Abstract
Background
A methotrexate autoinjector (MTXAI) was developed for self-administration of subcutaneous (SC) methotrexate by patients with rheumatoid arthritis (RA). The MTXAI circumvents the need for vials, needles, and syringes and may therefore improve dosing accuracy, handling risks, and patient adherence.
Objectives
The objective of this study was to evaluate actual human use of the MTXAI in patients with RA and determine its reliability, robustness, safety, local tolerance, and ease of use.
Methods
In this phase 2, multicenter, open-label, single-dose, single-arm, in-clinic US study, adults (N = 101) treated with methotrexate for 3 months or longer were trained to use the MTXAI and assigned to 10, 15, 20, or 25 mg methotrexate based on previous treatment and disease status. Patients completed training confirmation and ease-of-use questionnaires. Pain was evaluated immediately after self-administration and at follow-up with a 100-mm visual analog scale (0 = no pain, 100 = worst possible pain).
Results
At screening, 90.1% of patients had moderate to severe functional limitations (class II–IV). All patients successfully completed the study. All devices functioned correctly and as intended. The device was rated easy to use by 98%, and instructions clear and easy to follow by 100% of patients. On the visual analog scale, mean and median pain scores were 3.6/100 and 1.0/100 mm, respectively, immediately after self-administration, and were lower at follow-up. Most patients (92.3%) had no administration-site erythema; 7.7% had minimal erythema.
Conclusions
The SC MTXAI was well tolerated and considered easy to use by patients with RA. Improving SC methotrexate delivery may increase patient tolerance of self-administration, possibly improving adherence.
Key Words: methotrexate, rheumatoid arthritis, pain, self-administration, subcutaneous
As supported by the European League Against Rheumatism and the American College of Rheumatology recommendations for the pharmacologic management of rheumatoid arthritis (RA), the cornerstone of therapy for RA is the disease-modifying antirheumatic drug methotrexate.1,2 Multinational recommendations advocate the use of oral methotrexate, with the possibility for parenteral administration in cases of intolerance or inadequate clinical response to oral methotrexate, owing to enhanced bioavailability of parenteral administration, as an optimal, evidence-based approach.3 Although it is recognized that oral methotrexate doses of 20 to 30 mg weekly are often administered for disease control,1,4 the bioavailability of oral methotrexate has been found to plateau at doses 15 mg weekly or greater,5–8 and the use of oral methotrexate is not infrequently limited by gastrointestinal adverse events (AEs).4,9,10 Improved clinical outcomes, including improvements in tolerability, have been seen in several studies of patients who switched from oral methotrexate to parenteral methotrexate for inefficacy or intolerance.10–13 Consequently, subcutaneous (SC) administration appears to have advantages over oral administration of methotrexate in patients with RA.
Oral administration of methotrexate is the most common route of administration in the United States, largely because of convenience.4,10 It is estimated that the use of parenteral methotrexate is limited to less than 5% of patients with RA in the United States.14 The drawbacks of SC methotrexate administration include its cytotoxicity and the need for patient or caregiver manipulation of vials, needles, and syringes. Methotrexate is classified as a hazardous drug by the US Department of Labor, Occupational Safety & Health Administration Hazard Communication Standard.15 Therefore, all individuals who will be in contact with methotrexate require formal training, specific protocols are followed for cleanup of spills, and individuals exposed to methotrexate must be monitored to identify any biological effects.16 The functional limitations imposed by RA may affect manual dexterity such that patients may have difficulty using vials, needles, and syringes, and caregivers who use these items are at risk for exposure and needlestick injuries. Patients may have anxiety over self-injection and may experience pain while caregivers may have anxiety about performing injections and causing the patient pain.
We have developed a prefilled, single-use, pressure-assisted methotrexate autoinjector (MTXAI) for the self-administration of SC methotrexate (Fig. 1). The MTXAI (Otrexup™ [methotrexate], Antares Pharma, Ewing, NJ) was approved by the Food and Drug Administration for the management of patients with severe, active RA, who are intolerant of or had an inadequate response to first-line therapy.17 This device enables SC administration without the need for opening a vial, handling a needle, or preparing and operating a syringe and can be stored conveniently at room temperature, protected from light. Self-administration involves only the removal of a cap, a safety, and 1-click autoinjection of methotrexate. In this phase 2 study, the actual human use of this autoinjector was evaluated in patients with RA with regard to safe usability, ease of use, and administration-site pain and appearance.
