Abstract
Porcine circovirus type 2 (PCV2) is associated with postweaning multisystemic wasting syndrome in pigs, whereas PCV1 is nonpathogenic. We previously demonstrated that a chimeric PCV1-2 virus (with the immunogenic capsid gene of PCV2 cloned into the backbone of PCV1) induces an antibody response to the PCV2 capsid protein and is attenuated in pigs. Here, we report that the attenuated chimeric PCV1-2 induces protective immunity to wild-type PCV2 challenge in pigs. A total of 48 specific-pathogen-free piglets were randomly and equally assigned to four groups of 12 pigs each. Pigs in group 1 were vaccinated by intramuscular injection with 200 μg of the chimeric PCV1-2 infectious DNA clone. Pigs in group 2 were vaccinated by intralymphoid injection with 200 μg of a chimeric PCV1-2 infectious DNA clone. Pigs in group 3 were vaccinated by intramuscular injection with 103.5 50% tissue culture infective doses (TCID50) of the chimeric PCV1-2 live virus. Pigs in group 4 were not vaccinated and served as controls. By 42 days postvaccination (DPV), the majority of pigs had seroconverted to PCV2 capsid antibody. At 42 DPV, all pigs were challenged intranasally and intramuscularly with 2 × 104.5 TCID50 of a wild-type pathogenic PCV2 virus. By 21 days postchallenge (DPC), 9 out of the 12 group 4 pigs were viremic for PCV2. Vaccinated animals in groups 1 to 3 had no detectable PCV2 viremia after challenge. At 21 DPC the lymph nodes in the nonvaccinated pigs were larger (P < 0.05) than those of vaccinated pigs. The PCV2 genomic copy loads in lymph nodes were reduced (P < 0.0001) in vaccinated pigs. Moderate amounts of PCV2 antigen were detected in most lymphoid tissues of nonvaccinated pigs but in only 1 of 36 vaccinated pigs. Mild-to-severe lymphoid depletion and histiocytic replacement were detected in lymphoid tissues in the majority of nonvaccinated group 4 pigs but in only a few vaccinated group 1 to 3 pigs. The data from this study indicated that when given intramuscularly in pigs, the attenuated chimeric PCV1-2 live virus, as well as the chimeric PCV1-2 infectious DNA clone, induces protective immunity against PCV2 infection and could potentially serve as an effective vaccine.
Postweaning multisystemic wasting syndrome (PMWS) was first observed in piglets of a high-health herd in Canada in 1991 (14). Since then PMWS has been reported in many pig-producing regions of North America, Europe, and Asia (2, 5, 6, 21, 28). The primary causative agent of PMWS is thought to be type 2 porcine circovirus (PCV2) (2, 7, 9, 10, 15, 20, 29).
The type 1 porcine circovirus (PCV1) was initially discovered as a persistent contaminant of a porcine kidney cell culture (PK-15) (36). PCV1 is a small nonenveloped icosahedral virus, with a single-stranded circular DNA genome of about 1.76 kb. PCV1 has not been found to cause disease and is generally considered to be nonpathogenic (1, 35). Pathogenic PCV2 and nonpathogenic PCV1 share only about 76% nucleotide sequence identity but have similar genomic organizations (10). Two open reading frames (ORFs) have been characterized: ORF1 encodes rep proteins required for viral replication (4) and ORF2 encodes the immunogenic capsid protein (25). Both PCV1 and PCV2 are members of the Circoviridae family, along with psittacine beak and feather disease virus (3) and the tentative members columbid circovirus, goose circovirus, and canary circovirus (23, 31, 37). The human circoviruses TT virus, TT virus-like minivirus, and the SEN virus have genomic organization similar to that of PCV (27, 34, 38).
Accumulated evidence indicates that PCV2 is the primary, but not the sole, causative agent of PMWS (2, 7, 9, 10, 15, 20, 29, 30). Clinical PMWS has been reproduced in conventional pigs coinfected with PCV2 and either porcine parvovirus or porcine reproductive and respiratory syndrome virus (PRRSV) (15, 29). Ladekjaer-Mikkelsen et al. (20) recently reproduced PMWS in 3-week-old specific-pathogen-free (SPF) piglets inoculated with PCV2 alone. PMWS was also reproduced in PCV2-inoculated piglets immunostimulated with keyhole limpet hemocyanin in incomplete Freund's adjuvant (18). Opriessnig et al. (30) showed that pigs vaccinated with Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae prior to PCV2 inoculation had increased length of PCV2 viremia and more-severe lymphoid lesions compared to unvaccinated pigs. It is generally believed that immunostimulation either by vaccination or secondary viral infection plays a role in the occurrence of PMWS (12, 17, 18, 19). However, the exact role of immunostimulation in the progression to clinical PMWS is not known.
There is a need for a vaccine to prevent PCV2 infections and its role in the progression to clinical PMWS. We previously showed that a chimeric PCV1-2 infectious DNA clone (with the immunogenic capsid gene of PCV2 cloned into the backbone of the nonpathogenic PCV1) is infectious when injected directly into the lymph nodes of SPF piglets and induces a strong antibody response to PCV2 capsid antigen while remaining attenuated in pigs (11). Therefore, chimeric PCV1-2 appears to be a good candidate vaccine. In this study, we evaluated the efficacy of this candidate vaccine by subjecting SPF pigs to intramuscular and intralymphoid immunization with chimeric PCV1-2 virus and an infectious chimeric PCV1-2 DNA clone followed by challenge with wild-type pathogenic PCV2. We showed that the PCV1-2 chimeric virus and the infectious DNA clone both induce protective immunity against wild-type PCV2 challenge in SPF pigs.
MATERIALS AND METHODS
PCV2 and chimeric PCV1-2 infectious DNA clones.
