Abstract
Nabilone is a synthetic cannabinoid that has shown promise for the treatment of posttraumatic stress disorder (PTSD)–related insomnia and nightmares as well as efficacy in the management of chronic pain. It has also been proposed for harm reduction in cannabis dependence. Its effectiveness for management of concurrent disorders in seriously mentally ill correctional populations has not been evaluated. This retrospective study of 104 male inmates with serious mental illness prescribed nabilone analyzes the indications, efficacy, and safety of its use. Medications discontinued with the initiation of nabilone were also reviewed. The results showed nabilone targeting a mean of 3.5 indications per patient, thus likely reducing polypharmacy risk. The mean final dosage was 4.0 mg. Results indicated significant improvement in PTSD-associated insomnia, nightmares, PTSD symptoms, and Global Assessment of Functioning and subjective improvement in chronic pain. Medications associated with greater risk for adverse effects or abuse than nabilone were often able to be discontinued with the initiation of nabilone, most often antipsychotics and sedative/hypnotics. There was no evidence of abuse within this high-risk population or reduction of efficacy when nabilone was given in powder form with water rather than as a capsule. This study supports the promise of nabilone as a safe, effective treatment for concurrent disorders in seriously mentally ill correctional populations. Prospective, randomized controlled trials are required to confirm our preliminary results. Follow-up in the community will be required to confirm effectiveness in harm reduction.
Key Words: seriously mentally ill offenders, synthetic cannabinoid nabilone, PTSD-related insomnia and nightmares, chronic pain, harm reduction
Cannabis and its derivatives have been reported to have medicinal benefits going back thousands of years.1 In recent decades, there has been increasing evidence supporting the use of cannabinoids for a variety of indications.2,3 Nabilone is a synthetic cannabinoid C1 receptor agonist that was approved for use by Health Canada in 1982 for the treatment of chemotherapy-induced nausea.4 Over the years, several randomized controlled studies have shown cannabinoids, including nabilone, to be effective in the treatment of chronic pain.5,6 More recently, in an open study of 47 patients, Fraser7 found nabilone to be of benefit in the treatment of refractory nightmares in posttraumatic stress disorder (PTSD). Fraser7 also noted in this study that nabilone increased sleep time and was not associated with the development of tolerance. Another study by Lile et al8 found similar interoceptive effects of nabilone compared with delta-9-tetrahydrocannabinol, which lead the authors to conclude that nabilone may be useful as a harm reduction approach to reduce use of smoked cannabis in cannabis-dependent individuals similar to the use of agonists in tobacco and opioid dependence. Of note, in an extensive search of scientific literature, popular press, and focused interviews with medical professionals and law enforcement officials, Ware and St Armand9 found little evidence for nabilone abuse, including in Canada where it has been available for over 30 years. Ware and St Armand9 noted that addicts typically reported nabilone to have less of a high, more adverse effects, delayed onset of action, and be more expensive compared with cannabis. The authors also found law enforcement had a dearth of reports of abuse or diversion and reported nabilone to have no street value.
CB1 receptors are widely distributed and abundant in the brain yet relatively absent in the cardiorespiratory areas of the brain stem, so agents acting on this system may have a wide range of potential therapeutic effects with little risk of cardiorespiratory suppression.10 Hill et al11 found that those with PTSD following the World Trade Centre attacks in 2001 (n = 24) had lower circulating levels of the endocannabinoid 2-arachidonoylglycerol than those without (n = 22). Hill et al11 also found circulating levels of the endocannabinoid, anandamide, to be positively correlated with circulating cortisol and to have a negative relationship with the degree of intrusive symptoms. These findings seem to point to a potential role for cannabinoids in the treatment of PTSD. Jiang et al12 found in rat models that cannabinoids promote hippocampal neurogenesis and have anxiolytic and antidepressant effects. Given that individuals with PTSD are known to have smaller hippocampi than non-PTSD controls,13–15 Jiang and colleagues’ findings additionally suggest a potential role for cannabinoids in the treatment of PTSD.