FIGURE 1.
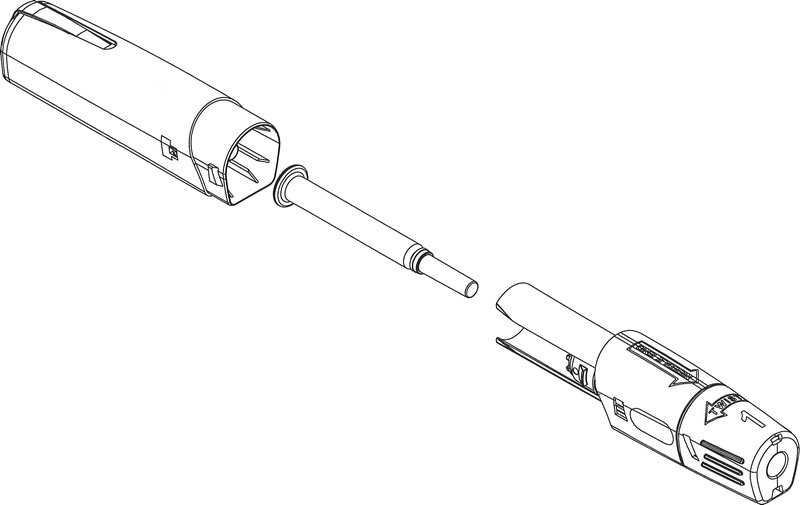
Schematic diagram of the SC MTXAI.
MATERIALS AND METHODS
Patients
Eligible patients were at least 18 years of age, had been diagnosed with adult RA (controlled or uncontrolled), and had been treated with methotrexate for 3 months or longer before enrollment. Women of childbearing potential were included only if they had negative pregnancy tests at screening and day of treatment and were using effective contraception. All patients were required to be capable of administering SC self-injections and of understanding verbal or written English. All patients signed and dated an approved informed consent form before the initiation of any study procedures.
Patients were excluded from the study if they were pregnant or lactating, had a history of malignancy or neoplastic disease (except successfully treated basal or squamous cell carcinoma of the skin >1 year ago or patients who were cancer-free for >5 years), had a skin condition or disorder preventing SC administration of methotrexate, or had other clinically significant disease. Other exclusion criteria were acute illness within 7 days or major illness/hospitalization within 1 month of study drug administration, history of drug or alcohol abuse within the past year, history of human immunodeficiency virus or hepatitis B or C virus infection, or the use of any other investigational agent within 1 month before enrollment.
Study Design
This was a phase 2, open-label, single-dose, single-arm, in-clinic study conducted at 8 sites in the United States. The total duration of the trial was 8 weeks, and it contained a screening period, a 1-day treatment period, and a 1-day follow-up period. During the screening period, eligible patients were enrolled and instructed to discontinue their previously prescribed methotrexate therapy. They were assigned by the investigator to 1 of 4 methotrexate dose levels (10, 15, 20, or 25 mg) according to their baseline methotrexate therapy and RA disease status (controlled or uncontrolled). At least 7 days had to have elapsed between the patient’s last previously prescribed methotrexate dose and the start of study treatment. Patients were to restart their previous methotrexate therapy 1 week after the treatment visit.
The protocol was approved by the institutional review board of each study site before study initiation. The study was conducted in accordance with the Declaration of Helsinki and was in compliance with Good Clinical Practice Guidelines. This trial is registered with ClinicalTrials.gov (NCT01618955).
Standardized Patient Training and Assessments
Patients received standardized SC self-administration training from nursing staff at the investigator sites that involved verbal instructions and a demonstration of proper use of the MTXAI through the use of a standardized script, as well as a review of written patient instructions. Self-administration was carried out independently, in the clinic, with the aid of written patient instructions. Patients used the MTXAI to administer their assigned dose of methotrexate into the anterior abdominal wall, and site personnel observed the procedure.
The primary end point for determination of safe usability was successful SC administration with the MTXAI. Successful self-administration was determined by the following: (1) SC self-administration was intentional; (2) the SC dose was administered by the patient; (3) SC self-administration was in the appropriate location on the abdomen; and (4) the device functioned appropriately as determined by inspection of used devices, including confirmation that the window was obstructed, the ram was released, and the needle guard was no longer retracted.
