Abstract
A new strategy for the rapid identification of new malaria antigens based on protein structural motifs was previously described. We identified and evaluated the malaria vaccine potential of fragments of several malaria antigens containing α-helical coiled coil protein motifs. By taking advantage of the relatively short size of these structural fragments, we constructed different poly-epitopes in which 3 or 4 of these segments were joined together via a non-immunogenic linker. Only peptides that are targets of human antibodies with anti-parasite in vitro biological activities were incorporated. One of the constructs, P181, was well recognized by sera and peripheral blood mononuclear cells (PBMC) of adults living in malaria-endemic areas. Affinity purified antigen-specific human antibodies and sera from P181-immunized mice recognised native proteins on malaria-infected erythrocytes in both immunofluorescence and western blot assays. In addition, specific antibodies inhibited parasite development in an antibody dependent cellular inhibition (ADCI) assay. Naturally induced antigen-specific human antibodies were at high titers and associated with clinical protection from malaria in longitudinal follow-up studies in Senegal.
Introduction
It is estimated that there are up to 500 million cases of malaria every year and that about one million children living in sub-Saharan Africa die within the same period.1 Over the past few years, appreciable progress has been made in the control of malaria infection in some parts of sub-Saharan Africa.2 Further reduction in morbidity and mortality as well as possible eradication of the disease will depend to a large extent on safe and effective vaccines. However there is currently no vaccine against malaria and only handful vaccine candidates are currently being evaluated.
The publication of the full genome of Plasmodium falciparum3 opened new opportunities for the development of novel drugs and vaccines against malaria. The aim of our group is to explore these vastly untapped data and discover new malaria antigens by combining bioinformatics and chemical peptide synthesis by using in vivo/in vitro parameters that are associated with protection against malaria. In our previous work4 we described the identification and production of 95 segments derived from 70 P. falciparum-3D7 erythrocytic stage proteins predicted to have α-helical coiled coil protein motifs. These α-helical coiled coil protein motifs consist of heptad repeats (abcdefg)n with hydrophobic residues at a and d positions while the other residues are generally hydrophilic. The synthesized fragments readily assume their native oligomeric structure.5
Out of the 95 segments synthesized, 12 polypeptides were found to be targets of in vivo parasite growth inhibition in an ADCI assay. In order to maximize the proportion of the general host population that will respond to such a candidate vaccine while conserving the individual functional capacities of the constituent polypeptides (with additional likelihood of synergism), we then synthesized constructs consisting of 2-4 polypeptides joined together by the non-immunogenic a modified diethylene glycol linker (DEG). Selection of the constituent polypeptides was based on the length of each fragment, sequence conservation, antigenic recognition by semi-immune adult sera, immunogenicity in mice and biological activities of affinity purified specific human antibodies in ADCI assays.
Of the different poly-epitopes we constructed, we report here the results for P181, which is composed of the 3 fragments, P90, P77 and P27 that are derived from the proteins PFD0520c (25 kD), PF08_0048 (247 kD), PFF0165c (160 kD), respectively [Plasmodb.org; manuscript submitted]. These peptides had been identified as the most promising candidates in our previous analysis.4
Materials and Methods
Peptide synthesis and antigen characterization
The polypeptides were synthesized using solid-phase Fmoc chemistry6 with Applied Biosystem synthesizer 431A and 433A (Foster City, 179 CA). Derivatized diethylene glycol (DEG, Merck Chemicals Ltd, Nottingham, UK) was inserted in between the synthesis of the three fragments P90, P77 and P27 (TKKLNKELSEGNKELEKLEKNIKELEETNNTLENDIKV-DEG-EKLKKYNNEISSLKKELDILNEKMGKCT-DEG-KKRNVEEELHSLRKNYNIINEEIEEIT). The resulting construct was HPLC purified and the purity (>90%) was confirmed by analytic C18 HPLC and mass spectrometry (MALDI-TOF; Applied Biosystem) All reagents used were purchased from Fluka (Buchs, Switzerland) and Novabiochem (Laufelfingen, Switzerland). A custom-made synthesis was performed by Almac Sciences, Craigavon, Northern Ireland. Purity was >95% as judged by analytical HPLC and mass spectrometry analysis shows material with the predicted MW of 11945.9 (data not shown).
