Introduction
Renal cell carcinoma (RCC) accounts for approximately 95% of neoplasms arising from the kidney. Despite the availability of several new agents for the treatment of advanced kidney cancer since late 2005, mortality from kidney cancer remains fairly stable.1 With the exception of high dose interleukin-2, agents used to treat advanced kidney cancer are not curative and seldom lead to long-term durable responses. This underscores the need to continue the search for new and effective RCC therapies.
While once considered a single disease, several histologic subtypes of kidney cancer characterized by classic morphologic features have been established. Individual subtypes of kidney cancer are characterized by distinct genetic alterations and metabolic properties, and therefore one therapy is not likely to be effective for all tumor types. With improved understanding of the unique biology of clear cell RCC (VHL inactivation), agents targeting key components of the VHL-Hypoxia inducible Factor (HIF)- vascular endothelial growth factor (VEGF) axis have been developed and form the mainstay of therapy against this subtype of kidney cancer.2 Applying this strategy in patients with non-clear cell RCC is not supported by similar biologic rationale and at this time the clinical benefit of VEGF-targeted therapy in these cancers appears to be modest.3 Recent insights into the biology of kidney cancer suggest that dysregulated metabolic pathways play an important role in many subtypes of this disease.4 An improved understanding of the metabolic changes underlying different forms of kidney cancer is likely to lead to innovative therapeutic strategies for the management of this disease.
1) Aerobic Glycolysis in Cancer
Warburg Effect
Glycolysis is a multistep process where glucose is converted to pyruvate. Most differentiated human tissues oxidize pyruvate to acetyl-coA by pyruvate dehydrogenase for entry into the mitochondrial Krebs cycle, the primary means of energy (ATP) production via oxidative phosphorylation. In the absence of oxygen, cells are able to generate ATP, albeit less efficiently, by converting pyruvate into lactate by the enzyme lactate dehydrogenase (LDH). In the early half of the 20th century, Dr. Otto Warburg first noted that compared to normal cells, cancer cells consumed large amounts of glucose and produced lactate even in the presence of oxygen (aerobic glycolysis). This phenomenon was named the ‘Warburg effect” and was hypothesized to be caused by mitochondrial dysfunction, thereby rendering the cells deficient in oxidative phosphorylation and dependent on aerobic glycolysis for generation of ATP.5 During his Nobel Laureate Meeting Lecture in 1966, Warburg concluded that “the prime cause of cancer is the replacement of the respiration of oxygen in normal body cells by a fermentation of sugar.”6 The validity of this theory has been much debated over the last several decades, and while several lines of evidence suggest that tumors do indeed preferentially use aerobic glycolysis, support for a concomitant or causative defect in mitochondrial function is lacking in all but a few models.7