After self-administration on day 1, patients completed an ease-of-use questionnaire, which contained 5 statements that assessed the device and the standardized patient training for ease of use by the patient. Immediately after self-administration on day 1 and at the follow-up visit on day 2, patients rated any pain at the injection site with a visual analog scale (VAS) (0 mm = no pain, 100 mm = worst possible pain).
Secondary end points included ease-of-use questionnaire scores regarding the device, ease-of-use and training confirmation questionnaire scores regarding written patient instructions and SC self-administration training, assessment of essential tasks questionnaire scores, self-reported VAS scores for pain at the injection site, and injection-site assessment numeric grades. Injection sites were assessed predose and at 0.25, 1, 6, and 24 hours postdose. Erythema severity was graded on a scale of 0 = none, 1 = very slight/barely perceptible, 2 = obvious, but well defined, 3 = moderate to severe, and 4 = severe.
Adverse events and medical and surgical history were coded to system organ class and preferred term using the Medical Dictionary for Regulatory Activities version 14.1. An AE was considered to be a treatment-emergent AE (TEAE) if it started on or after self-administration with the MTXAI. Vital signs (including heart rate, respiratory rate, and blood pressure) were measured at screening and at 1, 6, and 24 hours postdose.
Statistical Analysis
The safety population was defined as all patients who received study drug and carried out a successful or an unsuccessful self-administration. All analyses were performed on the safety population. A sample size of approximately 100 patients (approximately 25 patients per dose level with a minimum enrollment of 20 patients per group) was planned to provide a sufficient number of patients to determine the safety and tolerability of the MTXAI, based on correspondence with the US Food and Drug Administration.
RESULTS
Patients