The construction of PCV2 and the chimeric PCV1-2 infectious DNA clone was reported previously (9, 11). The original wild-type PCV2 was from a pig with naturally occurring PMWS on an Iowa farm (isolate 40895) (10). The PCV2 infectious DNA clone was constructed by cloning two tandem copies of the complete PCV2 genomes into pBluescript vector. The PCV2 infectious DNA clone and the PCV2 virus generated by transfection of PK-15 cells with the PCV2 infectious DNA clone have been shown to induce the hallmark pathological lesions of PMWS (9). The chimeric PCV1-2 infectious DNA clone was constructed by replacing the ORF2 capsid gene of nonpathogenic PCV1 with that of PCV2 in the genomic backbone of PCV1 (11). The PCV2 and PCV1-2 infectious clone plasmids used in this study were prepared essentially as previously described (9, 11). The concentration of the plasmid DNA used in vaccination of pigs was determined by spectrophotometry.
Generation and infectivity titration of PCV1-2 and PCV2 virus stocks.
PCV2 and chimeric PCV1-2 live viruses were generated by transfection of PK-15 cells with the respective infectious DNA clone as previously described (9, 11). To determine the infectivity titers of the PCV2 and chimeric PCV1-2 virus stocks, PK-15 cells were cultivated on 8-well LabTek chamber slides (Nalge Nunc International). When the PK-15 cells reached 70 to 80% confluency, the cells were infected with a 10-fold serial dilution of either PCV2 or PCV1-2 virus stock. After 3 days of incubation, the infected cells were stained in an immunofluorescence assay to determine the infectivity titers as previously described (9, 11). Briefly, the infected cells were fixed to the LabTek chamber slides through the use of an 80% acetone and 20% methanol fixing solution. Both PCV2- and PCV1-2-infected cells were then incubated with PCV2 polyclonal rabbit antibody. After washing three times with phosphate-buffered saline buffer, the cells were incubated with a secondary fluorescein isothiocyanate-labeled goat anti-rabbit IgG antibody (KPL Inc., Gaithersburg, Md.). Slides were mounted using Fluoromount-G and coverslipped. Viral infectivity titers were calculated using the Kärber method.
Vaccination of SPF pigs with a chimeric PCV1-2 infectious DNA clone as well as PCV1-2 chimeric live virus.
A total of 48 9-week-old SPF piglets were randomly assigned to four groups of 12 pigs each, and each group was housed separately. Pigs in group 1 were vaccinated with 200 μg of chimeric PCV1-2 infectious DNA clone by intramuscular injection. Pigs in group 2 were vaccinated with 200 μg of chimeric PCV1-2 infectious DNA clone by intrasuperficial inguinal lymph node injections. Pigs in group 3 were vaccinated with 103.5 50% tissue culture infective doses of the chimeric PCV1-2 live virus by intramuscular injection. Pigs in group 4 were not vaccinated and served as controls. All animals were monitored daily for clinical signs, and serum samples were collected at −1, 7, 14, 21, 28, 35, and 42 days postvaccination (DPV) and weekly on the appropriate days postchallenge (DPC) until necropsies were performed at 21 DPC.
Challenge of vaccinated pigs with wild-type pathogenic PCV2.
At 42 DPV, all pigs were challenged with 2 × 104.5 50% tissue culture infective doses of the wild-type pathogenic PCV2. To maximize the challenge conditions, each animal received one-third of the PCV2 challenge inoculum intramuscularly and two-thirds intranasally. All animals were subjected to necropsies at 21 DPC (63 DPV).
Clinical evaluation.
Pigs were weighed at DPV 0 and at the time of necropsy. Rectal temperatures and clinical respiratory scores ranging from 0 to 6 (0, normal; 6, severe) (13) were recorded every other day from −1 to 63 DPV (21 DPC). Clinical observations, including evidence of central nervous system disease, icterus, musculoskeletal disease, and changes in body condition, were recorded daily.
Gross pathology and histopathology.
The necropsy team was blinded to the vaccination status of the pigs at necropsy. Complete necropsies were performed on all pigs at 21 DPC. On the basis of a previously described scoring system (13), an estimated percentage of the lung with grossly visible pneumonia was recorded for each pig. The degree of enlargement of lymph nodes (range: 0, normal size; 3, three times larger than normal) was estimated. Sections for histopathologic examination were taken from lungs (five sections), heart, lymph nodes (tracheobronchial, mediastinal, mesenteric, subiliac, and superficial inguinal), tonsil, thymus, liver, spleen, small intestine, colon, pancreas, and kidney. The tissues were examined in a blinded fashion, and each section was given a score for severity of lung, lymph node, and liver lesions (13). Lung scores ranged from 0 (normal) to 6 (severe lymphohistiocytic interstitial pneumonia). Liver scores ranged from 0 (normal) to 3 (severe lymphohistiocytic hepatitis). Lymph nodes were scored for the estimated amount of lymphoid depletion (LD) of follicles ranging from 0 (normal or no LD) to 3 (severe LD) and for the degree of histiocytic replacement (HR) of follicles (0, none; 3, large amount) (13).
IHC.
Immunohistochemistry (IHC) detection of PCV2-specific antigen was performed on lymph node, spleen, tonsil, and thymus tissues collected during necropsies at 21 DPC. A rabbit polyclonal antiserum against PCV2 was used for IHC in accordance with procedures described previously (33). The amount of PCV2 antigen distributed in the lymphoid tissues was scored in a blinded fashion by assigning a score ranging from 0 for no signal to 3 for a strong positive signal.
Serology.
Serum samples were collected from all pigs at −1, 7, 14, 21, 28, 35, and 42 DPV and at 7, 14, and 21 DPC. Serum antibodies to PRRSV were assayed using a Herd Check PRRSV enzyme-linked immunosorbent assay (ELISA) (IDEXX Laboratories, Westbrook, Mass.). Serum antibodies to porcine parvovirus were detected by a hemagglutination inhibition assay (16). Serum antibodies to PCV2 were detected by a modified indirect ELISA based on the recombinant ORF2 capsid protein of PCV2 (24). Serum samples with a sample/positive (S/P) ratio above 0.20 were considered seropositive (24).
Quantitative real-time PCR assay.