Critics of the Warburg effect initially argued that glycolytic shift in cancer resulted from tumor hypoxia, with a consequent switch to anaerobic glycolysis and enhanced lactate production. As solid tumors frequently outgrow their oxygen supply, anaerobic glycolysis is a common occurrence. Induction of hypoxia inducible factor (HIF) acts to restore oxygen balance by induction of glycolysis, erythropoiesis, and stimulation of angiogenesis.8, 9 Whether tumor glycolysis happened independently of hypoxia (the Warburg effect) was a big question. Over the last few years, interest in this theory has been rekindled by evidence suggesting that oncogene activation or loss of tumor suppressor function could lead to a metabolic shift towards aerobic glycolysis.10-12
Role of HIF in glycolysis
Several cancers are associated with HIF overexpression despite normoxia. HIF1α is believed to play a key role in the induction of glycolysis, and stabilization of this protein either due to hypoxia or oncogenic alterations can promote glycolysis.13 In clear cell kidney cancer, VHL alteration by mutation or hypermethylation inhibits ubiquitination and subsequent proteosomal degradation of HIF alpha subunits.9, 14-17 Other mechanisms of normoxic HIF stabilization include ras activation, accumulation of Krebs cycles substrates such as fumarate, as well as activation of the PI3K/AKT/mTOR pathway.18 While HIF expression plays an important role in the regulation of glycolysis, there are multiple mediators of this process. Mechanisms of HIF-independent aerobic glycolysis have been demonstrated in cancer and were first linked to activation of c-Myc.12 Overexpression of c-Myc results in increased expression of several genes that promote glycolysis such as LDH-A, PKM2, GLUT-1 and hexokinase-2.12, 19, 20 Other alterations in oncogenenic pathways such as PI3K/AKT/mTOR also appear to be key mediators of a glycolytic switch in cancer.10
Aerobic Glycolysis and Macromolecule Generation
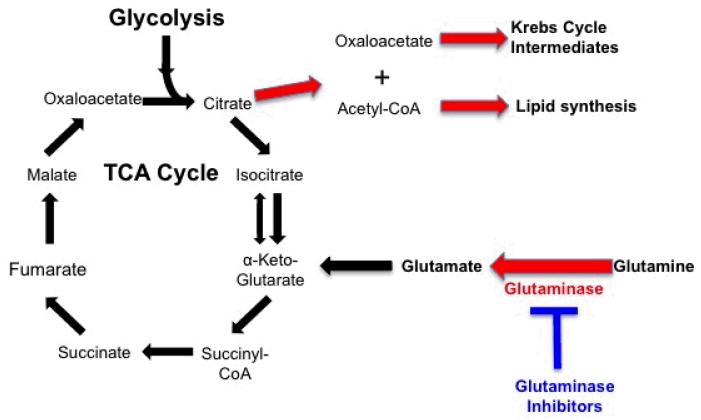
Cells require a constant pool of macromolecules such as proteins, lipids, and nucleotides to support growth and proliferation. Cells with a high proliferative rate, such as tumor cells, acquire many alterations that help satisfy their exaggerated metabolic needs. Glucose and glutamine are the major source of carbon and nitrogen molecules necessary for macromolecule synthesis. Aerobic glycolysis, by engendering a high glucose flux into cells, may help provide an abundant supply of the necessary substrates for macromolecule synthesis. Krebs cycle components such as citrate are important intermediaries in the synthesis of macromolecules from glucose and glutamine. In cells where entry of glucose into the Krebs cycle is limited (as with inactivation of key Krebs cycle enzymes such as fumarate hydratase), glutamine, by a process known as glutaminolysis, serves as the major source of biosynthetic intermediaries (Figure 2). Approaches aimed at targeting tumor glutaminolysis are being actively explored and may complement strategies designed to interfere with glycolysis.
Figure 2.
Macromolecule generation using glutamine as a biologic precursor.
2) What Drives Aerobic Glycolysis in Kidney Cancer
Interruption of the Krebs Cycle
At least two forms of hereditary kidney cancer appear to arise as a result of germline inactivating mutations in genes encoding Krebs cycle enzymes. The resulting incapacitation of the Krebs cycle forces the cells to use glycolysis as the major source of energy production. While these provide perhaps the best examples of the Warburg effect in human cancer, other tumors including sporadically occurring renal tumors may have similar metabolic dysfunction (such as mutations in isocitrate dehydrogenase in CNS tumors and leukemias). Mitochondrial mutations in complex I have also been described in oncocytoma, the most common benign renal tumor.21 Additionally, somatic alterations in mitochondrial genes have also been reported in RCC.22, 23