Of 104 patients screened, 101 were enrolled and completed the study (20, 30, 31, and 20 patients in the methotrexate 10-, 15-, 20-, and 25-mg groups, respectively). No patient discontinued from the study. Most patients were women (79.2%), and their mean age was 60.9 years. The mean duration of RA at informed consent was 13.3 years, and the majority had a functional status of class II or III (89.1%), indicating functional limitations in work and/or other activities. Most patients (80.2%) had previous experience with SC administration; 31 had used an autoinjector, 24 had used a pen device, and 53 had used a needle and syringe with a vial. Approximately 77% were receiving oral tablets of methotrexate at enrollment.
Outcomes
All patients performed successful self-administration with the MTXAI. Responses to the ease-of-use questionnaire (Fig. 2) indicated that 98% of patients agreed or strongly agreed that the device was easy to use, and all patients agreed that the written instructions and standardized training were clear and easy to follow. Results of the training confirmation questionnaire showed that almost all patients (94%) answered all 5 questions correctly.
FIGURE 2.
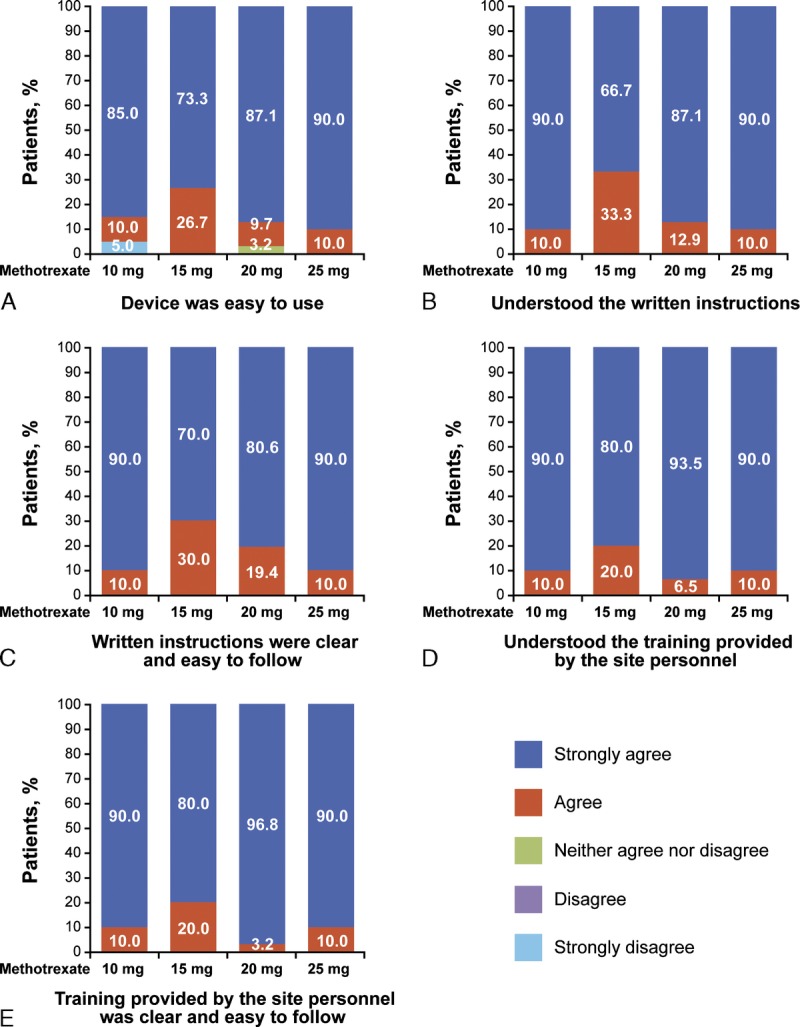
Patient ratings of ease of use of the MTXAI. Patients rated the ease of use of the MTXAI by strongly agreeing, agreeing, neither agreeing nor disagreeing, disagreeing, or strongly disagreeing that (A) the device was easy to use, (B) they understood the written instructions, (C) the written instructions were clear and easy to follow, (D) they understood the training provided by the site personnel, and (E) the training provided by the site personnel was clear and easy to follow.
Patients experienced minimal injection-site pain during the study, as reflected by mean and median VAS pain scores (Fig. 3). On day 1, immediately after administration, 34.3% of patients had VAS scores of 0 mm, and 20.2% of patients had VAS scores of 1 mm. The overall mean (median) severity of injection-site pain was 3.6 mm (1.0 mm) immediately after administration. On day 2, 55.6% of patients had VAS scores of 0 mm, and 17.2% of patients had VAS scores of 1 mm. The overall mean (median) severity of injection-site pain was 1.4 mm (0.00 mm) on day 2.
FIGURE 3.
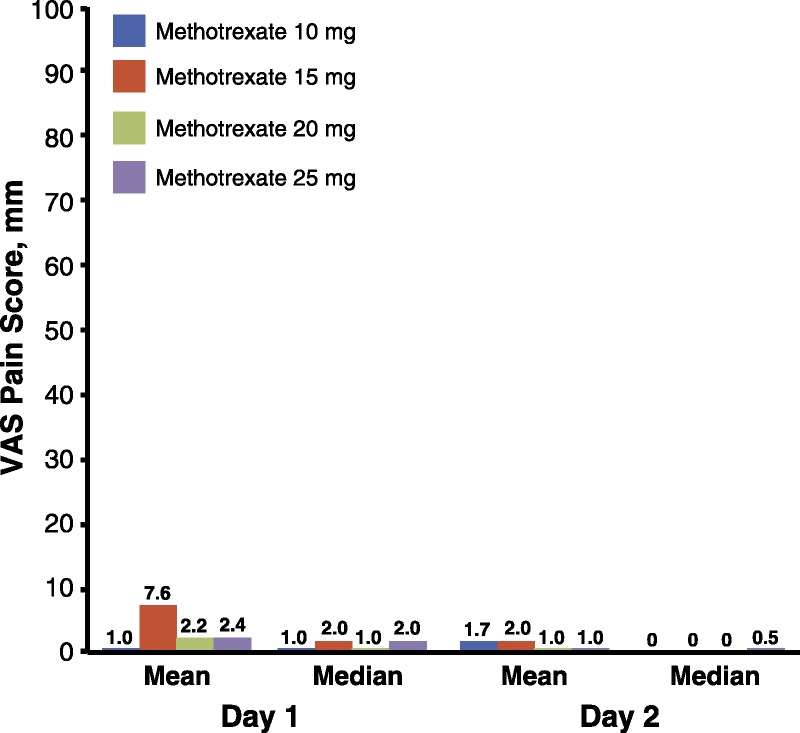
Patient-reported injection site pain. Visual analog scale pain scores immediately (day 1) and 24 hours (day 2) after SC methotrexate self-administration with the MTXAI.
