Abstract
On February 2, 2012, the National Cancer Institute (NCI) sponsored a 2-day workshop with the NCI Thoracic Malignancies Steering Committee and the Food and Drug Administration to bring together leading academicians, clinicians, industry and government representatives to identify challenges and potential solutions in the clinical development of novel targeted therapies for lung cancer. Measures of success are rapidly evolving from a scientific and regulatory perspective and the objectives of this workshop were to achieve initial consensus on a high priority biomarker-driven clinical trial designed to rapidly assess the activity of targeted agents in molecularly defined lung cancer subsets and to facilitate generation of data leading to approval of these new therapies. Additionally, the meeting focused on identification of the barriers to conduct such a trial and the development of strategies to overcome those barriers. The “Lung Master Protocols” recently launched by NCI were the direct outcome of this workshop.
Keywords: NCI/FDA workshop leading to the Inception of "Master Protocols" in Lung Cancer
Despite rapid progress in identifying molecular abnormalities driving lung cancer, this has not yet been translated into improvement in long-term outcome for patients.1 Treatment for non–small-cell lung cancer (NSCLC) that comprises about 80% of the lung cancer cases is shifting toward regimens targeting relatively uncommon molecular alterations rather than traditional histologic subtypes.2 This new complexity raises patient selection, accrual and funding challenges, so new more efficient approaches are essential.3
The predominance of negative Phase III trials4,5 conducted in unselected patient populations and the recent findings that the majority of NSCLC patients’ tumors harbor molecular “drivers” are changing the paradigm for rapid clinical development of new therapies.6 By incorporating potential biomarkers and assessing pharmacokinetic (PK)/pharmacodynamic (PD) effects, the next generation of early clinical trials can quickly establish agent activity with smaller sample sizes resulting in rapid and cost-effective biomarker-driven development.
While the potential utility of “companion” diagnostics opens the door to optimal patient selection,7 rapid development is hindered by intratumoral heterogeneity, low frequency of responsible driver mutations, and the variety of tests available to assess each potential target (analyte).8 The limited amount of tissue available for diagnostic use and the high costs of individual tests mean that sequential individual tests are not likely to be feasible and that multiplexed assays or next-generation DNA sequencing may be required.
TRIAL DESIGN ISSUES
A biomarker used in a clinical trial can be prognostic or predictive, and it is important to distinguish between these two types of biomarkers. A prognostic biomarker is associated with how well patients will do with a given treatment. A predictive biomarker predicts what therapy will work best for an individual patient. Predictive biomarkers tend to be more useful because they can improve outcomes for patients by optimizing choice of treatment. For a single predictive biomarker, there are various trial designs that can be considered.6,9–11 The appropriate trial design and analysis strategy should depend on the preexisting evidence that the biomarker can successfully identify patients who will benefit from the treatment.12 If one is confident that the targeted therapy will not work in the biomarker-negative population, then an enrichment design (also known as a targeted design or a marker-positive design), which only enrolls and randomizes patients who are positive for the biomarker can be used. For example, crizotinib was tested versus standard chemotherapy in NSCLC patients with alteration in anaplastic lymphoma kinase (ALK) gene.13 If one is less confident about the biomarker’s predictive ability, then a biomarker-stratified design (also known as an all-comers design) in which all patients are randomized can be used. With this design, the treatment effect can be assessed in both the biomarker-positive and biomarker-negative patient populations. For example, the trial BR.21 examined epidermal growth factor receptor (EGFR) expression status (in the patients for whom tumor tissue was available) and the effectiveness of erlotinib in NSCLC.14 One can also examine the effect of treatment overall not taking into account the biomarker in the all-comers design; this is a traditional randomized trial design. A third design, the biomarker strategy design, randomizes patients between a control treatment and a treatment that is chosen for each patient on the basis of his or her biomarker status. An example is the trial conducted by the Spanish Lung Cancer Group in which NSCLC patients were randomized between a control arm of docetaxel/cisplatin and an experimental arm of either docetaxel/cisplatin or docetaxel/gemcitabine based on their ERCC1 mRNA levels.15 The biomarker strategy design can be inefficient because many patients on both treatment arms may receive the same treatment; it is generally not recommended unless all (or almost all) the patients on the experimental treatment arm will receive many different treatments that are not the same as the control-arm treatment.