To determine virus genomic copy loads of chimeric PCV1-2 and PCV2 in sera and tissues of vaccinated and challenged swine, serum samples were tested with a quantitative real-time PCR (22) at −1, 7, 14, 21, 28, 35, and 42 DPV as well as at 7, 14, and 21 DPC. Briefly, a pair of primers, MCV1 (5′-GCTGAACTTTTGAAAGTGAGCGGG-3′) and MCV2 (5′-TCACACAGTCTCAGTAGATCATCCCA-3′), was synthesized (10) and used for the quantitative real-time PCR. The MCV1 and MCV2 primer pair was designed to amplify known PCV1 and PCV2 sequences, including the chimeric PCV1-2 sequence (11). Primers MCV1 and MCV2 amplify a 220-bp fragment when chimeric PCV1-2 or PCV2 is used as a template. Viral DNA was extracted from a 100-μl serum sample or 50 μg of homogenized tracheobronchial lymph node (TBLN) tissues through the use of DNAzol reagent according to the protocol of the manufacturer (Molecular Research Center, Cincinnati, Ohio). The DNA extracted from serum samples was resuspended in 100 μl of DNase-RNase-proteinase-free water. The DNA extracted from TBLN tissues was resuspended in 300 μl of DNase-RNase-proteinase-free water. The PCR was performed in the presence of intercalating SYBR green dye (Molecular Probes Inc., Eugene, Oreg.). The PCR parameters consisted of 38 cycles of denaturation at 94°C for 15 s, annealing at 48°C for 15 s, and extension at 72°C for 30 s. To quantify the viral genomic copy numbers, a standard dilution series with a known amount of plasmid containing a single copy of the PCV2 genome (9) was run simultaneously with samples in each reaction. After the reaction was completed, a melt curve cycle was included to confirm the size of the PCR product. The geometric mean of viral genomic copies per reaction on TBLN homogenates was calculated for each group after setting results for negative samples to 1 copy per sample.
PCR.
DNA extracts from TBLNs of selected animals in each group were amplified using PCR primer sets specific for PCV2 or PCV1-2. To amplify chimeric PCV1-2-specific sequences, the PCR employed primer pair Gen.PCV1 (5′-GTGGACCCACCCTGTGCC-3′) and Orf.PCV2 (5′-CAGTCAGAACGCCCTCCTG-3′) to amplify a fragment of 580 bp (11). To amplify PCV2-specific sequences, the PCR employed primer pair Gen.PCV2 (5′-CCTAGAAACAAGTGGTGGGATG-3′) and Orf.PCV2 (5′-CAGTCAGAACGCCCTCCTG-3′) to amplify a fragment of 900 bp (11). The PCR products were sequenced to confirm the identity of the virus recovered from pigs.
Statistical analyses.
All statistical analyses described below were performed using an SAS system (version 8.02; SAS Institute Inc., Cary, N.C.). Serum sample S/P ratios determined by ELISA were compared between vaccinated and nonvaccinated groups by analysis of variance using the MIXED procedure. The model included the effects of vaccine, DPV, and their interaction. Chimeric PCV1-2 vaccination effects for each DPV were evaluated by the slice option. S/P ratios were dichotomized to the presence or absence of seroconversion at S/P = 0.20 and analyzed by logistic regression using the LOGISTIC procedure. Mean viral genomic copy loads in lymph nodes were compared by the Kruskal-Wallis test using the NPAR1WAY procedure and/or by analysis of variance of ranked data followed by a Bonferroni test of multiple mean ranks, using the GLM procedure. Gross pathological and histopathologic lesion scores were compared by the Kruskal-Wallis test with the NPAR1WAY procedure and/or by analysis of variance using the GLM procedure followed by a Bonferroni test of multiple means. The proportions of pigs with gross and histopathologic lesions in various tissues were compared between groups by Fisher's exact test, using the FREQ procedure.
RESULTS
Chimeric PCV1-2 live virus and the chimeric PCV1-2 infectious DNA clone both replicate in pigs when injected intramuscularly or intralymphoid and induce specific antibody response against PCV2 capsid antigen.
Prior to inoculation at −1 DPV, serum samples from all animals tested negative by real-time PCR for PCV1 or PCV2 nucleic acids.
Group 1 pigs vaccinated intramuscularly with the chimeric PCV1-2 DNA clone did not develop PCV1-2 viremia for the duration of the study, as no PCV1-2 DNA was detected in sera (Table 1). Three pigs in group 1 had detectable PCV2 maternal antibody titers at −1 DPV; the titer levels waned by DPV 14. Seroconversion to PCV2-specific antibodies occurred in 7 out of 12 pigs by DPV 42, the day of challenge with wild-type pathogenic PCV2 (Table 2).
TABLE 1.
Detection of chimeric PCV1-2 and PCV2 viremia by real-time PCR in sera of vaccinated and nonvaccinated pigs
| Group | Vaccine | Route of vaccination | No. of pigs with viremia/total on DPVa:
|
Total (PCV1-2) | No. of pigs with viremia/total on DPCa,b
|
Total (PCV2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | 7 | 14 | 21 | 28 | 35 | 42 | 7 | 14 | 21 | |||||
| 1 | PCV1-2 DNAc | Intramuscular | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| 2 | PCV1-2 DNA | Intralymphoid | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| 3 | PCV1-2 virusd | Intramuscular | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 |
| 4 | None | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 0/12 | 1/12 | 9/12 | 4/12 | 9/12 | |
Results represent detection by real-time PCR of chimeric PCV1-2 and wild-type PCV2 nucleic acid.
At 42 DPV, the animals in all four groups were challenged with the wild-type PCV2 virus.
Cloned PCV1-2 genomic DNA in pSK plasmid.
A PCV1-2 live vaccine virus stock generated by transfection of PK-15 cells with PCV1-2 infectious DNA clone.
TABLE 2.