The circular dichroism spectrum of the constructs was assessed with a JASCO J-810 spectrometer (JASCO Corporation, Japan). The measurements were with 0.2 mg/ml of the construct dissolved in water at 22°C and at pH 7.3.
Analytical ultracentrifugation was carried out in ProteomeLab XL-I analytical ultracentrifuges (Beckman Coulter, Palo Alto, CA). Sedimentation velocity experiments followed the standard protocol.7-9 In brief, peptide P181 samples at final concentrations of 0.1, 0.2, 0.4, and 0.8 mg/ml were dialyzed into a buffer composed of 14 mM NaCl, 0.3 mM KCl, 0.4 mM sodium phosphate, 0.2 mM potassium phosphate, pH 7.4 and sedimented at 59,000 rpm at 10°C. Interference optical fringe shift profiles were fitted with the c(s) model.10 For the partial-specific volume, a value of 0.739 ml/g was predicted for the temperature-corrected partial specific volume from the amino acid composition and the tabulated data from Cohn & Edsall11 and the density of PEG.12 The density and viscosity of the buffer were measured using a DMA5000 densimeter and an AMVn automated micro viscometer (both from Anton Paar, Graz, Austria), respectively. The prediction of hydrodynamic parameters from model structures was based on the program BEST [please insert new reference to the following paper http://www.ncbi.nlm.nih.gov/pubmed/15116362].
Sedimentation equilibrium experiments were conducted following the standard protocol.7 In brief, 180 microliter samples at concentrations of 0.1, 0.3, and 1.0 mg/ml were sedimented to attain equilibrium at 25,000 rpm and 34,000 rpm at 10°C. Absorbance optical scans were acquired at 280 nm. The global analysis of all data sets was performed with the software SEDPHAT10,13 using the principle of soft mass conservation,14 and a model for monomer-dimer self-association in chemical and sedimentation equilibrium following mass action law, accounting for a non-participating species. Error estimates were obtained from Monte-Carlo analysis using 1000 iterations and a confidence level of one standard deviation.
Human sera
Anonymized archived human serum samples from malaria endemic areas and from Switzerland were used. The sera samples were collected from malaria-endemic regions of Burkina Faso, Tanzania, Papua New Guinea and Senegal and were the same samples used in our previous study.4,15 Briefly, the samples from Burkina Faso were collected from donors living in the village of Goundry in the province of Oubritenga. Ethical approval was obtained from the Ministry of Health, Burkina Faso. The Tanzanian samples were obtained from donors in Kikwawila village in Morogoro region. Ethical approval was obtained from the Tanzanian Commission for Science and Technology. Additional sera samples obtained from Papua New Guinea were collected in the Maprik District of the East Sepik Province, during a cross sectional survey in July 1992 within the framework of the Malaria Vaccine Epidemiology and Evaluation Project (MVEEP) supported by the United States Agency for International Development. The area is highly endemic for malaria. Ethical clearance for MVEEP was obtained from the Papua New Guinea Medical Research Advisory Committee. Blood samples were obtained by venipuncture into tubes containing EDTA. The study design in Senegal received clearance from the national Senegalese ethical committee. Negative control samples were Swiss adult donors with no history of malaria and no previous travel to malaria-endemic areas.
Human PBMCs
PBMC were obtained from adult donors living in Lagos, South-West Nigeria where malaria transmission is high all year round with seasonal peaks during the rainy season. Ethical approval was obtained from Lagos State University Teaching Hospital (LASUTH) ethical review committee.
Antigen recognition by human sera
The recognition of P181 and each of its constituent polypeptide was assessed by ELISA with sera of adults living in malaria endemic regions of Burkina Faso, Tanzania, Papua New Guinea and Senegal. Each well in a 96-well microtiter plate (Maxisorb F96, Nunc, Roskilde, Denmark) was coated with 50 μl of each antigen (concentration 1 or 5 μg/ml for P181 and single fragments, respectively) and incubated overnight in humid chamber at 4°C. Wells were washed 4 times with PBS-0.05% Tween-20 and blocked for 1 h with 200 μl per well of 5% milk PBS-0.05% Tween-20. Sera of adults living in Burkina Faso (n=37) and Tanzania (n=42) were added at 1:200 diluted in 2.5% milk PBS-0.05% Tween-20. This was incubated for 1 h at room temperature. The plates were then washed and 50 μl/well of alkaline phosphatase-conjugated goat anti-human polyvalent immunoglobulin (Sigma, MO, USA) was added at 1:1000 dilutions. Wells were washed before 50 μl of nitrophenylphosphate solution (Sigma, MO, USA) was added. OD was measured at 405 nm. The negative controls (n=8) were sera of adult Europeans with no previous history of malaria or travel to malaria-endemic region. Test samples were considered positive when the measured OD was higher than that of the sum of average OD and 3SD of the negative controls.