When there is more than one biomarker or more than one targeted therapy of interest, the trial designs and analyses can become more complicated. For example, one can combine an enrichment design with a biomarker-stratified design such as in the TAILOR trial.16 With multiple possible biomarkers (or high-dimensional genomic data), cross-validation in which combinations of markers are developed on subsets of the data and their predictive ability tested on other subsets of the data can be useful to guard against overfitting the data.17 When there are multiple targeted treatments, for example, in the BATTLE-1 trial18 and the I-SPY2 trial,19 the analyses of the trials can be considered exploratory. For a study with the potential to lead to agent registration, it is critical to have a matched control arm for each substudy to permit comparative assessment of efficacy and toxicity. It is desirable to have interim monitoring and analysis plans incorporated in clinical trials so that treatment arms that are not promising can be dropped early. Outcome-adaptive randomization is a different design strategy that aims to put more patients on better performing treatment arms. However, it is complex to implement and requires a meaningful short-term clinical end point and a fast turnaround for biomarker assays. Potential disadvantages of outcome-adaptive randomization designs are that they may produce biased results if the prognostic characteristics of patients entering the trial change over the period of accrual, and they may lead to a larger total sample size than simpler interim monitoring methods, potentially resulting in a greater absolute number of patients having bad outcomes.20,21
Regardless of the incorporation of a biomarker, trial designs need end points relevant to the questions being asked. Traditionally, the efficacy end point of trials leading to Food and Drug Administration (FDA) approval of therapies in lung cancer has been overall survival (OS), but progression-free survival (PFS) is gaining importance as a primary end point. The growing popularity of crossover designs and the increasing availability of multiple lines of therapy have largely undermined OS as a sole definitive end point. Nonetheless, assessment of OS is still critical even when there are effective subsequent therapies.22
PFS results should be statistically significant and clinically meaningful, and ideally should be associated with an improvement in patient reported outcomes and Quality of Life. What is clinically meaningful to patients will depend on the clinical stage of their cancer and the toxicity of the drug. For example, a patient with early stage lung cancer receiving adjuvant therapy with intention to achieve a cure may be willing to accept a higher level of toxicity than a patient who is receiving the therapy in a metastatic setting where cure is not possible. There are no FDA-approved patient reported outcomes and Quality of Life tools for lung cancer and the development and validation of these tools is urgently needed.
REGULATORY CHALLENGES
Many regulatory challenges have arisen as a result of increased knowledge of the molecular aberrations in lung cancer and subsequent development of novel agents to target these abnormalities. There has been a tendency by drug developers and investigators toward overly strict interpretation of the regulations and a general reluctance to change current practice for fear of adverse regulatory consequences. The trials submitted sometimes require excessive data collection and may be poorly designed (e.g., too complicated or too many objectives). Earlier and more continuous engagement between regulatory authorities and pharmaceutical and diagnostic industry partners are crucial for success in this rapidly developing field.
FDA regulations state that approval should be based on two adequate and well-controlled studies. A single study may be acceptable to the FDA if it is a large multicenter study where one or a few institutions did not contribute a disproportionate number of patients, it has consistency among various subsets, and it has multiple end points evaluated such as response rate, PFS, and OS such that the findings are robust with clinically and statistically persuasive outcomes.
An optimal drug/device co-development plan requires simultaneous development of biomarker assay methodology and clinical trials to determine drug efficacy. Clinical data for biomarker development can come from retrospective or prospective clinical trials.23 Retrospective trial analysis requires that adequate biomarker specimens were collected in most of the study patients and analyzed according to a prespecified plan.24 The advantages of retrospective analysis include availability of adequate follow-up, the inclusion of patients with biomarker-positive and biomarker-negative cancers in the data set, and a cost savings over conducting an additional prospective trial. Disadvantages include multiplicity issues in biomarker selection and, depending on assay requirements, the possibility of inadequate specimen collection, handling, or storage. In addition, a confirmatory trial will still likely be needed as retrospective trial data are often incomplete.
In contrast to retrospective analyses, a prospective trial requires early development of an in vitro diagnostic to identify the presence or absence of a specific predictive biomarker. All study patients will have known biomarker status before randomization and biomarker status can be used as a stratification variable. Both biomarker-positive and biomarker-negative patients should be enrolled if it is desired to determine whether the biomarker and its assay are truly predictive.
Ideally, the tissue sample should be obtained as close to the time of treatment as possible because a patient’s cancer may develop new competitive advantage and/or resistance mutations over time. However, this may not be always possible due to the morbidities and the cost associated with biopsies. As might be expected, tissue often runs out before all the planned later assays can be performed and may require biomarker prioritization.