a) Hereditary Leiomyomatosis Renal Cell Carcinoma (HLRCC)
Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) is an autosomal dominant condition characterized by the development of cutaneous and uterine leiomyomas and kidney cancer.24-28 Germline inactivating mutations in Fumarate Hydratase (FH) are responsible for HLRCC.29 This key enzyme is responsible for the conversion of fumarate to malate in the Krebs cycle. Loss of FH activity leads to impairment of oxidative phosphorylation and reliance on aerobic glycolysis to meet the bioenergetic needs of the cell.30 Approximately 15-30% of individuals affected with HLRCC develop kidney cancer that is a clinically aggressive papillary variant with large orangiophilic nuclei and a characteristic perinucelar halo.26, 28, 31
b) Succinate dehydrogenase Deficiency
Hereditary Paraganglioma (PGL) is a syndrome manifested by head and neck PGL and pheochromocytoma (PCC). Germline alterations in genes that encode the subunits of succinate dehydrogenase (SDH), are associated with this entity.32-34 SDH, an enzyme located on the inner mitochondrial membrane, consists of SDHA, SDHB, SDHC, and SDHD subunits. SDH catalyzes the oxidation of succinate to fumarate in the Krebs cycle and serves as complex II in the electron transport chain. The identification of germline mutations in SDHB in familial kidney cancer patients led to the realization that RCC is another disease manifestation.35, 36 Renal tumors associated with this syndrome demonstrate impaired oxidative phosphorylation with reliance on aerobic glycolysis, and are clinically aggressive.37, 38 Tumors associated with SDHB appear to have a distinct morphology with cuboidal cells having “bubbly, eosinophilic cytoplasm” with indistinct cell borders.39
Upregulation of HIF
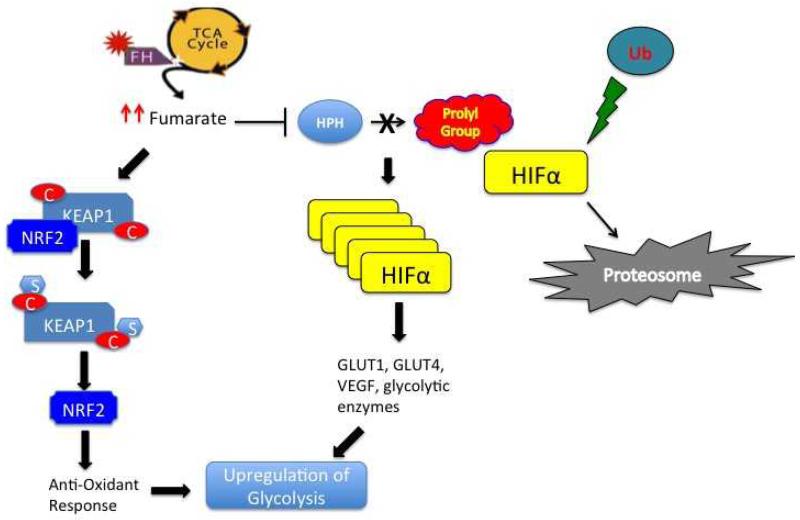
Inactivating mutations in VHL and upregulation of HIF are the hallmark of both sporadic and hereditary clear cell renal cell carcinoma (ccRCC).14, 16, 17 Increased intracellular HIF, particularly HIF-1α, leads to transcriptional upregulation of a variety of genes including several-GLUT1, PFK2, PDH, LDH-A- that promote aerobic glycolysis.HIF-1α can also be upregulated by VHL-independent mechanisms. Tumors associated with HLRCC and those resulting from SDH mutations express high levels of HIF 1α.40, 41In patients with HLRCC, biallelic inactivation of FH leads to accumulation of fumarate. Similarly, in cancers associated with SDH mutations, intracellular levels of succinate accumulate. Both fumarate and succinate appear to interfere with the hydroxylation of HIF by HIF prolyl hydroxylases (HPHs), leading to intracellular HIF accumulation (Figure 1).
Figure 1.
Proposed mechanisms of tumorigenesis in HLRCC. Due to loss of function of fumarate hydratase, fumarate accumulates, inhibiting HIF prolyl hydroxylases (HPHs). Inactivation of HPH leads to intracellular HIF accumulation and transcription of important mediators of angiogenesis, growth and proliferation. In addition, upregulation of fumarate can lead to post-translational modification (succination of cysteine residues) and inactivation of KEAP1. This results in NRF2 dysregulation and transcriptional activation of several NRF2-dependant genes that mediate the cellular anti-oxidant response, Both HIF and NRF2 contribute to the aberrant metabolic signature associated with FH inactivation.