Of 404 injection-site assessments carried out postdose, 373 (92.3%) noted no erythema (severity of 0), and 31 (7.7%) noted very slight, barely perceptible erythema (severity of 1). All but 2 patients with grade 1 assessments were described as having erythema the size of a pinprick or erythema consistent with a pressure mark from the MTXAI. Of these 2 exceptions, 1 patient had grade 1 erythema that was 10 mm in diameter and resolved by 6 hours after administration, and the other had grade 1 erythema that was 3 mm in diameter and resolved by 1 hour after administration. Neither patient had associated induration or ecchymosis. Two patients with pinprick-sized erythema had accompanying induration/swelling the size of a pinprick at the injection site at the 0.25-hour postdose assessment. No patient experienced induration/swelling at later time points, and no patient with grade 1 erythema experienced bleeding, ecchymosis, or hematoma at the injection site or required countermeasures at any assessment time point. No patient had erythema of severity 2, 3, or 4 at any injection-site assessment. Two patients had mild ecchymoses at the injection site, but neither had accompanying erythema. No dose-related trends were noted for injection-site assessments, and no patient had erythema worsening of more than 1 point from previous injection-site assessments at any injection-site assessment.
Two patients (6.5%) in the methotrexate 20-mg group and 1 patient (5.0%) in the methotrexate 25-mg group had a TEAE during the study. One patient receiving methotrexate 20 mg had a mild headache that was considered by the investigator to be related to study drug. The other patient receiving methotrexate 20 mg had exostosis that was considered unrelated to study drug. The TEAE in the methotrexate 25-mg group was a serious AE of sick sinus syndrome, which was considered by the investigator to be severe but unrelated to study drug. No action was taken, and the event resolved after 8 days. There were no injection-site reaction AEs. No deaths were reported during the study. There were no clinically meaningful changes in vital signs from predose to any postdose time point.
DISCUSSION
The results of this phase 2 study demonstrate the safe usability of the SC MTXAI in adult patients with RA after standardized training by site personnel and review of written instructions. All patients performed successful self-administration and completed all essential tasks successfully. Reliability and robustness of the device were demonstrated by the correct functioning of all devices as intended. Patients reported no or minimal administration-site pain. Taken together, these results suggest that patients with RA can successfully use the MTXAI for self-administration of SC methotrexate, that administration is safe and well tolerated, and that patient education tools are clear and easy to follow.
Almost all patients (90.1%) in this study had moderate to severe functional limitations (functional status of class II–IV); however, these limitations did not affect their ability to self-administer SC methotrexate with the MTXAI. Because 80% of the population had prior experience with SC injection, it is possible that some patients with no prior injection experience had declined participation, biasing the population toward experienced patients. However, 98% of all patients agreed with the statement “The device was easy to use,” suggesting that prior experience did not influence ease of use in this study.
Subcutaneous MTX may provide important clinical benefits compared with oral MTX with respect to both gastrointestinal tolerability and bioavailability,7,10 and the consideration of SC methotrexate when oral methotrexate is intolerable or inadequately effective is recommended by both Canadian guidelines and European experts.3,18 Difficulties with traditional methods of SC administration of methotrexate may, however, affect adherence. Administration of a single-use autoinjector has been shown in other disease states to help patients overcome needle anxiety,19 decrease the incidence of needlestick injury,20 and improve treatment adherence and persistence.21 For patients with RA, the MTXAI represents an alternative to oral administration and an alternative to the need for vials, needles, and syringes. Its design is such that the needle is not visible to the patient, which should minimize needle anxiety; it is prefilled, which should minimize the risk of spillage and accidental exposure; and it is easy to use and nearly pain-free, which should promote treatment adherence and persistence. The commercially available version of the MTXAI is a prefilled device (in dosage strengths of 10, 15, 20, and 25 mg) that matches those doses used in the current trial.17
In conclusion, delivery of SC methotrexate with the MTXAI is easily performed by patients with RA who have moderate to severe dexterity limitations and is associated with no or minimal pain and erythema. Improving SC methotrexate delivery may increase patient tolerance of self-administration, possibly improving adherence.
Figure.

No caption available.