Pharmaceutical industry representatives at the meeting discussed their challenges. These include the global burden of developing drugs, establishing unmet medical need in a newly defined molecular group, differences in the evidence required for US and EU accelerated/conditional approval, and the costs of companion diagnostic development. In addition, when there is preliminary evidence of promising efficacy, not allowing crossover has been found to be unacceptable to investigators and patients.
DRUG AND BIOMARKER CO-DEVELOPMENT IN LUNG CANCER
Biomarker-targeted drug development presents opportunities for “personalized medicine,” although clinical trial design is complicated by the need to address simultaneously both diagnostics and treatment. Well-planned development and evaluation of the diagnostic device is essential to understanding its value in guiding use of the therapeutic product. Clinicians will rely on this test information to help make critical treatment decisions.
A clinical assay is a system that includes both preanalytical treatment of the specimen (i.e., how the specimen is obtained, stored, shipped, and preprocessed for the assay) and conduct of the assay itself, which includes monitoring for quality and reproducibility and the methods for data capture, assessment, and reporting. A clinical assay must be demonstrated to perform reproducibly in the intended clinical environment.
Biomarker testing should be standardized and reproducible, should have a quick enough turnaround time to be useful for clinical decision-making, should be cost-effective, and ideally should involve little risk to patients.
Concerns regarding the availability of tissue needed to test for multiple markers and the time lost to treat patients while testing different markers are being addressed by the current effort to develop multiplex assays that interrogate multiple signaling pathways. Approval of these assays by the FDA will require proper validation.25,26
LESSONS LEARNED: PERSPECTIVES MOVING FORWARD
Bevacizumab was approved by the FDA for the first-line therapy of advanced nonsquamous NSCLC based on a modest survival benefit noted in a Phase III study E4599.27 However, several other agents that target the angiogenic pathway have not demonstrated therapeutic benefit in NSCLC. The main problem has been the lack of a predictive biomarker that could identify patients who stand to benefit. None of the biomarkers tested to date have consistently demonstrated predictive potential. The panel concluded that in the absence of a promising biomarker, conducting further large trials with anti-angiogenic agents in NSCLC is not warranted.
The development of EGFR inhibitors represents a remarkable tale of drug development followed by identification of a predictive biomarker.28–33 As new EGFR inhibitors are developed, the main areas of need are to improve upon the efficacy of existing agents, to delay the emergence of resistance, and to overcome resistance. It will be important to obtain tumor tissues in clinical trials of EGFR inhibitors to conduct biomarker studies that will enhance our knowledge regarding resistance pathways. As the importance of developing biomarkers is clear, physicians should be open to obtaining a tumor biopsy at various time points during the course of the treatment of lung cancer. The BATTLE study showed that such an approach could aid in development of individualized treatment approaches. There is a need to adopt standard practices for specimen preparation and storage in order to obtain maximal yield from the biopsy.
Increasingly, clinical trials require archived tumor specimens and in some instances, fresh frozen tumor tissue, as part of the evaluation of new agents. This represents a challenge to trial patient accrual through community oncology practices. In addition to logistical challenges, such procedures are often not compensated adequately by the study sponsor or payers. Standardized specimen acquisition and storage practices are also necessary to ensure submission of high-quality specimens from the community setting. The panel agreed that community sites would greatly benefit from guidance on these important issues in order to increase the proportion of patients enrolled to lung cancer trials.
As clinical trials move toward patient selection based on predictive biomarkers, patients with cancers negative for these markers are often excluded. This causes anxiety and disappointment for patients who are unable to participate in clinical trials of exciting new agents. It will be important to allow for testing promising drugs in biomarker-negative patients, provided there is an appropriate scientific rationale. Another possibility is to include patients without the biomarker in a concurrent trial of a non-targeted agent. This will enhance patient enthusiasm about participation in such clinical trials.
DEVELOPMENT OF FUTURE LUNG CANCER TRIALS
A number of controversies remain including how to prioritize treatment arms, when there are multiple, targeted agents, and how to select a combination of two targeted agents versus the addition of targeted agents to conventional therapy such as cytotoxic chemotherapy and/or radiotherapy.
The panel agreed that future proposals should result in a paradigm shift, be biologically based with innovative designs and that patients should be selected based upon molecular characteristics if progress in NSCLC is to occur. There was general agreement regarding the desire to integrate validated biomarkers into future clinical trial designs, to include assessment of toxicity in conjunction with pharmacogenomics, to validate imaging as an early indicator of treatment effectiveness, and to assess the utility of serial biopsies whenever possible, particularly at the time of clinical or radiographic progression.