HIF can also be upregulated by activation of the mTOR pathway. mTOR is a key regulator of cell growth and proliferation and is commonly upregulated in many cancers. In clear cell RCC, mutations in an upstream regulator of mTOR function (PTEN) are infrequently observed.42, 43 Decreased expression of PTEN in the absence of a discernible mutation is common and is believed to contribute to an activated downstream AKT/mTOR pathway.42, 44 Mutations in TSC1 and TSC2 can also upregulate mTOR and have been associated with renal angiomyolipomas and kidney cancer. The mTOR pathway is also activated in tumors associated with BHD, a hereditary kidney cancer syndrome resulting from germline inactivating mutations in folliculin (FLCN) and characterized by bilateral multifocal renal tumors of varied histology.45, 46
Reactive Oxygen Species
Reactive oxygen species (ROS) are oxygen free radicals characterized by unpaired electrons. These highly reactive molecules are byproducts of normal oxidative metabolism and function in normal homeostasis and signaling. However with increased levels of ROS, cellular damage and dysfunctional signaling can ensue. In HLRCC, increased cellular glucose levels stimulate NADPH-mediated ROS production.47 The increased levels of ROS can directly stabilize HIF1, contributing to a metabolic shift towards aerobic glycolysis.48, 49 In HLRCC, ROS are believed to stabilize HIF1 by direct inhibition of HPHs.47
In SDH, increased levels of ROS may contribute to HIF1 stabilization and tumorigenesis.50 In yeast models, loss of SDH function generates increased levels ROS and the accumulation of oxidized proteins.51 However data from SDH knockdown models in mammalian cells demonstrates that increased levels of ROS do not occur.41
NRF2/KEAP1
Although HIF is upregulated in both VHL null and FH deficient kidney cancer, these two entities demonstrate significant histological and clinical differences. In a bid to account for these differences, several groups have investigated the role of HIF-independent pathways in FH associated renal tumors. Animal models attempting to recapitulate HLRCC have been developed using conditional FH (−/−) knockout mice, which develop large renal cysts and renal failure.52 Crossing these mice with HIF1 or HIF2 knockout mice fails to ameliorate this renal phenotype, suggesting that in this model, the renal abnormalities associated with FH loss are independent of HIF 1 and HIF 2.53 Analyses of renal cysts from these FH-/mice demonstrate an aberrant anti-oxidant response pathway that may contribute to HLRCC associated renal tumorigenesis. At least two groups investigating the mechanism by which FH loss leads to upregulation of anti-oxidant genes have demonstrated that elevated intracellular fumarate leads to post-translational modification of Kelch-like ECH-associated protein 1(KEAP1). KEAP1 is a regulator of Nuclear factor (erythroid-derived 2)-like 2 (NRF2), a master transcriptional regulator of the response pathway to oxidative stress. Under normal conditions, KEAP1 is believed to bind to and ubiquinate NRF2, resulting in its degradation. The interaction between KEAP 1 and NRF2 is disrupted following succination of the former in the presence of elevated levels of fumarate, leading to intracellular accumulation of NRF2 and activation of antioxidant signaling pathways.53, 54 Recent evidence suggests that NRF2 is an important regulator in cancer cell metabolism. Mitsuishi and colleagues recently demonstrated that activation of this pathway results in the redirection of glucose and glutamine into anabolic pathways such as glycolysis and the pentose phosphate pathway.55 In addition to HLRCC tumors, dysregulation of the KEAP1/NRF pathway appears has also been reported in the morphologically similar sporadic papillary type II RCC.54
Other Oncogenic Pathways
The PI3K/AKT/MTOR and myc proto-oncogene pathways are activated in different subtypes of kidney cancer. Both pathways play critical roles in the altered cellular metabolism seen in kidney cancer. Myc has been shown to upregulate the expression of various pro-glycolytic enzymes such as PKM2, hexokinase 2, and LDH-A. In addition, both the glucose transporter GLUT-1 and the glutamine transporter ASCT2 are regulated by Myc. The AKT/mTOR pathway stimulates glycolysis directly by upregulating several key glycolytic enzymes and indirectly by translational upregulation of HIF.
3) Targeting aerobic glycolysis
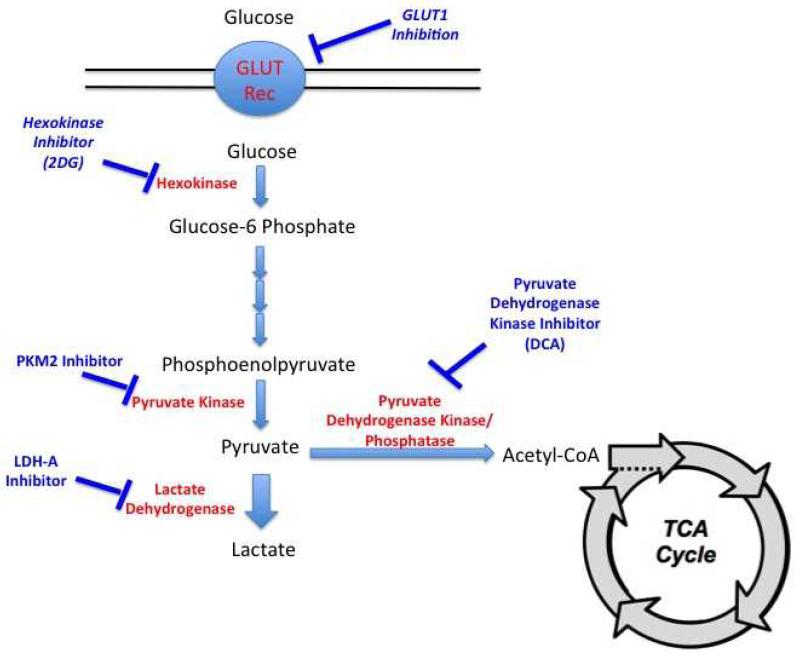
A variety of rational ‘metabolic’ targets have been proposed and are being evaluated in preclinical/clinical studies. These targets include critical components of aerobic glycolysis as well pathways involved in biosynthesis of macromolecules (Figure 3). The following discussion is restricted to those targets considered relevant to kidney cancer.
