate can be hyperpolarized via PHIP to %P13C > 15%.46 When injected into living organisms, 1-13C-phospholactate is rapidly dephosphorylated (within seconds) to 1-13C-lactate47 and therefore can be potentially used for cancer imaging in similar fashion to DNP hyperpolarized 1-13C-lactate and 1-13C-pyruvate.48,49 However, one advantage for hyperpolarized 13C-lactate compared to 13C-pyruvate is the fact that it can be used within a physiologically relevant in vivo concentration range.48
Other technical improvements in low-field rf coil development involve the use of superconducting or cryogenically cooled rf coils to increase the detection sensitivity by several fold,50,51 leading to concomitant improvements in the resolution limits. Furthermore, the use of compressed sensing in conjunction with faster and more sensitive imaging sequences30 can further improve the detection sensitivity and accelerate total imaging time.
Methods
PHIP/SABRE Polarizer
A 5.75 mT electromagnet-based polarizer produced the SABRE-polarized pyridine and PHIP-polarized 1-13C-succinate-d2. Details of the polarizer are summarized in Supporting Information text. Briefly, the polarizer is fully automated with a console-based graphical user interface. The polarizer controller unit employs open-source computer software applied in previous work8,52,53 and utilizes a chemical reactor, mixing manifold, and rf probe design similar to ones reported earlier.21,54
47.5 mT MRI System
Low-field MR spectra and images were acquired using a 47.5 mT MR scanner (89 mm i.d. bore) equipped with Prospa software (version 3.12, Magritek, Wellington, New Zealand). Previously developed, 38 mm i.d. dual-channel rf probes were used for imaging in 13C/1H and 1H/13C configurations. These probes have crystal-radio solenoid rf coils whose detection sensitivity was optimized for the primary (first-listed) channel’s resonance frequency ω0 (2.02 MHz for 1H, X tuned to 0.508 MHz for 13C).19
Production and Transfer of HP Contrast Media
An N-heterocyclic carbene complex-based Ir catalyst55,56 was prepared as described previously.57,58 Solutions (∼2 mL) of the Ir-catalyst and Py substrate in methanol-d4 were used for SABRE hyperpolarization in a standard 10 mm NMR tube with ∼7 mM [IrCl(COD)(IMes)]57 [IMes = 1,3-bis(2,4,6-trimethylphenyl), imidazole-2-ylidene; COD = cyclooctadiene] catalyst concentration and ∼100 mM Py substrate concentration. For SABRE, the polarizer was operated with the rf probe removed with an estimated solution temperature of ∼40 °C. Ultrahigh purity (>99.999% H2) parahydrogen gas with >90% para- state59 was bubbled through the solution for 2 min to generate HP pyridine.56,60 Immediately after cessation of bubbling, the HP Py was transferred through Earth’s field to the imaging system (see the Supporting Information for details), where images were obtained ∼12 s after hyperpolarization, a time sufficient to significantly depolarize HP Ir-hydride and HP ortho-H2 (T1 ∼2–3 s at 9.4 T in these solutions) but not HP Py (T1 ∼ 21 s at 9.4 T58 and T1 = 11.1 ± 0.1 s at 47.5 mT).
13C HP 1-13C-succinate-d261 was produced using the PASADENA62 method, employing 30 mM 1-13C-fumaric acid-d2 precursor in aqueous medium at pH > 9 and 5 mM Rh(I) catalyst. The Rh(I) catalyst was prepared as described previously63 using bis(norbornadiene)rhodium(I) tetrafluoroborate (Strem no. 45-0230, Newburyport, MA) and 1,4-bis[(phenyl-3-propanesulfonate) phosphine]butane (no. 717347, Sigma-Aldrich-Isotec, OH). The % polarization of the HP 1-13C-succinate-d2 was tested in situ(21) of the PHIP polarizer. All handling of the HP 1-13C-succinate-d2 prior to sample insertion into the low-field imaging system took place in magnetic fields generated by the “HyperBridge” and “HyperGate” to preserve hyperpolarization (Supporting Information text), Figure 1. Images were obtained from ∼45 μmol of HCA in 1.5 mL of water loaded into a 15 mL Falcon tube (part 14-959-70C, Fisher Scientific). Because only one low-field MR console was available for PHIP 1H → 13C hyperpolarization transfer and low-field MRI, the rf cables had to be switched (from PHIP probe to MRI probe) after 1-13C-succinate-d2 PHIP in order to acquire images. Further details are given in the Supporting Information text.