T-cell proliferation
Frozen PBMCs collected from adult donors living in Nigeria were thawed and counted. Five replicates containing 2×105 cells/well were cultured with 10 μM of each of the constituent polypeptide and 5 μM of P181, respectively. The complete culture medium used consisted of RPMI (Sigma) supplemented with glutamine, 100 IU/ml Penicillin-Streptomycin, 100 μM non-essential amino acids, Kanamycin (Invitrogen, Paisley, UK), 2 mM sodium pyruvate (Invitrogen) and 8% human AB serum (Blutspendendienst SRK, Bern, Switzerland). The positive control was a mixture of tetanus toxoid (Pasteur Merieux, Lyon, France), Mycobacterium tuberculosis purified protein derivatives (PPD) (SSI, Copenhagen, Denmark) and Candida (NIBSC, London, UK).
On day 5, 1 μCi of thymidine diluted in the culture medium was added. The cultures were harvested 24 hours later, and radioactivity was measured as count/min. The stimulation index (SI) was calculated as (average count/min)/(count/min in culture without antigens). SI >2 was considered significant.
Immunogenicity in mice
Two syngeneic and one outbred strains of mice, C3H, CBF1 and ICR respectively (mice, 4 mice/group) were injected with 20 μg of the poly-epitope P181 or with each of the constituent polypeptides (P90, P77 and P27) formulated in Montanide ISA 720 (35 μg/mouse). Immunogenicity of P181 formulated with Alhydrogel (1 mg/mouse) and GLA-SE (20 μg/mouse), an oil-in water stable emulsion containing a lipid A-like synthetic compound16-17 was also assessed in C3H, CBF1 and ICR mice. Each mouse was injected either subcutaneously at the base of the tail (Montanide and GLA-SE, 50 μl/mouse) or intraperitoneally (Alum, 500 μl/mouse) at 0, 3 and 8 weeks. The induced antibody responses were assessed by ELISA 10 days following second and third immunizations. Antibody titers were assessed by 1:3 serial dilutions of the sera starting from 1:100. The ELISA protocol used was same as that described above for human antibodies with the exception for mouse polyvalent immunoglobulin (Sigma, MO, USA) as second antibody. The end point is taken as the last dilution with OD value greater than average OD of control sera + 3SD.
Affinity purified human antibodies
Human antibodies specific for the poly-epitope P181 and the constituent polypeptides were purified from pooled sera (50 ml) of adults living in Papua New Guinea. The pooled serum was centrifuged at 3,000 rpm for 5 min and the supernatant was filtered and further diluted at 1:5 in PBS/0.5 M NaCl. Two to five mg of each polypeptide was coupled with CNBr-activated Sepharose 4B (Amersham Biosciences, Büdendorf, Switzerland). The serum solution was then mixed with the antigen-coupled beads and stirred on wheel overnight at 4°C. The solution was then centrifuged at 3,000 rpm for 20 min and the beads were washed twice first with 5 ml of 20 mM Tris/0.5 M NaCl and then with 20 mM Tris. The specific antibodies were eluted with 0.1M glycine pH 2.5 at room temperature. Three fractions were collected in 100 μl of neutralization solution (10 PBS, pH 7.4). Antibodies were filtered through a 0.22 μm syringe filter, aliquoted and stored at -80°C. The antibody concentration was determined by the absorbance of each fraction at 280 nm.