Footnotes
Financial support was provided by Antares Pharma Inc.
Medical writing services were provided by Stephanie Leinbach, PhD, and Judy Fallon, PharmD, CMPP (C4 MedSolutions, LLC. A CHC Group company, Yardley, PA), with funding from Antares Pharma Inc, Ewing, NJ.
B.F. is an employee of Antares Pharma, Inc, a shareholder of Pfizer, and was a consultant for Celgene and Bristol-Myers Squibb. A.K. is a consultant for AbbVie, UCB, Amgen, Janssen, Bristol-Myers Squibb, Pfizer, and Celgene. J.S.J. is an employee of Antares Pharma, Inc.
REFERENCES
- 1. Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010; 69: 964– 975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012; 64: 625– 639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009; 68: 1086– 1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis. 2009; 68: 1094– 1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004; 31: 645– 648 [PubMed] [Google Scholar]
- 6. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997; 36: 86– 90 [DOI] [PubMed] [Google Scholar]
- 7. Schiff MH, Simon LS, Freundlich B, et al. Drug exposure limitations of oral methotrexate (MTX) at doses >15mgs may be overcome by using a subcutaneous MTX auto-injector in patients with rheumatoid arthritis (RA). Presented at the American College of Rheumatology Annual Meeting. San Diego, CA, 2013. [Google Scholar]
- 8. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Joint Dis. 2007; 65: 168– 173 [PubMed] [Google Scholar]
- 9. Furst DE, Koehnke R, Burmeister LF, et al. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. J Rheumatol. 1989; 16: 313– 320 [PubMed] [Google Scholar]
- 10. Wegrzyn J, Adeleine P, Miossec P. Better efficacy of methotrexate given by intramuscular injection than orally in patients with rheumatoid arthritis. Ann Rheum Dis. 2004; 63: 1232– 1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakker MF, Jacobs JW, Welsing PM, et al. Are switches from oral to subcutaneous methotrexate or addition of ciclosporin to methotrexate useful steps in a tight control treatment strategy for rheumatoid arthritis? A post hoc analysis of the CAMERA study. Ann Rheum Dis. 2010; 69: 1849– 1852 [DOI] [PubMed] [Google Scholar]
- 12. Hameed B, Jones H. Subcutaneous methotrexate is well tolerated and superior to oral methotrexate in the treatment of rheumatoid arthritis. Int J Rheum Dis. 2010; 13: e83– e84 [DOI] [PubMed] [Google Scholar]
- 13. Mainman H, McClaren E, Heycock C, et al. When should we use parenteral methotrexate? Clin Rheumatol. 2010; 29: 1093– 1098 [DOI] [PubMed] [Google Scholar]
- 14. Curtis JR, Xie F, Zhang J, et al. Patterns of use of oral and subcutaneous methotrexate use in rheumatoid arthritis patients enrolled in the U.S. Medicare program. Presented at the American College of Rheumatology Annual Meeting. San Diego, CA, 2013. [Google Scholar]
- 15.Occupational Safety and Health Administration. Controlling occupational exposure to hazardous drugs. 1999. Available at: https://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html Accessed August 12, 2013 [DOI] [PubMed]
- 16.Bedford Laboratories. Material safety data sheet. 2007. Available at: http://www.bedfordlabs.com/content/dam/internet/opu/bedfordlabs/com_EN/documents/products/Methotrexate/Methotrexate-Lyo-8-23-05Rev07.pdf Accessed August 12, 2013
- 17.OTREXUP. Methotrexate Injection, for Subcutaneous Use. Ewing, NJ: Antares Pharma, Inc; 2013 [Google Scholar]
- 18. Katchamart W, Bourre-Tessier J, Donka T, et al. Canadian recommendations for use of methotrexate in patients with rheumatoid arthritis. J Rheumatol. 2010; 37: 1422– 1430 [DOI] [PubMed] [Google Scholar]
- 19. Phillips JT, Fox E, Grainger W, et al. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011; 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ward LG, Aton SS. Impact of an interchange program to support use of insulin pens. Am J Health Syst Pharm. 2011; 68: 1349– 1352 [DOI] [PubMed] [Google Scholar]
- 21. Xie L, Zhou S, Wei W, et al. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013; 15: 230– 236 [DOI] [PubMed] [Google Scholar]