Imaging Protocol/Parameters
One 2D gradient echo (GRE) image without slice selection was acquired from each freshly polarized individual HCA batch using a planar GRE imaging sequence provided in Prospa software, where the parameters varied for MRI acquisition in two mutually orthogonal imaging planes. Proton Py images used 128 × 128 or 256 × 256 pixel matrices over fields of view (FOV) ranging from 24 × 24 mm2 to 64 × 64 mm2 with corresponding k-space under-sampling (defined as percentage of acquired k-space projections). Py GRE imaging parameters for 375 × 375 μm2 in-plane pixel resolution were echo time (TE) = 7 ms, repetition time (TR) = 60 ms (limited by the electronics response time), and time of acquisition (tAcq) = 6.4 ms. Imaging parameters for 188 × 188 μm2 and 94 × 94 μm2 in-plane pixel resolution images were TE = 13 ms, TR = 60 ms, and tAcq = 12.8 ms. Other imaging parameters are provided in Figures 2 and 3. Quality assurance reporting on the level of %PH of the HCA before imaging at 47.5 mT was performed with 90° square excitation rf pulses in a separate experimental series. For the 13C HCA, %P13C was measured spectroscopically in the MRI scanner immediately after the sample transfer from PHIP polarizer via the HyperBridge and HyperGate (using a 15° square rf excitation pulse). GRE imaging followed immediately after spectroscopic polarization measurements. All 13C imaging of 1-13C-succinate-d2 was performed with a 64 × 64 mm2 FOV, TE = 26 ms, TR = 110 ms (limited by the electronics response time), tAcq = 25.6 ms, and a fixed pulse angle of 30°. Other imaging parameters are provided in Figure 4.
Acknowledgments
This work was supported by the RAS (5.1.1), RFBR (Grants 14-03-00374-a, 14-03-31239-mol-a, 12-03-00403-a), SB RAS (Grants 57, 60, 61, 122), the Ministry of Education and Science of the Russian Federation, and the Council on Grants of the President of the Russian Federation (Grant MK-4391.2013.3). We thank for funding support NIH Grants ICMIC 5P50 CA128323-03, 5R00 CA134749-03, 3R00CA134749-02S1, and DoD CDMRP Breast Cancer Program Era of Hope Award W81XWH-12-1-0159/BC112431.
Supporting Information Available
Additional details regarding PHIP hyperpolarizer, low-field MRI system, hyperpolarized contrast agent production and transfer, and MRI imaging. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Weissleder R. Science 2006, 312, 1168–1171. [DOI] [PubMed] [Google Scholar]
- Gambhir S. S. Nat. Rev. Cancer 2002, 2, 683–693. [DOI] [PubMed] [Google Scholar]
- Kobayashi H.; Choyke P. L. Acc. Chem. Res. 2010, 44, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurhanewicz J.; Vigneron D. B.; Brindle K.; Chekmenev E. Y.; Comment A.; Cunningham C. H.; DeBerardinis R. J.; Green G. G.; Leach M. O.; Rajan S. S.; Rizi R. R.; Ross B. D.; Warren W. S.; Malloy C. R. Neoplasia 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. J.; Kurhanewicz J.; Vigneron D. B.; Larson P. E. Z.; Harzstark A. L.; Ferrone M.; van Criekinge M.; Chang J. W.; Bok R.; Park I.; Reed G.; Carvajal L.; Small E. J.; Munster P.; Weinberg V. K.; Ardenkjaer-Larsen J. H.; Chen A. P.; Hurd R. E.; Odegardstuen L. I.; Robb F. J.; Tropp J.; Murray J. A. Sci. Transl. Med. 2013, 5, 198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abragam A.; Goldman M. Rep. Prog. Phys. 1978, 41, 395–467. [Google Scholar]