Seroconversion to PCV2-specific antibodies in pigs vaccinated with PCV1-2 live virus or with PCV1-2 infectious DNA clone before and after PCV2 challenge
| Group | Vaccine | Route of vaccination | Maternal antibodya | No. of pigs testing positive/total no. of pigs on DPVb:
|
No. of pigs testing positive/total no. of pigs on DPCb,c:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 7 | 14 | 21 | 28 | 35 | 42 | 7 | 14 | 21 | ||||
| 1 | PCV1-2 DNAd | Intramuscular | No | 0/9 | 0/9 | 1/9 | 1/9 | 1/9 | 1/9 | 6/9 | 8/9 | 9/9 | 9/9 |
| Yes | 3/3 | 1/3 | 0/3 | 0/3 | 0/3 | 2/3 | 1/3 | 2/3 | 2/3 | 2/3 | |||
| 2 | PCV1-2 DNAd | Intralymphoid | No | 0/9 | 0/9 | 0/9 | 1/9 | 2/9 | 5/9 | 5/9 | 8/9 | 9/9 | 8/9 |
| Yes | 3/3 | 1/3 | 0/3 | 1/3 | 1/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 | |||
| 3 | PCV1-2 viruse | Intramuscular | No | 0/8 | 0/8 | 0/8 | 0/8 | 2/8 | 6/8 | 8/8 | 7/8 | 8/8 | 8/8 |
| Yes | 4/4 | 0/4 | 0/4 | 0/4 | 2/4 | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 | |||
| 4 | None | No | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 5/9 | 9/9 | |
| Yes | 3/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 3/3 | |||
Some animals in each group had maternal antibodies at −1 DPV.
PCV2 antibody was measured with an ELISA with the recombinant PCV2 capsid antigen: results represent the number of pigs testing positive/number tested.
At 42 DPV, the animals in all four groups were challenged with the wild-type PCV2 virus.
Cloned chimeric PCV1-2 genomic DNA in pSK plasmid.
PCV1-2 candidate vaccine live virus stock generated by transfection of PK-15 cells with the chimeric PCV1-2 infectious DNA clone.
Fenaux et al. have previously shown that pigs can be infected by intralymphoid injection of PCV2 or PCV1-2 infectious DNA clone (9, 11). Therefore, group 2 pigs were made positive controls by vaccination through intralymphoid injection of the chimeric PCV1-2 infectious DNA clone. Like the pigs in group 1, none of the vaccinated pigs in group 2 developed PCV1-2 viremia (Table 1). Of the 12 pigs, 3 had detectable PCV2 maternal antibody titers at DPV −1; the titer levels waned in all by DPV 14. Seroconversion to PCV2 capsid antibodies was first detected at DPV 21, and 7 of the 12 pigs had seroconverted by DPV 42 (Table 2).
Animals in group 3 were vaccinated with the chimeric PCV1-2 live virus by intramuscular injection. PCV1-2 viremia was not detected in any of the immunized pigs for the duration of the study. Four pigs in group 3 had detectable maternal PCV2 antibodies at −1 DPV; the titer levels waned in all by 7 DPV. Seroconversion to PCV2 capsid antibodies was first detected at 28 DPV in 4 of the 12 animals, and by 42 DPV all pigs in group 3 had seroconverted (Table 2).
PCV1-2 viremia was not detected in the nonvaccinated pigs in group 4, and none of the animals seroconverted to PCV2 antibodies prior to PCV2 challenge (Table 1 and Table 2). Three pigs in group 4 had detectable PCV2 maternal antibodies at DPV −1. By DPV 7, the maternal antibody had waned in all but one animal.
PCV2 antibody S/P ratios differed between treatment groups (P < 0.0001) and over time (P < 0.0001) (data not shown). Following vaccination, up to DPV 42 (the day of PCV2 challenge) pigs in groups 1, 2, and 3 were 1.9 (95% confidence interval [CI] [0.7; 5.1]), 3.51 (95% CI [1.3; 9.1]), and 5.4 (95% CI [2.0; 14.1]) times more likely to have an S/P ratio greater than 0.20 than nonvaccinated pigs in group 4 (overall vaccine effect; P = 0.004).
The chimeric PCV1-2 candidate vaccine prevents PCV2 viremia and reduces virus load in lymph nodes after challenge with wild-type pathogenic PCV2.
After the vaccinated pigs were challenged with wild-type pathogenic PCV2, PCV2 viremia was not detected by real-time PCR in any of the vaccinated pigs in groups 1, 2, and 3 (Table 1). In group 4 nonvaccinated pigs, PCV2 viremia was first detected at DPC 7 in 1 of the 12 pigs and 9 of the 12 pigs had detectable PCV2 viremia by 14 DPC (Table 1). Seroconversion to PCV2 antibodies was first detected at DPC 14 in 6 of the 12 group 4 pigs, and by DPC 21 all pigs in group 4 had seroconverted to PCV2 (Table 2). The PCV2 viral genome loads in serum samples of group 4 pigs peaked at DPC 14; the loads ranged from 2,800 to 240,800 PCV2 genomic copies per ml of serum.
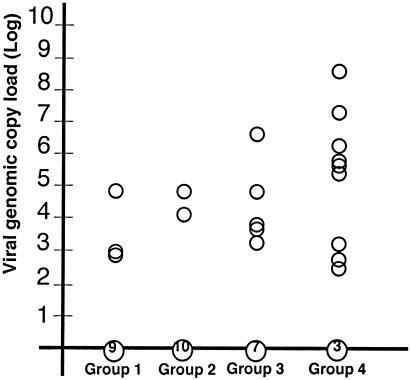
At necropsy (DPC 21), PCV2 genomic DNA was detected in the tracheobronchial lymph nodes (TBLN) in 3 of 12 group 1 pigs, 2 of 12 group 2 pigs, 5 of 12 group 3 pigs, and 9 of 12 group 4 pigs (P = 0.057) (Fig. 1). The range of PCV2 viral genomic copy loads per 10 μg of homogenized TBLN in positive-testing samples was 1,023 to 61,119 in group 1, 10,532 to 82,152 in group 2, 1,652 to 5,419,774 in group 3, and 363 to 621,285,534 in group 4 (Fig. 1). The median genomic copy loads differed between groups 1, 2, 3, and 4 (P = 0.012). Median PCV2 copy loads in TBLN did not differ between groups 1, 2, and 3 or between groups 3 and 4 (P > 0.05); however, loads did differ between groups 1 and 4 and between groups 2 and 4 (P < 0.05). PCR amplification using PCV2- and PCV1-2-specific primers followed by DNA sequencing confirmed that the genomic sequence detected by real-time PCR in the TBLN of animals in groups 1, 2, 3, and 4 originated from the pathogenic PCV2 and not from the chimeric PCV1-2 vaccine virus.