The panel was asked to consider three overriding questions when evaluating a proposal presented: (1) Is the proposal novel and feasible? (2) Are the study end points indicative of adequate clinical benefit and are the statistical underpinnings of the trial robust? (3) Is there adequate evidence to support the use of a predictive biomarker and are the selected assays appropriately validated?
Concepts presented included first-in-man targets to be studied with response rate as end point, combination chemo/radiation trials for locally advanced lung cancers, and target-based adjuvant trials. An adaptive trial for patients with regionally advanced NSCLC was discussed by RTOG. The proposal would test chemoradiation following the appropriate molecular-targeted agent (EGFR mutated or ALK-positive patients).
Two adjuvant trials (stages I–III) were discussed, one proposed by ECOG (Now ECOG-ACRIN) in patients with ALK gene rearrangements to study crizotinib with an end point of disease-free survival (DFS) and a similar trial proposed by CALGB (now Alliance) in patients with EGFR mutations to study erlotinib with an end point of OS. It was noted that a large number of patients would need to be screened, but only about 5% to 15% of screened patients would be enrolled in each trial making upwards of 85% of the screened specimens irrelevant. A call was made by the attendees to combine the efforts to employ a common screening step with central testing and the provision to add study arms in future. The outcome of this discussion is the current design of ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial).
The ALCHEMIST trial will enroll patients with resectable (stage IB ≥ 4 cm, II and IIIA) adenocarcinoma of the lung, with OS as the primary end point (Figure 1).
FIGURE 1.
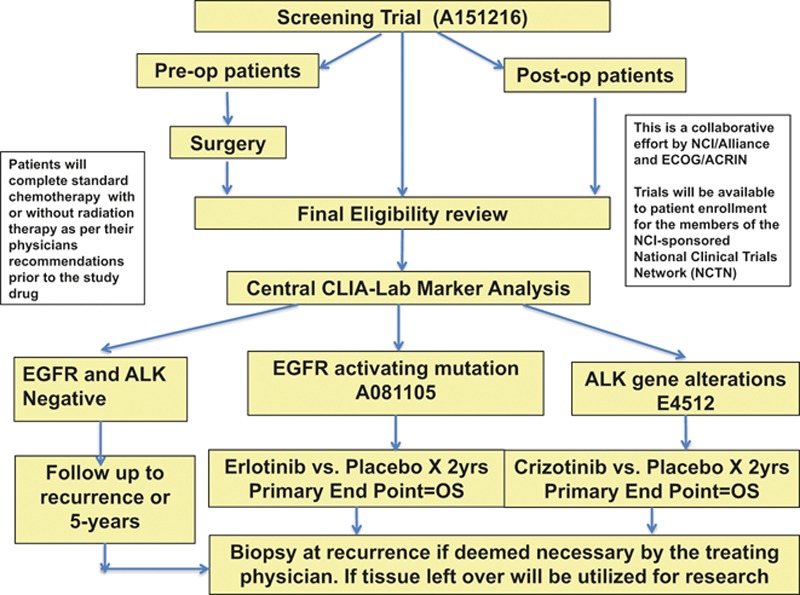
ALCHEMIST study schema. ALCHEMIST—A151216, screening trial; ALCHEMIST—EGFR A081105, erlotinib treatment arm; ALCHEMIST—ALK E4512, crizotinib treatment arm.
In an umbrella screening protocol A151216, patients’ tumors will be screened for EGFR mutations using a polymerase chain reaction-based assay and ALK fusion alterations using the FDA-approved break-apart fluorescence in situ hybridization assay. If genetic alterations are detected in their tumors, these patients will be eligible for separate adjuvant studies A081105 comparing the addition of erlotinib (for activating EGFR mutations) or E4512 adding crizotinib (for ALK fusions) after standard treatment versus standard treatment and placebo. Patients will receive protocol therapy for up to 2 years unless development of unresolved toxicities, patient withdrawal or physician decides to stop the therapy in the best interest of the patient. In order to enroll 410 patients in two arms of erlotinib versus placebo and 300 in crizotinib versus Placebo, it is estimated that about6000 to 8000 patients will be needed to enroll in screening protocol. All patients, including those who discontinue protocol therapy early, will be followed for survival for 10 years from the date of registration.