Immunofluorescence assay (IFA)
For indirect immunofluorescence microscopy, infected RBCs were smeared onto glass slides and fixed in ice-cold acetone:methanol (1:1, vol/vol) for 10 min. Slides were blocked for 1h in 3%BSA/PBS and probed with one of the following primary and secondary antibodies: mouse anti-P27 (1:200), mouse anti-P90 (1:200) mouse anti-P77 (1:200), human affinity purified sera specific to P27 (1:2000) P90 (1:1000) and P77 (1:500), Cy3-labelled anti-mouse IgG (1:500), Cy3-labelled anti-human IgG (1:500; Jackson ImmunoResearch, Suffolk, UK) and Alexa 488-conjugated anti-rabbit IgG (1:200; Invitrogen). The slides were mounted in vectashield (Vector Laboratories, Burlingame, CA, USA) supplemented with DAPI for nucleus staining. Fluorescence microscopy was performed on a Leica fluorecence microscope DM-5000B using the × 60 oil immersion lens and documented with Leica DC200 digital camera system.
Western Blot analysis
Protein extracts were separated on a 10% SDS-polyacrylamide gel and transferred to nitrocellulose (0.2 mm, Whatman Schleicher + Schuell, Florham Park, NJ) under cooling conditions for 1 h at 80V and an additional hour at 100V. The membrane was blocked for 1 h in PBS/3% milk powder at room temperature and the primary antibody was diluted 1:500 in PBS and 1.5% milk powder. The membrane was incubated in the primary antibody solution overnight at 4°C, washed 8× in PBS, 0.05% Tween 20 and incubated with a peroxidase-conjugated goat anti-mouse IgG antibody (1:3000, Sigma). Bound secondary antibodies were visualized using Western Lightning® (PerkinElmer Life Sciences, Schwerzenbach, Switzerland).
Antibody dependent cellular inhibition (ADCI) assay
The inhibition of P. falciparum (3D7 strain) growth in vitro in the presence of human monocytes (MN) and antigen-specific antibodies was carried out by methods described elsewhere.18-19 Mature schizonts from a synchronized parasite culture were diluted at starting parasitemia of 0.5 % in normal human AB+ type RBCs and the hematocrit was adjusted to 2.5% with RPMI 1640 culture medium. Duplicate assays were set up in preheated 96-wells flat bottom sterile plastic plates (TPP, Trasadingen, Switzerland) containing 2×103 MN/well and by addition of 50 μl of parasite culture mixed with 50 μl of RPMI and various dilutions of each pool of sera at final concentration of 0.5%-10%. Control wells with parasite culture and RPMI were done in parallel. The plates were incubated in a candle jar at 37°C, in a 5% CO2 incubator for 96 h. Thin blood smears for each well were fixed in methanol and stained in eosin and methylene blue. The parasitemia was determined by microscopic examination and counting of at least 5,000 RBCs in duplicate. The Specific Growth Inhibitory index (SGI) which estimates the parasite growth inhibition due to the effect of test Abs cooperating with MN was calculated as follows: SGI = 100 × (1 - (% parasitemia with MN and test Abs/% parasitemia test Abs)/(% parasitemia with MN and N-IgG/% parasitemia N-IgG)).
For each Ab tested, duplicate wells included the following controls: 1) non-specific monocytic inhibition, both MN + parasite, and MN + normal (N)-IgG + parasites and 2) direct inhibition by control or test IgG, both N-IgG + parasites, and test Abs + parasites. Pooled immunoglobulins of Africans (PIAG) and N-IgG were used at a final concentration of 1 mg/ml and as positive and negative controls, respectively. Immunopurified test human Abs were used at 15μg/ml while mouse sera were used at different dilutions.