FIG. 1.
Quantitative real-time PCR results of determinations of wild-type PCV2 viral genomic copy loads in TBLN tissues collected during necropsy at DPC 21 from vaccinated pigs in groups 1, 2, and 3 and from nonvaccinated pigs in group 4. PCV2 DNA was extracted from 50 μg of homogenized TBLN lymph node tissues through the use of DNAzol reagent and subjected to real-time PCR amplification. Pigs positive for PCV2 genomic DNA in each group are indicated with an open circle. The numbers 9, 10, 7, and 3 within circles along the x axis indicate the numbers of animals in each group that were negative for PCV2 genomic DNA in the TBLN. The PCV2 genomic copy loads were determined by real-time PCR and are represented as a log value of PCV2 genomic copy loads per 10 μg of TBLN tissue (y axis).
The chimeric PCV1-2 candidate vaccine reduces macroscopic and microscopic lesions as well as viral antigen load in tissues of vaccinated pigs after challenge with wild-type pathogenic PCV2. (i) Clinical evaluation.
Clinical signs characteristic of PMWS were not observed in any animals of groups 1, 2, 3, and 4 for the duration of the study.
(ii) Gross lesions.
All pigs were subjected to necropsy at DPC 21. The enlargement of lymph nodes of the vaccinated pigs in groups 1, 2, and 3 generally ranged from mild to moderate, with 1 or 2 pigs with severely enlarged (3 times normal size) lymph nodes in each group (Table 3). The lymph nodes of all nonvaccinated group 4 pigs were moderately to severely enlarged (Table 3). The mean gross lesion scores of the lymph nodes differed between groups 1, 2, 3, and 4 (P = 0.0007). The values for vaccinated groups 1, 2, and 3 did not differ (P > 0.05) among those groups but were less than the mean score of the nonvaccinated group 4 pigs (P < 0.05; Table 3).
TABLE 3.
Gross lymph node lesions in vaccinated and nonvaccinated pigs
| Group | Vaccinea | Route of vaccination | No. of pigs with gross lymph node lesions/total no. of pigsb |
|---|---|---|---|
| 1 | PCV1-2 DNA | Intramuscular | 12/12 (2.0)I |
| 2 | PCV1-2 DNA | Intralymphoid | 12/12 (1.8)I |
| 3 | PCV1-2 virus | Intramuscular | 12/12 (1.6)I |
| 4 | None | 12/12 (2.7)II |
Animals in group 1 to 3 were vaccinated with chimeric PCV1-2 infectious DNA clone or chimeric PCV1-2 live virus. At 42 DPV, all animals in groups 1 to 4 were challenged with wild-type pathogenic PCV2.
All 12 pigs in each group were subjected to necropsy at 21 DPC. Results represent the number of animals with enlarged lymph nodes/number of animals in each group (numbers in parentheses represent mean scores of estimated enlargement). Different superscripts Roman numerals (I and II) indicate different mean value scores between groups (P < 0.05).
(iii) Microscopic lesions.
Microscopic lung lesions characterized as mild peribronchiolar lymphoplasmacytic and histiocytic bronchointerstitial pneumonia and liver lesions characterized as mild lymphoplasmacytic hepatitis were detected in pigs in all groups (Table 4). Mild LD of lymph node follicles was detected in 4 of 12 group 1 pigs, 1 of 12 group 2 pigs, and 1 of 12 group 3 pigs, and mild to moderate LD was detected in 11 of 12 pigs in group 4 (P < 0.001; Table 4). Mild HR of lymph node follicles was observed in 3 of 12 pigs in group 1 and 1 of 12 pigs in both groups 2 and 3. Mild to moderate HR was detected in 10 of 12 pigs in group 4 (P = 0.001; Table 4). Mild LD and HR of the tonsil follicles were found in 1 of 12 pigs in group 1, 0 of 24 pigs in groups 2 and 3, and 7 of 12 pigs in group 4 (P = 0.0003; Table 4). Mild LD and HR of the spleen follicles were observed in 2 of 12 pigs in group 1 and in none of the pigs in groups 2 and 3, but 10 of 12 group 4 pigs exhibited mild to moderate LD and 9 of 12 group 4 pigs exhibited mild to moderate HR (P < 0.0001; Table 4). Results with respect to the presence of lesions in other tissues and organs are summarized in Table 4.
TABLE 4.
Distribution of histological lesions in different tissues and organs from vaccinated and nonvaccinated pigs challenged with PCV2
| Group | Vaccinea | Route of vaccination | No. of pigs with lesions/no. of pigs examinedb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Liver | Lymph node
|
Tonsil
|
Spleen
|
Kidney | Heart | Thymus | Intestine | ||||||
| LD | HR | LD | HR | LD | HR | |||||||||
| 1 | PCV1-2 DNA | Intramuscular | 4/12 (0.33)I,II | 2/12 (0.16)I | 4/12 (0.33)I | 3/12 (0.25)I | 1/12 (0.08)I | 1/12 (0.08)I | 2/12 (0.17)I | 1/12 (0.08)I | 1/12 | 0/12 | 0/12 | 0/12 |
| 2 | PCV1-2 DNA | Intralymphoid | 1/12 (0.08)I | 3/12 (0.25)I | 1/12 (0.08)I | 1/12 (0.08)I | 0/12I | 0/12I | 0/12I | 0/12I | 0/12 | 0/12 | 0/12 | 0/12 |
| 3 | PCV1-2 virus | Intramuscular | 2/12 (0.17)I | 2/12 (0.16)I | 1/12 (0.08)I | 1/12 (0.08)I | 0/12I | 0/12I | 0/12I | 0/12I | 2/12 | 0/12 | 0/12 | 0/12 |
| 4 | None | 8/12 (0.67)II | 6/12 (0.5)I | 11/12 (0.92)II | 10/12 (0.83)II | 7/12 (0.58)II | 7/12 (0.58)II | 10/12 (0.83)II | 9/12 (0.75)II | 4/12 | 0/12 | 0/12 | 0/12 | |
The candidate vaccines were chimeric PCV1-2 live virus or chimeric PCV1-2 infectious DNA clone.