- Ardenkjaer-Larsen J. H.; Fridlund B.; Gram A.; Hansson G.; Hansson L.; Lerche M. H.; Servin R.; Thaning M.; Golman K. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou P.; Coffey A. M.; Walkup L. L.; Gust B. M.; Whiting N.; Newton H.; Barcus S.; Muradyan I.; Dabaghyan M.; Moroz G. D.; Rosen M.; Patz S.; Barlow M. J.; Chekmenev E. Y.; Goodson B. M. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannin S.; Bornet A.; Melzi R.; Bodenhausen G. Chem. Phys. Lett. 2012, 549, 99–102. [Google Scholar]
- Eisenschmid T. C.; Kirss R. U.; Deutsch P. P.; Hommeltoft S. I.; Eisenberg R.; Bargon J.; Lawler R. G.; Balch A. L. J. Am. Chem. Soc. 1987, 109, 8089–8091. [Google Scholar]
- Adams R. W.; Aguilar J. A.; Atkinson K. D.; Cowley M. J.; Elliott P. I. P.; Duckett S. B.; Green G. G. R.; Khazal I. G.; Lopez-Serrano J.; Williamson D. C. Science 2009, 323, 1708–1711. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Phys. Rev. Lett. 1986, 57, 2645–2648. [DOI] [PubMed] [Google Scholar]
- Golman K.; in’t Zandt R.; Thaning M. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11270–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golman K.; Petersson J. S. Acad. Radiol. 2006, 13, 932–942. [DOI] [PubMed] [Google Scholar]
- Brindle K. M.; Bohndiek S. E.; Gallagher F. A.; Kettunen M. I. Magn. Reson. Med. 2011, 66, 505–519. [DOI] [PubMed] [Google Scholar]
- Day S. E.; Kettunen M. I.; Gallagher F. A.; Hu D. E.; Lerche M.; Wolber J.; Golman K.; Ardenkjaer-Larsen J. H.; Brindle K. M. Nat. Med. 2007, 13, 1382–1387. [DOI] [PubMed] [Google Scholar]
- Albers M. J.; Bok R.; Chen A. P.; Cunningham C. H.; Zierhut M. L.; Zhang V. Y.; Kohler S. J.; Tropp J.; Hurd R. E.; Yen Y.-F.; Nelson S. J.; Vigneron D. B.; Kurhanewicz J. Cancer Res. 2008, 68, 8607–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen J. H.; Leach A. M.; Clarke N.; Urbahn J.; Anderson D.; Skloss T. W. NMR Biomed. 2011, 24, 927–932. [DOI] [PubMed] [Google Scholar]
- Coffey A. M.; Truong M. L.; Chekmenev E. Y. J. Magn. Reson. 2013, 237, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. E.; Bidinosti C. P.; Chapple E. M. Concepts Magn. Reson., Part A 2012, 40A, 281–294. [Google Scholar]
- Waddell K. W.; Coffey A. M.; Chekmenev E. Y. J. Am. Chem. Soc. 2011, 133, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornet A.; Melzi R.; Linde A. J. P.; Hautle P.; van den Brandt B.; Jannin S.; Bodenhausen G. J. Phys. Chem. Lett. 2013, 4, 111–114. [DOI] [PubMed] [Google Scholar]
- Cheng T.; Mishkovsky M.; Bastiaansen J. A.; Ouari O.; Hautle P.; Tordo P.; van den Brandt B.; Comment A. NMR Biomed. 2013, 26, 1582–1588. [DOI] [PubMed] [Google Scholar]
- Venkatesh A. K.; Zhang A. X.; Mansour J.; Kubatina L.; Oh C. H.; Blasche G.; Unlu M. S.; Balamore D.; Jolesz F. A.; Goldberg B. B.; Albert M. S. Magn. Reson. Imaging 2003, 21, 773–776. [DOI] [PubMed] [Google Scholar]