Human antibody response and association with protection
The inhabitants of Ndiop village, in Senegal (with 300-350 villagers), where malaria transmission is seasonal, were involved in a prospective study using a stringent protocol of clinical follow-up including the daily active surveillance of each villager by a medical staff present seven days a week and 24 hours a day. The presence of each individual was checked on a daily basis and each febrile episode was recorded. A malaria attack was defined as an episode of fever with temperature ≥38.5°C associated with a parasite density exceeding a parasite threshold of 3000 parasites/μl. Therefore both the occurence of clinically defined malaria attacks and the actual time spent in Ndiop by each villager were available and included in statistical analyses. The informed consent of each villager enlisted in this protocol (or that of the parents in the case of children) was obtained at the beginning of the study and was renewed at the beginning of each year of the survey. The study design received clearance from the national Senegalese ethical committee.20
Antibody responses determined in Ndiop village were tested by multivariate analysis using stepwise regression process available in the JMP® (SAS) software. The number of malaria attacks identified during the 3 years of active follow-up following the plasma sampling was used as a Log10+1-transformed continuous variable. Both age and Log10-transformed antibody responses were simultaneously tested as explanatory variables and controlled for to explain the number of malaria attacks identified for each individual. The F ratios, (i.e., the ratio of the mean square for the effect divided by the mean square of the error) were calculated as well as probabilities associated with F ratio values (i.e., the probability was tested that, given that the null hypothesis was true, an even larger F statistic would occur due to random error).21
Results
Synthetic polypeptides consisting of 2-4 fragments with α-helical coiled coil protein motif described in our previous publication4 were co-linearly synthesized by using a non-immunogenic linker, di-ethylene glycol (DEG). The P181 construct is composed of P90 (38 amino acids), P77 (28 amino acids) and P27 (27 amino acids) fragments with a modified diethylene glycol inserted between the fragments (see Materials and Methods). The selection of these 3 among 12 candidates that were previously identified,4 was guided by the frequency of recognition by human antibodies, their in vitro biological activities, immunogenicity in mice and fragment length to obtain a poly-epitope amenable to peptide synthesis.22 The linking of the 3 epitopes stabilizes the α–helical conformation of the single epitopes as seen in Fig. 1. In fact, the CD profile of P181 with minima at 207 and 222 nm and a high intensity band at 207 nm indicates a high content of α-helical structures in contrast to those of the single epitopes.
Fig. 1. CD profile of P181 and its constituents.

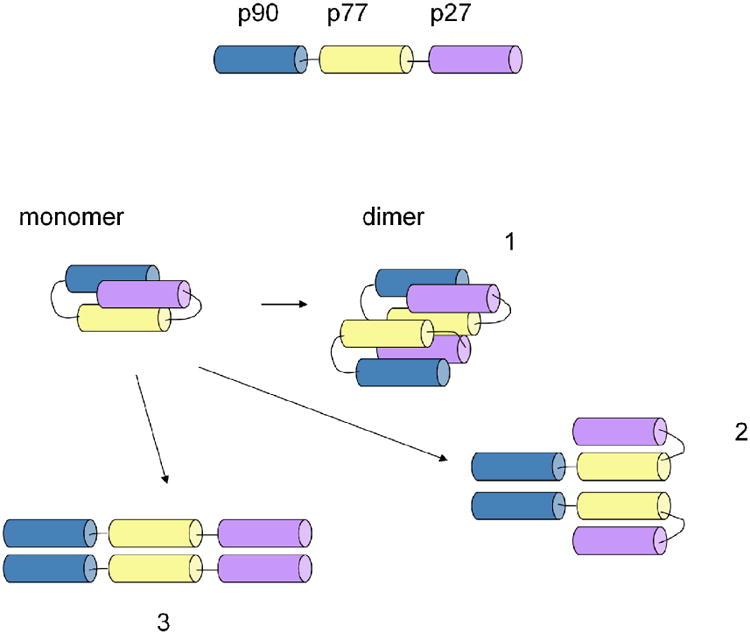
The sedimentation coefficient distribution c(s) exhibited distinct peaks of approximately equal areas at s20,w values of ∼1.2 S and ∼1.8 S, with a best-fit average frictional ratio of ∼1.41 corresponding to species of ∼ 10 kDa and 18 kDa. Within the typical error of this analysis, this strongly suggests the presence of monomers and dimers. The measured frictional ratio is best consistent with hydrodynamic properties of the moderately compact structures in Figure 2, 1 and 2, rather than the most extended form 3.
Fig.2. Structural model of P181.
In order to examine the association state in more detail, we next performed sedimentation equilibrium experiments with samples at several loading concentrations. Cell-average molecular weights were in between that of the monomer and dimer. The global analysis with a monomer-dimer self-association model resulted in an estimated equilibrium constant of KD = 115 (±15) μM.
Antigenic recognition
Sera
The majority of the adult donor population living in Burkina Faso. Tanzania and PNG recognized the constituent fragments (range 52-83%, Table 1). The prevalence of recognition of the poly-epitope was similar or higher than the individual components (73%, 79% and 90% respectively for BF, TZ and PNG samples). As expected, average ELISA OD values for P181 were higher than those observed for the individual epitopes.