Values in parentheses are mean histological scores (0, normal; 6, severe multifocal interstitial pneumonia) for interstitial pneumonia in lung and scores (0, normal; 1, mild; 2, moderate; 3, severe) for hepatitis in liver and for lymphoid depletion (LD) and histiocytic replacement (HR) of follicles in lymphoid tissues. Different superscript Roman numerals (I and II) within each column indicate mean value score differences between groups (P < 0.05).
(iv) Detection of PCV2 antigen.
At necropsy (DPC 21), PCV2 antigen was not detected in the lymph node tissues of pigs in vaccinated groups 1, 2, and 3 except for one pig in group 3. In the nonvaccinated group 4 pigs, low-to-high amounts of PCV2 antigen were detected in the lymph nodes (P < 0.0001; Table 5). No PCV2 antigen was detected in the tonsil of group 1, 2, and 3 pigs. However, low-to-high amounts of PCV2 antigen were detected in the tonsil of 8 of 12 nonvaccinated group 4 pigs (P < 0.0001; Table 5). PCV2 antigen was not detected in spleen tissues of vaccinated group 1, 2, or 3 pigs. Low-to-moderate amounts of PCV2 antigen were detected in the spleen tissue of 5 of 12 pigs in nonvaccinated group 4 (P = 0.002; Table 5). No PCV2 antigen was detected in thymus tissues of any pigs (Table 5).
TABLE 5.
Immunohistochemical detection of PCV2 antigen in lymph nodes, tonsils, spleen, and thymus of vaccinated and nonvaccinated pigs at 21 DPC with wild-type PCV2
| Group | Vaccinea | Route of vaccination | No. of pigs testing positive/no. testedb
|
|||
|---|---|---|---|---|---|---|
| Lymph node | Tonsils | Spleen | Thymus | |||
| 1 | PCV1-2 DNA | Intramuscular | 0/12 (0.0)I | 0/12 (0.0)I | 0/12 (0.0)I | 0/12 (0.0) |
| 2 | PCV1-2 DNA | Intralymphoid | 0/12 (0.0)I | 0/12 (0.0)I | 0/12 (0.0)I | 0/12 (0.0) |
| 3 | PCV1-2 virus | Intramuscular | 1/12 (0.08)I | 0/12 (0.0)I | 0/12 (0.0)I | 0/12 (0.0) |
| 4 | None | 9/12 (0.75)II | 8/12 (0.67)II | 5/12 (0.42)II | 0/12 (0.0) | |
The candidate vaccine was either chimeric PCV1-2 infectious DNA clone or chimeric PCV1-2 live virus.
All pigs were subjected to necropsy at 21 DPC. Values in parentheses are the mean estimated amounts of PCV2 antigen in lymphoid tissue (range: 0, no antigen detected; 3, high amounts of antigen). Different superscripts Roman numerals (I and II) indicate mean value score differences between groups (P < 0.05).
DISCUSSION
PMWS has become a serious global pig disease; hence, there is an urgent need for the development of a vaccine against PCV2-associated diseases, including PMWS. Fenaux et al. previously reported that a chimeric PCV1-2 virus is attenuated when inoculated into SPF pigs but is capable of inducing a humoral immune response against PCV2 capsid protein, suggesting that the chimeric PCV1-2 may serve as a candidate vaccine against PCV2 infection (11). In this study, we demonstrated that pigs vaccinated with the chimeric PCV1-2 candidate vaccine developed protective immunity against wild-type pathogenic PCV2 challenge. We also demonstrated that pigs can be effectively vaccinated using an intramuscular route by injections with both the infectious chimeric PCV1-2 DNA clone and chimeric PCV1-2 live virus.
The majority of the vaccinated pigs in all 3 groups seroconverted to PCV2 antibodies within 4 to 6 weeks postvaccination. The remaining seronegative pigs at the time of challenge had elevated PCV2 antibody titers with rising S/P ratios. Statistical analysis showed that there is a significant overall vaccine effect on S/P ratios (P = 0.004). No chimeric PCV1-2 viremia was detected throughout the study in the vaccinated pigs, which is in agreement with our earlier study (11). Most importantly, no PCV2 viremia was detected in vaccinated pigs after challenge with wild-type pathogenic PCV2. In contrast, PCV2 viremia was detected in 9 of 12 nonvaccinated pigs after challenge. After PCV2 challenge, PCV2 antigen was detected in low-to-high amounts in lymph node, tonsil, and spleen tissues of nonvaccinated pigs but not in the vaccinated pigs with the exception of one pig in group 3. Vaccinated pigs also had reduced PCV2 genomic copy viral loads in the lymph nodes. These data indicate that the chimeric PCV1-2 candidate vaccine can prevent PCV2 viremia and significantly reduce the amount of PCV2 virus in the lymphoid tissues, which are important factors in pathogenesis of PCV2-associated diseases.
The mean scores of microscopic lesions in lymph node, spleen, and tonsil tissues of the three vaccinated groups showed that the lesions were less severe (P < 0.05) than those of the nonvaccinated group, indicating protection against PCV2 challenge by the candidate vaccine. The vaccinated pigs had significantly less enlargement of lymph nodes than the nonvaccinated pigs. The enlargement of the lymph nodes observed in vaccinated pigs may be attributed to normal vaccine activation of the immune system in response to the PCV2 challenge, since the enlarged lymph nodes in vaccinated pigs had no detectable microscopic lesions or viral DNA. In nonvaccinated pigs, LD and HR of follicles associated with PCV2 antigen were observed in lymph node, spleen, and tonsil consistent with the hallmark PCV2-associated pathological lesions observed in natural and experimental cases of PMWS. In contrast, only one of the vaccinated pigs had detectable PCV2 antigen in lymphoid tissues. These results strongly indicate that chimeric PCV1-2 candidate vaccine is effective in protecting pigs from PCV2-associated lymphoid lesions and thus in preventing the detrimental effects on the immune system.