- Matter N. I.; Scott G. C.; Venook R. D.; Ungersma S. E.; Grafendorfer T.; Macovski A.; Conolly S. M. Magn. Reson. Med. 2006, 56, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Ruset I. C.; Tsai L. L.; Mair R. W.; Patz S.; Hrovat M. I.; Rosen M. S.; Muradian I.; Ng J.; Topulos G. P.; Butler J. P.; Walsworth R. L.; Hersman F. W. Concepts Magn. Reson., Part B 2006, 29B, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krjukov E.; Fichele S.; Wild J. M.; Paley M. N. J. Concepts Magn. Reson., Part B 2007, 31B, 209–217. [Google Scholar]
- Tsai L. L.; Mair R. W.; Rosen M. S.; Patz S.; Walsworth R. L. J. Magn. Reson. 2008, 193, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheenen T. W. J.; Futterer J.; Weiland E.; van Hecke P.; Lemort M.; Zechmann C.; Schlemmer H. P.; Broome D.; Villeirs G.; Lu J. P.; Barentsz J.; Roell S.; Heerschap A. Invest. Radiol. 2011, 46, 25–33. [DOI] [PubMed] [Google Scholar]
- Sarracanie M.; Armstrong B. D.; Stockmann J.; Rosen M. S. Magn. Reson. Med. 2014, 71, 735–745. [DOI] [PubMed] [Google Scholar]
- Shchepin R. V.; Coffey A. M.; Waddell K. W.; Chekmenev E. Y. J. Am. Chem. Soc. 2012, 134, 3957–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. I.; Richards R. E. J. Magn. Reson. 1976, 213, 329–343. [DOI] [PubMed] [Google Scholar]
- Hoult D. I.; Lauterbur P. C. J. Magn. Reson. 1979, 34, 425–433. [Google Scholar]
- Sharma U.; Baek H. M.; Su M. Y.; Jagannathan N. R. NMR Biomed. 2011, 24, 700–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J.; Tkac I.; Provencher S. W.; Gruetter R. J. Magn. Reson. 1999, 141, 104–120. [DOI] [PubMed] [Google Scholar]
- Gallagher F. A.; Kettunen M. I.; Day S. E.; Lerche M.; Brindle K. M. Magn. Reson. Med. 2008, 60, 253–257. [DOI] [PubMed] [Google Scholar]
- Dorrius M. D.; Pijnappel R. M.; Jansen-van der Weide M. C.; Jansen L.; Kappert P.; Oudkerk M.; Sijens P. E. Radiology 2011, 259, 695–703. [DOI] [PubMed] [Google Scholar]
- Keshari K. R.; Tsachres H.; Iman R.; Delos Santos L.; Tabatabai Z. L.; Shinohara K.; Vigneron D. B.; Kurhanewicz J. NMR Biomed. 2011, 24, 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popota F. D.; Aguiar P.; Herance J. R.; Pareto D.; Rojas S.; Ros D.; Pavia J.; Gispert J. D. IEEE Trans. Nucl. Sci. 2012, 59, 1879–1886. [Google Scholar]
- Hövener J.-B.; Schwaderlapp N.; Borowiak R.; Lickert T.; Duckett S. B.; Mewis R. E.; Adams R. W.; Burns M. J.; Highton L. A. R.; Green G. G. R.; Olaru A.; Hennig J.; von Elverfeldt D. Anal. Chem. 2014, 86, 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkovsky M.; Cheng T.; Comment A.; Gruetter R. Magn. Reson. Med. 2012, 68, 349–352. [DOI] [PubMed] [Google Scholar]
- Truong M. L.; Coffey A. M.; Shchepin R. V.; Waddell K. W.; Chekmenev E. Y. Contrast Media Mol. Imaging 2014, 10.1002/cmmi.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin E.; Safiullin K.; Morgan S. W.; Nacher P. J. J. Phys.: Conf. Ser. 2011, 294, 012009. [Google Scholar]