Table 1. Prevalence of positive donors.
| Burkina Faso (N=37) | Tanzania (N=42) | Papua New Guinea (N=37) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean OD + 3SD (%) | OD Ratio (%) | Mean OD | Mean OD + 3SD (%) | OD Ratio (%) | Mean OD | Mean OD + 3SD (%) | OD Ratio (%) | Mean OD | |
| P181 | 73 | 65 | 0,549 | 79 | 74 | 0,493 | 90 | 83 | 0,871 |
| P90 | 62 | 41 | 0,340 | 83 | 43 | 0,270 | 86 | 62 | 0,450 |
| P77 | 73 | 51 | 0,295 | 71 | 55 | 0,371 | 71 | 55 | 0,403 |
| P27 | 63 | 43 | 0,310 | 52 | 31 | 0,279 | 83 | 64 | 0,520 |
Prevalence: % of donors whose serum gave an OD value in ELISA higher than the mean OD + 3 standard deviations of negative controls (naïve European donors) or OD ratio > or = 2. Sera dilution = 1:200. Mean OD comprises the mean of all OD.
T-cell proliferation
Peripheral blood mononuclear cells of adults living in malaria-endemic region of South-West Nigeria (12 out of 13) proliferated when incubated with P181 solution. This proportion was relatively higher than that of the single constituents (range 23-54%). In addition, the average SI obtained with P181 (9.8) was also higher as compared to that obtained with each peptide (2.2-3.2; Table 2).
Table 2. Human PBMC proliferation in the presence of different antigens.
| Prev - (%) | SI | |
|---|---|---|
| P181 | 92 | 9.8 |
| P90 | 23 | 3.2 |
| P77 | 54 | 3.1 |
| P27 | 38 | 2.2 |
PBMC proliferation of adult donors living in Nigeria (N=13). Each antigen was put in culture in 200 μl at 10 μM (5 μM for P181) together with 2×105 PBMC at 37°C for 5 days. The cultures were then pulsed overnight with 3H-thymidine, and stimulation index (SI) was regarded significant when the radioactivity of stimulated over unstimulated cultures was ≥ 2.
Human antibodies specific for P181
Human antibodies specific for P181 and each of its constituents were obtained from pools of sera obtained from adults living in Papua New Guinea. The reactivity and specificity of purified antibodies were confirmed by ELISA (Table 3). Antibodies specific for each of the components recognized the P181. Likewise, anti-P181 antibodies recognized each of the individual fragments.