Figure 3.
Metabolic flow of glucose and opportunities for therapeutic intervention in cancer
Glucose Uptake
Cellular glucose uptake is regulated by trans-membrane glucose transporters (GLUT receptors). Different genes encode for GLUT isoforms, which have variable distribution based on tissue type. Cancer cells frequently upregulate GLUT expression in an attempt to increase glucose uptake, especially important for cells that rely on aerobic glycolysis. Inhibiting this process could hinder cell proliferation by “glucose starvation” and make them more susceptible to cell death. This approach may be a viable option in clear cell RCC as a recent study demonstrated that VHL deficient cell lines demonstrate synthetic lethality with GLUT1 inhibition as did cancers with obligate aerobic glycolysis such as those associated with FH and SDH mutations.56 Inhibitors of glucose uptake are currently in development.
PKM2
Pyruvate kinase (PK) is the last enzyme involved in glycolysis, catalyzing the dephosphorylation of phosphoenolpyruvate (PEP) to pyruvate with the generation of a molecule of ATP. Several isoforms of PK have been identified and the expression of these isoforms is tissue-dependent. The PKM gene has two splice variants (M1 and M2) varying by the presence of one exon.57 The M2 isoform is present in embryonic tissues and in cells requiring rapid glucose turnover including muscle and brain cells. This enzyme is unique in that it can exist in both dimeric and tetrameric forms.58 In cancer tissue, PKM2 is preferentially expressed and is important to tumor metabolism and proliferation.59-61 The ratio of the dimeric to tetrameric forms determines enzyme activity and whether PEP is converted to pyruvate or is shuttled into biosynthesis of other molecules such as amino acids and nucleic acids.62
PKM2 is being explored as a therapeutic target in cancer cells dependent on aerobic glycolysis. Knockdown of PKM2 decreases in vitro cell proliferation and glucose metabolism and also inhibits growth of xenografts.63 Small molecule inhibitors targeting PKM2 are currently in development.64
Hexokinase
Hexokinase is the first enzyme involved in the glycolytic pathway and catalyzes the phosphorylation of glucose into glucose-6-phosphate. Once phosphorylated, glucose-6-phosphate cannot exit the cell. 2-deoxy-D-glucose (2DG) is a glucose analog that is taken up by cells via the same mechanisms mediating glucose uptake. Once in the cell, 2DG is phosphorylated by hexokinase. However, after this step, the resultant metabolite cannot be processed by glucose-6-phosphate isomerase, the second enzyme in glycolysis. Accumulation of phosphorylated-2DG leads to inhibition of hexokinase. Treatment with 2DG has long been known to inhibit in vitro tumor growth.65 2DG inhibits in vitro growth of UOK262, an FH deficient kidney cancer cell line derived from a patient with HLRCC. The agent has been evaluated in phase I and II studies. A case report of a patient with metastatic HLRCC treated with 2DG was recently reported; unfortunately, the patient did not appear to derive any clinical benefit.66 Treatment with 2DG may not be effective as monotherapy, as escape mechanisms such as activation of the PI3K/AKT pathway may overcome inhibition of hexokinase.67 Other pharmacologic mechanisms aimed at inhibiting hexokinase have been explored. Inhibitors such as 3-Bromopyruvate and Lonidamine can inhibit hexokinase function and may be a useful strategy for tumors relying on aerobic glycolysis.68 Lonidamine was in clinical development in the United States but these trials were terminated due to modest clinical activity. However, this drug was approved in Italy for cancer therapy over 20 years ago.68
LDH-A
Lactate dehydrogenase (LDH) catalyzes the reversible conversion of lactate to pyruvate. LDH exists in two major isoforms; LDH-A is the isoform that promotes conversion of pyruvate into lactate. As LDH-A is upregulated in solid tumors reliant on glycolysis, inhibition of this enzyme may be a valid therapeutic approach.69 Knockdown experiments in several models demonstrate decreased in vitro cell proliferation and in vivo tumorigenicity.69 HLRCC associated tumors appear to overexpress LDH-A and knockdown of this enzyme in UOK262 limits in vitro cell proliferation and in vivo growth inhibition.70