The occurrence of LD during initial PCV2 infection may be linked to the eventual occurrence of leukopenia prior to the onset of clinical PMWS (26, 32). Therefore, the chimeric PCV1-2 candidate vaccine may have the ability to stop the eventual progression to clinical PMWS by preventing the initial LD of lymphoid tissues.
No significant differences were found among the three different routes of vaccination with the PCV1-2 candidate vaccine. Intramuscular vaccination with PCV1-2 DNA clone, intralymphoid vaccination with PCV1-2 DNA clone, and intramuscular vaccination with PCV1-2 live virus were all effective in inducing protective immunity against PCV2 infection. However, the intramuscular vaccination route is the only route likely to be acceptable to swine producers. The intralymphoid route of vaccination with chimeric PCV1-2 infectious DNA clone was included as a positive control, since Fenaux et al. had previously shown that this route has the ability to induce an infection (9, 11).
Low levels of maternal antibody found in a few animals in groups 1, 2, and 3 had no apparent effect on the induction of protective immunity by the chimeric PCV1-2 candidate vaccine. Since there were only a few animals with low levels of maternal antibodies in this study, a definitive answer cannot be given to the question of whether or not the low level of maternal antibodies has any effect on vaccination with a live vaccine. Since many newborns in commercial swine farms have PCV2 maternal antibodies following colostrum uptake, future studies with larger numbers of animals with different levels of maternal antibody are warranted to confirm our preliminary results.
Although not all the vaccinated animals seroconverted to PCV2 by the time of challenge, they were all protected against the pathogenic PCV2 challenge, suggesting that high S/P ratios of PCV2 antibody responses are not absolutely required for protection. It is possible that the chimeric PCV1-2 candidate vaccine induces a cell-mediated immune response (8), which may be equally or more important for induction of protection against PCV2. Further research is warranted to determine the exact role and the extent of cell-mediated immunity induced by the candidate vaccines in protection against PCV2 infections.
Acknowledgments
We thank Mike Gill and Jay Srinivas of Fort Dodge Animal Health, Inc., and S. M. Boyle, L. A. Eng, R. B. Duncan, J. C. Sible, and T. E. Toth for their support and Jillian Fenaux for editorial assistance.
This study was supported in part by a grant from Fort Dodge Animal Health, Inc., Fort Dodge, Iowa, and by a grant from the U.S. Department of Agriculture National Research Initiative Competitive Grant Program (NRI 2004-35204-14213).
REFERENCES
- 1.Allan, G. M., F. McNeilly, J. P. Cassidy, G. A. Reilly, B. Adair, W. A. Ellis, and M. S. McNulty. 1995. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 44:49-64. [DOI] [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, S. Kennedy, B. Daft, E. G. Clarke, J. A. Ellis, and D. M. Haines. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig. 10:3-10. [DOI] [PubMed] [Google Scholar]
- 3.Bassami, M. R., D. Berryman, G. E. Wilcox, and S. R. Raidal. 1998. Psittacine beak and feather disease virus nucleotide sequence analysis and its relationship to porcine circovirus, plant circoviruses, and chicken anaemia virus. Virology 249:453-459. [DOI] [PubMed] [Google Scholar]
- 4.Cheung, A. K. 2003. Transcriptional analysis of porcine circovirus. Virology 305:168-180. [DOI] [PubMed] [Google Scholar]
- 5.Choi, C., C. Chae, and E. G. Clark. 2000. Porcine postweaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Investig. 12:151-153. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, S., and J. J. Sands. 1994. Evidence of circovirus infection in British pigs. Vet. Rec. 134:680-681. [DOI] [PubMed] [Google Scholar]
- 7.Ellis, J. A., A. Bratanich, E. G. Clark, G. Allan, B. Meehan, D. M. Haines, J. Harding, K. H. West, S. Krakowka, C. Konoby, L. Hassard, K. Martin, and F. McNeilly. 2000. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Investig. 12:21-27. [DOI] [PubMed] [Google Scholar]
- 8.Darwich, L., P. Sandrine, A. Rovira, J. Segales, M. Domingo, I. P. Oswald, and E. Mateu. 2003. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisystemic wasting syndrome. J. Gen. Virol. 84:2117-2125. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux, M., P. G. Halbur, G. Haqshenas, R. Royer, P. Thomas, P. Nawagitgul, M. Gill, T. E. Toth, and X. J. Meng. 2002. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathological lesions. J. Virol. 76:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenaux, M., P. G. Halbur, M. Gill, T. E. Toth, and X. J. Meng. 2000. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 38:2494-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaux, M., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2003. Immunogenicity and pathogenicity of the chimeric infectious DNA clones between pathogenic type 2 porcine circovirus (PCV2) and non-pathogenic PCV1 in weaning pigs. J. Virol. 77:11232-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilpin, D. F., K. McCullough, B. M. Meeham, F. McNeilly, I. McNair, L. S. Stevenson, J. C. Foster, J. A. Ellis, S. Krakowka, B. M. Adair, and G. M. Allan. 2003. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of porcine immune system. Vet. Immunol. Immunopathol. 94:149-161. [DOI] [PubMed] [Google Scholar]
- 13.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two U. S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648-660. [DOI] [PubMed] [Google Scholar]
- 14.Harding, J. C., and E. G. Clark. 1997. Recognizing and diagnosing post-weaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 5:201-203. [Google Scholar]