The St Lawrence Valley Correctional and Treatment Centre (Secure Treatment Unit [STU]) is a hybrid mental health center and correctional center whose mandate is to treat seriously mentally ill adult male offenders serving a provincial sentence (<2 years). All patients routinely undergo a comprehensive psychiatric assessment at the time of admission, which includes screening for PTSD, sleep time, nightmares, and alcohol and substance use. Patients also have weekly psychiatric sessions throughout their stay to monitor their symptoms. In addition, all patients also have a full medical assessment and physical examination at the time of admission by a family physician with regular follow-up throughout their stay for identified problems. In the course of their clinical work, the investigators noted a high prevalence of concurrent disorders in the STU population, including those with trauma disorders, chronic pain, and cannabis dependence. Of these, many were found to be reluctant to give up cannabis because of its purported benefits for anxiety, insomnia, nightmares, and chronic pain, although they were otherwise motivated to give up alcohol and other illicit drugs. Studies in other correctional populations show a similar high prevalence of PTSD, PTSD-related sleep problems, chronic pain, and cannabis misuse,16–20 so it is likely that the challenge of managing these complex patients is relatively common.
Based on the good evidence for its effectiveness in chronic pain, some evidence in PTSD insomnia and nightmares, and suggestions of its possible role in harm reduction, nabilone has been used for its off-label indications in the STU for the past 4 to 5 years (always with informed consent). Other cannabinoids were not used as they are not covered by the provincial drug plan, and nabilone has the advantage of not giving a positive urine enzyme multiplied immunoassay test for cannabis; thus, use of illicit cannabis can easily be identified. This study aimed to review the use of nabilone at the STU, including its indications, dosing, efficacy, adverse effects, and abuse. The overall objective is to add to the evidence base with regard to these off-label indications, which if effective could allow a number of comorbid conditions to be targeted by a single medication, thereby reducing polypharmacy and costs and improving medication compliance and outcomes. Its use might also reduce reliance on other medications with more serious potential for adverse effects (eg, antipsychotic-associated metabolic syndrome) or abuse (eg, benzodiazepines, opioids).
OBJECTIVES
(1) To determine the indications for which nabilone is being used in the STU
(2) To review the dosing being used for nabilone in the STU population
(3) To assess if nabilone powder mixed in water demonstrates comparable efficacy to whole capsules reported elsewhere in the literature
(4) To determine the efficacy of nabilone in seriously mentally ill adult male offenders for various off-label indications, including PTSD-related insomnia and nightmares and chronic pain
(5) To know if nabilone allows for other psychotropic, analgesic, or other medications to be stopped
(6) To gain insight into the understanding of adverse effects of nabilone in seriously mentally ill adult male offenders
(7) To gain insight into the abuse potential of nabilone within a correctional environment
(8) To know the reasons why nabilone trials are sometimes abandoned
MATERIALS AND METHODS
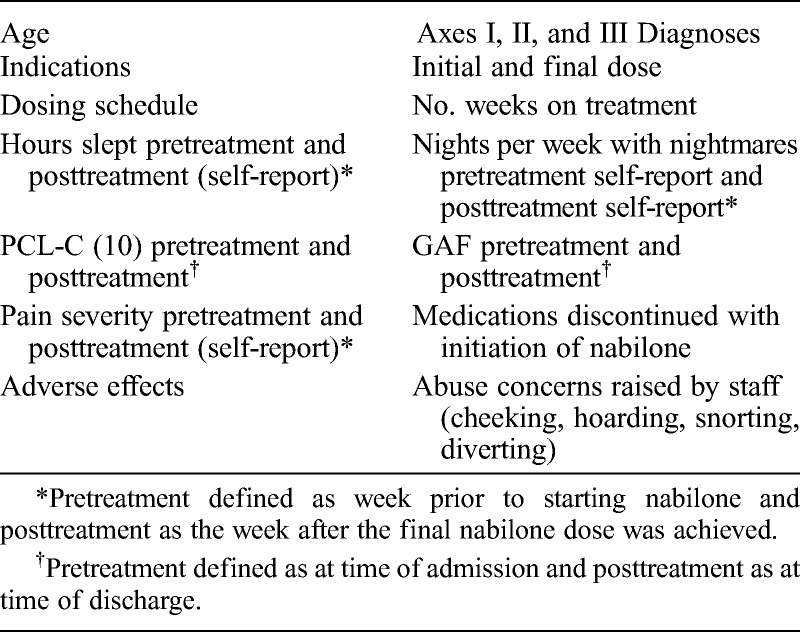
This study involves a retrospective chart review of all STU patients who were prescribed a single dose or more of nabilone for any indication from January 1, 2010, to July 31, 2013 (n = 104), as identified by the institutional pharmacy. Of note is that to minimize the risk of abuse and diversion, all patients in our facility who receive nabilone are given it only in powder form with water. Table 1 is a list of the data elements collected.