Patients whose tumors lack these alterations will enroll in a registry study and their tumors will undergo whole-exome sequencing. If they should relapse, every attempt will be made to re-sequence their tumor DNA at that time to determine the natural genomic history of these cancers.
To accelerate development of biomarker-driven trials, attendees agreed that it is critical to enhance coordination between pharmaceutical developers, FDA, academic and community-based clinical investigators, National Cancer Institute (NCI), and patients with the intention to decrease red tape, reduce competition, and enhance cooperation across both commercial and academic clinical trials organizations. I-SPY was cited as an example of such collaborative effort currently ongoing in patients with breast cancer. There was a consensus to reward participation and teamwork and to create a mechanism to eliminate competition among investigators.
In conclusion, the development of “Master Protocols” for different stages of lung cancer (adjuvant, neoadjuvant, stage III, advanced/metastatic) was identified as the most expeditious way to cut down the time and effort needed to test new agents. Extensive collaboration between the FDA, NCI, academia, community oncology programs, and the pharmaceutical industry is the key to developing such complex trials but the effort could be rewarded with more scientifically relevant trials that are attractive to patients and thus lead to more rapidly completed trials. This approach could revolutionize lung cancer treatment by bringing forward new drugs much more rapidly for approval and eventually leading to improved outcomes for patients.
The NCI “Master Protocol” Phase II/III trial for second-line treatment of squamous lung cancer, the Lung-MAP trial, is attempting such a novel approach. In this trial, patients will be screened for sets of specific molecular abnormalities identified by a combination of a customized next-generation DNA sequencing mutation panel and immunohistochemistry assays. For each set of abnormalities corresponding to targeted pathway, patients will be randomly assigned to new targeted therapy or standard therapy based on designated therapeutic biomarker–drug combinations for each biomarker-driven substudy. Patients whose tumors lack these specific abnormalities will be assigned to a “non-match” arm and will be randomly assigned to an immunotherapy agent versus standard chemotherapy. Each substudy will function autonomously and will open and close independently of the other substudies. The candidate drugs must have demonstrated biologic activity against the target associated with a proposed predictive biomarker(s). The primary objective within the Phase II component of each substudy is to evaluate whether there is sufficient evidence to continue to the Phase III component of the substudy by comparing PFS. Phase III components of the study has PFS as primary end point that has been prespecified to not only be statistically significant but also clinically meaningful (Figure 2).
FIGURE 2.
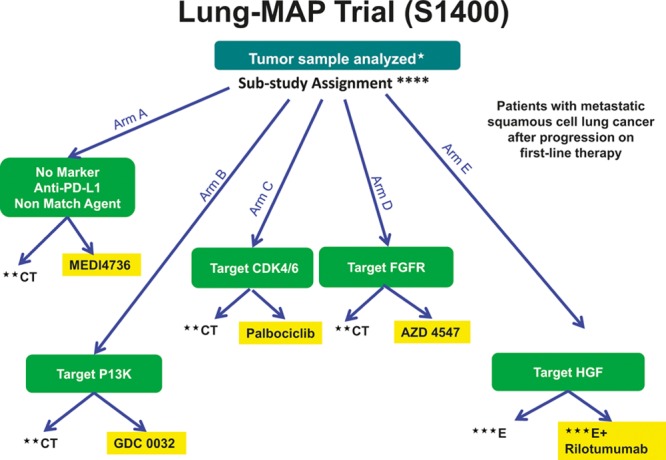
Phase II/III biomarker-driven master protocol for second-line therapy of squamous cell lung cancer (Lung-MAP Trial). *Archival formalin-fixed paraffin-embedded (FFPE) tumor, fresh core needle biopsy if needed. **CT = chemotherapy (docetaxel) control arm. ***E = erlotinib control arm. ****Substudy assignment will be determined based on randomization for patients eligible for multiple substudies.
The goal of this trial is to expeditiously provide evidence of agent efficacy to support FDA approval of new therapies in order to ultimately benefit patients.