Table 3. Cross reactivity of human antigen-specific antibodies.
| IgG anti- | ||||
|---|---|---|---|---|
|
|
||||
| P181 | P90 | P77 | P27 | |
| P181 | ++++ | ++ | + | +++ |
|
| ||||
| P90 | + | +++ | - | - |
|
| ||||
| P77 | + | - | ++ | - |
|
| ||||
| P27 | ++ | - | - | +++ |
Affinity purified antibodies specific for P181 and its constituents recognize P181 and its constituents and vice-versa (+ to ++++ represent positive ELISA at dilutions 1:300 to 1:8100).
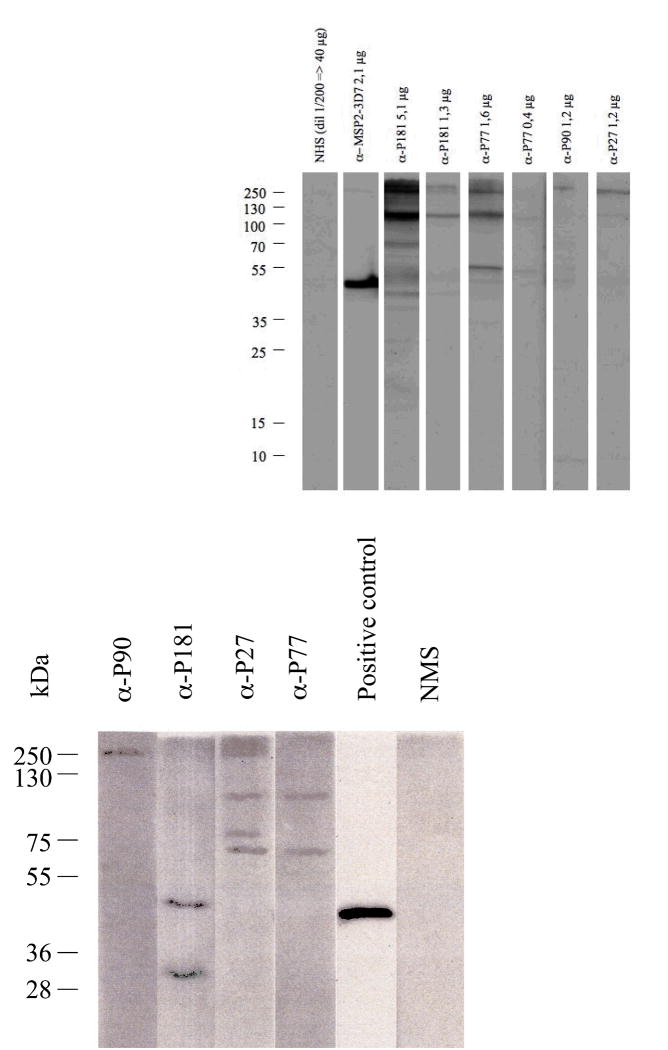
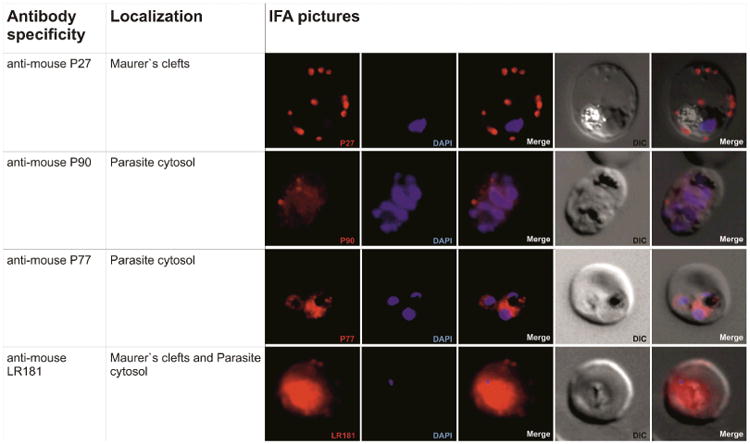
The P181-specific mouse or human antibodies recognized parasite structures on P. falciparum-infected erythrocytes in IFA (Fig. 3a). They also produced distinct bands by western blotting of infected erythrocyte lysates (Fig. 4a).
Fig. 3.
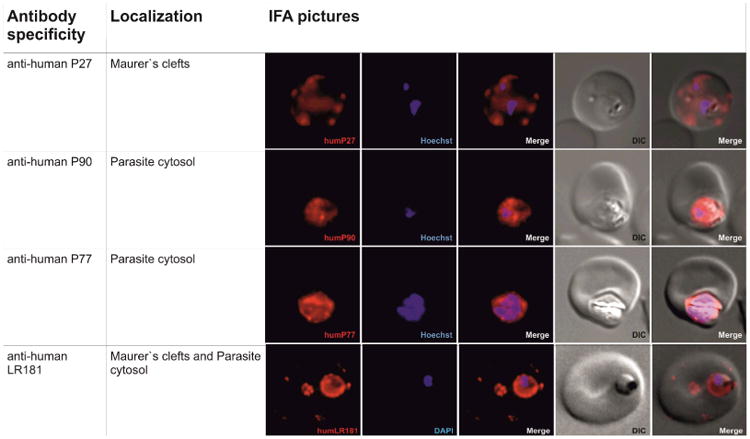
(a) Immunofluorescence staining of P. falciparum infected erythrocytes with affinity purified human antibodies (P27, P77, P90 and P181 in red). First row: P27 specific human antibodies indicate localization of the corresponding protein PFF0165c to the periphery of the RBC (in red). Nucleus stained with Hoechst (in blue), transmission light microscopy of the infected red blood cell (differential interference contrast, DIC) and merged picture of the nucleus and P27 specific antibody signal (merge).Second and third rows: P77 or P90 specific human sera indicate the localization of the corresponding proteins PF08_0048 or PFD0520c respectively to the cytoplasma of the parasite. Nucleus stained with Hoechst (in blue), transmission light microscopy of the infected red blood cell (DIC) and merged picture of the nucleus and P77 or P90 signal (merge). Fourth row: P181 specific human antibodies. Signal at the periphery of the RBC and the cytoplasm of the parasite indicates that sera raised against the poly-epitope recognizes the three corresponding proteins PFF0165c, PF08_0048 and PFD0520c. Nucleus stained with DAPI (in blue), transmission light microscopy of the infected red blood cell (DIC) and merged picture of the nucleus and P181 (merge).