TABLE 1.
Data Elements Collected
Repeated measure t Tests were used to compare pretreatment and posttreatment sleep hours per night, nights with nightmares per week, Posttraumatic Checklist–Civilian version (PCL-C),21 and Global Assessment of Functioning (GAF) (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [DSM-IV-TR], 2000). The independent variable was time (pre/post), and the dependent/experimental variables consisted of mean number of hours slept per night over the target week (sleep), number of nights during the week that the participant experienced nightmares (nightmares), PCL-C, and GAF. For sleep and nightmares, pretreatment is defined as the week before nabilone initiation and posttreatment as the week after the final dose of nabilone was achieved. For PCL-C and GAF, pretreatment is defined as at the time of admission and posttreatment as at the time of discharge. Participants with missing data were removed from the analysis. Because of the retrospective design of the study, a post hoc power analysis was used. It was assumed a moderate effect size (0.5) would be expected based on previous research.7 Given an α error probability of 0.05 with a 2-tailed distribution and a sample size of 104, the critical t value was set at 1.98. Therefore, the t value must be above 1.98 to indicate a significant difference between 2 means with 95% certainty (confidence interval = 0.95). Furthermore, with a moderate effect size (Cohen d = 0.5), power calculations suggest that a total sample size of 54 would be necessary to reveal any significant effects for the current study, and data from the 104 cases had at least this sample size for all data elements analyzed.
RESULTS
Population Profile
One hundred four men were identified as receiving nabilone at some point in their stay during the study period. None of these, however, were on nabilone at the time of their admission, so all cases identified were prescribed nabilone de novo. The mean age was 32.7 years (range, 19–55 years). Subjects were diagnosed clinically using DSM-IV-TR (2000) and were found to have a mean of 4.1 (SD, 1.6) Axis I disorders, including 95.2% with anxiety disorders (n = 99), 95.2% with either alcohol or substance use disorders (n = 99), 67.3% with mood disorders (n = 70), and 12.5% with psychotic disorders (n = 13) (Fig. 1). It should be noted that a maximum of 1 diagnosis was given for alcohol and substance use disorder even if the subject used multiple substances. Of note, fully 91% (n = 95) met the criteria for cannabis dependence prior to their admission to the facility. Of the 59 with alcohol use disorders, only 1 had an alcohol use disorder independent of another substance use disorder. Subjects were also found to have a mean of 1.3 (SD, 1.8) Axis II disorders, including 75.0% (n = 78) with antisocial personality disorder, 19.2% (n = 20) with borderline personality disorder, and 9.6% (n = 10) with intellectual disabilities.
FIGURE 1.
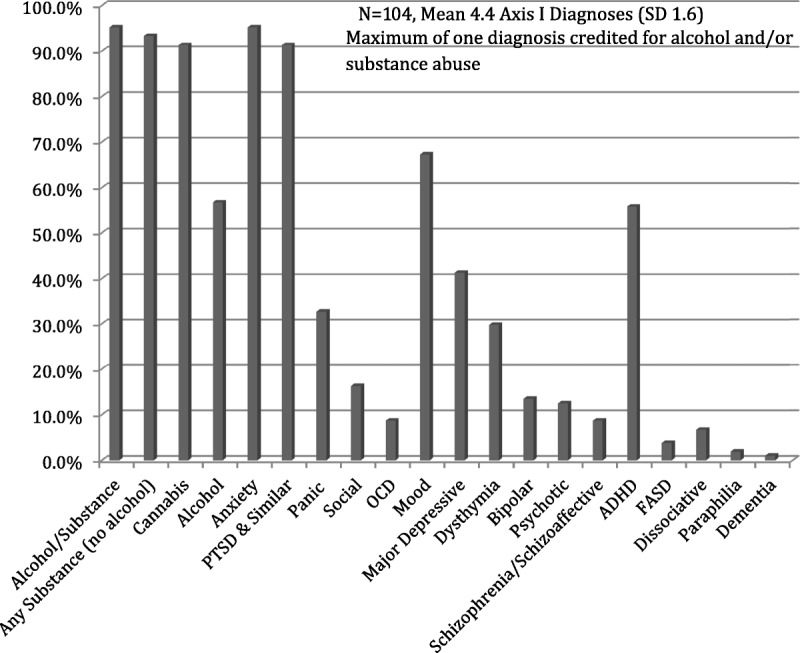
DSM-IV-TR Axis I diagnoses.