ACKNOWLEDGMENTS
Alex Adjei, Robert Becker, Christopher Bowden, Jeffrey Bradley, Paul Bunn, David Carbone, Hak Choy, Martin Cohen, Barbara Conley, John Crowley, Gregory Curt, Angela DeMichele, David Gandara, Richard Gaynor, Giuseppe Giaccone, Ramaswamy Govindan, Stephanie Haney, Roy Herbst, Pasi Janne, Patricia Keegan, Kemp Kernstine, Fadlo Khuri, Mark Kris, Rogerio Lilenbaum, Tracy Lively, Sumithra Mandrekar, Gregory Masters, Lisa McShane, James Ranger-Moore, Anthony Murgo, Ken O’Byrne, William Pao, Edward Patz, Francesco Pignatti, Robert Pirker, Suresh Ramalingam, Ravi Salgia, Giorgio Scagliotti, Joan Schiller, Frances Shepherd, David Spigel, Rajeshwari Sridhara, Robert Sweetman, Ming Tsao, Regina Vidaver, Mickey Williams, Ignacio Wistuba, Xiaofei Wang, and James Yang.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Accessed April. 2014. [Google Scholar]
- 2.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med. 2012;18:349–351. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 3.Collier R. Rapidly rising clinical trial costs worry researchers. CMAJ. 2009;180:277–278. doi: 10.1503/cmaj.082041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 5.Laleh A-K, Tito F. Why do phase III clinical trials in oncology fail so often? J Natl Cancer Inst. 2012;104:568–569. doi: 10.1093/jnci/djs180. [DOI] [PubMed] [Google Scholar]
- 6.Hoering A, LeBlanc M, Crowley J. Randomized, phase III clinical trial designs for targeted agents. Clin Cancer Res. 2008;14:4358–4367. doi: 10.1158/1078-0432.CCR-08-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansfield E, O’Leary TJ, Gutman SI. Food and Drug Administration regulation of in vitro diagnostic devices. J Mol Diagn. 2005;7:2–7. doi: 10.1016/S1525-1578(10)60002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ransohoff DF, Gourlay ML. Sources of bias in specimens for research about molecular markers for cancer. J Clin Oncol. 2010;28:698–704. doi: 10.1200/JCO.2009.25.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J Clin Oncol. 2009;27:4027–4034. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers design issues. J Natl Cancer Inst. 2010;102:152–160. doi: 10.1093/jnci/djp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SL, Wang X. Targeted clinical trials. In: Harrington D, editor. Designs for Clinical Trials: Perspectives on Current Issues. Springer; 2012. pp. Pp. 157–177. [Google Scholar]
- 12.Freidlin B, Korn EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol. 2014;11:81–90. doi: 10.1038/nrclinonc.2013.218. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 15.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 16.Farina G, Longo F, Martelli O, et al. Rationale for treatment and study design of tailor: a randomized phase III trial of second-line erlotinib versus docetaxel in the treatment of patients affected by advanced non-small-cell lung cancer with the absence of epidermal growth factor receptor mutations. Clin Lung Cancer. 2011;12:138–141. doi: 10.1016/j.cllc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Freidlin B, Jiang W, Simon R. The cross-validated adaptive signature design. Clin Cancer Res. 2010;16:691–698. doi: 10.1158/1078-0432.CCR-09-1357. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: an adaptive breast cancer trial in the setting of neoadjuvant chemotherapy. Clin Pharm Therap. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 20.Korn EL, Freidlin B. Outcome-adaptive randomization: Is it useful? J Clin Oncol. 2011;29:771–776. doi: 10.1200/JCO.2010.31.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn EL, Freidlin B. On the usefulness of outcome-adaptive randomization. J Clin Oncol. 2011;29:e393. doi: 10.1200/JCO.2010.31.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn EL, Freidlin B, Abrams JS. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol. 2011;29:2439–2442. doi: 10.1200/JCO.2011.34.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polley MY, Freidlin B, Korn EL, Conley BA, Abrams JS, McShane LM. Statistical and practical considerations for clinical evaluation of predictive biomarkers. J Natl Cancer Inst. 2013;105:1677–1683. doi: 10.1093/jnci/djt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansfield EA. FDA perspective on companion diagnostics: an evolving paradigm. Clin Cancer Res. 2014;20:1453–1457. doi: 10.1158/1078-0432.CCR-13-1954. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. Draft guidance for industry and food and drug administration staff. In vitro companion diagnostic devices. Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM262327.pdf. Accessed July 14, 2011. [Google Scholar]
- 27.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 28.Kris M G, Natale R B, Herbst R S, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA. 290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BE, Jänne PA. Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res. 2005;65:7525–7529. doi: 10.1158/0008-5472.CAN-05-1257. [DOI] [PubMed] [Google Scholar]
- 31.Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib and erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 32.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 33.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG- 0802): a multicenter, open-label, randomized, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]