Haem Oxygenase
Several Krebs cycle intermediates are needed for macromolecule biosynthesis and integrity of this cycle is essential for sufficient NADH production. In cells with Krebs cycle dysfunction, the mechanisms used to generate macromolecules and NADH is not well understood. Cells dependent on aerobic glycolysis are thought to rely on high levels of glutamine metabolism for these biochemical precursors.71 Experimental modeling and 13C labeling in mouse FH-deficient kidney cells determined that these cells utilize an alterative metabolic pathway involving heme biosynthesis and degradation for NADH production. Knockout of heme oxygenase 1, required for heme degradation, disrupts this pathway and is associated with synthetic lethality in FH negative cells and is being explored as a therapeutic target..72
Glutaminase
In addition to their reliance on aerobic glycolysis, cancer cells also demonstrate increased glutamine uptake, which serves as the major source of nitrogen for protein and lipid biosynthesis.71, 73 Interfering with glutamine metabolism may therefore starve the cancer cell of the necessary biosynthetic molecules required for continued growth and proliferation. Cells relying on a high rate of aerobic glycolysis are profoundly sensitive to glutamine withdrawal.74 Therapeutic strategies aimed at blocking glutamine metabolism by glutaminase inhibition are being explored. Small molecule inhibitors of this enzyme have been shown to block oncogenic transformation in several cell lines without affecting normal cells.75 In glioma cell lines with a mutated Krebs cycle enzyme (isocitrate dehydrogenase), genetic and pharmacologic inhibition of glutaminase can decrease cell proliferation.76 While this may be a promising strategy for targeting cancer metabolism, emerging data suggests that cells adapt to overcome glutaminase inhibition by activating alternative pathways for generating substrates for macromolecule synthesis. Further studies are required to determine the role of glutaminase inhibition in the treatment of cancer.77
Pyruvate Dehydrogenase Kinase
Pyruvate dehydrogenase (PDH) is a mitochondrial enzyme complex responsible for the decarboxylation and acetylation of pyruvate into acetyl-CoA, the starting point of the Krebs cycle. This complex is tightly regulated by two distinct enzymes, pyruvate dehydrogenase phosphatase and pyruvate dehydrogenase kinase (PDK). Phosphorylation of PDH inactivates the complex and shunts pyruvate towards lactate production in the cytoplasm. Methods aimed at inhibiting PDK are thought to shift metabolism towards the Krebs cycle. Sodium Dichloroacetate (DCA) is a pyruvate analogue that promotes entry of pyruvate into the Krebs cycle and has been extensively studied for cancer treatment. Cells with defects in the electron transport chain appear to be more sensitive than cells less reliant on glycolysis.68 The agent is in early clinical trials and appears to be well tolerated.
Angiogenesis Inhibitors
Prior to 2005 immunotherapy was the sole systemic modality available to patients with advanced kidney cancer. Unfortunately, only a small proportion of patients with clear cell RCC appear to benefit from immunotherapy while non-clear cell RCC are generally not responsive.78, 79 Newer agents targeting the VEGF or mTOR pathways show only modest activity in papillary RCC.80 Thus, no effective strategy exists for papillary variants of RCC. In a phase II study of erlotinib in papillary RCC, a low overall response rate was observed, although the median survival of 27 months was encouraging.81
We hypothesized that combining a VEGF pathway antagonist and an EGFR inhibitor would constrain glucose delivery to tumor cells by targeting its vasculature; targeting a growth factor pathway downstream from HIF would theoretically act in concert with the antiangiogenic approach. This approach was first evaluated in clear cell RCC using using bevacizumab and erlotinib therapy, a combination that was well tolerated.82 This approach is being currently evaluated in patients with papillary RCC in a phase II trial at the NCI (Trial ID, NCI01130519).