Axis III diagnoses included musculoskeletal pain, 56.7% (n = 59); neuropathic pain, 12.5% (n = 13); acquired brain injury, 27.9% (n = 29); posttraumatic headaches, 12.5% (n = 13); migraines, 8.7% (n = 9); seizure disorder, 4.8% (n = 5); hepatitis C, 28.8% (n = 30); metabolic syndrome, 18.3% (n = 19); diabetes mellitus, 4.8% (n = 5); gastrointestinal disorders, 14.4% (n = 15); asthma, 11.5%; (n = 12); hypertension, 3.8% (n = 4); and cardiac disease, 1.9% (n = 2).
Indications for Nabilone
The mean number of indications for nabilone was 3.5 (SD, 0.8) indications per patient, with the most common being insomnia (n = 101, 97.1%), nightmares (n = 90, 86.5%), and chronic pain (n = 68, 65.4%) (Fig. 2). Only 4 (3.8%) took nabilone as an antiemetic, but none received it for the on-label indication of chemotherapy-induced nausea. Five (4.8%) took it for anorexia, whereas 4 (3.8%) took for other indications, which included tinnitus, abdominal cramps, and anxiety. Harm reduction as a secondary indication was noted in the overwhelming majority (n = 95, 91.3%).
FIGURE 2.
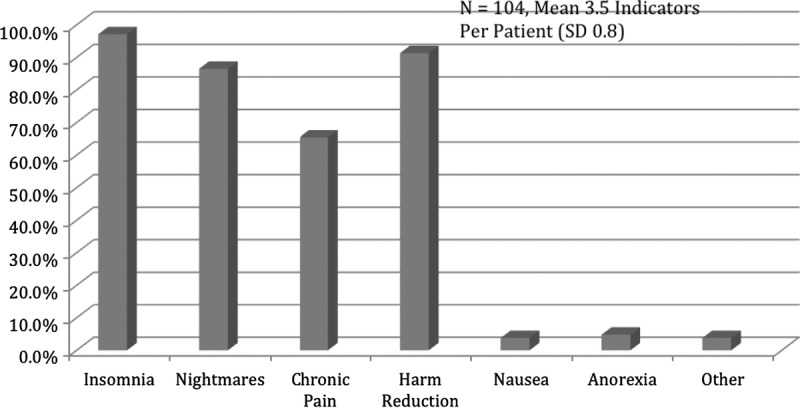
Indications for nabilone (all off-label).
Dosing and Schedule
The mean initial dose of nabilone was 1.4 mg daily (range, 0.5–2.0 mg), whereas the mean final dose was 4.0 mg (range, 0.5–6.0 mg). The mean length on nabilone was 11.2 weeks (range, 1 day to 36 weeks) for a total of 1229.9 weeks of nabilone treatment for the entire cohort. Of the 17 on nabilone for 20 weeks or more, the mean final dose was 4.6 versus 3.6 mg for those on it for 8 to 12 weeks. Patients with hepatitis C had a mean final dose of 4.2 mg, whereas cannabis-naive individuals had a mean final dose of 3.2 mg. Seventeen (16.3%) were prescribed divided doses, all of whom were taking nabilone for indications in addition to insomnia and nightmares. Of those taking it for insomnia or nightmares (n = 101), 8 reported better results, taking it after supper rather than bedtime because of a reported delay in onset of action.
Outcomes
Insomnia, Nightmares, PTSD Symptoms, and GAF
All the pretreatment/posttreatment measures indicated a significant improvement. Subjects (n = 101) reported a significant increase in average number of hours slept pretreatment (mean, 5.0 [SD, 1.4]) and posttreatment (mean, 7.2 [SD, 1.2]); t99 = 13.7, P < 0.001 (Fig. 3). Subjects (n = 90) also reported a significant reduction in the number of nights that they had nightmares per week from pretreatment (mean, 5.2 [SD, 2.2]) and posttreatment (mean, 0.9; SD, 1.8); t90 = 17.9, P < 0.001 (Fig. 3). Of note is that the improvements in sleep time, sleep quality, and nightmares were typically seen within the first 1 to 2 weeks of treatment and were maintained for the balance of the trial.