- 15.Harms, P. A., S. D. Sorden, P. G. Halbur, S. R. Bolin, K. M. Lager, L. Morozov, and P. S. Paul. 2001. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 38:528-539. [DOI] [PubMed] [Google Scholar]
- 16.Joo, H. S., C. R. Donaldson-Wood, and R. H. Johnson. 1976. A standardized haemagglutination inhibition test for porcine parvovirus antibody. Aust. Vet. J. 52:422-424. [DOI] [PubMed] [Google Scholar]
- 17.Krakowka, S., J. A. Ellis, F. McNeilly, D. Gilpin, B. Meeham, K. McCullough, and G. Allan. 2002. Immunologic features of porcine circovirus type 2 infection. Viral Immunol. 15:567-582. [DOI] [PubMed] [Google Scholar]
- 18.Krakowka, S., J. A. Ellis, F. McNeilly, S. Ringler, D. M. Rings, and G. Allen. 2001. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet. Pathol. 38:31-42. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakis, S. C., K. Saoulidis, S. Lekkas, Ch. C. Miliotis, P. A. Papoutsis, and S. Kennedy. 2002. The effects of immuno-modulation on the clinical and pathological expression of postweaning multisystemic wasting syndrome. J. Comp. Pathol. 126:38-46. [DOI] [PubMed] [Google Scholar]
- 20.Ladekjaer-Mikkelsen, A. S., J. Nielsen, T. Stadejek, T. Storgaard, S. Krakowka, J. Ellis, F. McNeilly, G. Allan, and A. Botner. 2002. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and non-immunostimulated 3-week-old piglets experimentally infected with porcine circovirus type-2 (PCV2). Vet. Microbiol. 89:97-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larochelle, R., M. Morin, M. Antaya, and R. Magar. 1999. Identification and incidence of porcine circovirus in routine field cases in Quebec as determined by PCR. Vet. Rec. 145:140-142. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Q., L. Wang, P. Willison, and L. A. Babiuk. 2000. Quantitative, competitive PCR analysis of porcine circovirus DNA in serum from pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 38:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mankertz, A., K. Hattermann, B. Ehlers, and D. Soike. 2000. Cloning and sequencing of columbid circovirus (CoCV), a new circovirus from pigeons. Arch. Virol. 145:2469-2479. [DOI] [PubMed] [Google Scholar]
- 24.Nawagitgul, P., P. A. Harms, I. Morozov, B. J. Thacker, S. D. Sorden, C. Lekcharoensuk, and P. S. Paul. 2002. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based ELISA for the detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawagitgul, P., I. Morozov, S. R. Bolin, P. A. Harms, S. D. Sorden, and P. S. Paul. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81:2281-2287. [DOI] [PubMed] [Google Scholar]
- 26.Nielson, J., I. E. Vincent, A. Bøtner, A.-S. Ladekjaer-Mikkelsen, G. Allan, A. Summerfield, and K. C. McCullough. 2002. Association of lymphopenia with porcine circovirus type 2 induced postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 92:97-111. [DOI] [PubMed] [Google Scholar]
- 27.Nishizawa, T., H. Okamoto, K. Konishi, H. Yoshizawa, Y. Miyakawa, and M. Mayumi. 1997. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 241:92-97. [DOI] [PubMed] [Google Scholar]
- 28.Onuki, A., K. Abe, K. Togashi, K. Kawashima, A. Taneichi, and H. Tsunemitsu. 1999. Detection of porcine circovirus from lesions of a pig with wasting disease in Japan. J. Vet. Med. Sci. 61:1119-1123. [DOI] [PubMed] [Google Scholar]
- 29.Opriessnig, T., M. Fenaux, S. Yu, R. B. Evans, D. Cavanaugh, J. M. Gallup, F. J. Pallares, E. L. Thacker, K. M. Lager, X. J. Meng, and P. G. Halbur. 2004. Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus. Vet. Microbiol. 98:209-220. [DOI] [PubMed] [Google Scholar]
- 30.Opriessnig, T., S. Yu, J. M. Gallup, R. B. Evans, M. Fenaux, F. J. Pallares, E. L. Thacker, C. W. Brockus, M. R. Ackermann, P. Thomas, X. J. Meng, and P. G. Halbur. 2003. Effects of vaccination with selective bacterins on conventional pigs infected with wild type 2 porcine circovirus. Vet. Pathol. 40:521-529. [DOI] [PubMed] [Google Scholar]
- 31.Phenix, K. V., J. H. Weston, I. Ypelaar, A. Lavazza, J. A. Smyth, D. Todd, G. E. Wilcox, and S. R. Raidal. 2001. Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus circovirus of the family Circoviridae. J. Gen. Virol. 82:2805-2809. [DOI] [PubMed] [Google Scholar]
- 32.Segales, J., C. Alfonso, C. Rosell, J. Pastor, F. Chianini, E. Campos, L. Lopez-Fuertes, J. Quinana, G. Rodriguez-Arrioja, M. Calsamiglia, J. Pujols, J. Dominguez, and M. Domingo. 2001. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 81:37-44. [DOI] [PubMed] [Google Scholar]
- 33.Sorden, S. D., P. A. Harms, P. Nawagitgul, D. Cavanaugh, and P. S. Paul. 1999. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J. Vet. Diagn. Investig. 11:528-530. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi, K., Y. Iwasa, M. Hijikata, and S. Mishiro. 2000. Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch. Virol. 145:979-993. [DOI] [PubMed] [Google Scholar]
- 35.Tischer, I., W. Mields, D. Wolff, M. Vagt, and W. Griem. 1986. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 91:271-276. [DOI] [PubMed] [Google Scholar]
- 36.Tischer, I., H. Gelderblom, W. Vettermann, and M. A. Koch. 1982. A very small porcine virus with single-stranded DNA. Nature 295:64-66. [DOI] [PubMed] [Google Scholar]
- 37.Todd, D., J. H. Weston, D. Soike, and J. A. Smyth. 2001. Genome sequence determinations and analyses of novel circoviruses from goose and pigeon. Virology 286:354-362. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, L. E., T Umemeru, J. Astembroski, S. C. Ray, H. J. Alter, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2001. Dynamics of SEN virus infection among injection drug users. J. Infect. Dis. 184:1315-1319. [DOI] [PubMed] [Google Scholar]