4) Conclusion
A variety of kidney cancer subtypes are characterized by dysregulated metabolic pathways. The dependence of some tumors on aerobic glycolysis is an Achilles heel that can be exploited in targeted therapeutic strategies. Multiple steps in the glycolytic pathway have been evaluated as possible anti-cancer targets in preclinical studies and some agents have entered early clinical trials for solid tumors. It is hoped that these approaches will spawn novel therapeutic options in the fight against kidney cancer.
Acknowledgements
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- 1.American Cancer Society: Cancer Facts & Figures 2010. Atlanta: 2010. [Google Scholar]
- 2.Linehan WM. Molecular targeting of VHL gene pathway in clear cell kidney cancer. J Urol. 2003;170:593. doi: 10.1097/01.ju.0000077210.05543.ae. [DOI] [PubMed] [Google Scholar]
- 3.Singer EA, Bratslavsky G, Linehan WM, et al. Targeted therapies for non-clear renal cell carcinoma. Targeted oncology. 2010;5:119. doi: 10.1007/s11523-010-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nature reviews. Urology. 2010;7:277. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269. [PubMed] [Google Scholar]
- 6.Warburg O. The Prime Cause and Prevention of Cancer; Presented at the 17th Annual Lindau Nobel Laureate Meetings; Lindau, Germany. 1967. [Google Scholar]
- 7.Garber K. Energy boost: the Warburg effect returns in a new theory of cancer. Journal of the National Cancer Institute. 2004;96:1805. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell PH, Dachs GU, Gleadle JM, et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer research. 2004;64:3892. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6658. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Molecular and cellular biology. 2003;23:9361. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 15.Herman JG, Latif F, Weng Y, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9700. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Pore N, Behrooz A, et al. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. The Journal of biological chemistry. 2001;276:9519. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Zeller KI, Wang Y, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Molecular and cellular biology. 2004;24:5923. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osthus RC, Shim H, Kim S, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. The Journal of biological chemistry. 2000;275:21797. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 21.Mayr JA, Meierhofer D, Zimmermann F, et al. Loss of complex I due to mitochondrial DNA mutations in renal oncocytoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:2270. doi: 10.1158/1078-0432.CCR-07-4131. [DOI] [PubMed] [Google Scholar]
- 22.Selvanayagam P, Rajaraman S. Detection of mitochondrial genome depletion by a novel cDNA in renal cell carcinoma. Laboratory investigation; a journal of technical methods and pathology. 1996;74:592. [PubMed] [Google Scholar]
- 23.Horton TM, Petros JA, Heddi A, et al. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes, chromosomes & cancer. 1996;15:95. doi: 10.1002/(SICI)1098-2264(199602)15:2<95::AID-GCC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:6479. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet. 2006;43:523. doi: 10.1136/jmg.2005.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino MJ, Torres-Cabala C, Pinto P, et al. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 27.Reed WB, Walker R, Horowitz R. Cutaneous leiomyomata with uterine leiomyomata. Acta dermato-venereologica. 1973;53:409. [PubMed] [Google Scholar]
- 28.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam NA, Bevan S, Churchman M, et al. Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. American journal of human genetics. 2001;68:1264. doi: 10.1086/320124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong WH, Sourbier C, Kovtunovych G, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer cell. 2011;20:315. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grubb RL, 3rd, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. The Journal of urology. 2007;177:2074. doi: 10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- 32.Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. American journal of human genetics. 2001;69:49. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 34.Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nature genetics. 2000;26:268. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 35.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100:1260. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 36.Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. American journal of human genetics. 2004;74:153. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bratslavsky G, Linehan WM. Long-term management of bilateral, multifocal, recurrent renal carcinoma. Nat Rev Urol. 7:267. doi: 10.1038/nrurol.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricketts CJ, Shuch B, Vocke CD, et al. Succinate Dehydrogenase Kidney Cancer: An Aggressive Example of the Warburg Effect in Cancer. The Journal of urology. 2012 doi: 10.1016/j.juro.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill AJ, Pachter NS, Chou A, et al. Renal Tumors Associated With Germline SDHB Mutation Show Distinctive Morphology. The American journal of surgical pathology. 2011;35:1578. doi: 10.1097/PAS.0b013e318227e7f4. [DOI] [PubMed] [Google Scholar]