FIGURE 3.
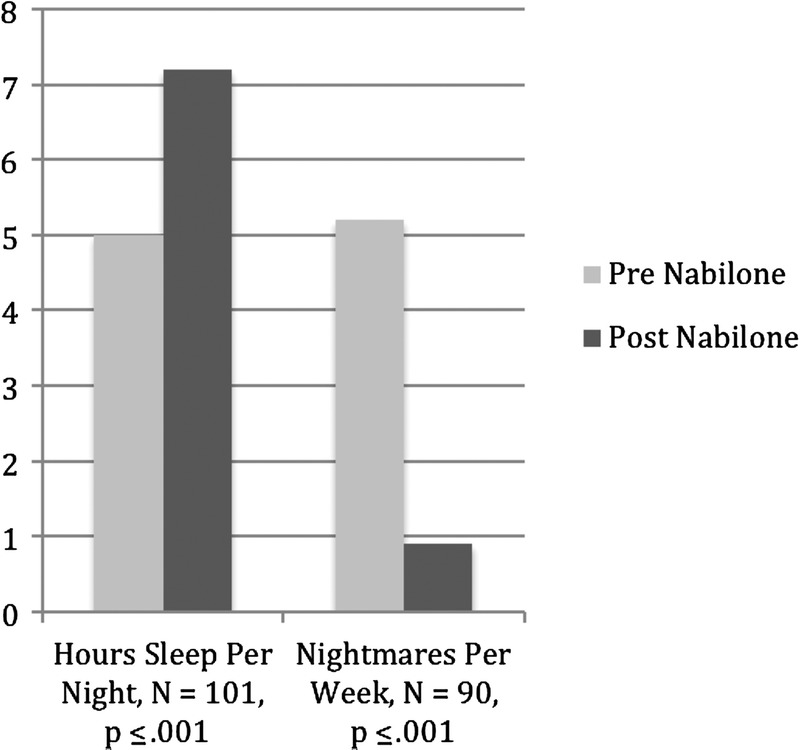
Sleep effects. Hours sleep per night = mean number of hours/night, nightmares per week = nights with nightmares/week. Pre nabilone = week prior to starting. Post nabilone = week after final dose was achieved.
The PCL-C scores (n = 58) decreased significantly (pretreatment: mean, 54.7 [SD, 13.0]; posttreatment: mean, 38.8 [SD, 7.1]; t57 = 10.2, P = 0.001) (Fig. 5) and were consistent with a change from moderate PTSD symptoms to borderline-mild symptoms.
FIGURE 5.
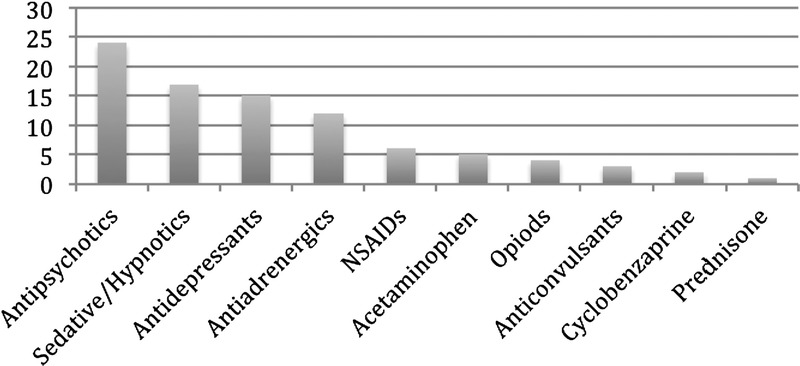
Number of medications discontinued.
Finally, GAF scores (n = 103) also increased significantly from serious to moderate impairments in functioning (pretreatment: mean, 45.0 [SD, 6.9]; posttreatment: mean, 58.2 [SD, 8.4]; t100 = 16.9, P = .001) (Fig. 4).
FIGURE 4.