- Bhattacharya P.; Chekmenev E. Y.; Perman W. H.; Harris K. C.; Lin A. P.; Norton V. A.; Tan C. T.; Ross B. D.; Weitekamp D. P. J. Magn. Reson. 2007, 186, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias N. M.; Chan H. R.; Sailasuta N.; Ross B. D.; Bhattacharya P. J. Am. Chem. Soc. 2012, 134, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Coffey A. M.; Waddell K. W.; Chekmenev E. Y. Anal. Chem. 2014, 86, 5601–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchepin R. V.; Pham W.; Chekmenev E. Y. J. Labelled Compd. Radiopharm. 2014, 57, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. W. C.; Kettunen M. I.; Hu D.-E.; Brindle K. M. J. Am. Chem. Soc. 2012, 134, 4969–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen M. I.; Kennedy B. W. C.; Hu D.-e.; Brindle K. M. Magn. Reson. Med. 2012, 70, 1200–1209. [DOI] [PubMed] [Google Scholar]
- Darrasse L.; Ginefri J. C. Biochimie 2003, 85, 915–937. [DOI] [PubMed] [Google Scholar]
- Resmer F.; Seton H. C.; Hutchison J. M. S. J. Magn. Reson. 2010, 203, 57–65. [DOI] [PubMed] [Google Scholar]
- Nikolaou P.; Coffey A. M.; Walkup L. L.; Gust B.; LaPierre C.; Koehnemann E.; Barlow M. J.; Rosen M. S.; Goodson B. M.; Chekmenev E. Y. J. Am. Chem. Soc. 2014, 136, 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou P.; Coffey A. M.; Walkup L. L.; Gust B. M.; Whiting N. R.; Newton H.; Muradyan I.; Dabaghyan M.; Ranta K.; Moroz G.; Patz S.; Rosen M. S.; Barlow M. J.; Chekmenev E. Y.; Goodson B. M. Magn. Reson. Imaging 2014, 32, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey A. M.; Shchepin R. V.; Wilkens K.; Waddell K. W.; Chekmenev E. Y. J. Magn. Reson. 2012, 220, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M. J.; Adams R. W.; Atkinson K. D.; Cockett M. C. R.; Duckett S. B.; Green G. G. R.; Lohman J. A. B.; Kerssebaum R.; Kilgour D.; Mewis R. E. J. Am. Chem. Soc. 2011, 133, 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.; Xu J.; Gillen J.; McMahon M. T.; Artemov D.; Tyburn J. M.; Lohman J. A.; Mewis R. E.; Atkinson K. D.; Green G. G.; Duckett S. B.; van Zijl P. C. J. Magn. Reson. 2013, 237C, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Serrano L. D.; Owens B. T.; Buriak J. M. Inorg. Chim. Acta 2006, 359, 2786–2797. [Google Scholar]
- Barskiy D. A.; Kovtunov K. V.; Koptyug I. V.; He P.; Groome K. A.; Best Q. A.; Shi F.; Goodson B. M.; Shchepin R. V.; Coffey A. M.; Waddell K. W.; Chekmenev E. Y. J. Am. Chem. Soc. 2014, 136, 3322–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B. B.; Coffey A. M.; Colon R. D.; Chekmenev E. Y.; Waddell K. W. J. Magn. Reson. 2012, 214, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak R.; Schwaderlapp N.; Huethe F.; Lickert T.; Fischer E.; Bar S.; Hennig J.; von Elverfeldt D.; Hovener J. B. Magn. Reson. Mater. Phys. 2013, 26, 491–499. [DOI] [PubMed] [Google Scholar]
- Chekmenev E. Y.; Hovener J.; Norton V. A.; Harris K.; Batchelder L. S.; Bhattacharya P.; Ross B. D.; Weitekamp D. P. J. Am. Chem. Soc. 2008, 130, 4212–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar]
- Cai C.; Coffey A. M.; Shchepin R. V.; Chekmenev E. Y.; Waddell K. W. J. Phys. Chem. B 2013, 117, 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