- 40.Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer cell. 2005;8:143. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer cell. 2005;7:77. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Abou Youssif T, Fahmy MA, Koumakpayi IH, et al. The mammalian target of rapamycin pathway is widely activated without PTEN deletion in renal cell carcinoma metastases. Cancer. 2011;117:290. doi: 10.1002/cncr.25402. [DOI] [PubMed] [Google Scholar]
- 43.Hara S, Oya M, Mizuno R, et al. Akt activation in renal cell carcinoma: contribution of a decreased PTEN expression and the induction of apoptosis by an Akt inhibitor. Ann Oncol. 2005;16:928. doi: 10.1093/annonc/mdi182. [DOI] [PubMed] [Google Scholar]
- 44.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 45.Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci U S A. 2009;106:18722. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sudarshan S, Sourbier C, Kong HS, et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol. 2009;29:4080. doi: 10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finley LW, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer cell. 2011;19:416. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi DY, Xie FZ, Zhai C, et al. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Molecular cancer. 2009;8:32. doi: 10.1186/1476-4598-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eng C, Kiuru M, Fernandez MJ, et al. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nature reviews. Cancer. 2003;3:193. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 51.Smith EH, Janknecht R, Maher LJ., 3rd Succinate inhibition of alpha-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Human molecular genetics. 2007;16:3136. doi: 10.1093/hmg/ddm275. [DOI] [PubMed] [Google Scholar]
- 52.Pollard PJ, Spencer-Dene B, Shukla D, et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer cell. 2007;11:311. doi: 10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Adam J, Hatipoglu E, O’Flaherty L, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer cell. 2011;20:524. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ooi A, Wong JC, Petillo D, et al. An Antioxidant Response Phenotype Shared between Hereditary and Sporadic Type 2 Papillary Renal Cell Carcinoma. Cancer cell. 2011;20:511. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 55.Mitsuishi Y, Taguchi K, Kawatani Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer cell. 2012;22:66. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Chan DA, Sutphin PD, Nguyen P, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Science translational medicine. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. The Journal of biological chemistry. 1986;261:13807. [PubMed] [Google Scholar]
- 58.Mazurek S, Boschek CB, Hugo F, et al. Pyruvate kinase type M2 and its role in tumor growth and spreading. Seminars in cancer biology. 2005;15:300. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 60.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider J, Neu K, Grimm H, et al. Tumor M2-pyruvate kinase in lung cancer patients: immunohistochemical detection and disease monitoring. Anticancer research. 2002;22:311. [PubMed] [Google Scholar]
- 62.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. The international journal of biochemistry & cell biology. 2011;43:969. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Christofk HR, Vander Heiden MG, Wu N, et al. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 64.Vander Heiden MG, Christofk HR, Schuman E, et al. Identification of small molecule inhibitors of pyruvate kinase M2. Biochemical pharmacology. 2010;79:1118. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism: clinical and experimental. 1962;11:1098. [PubMed] [Google Scholar]
- 66.Yamasaki T, Tran TA, Oz OK, et al. Exploring a glycolytic inhibitor for the treatment of an FH-deficient type-2 papillary RCC. Nature reviews. Urology. 2011;8:165. doi: 10.1038/nrurol.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong D, Liu X, Schafer-Hales K, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Molecular cancer therapeutics. 2008;7:809. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 68.Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Advanced drug delivery reviews. 2009;61:1250. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer cell. 2006;9:425. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 70.Xie H, Valera VA, Merino MJ, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frezza C, Zheng L, Folger O, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 73.Mullen AR, Wheaton WW, Jin ES, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011 doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang JB, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell. 2010;18:207. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer research. 2010;70:8981. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng T, Sudderth J, Yang C, et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8674. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upton MP, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother (1997) 2005;28:488. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 79.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 80.Plimack E, Jonasch E, Bekele B, et al. Sunitinib in papillary renal cell carcinoma (pRCC): Results from a single-arm phase II study. J Clin Oncol. 2010;28 [Google Scholar]
- 81.Pan C, Hussey M, Lara P, et al. Encouraging survival with erlotinib in advanced papillary renal cell carcinoma (pRCC): Final results from Southwest Oncology Group study 0317. J Clin Oncol. 2008;26 [Google Scholar]
- 82.Hainsworth JD, Sosman JA, Spigel DR, et al. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:7889. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]