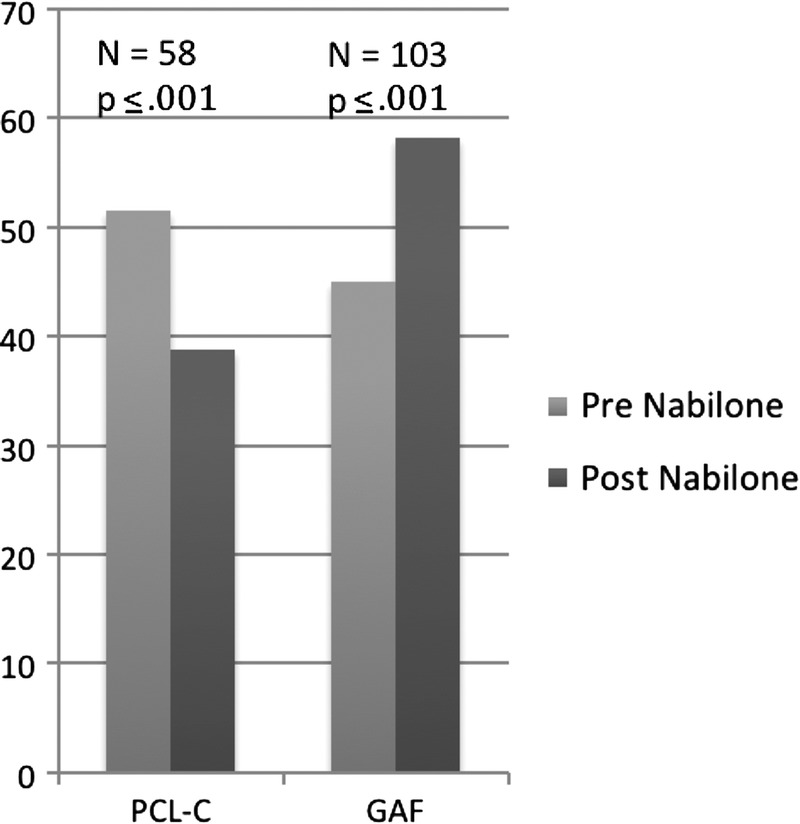
Posttraumatic Checklist–Civilian version and GAF Scores. Pre nabilone = time of admission. Post nabilone = time of discharge.
Chronic Pain
Of the 68 who took nabilone for chronic pain, 61 (89.6%) reported a subjective improvement in their pain, typically within the first 1 to 2 weeks of treatment, and this was largely maintained for the balance of the trial. The improvement reported was seen regardless of whether the pain was of neuropathic, musculoskeletal, or other origin.
Harm Reduction
Not a single subject while on nabilone had a positive urine test for cannabis during their incarceration. It was not possible, however, to draw conclusions from this given the relative lack of access to illicit cannabis in the STU where cannabis abuse has not historically been noted to be a problem.
Polypharmacy Concerns
Ninety medications were discontinued with the initiation of nabilone (mean of 0.87 drugs per patient) as they had been found to be of limited efficacy, and nabilone was deemed to adequately target the same symptoms as the drugs which were discontinued. Many of the medications stopped were felt to have a greater risk of serious adverse effects or abuse (antipsychotics, sedative hypnotics, opioids). The most frequent class of medication stopped was antipsychotics (being used off-label for sleep) with their associated risk of obesity and metabolic and other concerns. Sedative hypnotics (benzodiazepines and cyclopyrrolones), with their high risk for tolerance, dependence, and abuse, were next in number to be stopped followed by antidepressants (used off-label for insomnia or pain), antiadrenergics (prazosin, clonidine, and propranolol) (used off-label for nightmares or somatic anxiety), nonsteroidal anti-inflammatory drugs, acetaminophen, opioids (methadone and codeine), anticonvulsants (gabapentin, divalproex) (used for neuropathic pain), and prednisone (for inflammatory bowel disease) (Fig. 5). No subjects were noted to require additional new medications as a result of receiving nabilone, although 1 subject with schizoaffective disorder did require an increased dose of his antipsychotic because of breakthrough psychotic symptoms.
Adverse Effects, Reasons for Termination, and Abuse Concerns
Thirty-one subjects (29.8%) reported adverse effects, of which 10 (9.6%) chose to abandon the trial. Psychosis was the most serious adverse effect, but occurred in only 2 patients (1.9%); both of which had a preexisting psychotic illness. One of these was later resumed on nabilone without any recurrence of psychotic symptoms after his antipsychotic medication was adjusted. The 10 others in our cohort with a history of psychotic illness, all of whom were maintained on antipsychotic medication, responded well to nabilone without recurrence of psychotic symptoms or other adverse effects. The other adverse effects reported tended to be fairly minor (sedation, 12.5%; dry mouth, 6.7%; feeling “stoned,” 3.8%; orthostatic hypotension, 1.9%; agitation, 1.9%; and headache, 1.0%).
In 20 cases (19%), the nabilone trial was abandoned: 10 because of adverse effects, 4 because of abuse of other medications, 2 because patients were going to a treatment center that prohibited its use, 2 because patients preferred to go without, 1 because of lack of efficacy, and 1 because the patient had no drug coverage for it in his home province. Of the 10 subjects who stopped because of adverse effects, most (80%) did so in the first month (mean, 3.7 weeks) and were on a mean dose of 1.5 mg. Two of the 10 subjects were cannabis-naive before starting nabilone. Four (44%) of the 9 cannabis-naive patients experienced adverse effects versus 27 (28%) of 95 of cannabis-dependent individuals.
There was not a single report from staff about nabilone being cheeked, diverted, or snorted; thus, giving it as a powder in water seemed to effectively mitigate against this risk. It is unclear, however, if this would have been a problem had the nabilone been given in capsule form. It should be noted that 4 patients did have their nabilone discontinued because of abusing or diverting other drugs.
SUMMARY AND IMPLICATIONS
The findings of our retrospective chart review are consistent with the existing literature, namely, that nabilone holds promise as an agent in the treatment of PTSD-related insomnia and nightmares7 and is an effective treatment for chronic pain.5,6 It also may incidentally benefit as a harm reduction technique in cannabis-dependent individuals, although this study was not able to speak to this. It is particularly noteworthy that in virtually all subjects, nabilone was targeting a number of symptoms simultaneously, which may have helped to limit the risks associated with polypharmacy. The most common indications in the study population were PTSD-related insomnia and nightmares, chronic pain, and harm reduction. Adverse effects were for the most part minor and easy to manage, and when they did occur, tended to occur early within the first month of treatment. Adverse effects were also relatively more common in cannabis-naive individuals than in those with preexisting cannabis dependence and occurred at relatively low doses. This finding suggests that in cannabis-naive individuals the initial dose should be lower than the mean 1.4-mg starting dose in this study and titrated more slower than for those who are cannabis dependent. This study reinforced the importance of proceeding with caution when nabilone is given to individuals with a history of psychosis. The data also suggest that tolerance may be a factor in trials lasting over several months, and this is something that seems to merit further investigation. Also of note was that when given as a powder in water, there did not appear to be a loss of efficacy compared with what is reported in the literature for nabilone in capsule form. In addition, administering nabilone as a powder in water showed no risk of abuse and diversion.
Negative attitudes on the part of some professional staff both in the STU and the community were sometimes a problem. There were those who perceived the prescription of a cannabinoid agent as an implicit endorsement of marijuana use, and often this had to be addressed through education. It was evident, however, that there remains a lack of knowledge about the endocannabinoid system and the therapeutic potential for drugs acting on this.
Limitations
No doubt, this study has several limitations that prevent drawing firm conclusions, not the least of which are the retrospective design and lack of control group. Sleep, nightmares, and pain measures were less than ideal as they were entirely based on patient self-report and, in the case of pain, were quite crude (subjective report of improvement or not). In addition, all patients were receiving concurrent treatments with other psychotropic medication(s) and a range of individual and group psychotherapies, so it is not possible to exclude that these too may have contributed to the therapeutic benefits noted, especially with respect to the improvement in PCL-C and GAF scores, which were measured only on admission and discharge. That the therapeutic benefits for sleep hours, nightmares, and pain were typically dramatic and noted within 1 to 2 weeks of starting on the drug and maintained throughout the trial suggests, however, that the nabilone was in large measure responsible for these therapeutic benefits. The crudeness of the pain measure is likely mitigated, given the already strong evidence for nabilone’s effectiveness in chronic pain.5,6 Given the lack of illicit cannabis within the STU, the effectiveness for nabilone as a harm reduction approach was not possible to test.
Future Directions
Prospective, randomized controlled studies comparing nabilone to placebo and to prazosin for PTSD-related insomnia and nightmares seem to be reasonable next steps, as well as looking at its effect on other PTSD symptoms. In addition, a randomized placebo-controlled study looking at nabilone for harm reduction also seems warranted. Other agents acting on the endocannabinoid system also seem to merit investigation. If our hypothesis is correct that cannabinoids can be safely and effectively used to target comorbid PTSD-related insomnia and nightmares, chronic pain, and harm reduction in cannabis-dependent individuals, the challenge faced by clinicians working with these often complex patients may become greatly facilitated.
AUTHOR DISCLOSURE INFORMATION
The authors declare no conflicts of interest.
Footnotes
Ethics approval was provided by the Research and Ethics Board of the Royal Ottawa Health Care Group (2013).
No funding was received for this study.
REFERENCES
- 1. Earlywine M. Chapter 1: Highlights in the history of cannabis. In: Understanding Marijuana: A New Look at the Scientific Evidence. Oxford: Oxford University Press; 2002; 3– 28 [Google Scholar]
- 2. Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 2012; 109; 29– 30:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006; 105; 1– 2:1-25 [DOI] [PubMed] [Google Scholar]
- 4.Repchinsky C. Compendium of Pharmaceuticals & Specialities. Ottawa, Ontario, Canada: Canadian Pharmacists Association; 2013 [Google Scholar]
- 5. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain: a systematic review of randomized trials. Br J Clin Pharmacol. 2011; 72 (5): 735– 744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns TL, Ineck JR. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann Pharmacother. 2006; 40 (2): 251– 260 [DOI] [PubMed] [Google Scholar]
- 7. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009; 15 (1): 84– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lile JA, Kelly TH, Hays LR. Substitution profile of the cannabinoid agonist nabilone in human subjects discriminating delta-9-tertrahydrocannabinol. Clin Neuropharmacol. 2012; 33 (5): 235– 242 [DOI] [PubMed] [Google Scholar]
- 9. Ware MA, St Armand E. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010; 105 (3): 494– 503 [DOI] [PubMed] [Google Scholar]
- 10. Svízenská I, Dubový P, Sulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures–a short review. Pharmacol Biochem Behav. 2008; 90 (4): 501– 511 [DOI] [PubMed] [Google Scholar]
- 11. Hill MN, Bierer LM, Makotkine I, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013; 38 (12): 2952– 2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang W, Zhang Y, Xiao L, et al. Cannabinoids promote embryonic and adult hippocampal neurogenesis and produce anxiolytic and antidepressant-like effect. J Clin Invest. 2005; 115 (11): 3104– 3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bremner JD, Randall P, Scott TM, et al. MRI-based measurements of hippocampal volume in combat-related post-traumatic stress disorder. Am J Psychiatry. 1995; 152: 973– 978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002; 5: 1242– 1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related post-traumatic stress disorder. Biol Psychiatry. 1996; 40: 1091– 1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goff A, Rose E, Rose S, et al. Does PTSD occur in sentenced prison populations? A systematic review. Crim Behav Ment Health. 2007; 17: 152– 162 [DOI] [PubMed] [Google Scholar]
- 17. Maher MJ, Rigo SA, Asnis GM. Sleep disturbances in patients with posttraumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006; 20 (7): 567– 590 [DOI] [PubMed] [Google Scholar]
- 18. Fazel S, Bains P, Doll H. Substance abuse and dependence in prisoners: a systematic review. Addiction. 2006; 101 (2): 181– 191 [DOI] [PubMed] [Google Scholar]
- 19. Pacella ML, Hruska B, Delahanty DL. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J Anxiety Disord. 2012; 13 (27): 33– 46 [DOI] [PubMed] [Google Scholar]
- 20. Swartz JA. Chronic medical conditions among jail detainees in residential psychiatric treatment: a latent class analysis. J Urban Health. 2011; 88 (4): 700– 717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weathers FW, Litz BT, Huska JA, et al. Posttraumatic Checklist Civilian Version (PCL-C). National Centre for PTSD—Behavioural Science Division. 1994. Available at: http://www.mirecc.va.gov/docs/visn6/3_PTSD_CheckList_and_Scoring.pdf. Accessed February 22, 2014