(b) Immunofluorescence staining of P. falciparum infected erythrocytes with murine antibodies against P27, P77, P90 or P181,respectively. First row: P27 specific human antibodies indicate localization of the corresponding protein PFF0165c to the periphery of the RBC (in red). Nucleus stained with Hoechst (in blue), transmission light microscopy of the infected red blood cell (DIC) and merged picture of the nucleus and P27 specific serum signal (merge). Second and third rows: Localization of PF08_0048 and PFD0520c using mouse sera raised against P77 and P90 respectively (in red). Staining indicates localization of both proteins to the parasite cytoplasm. Nucleus stained with DAPI (in blue), transmission light microscopy of the infected red blood cell (DIC) and merged picture of the nucleus and P77 or P90 respectively. Fourth row: Signal in the infected RBC using mouse sera raised against the poly-epitope P181 (in red). Signal at the periphery of the RBC and the cytoplasm of the parasite indicates that sera raised against the poly-epitope recognizes the three corresponding proteins PFF0165c, PF08_0048 and PFD0520c. Nucleus stained with DAPI (in blue), transmission light microscopy of the infected red blood cell (DIC) and merged picture of the nucleus and P181 (merge).
Fig 4.
(a) Immunoblot from saponin-lysed 3D7 late stage parasite extracts electrophoresed under reducing conditions. Immunoblot was probed with affinity purified human α-P181, α-P27, α-P77, α-P90, normal human serum (NHS) and affinity purified human α-MSP-2-3D7 which was used as a positive control. Relative size standards are indicated on the left in kDa.
(b) Immunoblot from saponin-lysed 3D7 late stage parasite extracts electrophoresed under reducing conditions. Immunoblot was probed with mouse α-P181, α-P27, α-P77, α-P90, normal mouse serum (NMS) and α-R634 which was used as a positive control. α-R634 was a second bleed sera obtained from rabbits immunized with recombinant MSP-2. Relative size standards are indicated on the left in kDa.
Immunogenicity in mice
High antibody titers were induced in inbred and outbred mice immunized with P181 formulated with Alhydrogel, GLA-SE and Montanide AS 720 in several strains of mice. The induced antibodies also recognized all or part of the individual constituents depending on the adjuvant or mouse strain used (Table 4a and b). Similarly, anti-sera specific for P27, P77 and P90 recognize the poly-epitope.
Table 4. a. Immunogenicity of P181 and its constituents in mice immunized with Montanide ISA 720 and cross-reactivity of specific sera.
| Immunogen | Mice | Antigen | GMT | SD | Responders |
|---|---|---|---|---|---|
| P181 | CB6F1 | P181 | 5.10 | 0.28 | 4/4 |
| P90 | 3.20 | 0.90 | 3/4 | ||
| P27 | 4.50 | 0.24 | 4/4 | ||
| P77 | 4.46 | 0.72 | 4/4 | ||
| C3H | P181 | 5.34 | 0.00 | 3/3 | |
| P90 | 4.54 | 0.28 | 3/3 | ||
| P27 | 4.04 | 0.55 | 3/3 | ||
| P77 | 4.70 | 0.28 | 3/3 | ||
| ICR | P181 | 4.97 | 0.46 | 4/4 | |
| P90 | 2.79 | 1.17 | 2/4 | ||
| P27 | 4.32 | 0.87 | 4/4 | ||
| P77 | 3.77 | 0.46 | 4/4 | ||
|
| |||||
| P27 | CB6F1 | P27 | 4.08 | 0.43 | 5/5 |
| P181 | 4.68 | 0.55 | 5/5 | ||
| C3H | P27 | 5.10 | 0.34 | 4/4 | |
| P181 | 5.34 | 0.00 | 4/4 | ||
| ICR | P27 | 3.90 | 0.39 | 4/4 | |
| P181 | 4.73 | 0.46 | 4/4 | ||
|
| |||||
| P77 | CB6F1 | P77 | 4.70 | 0.72 | 4/4 |
| P181 | nd | ||||
| C3H | P77 | 5.00 | 0.55 | 3/3 | |
| P181 | 4.22 | 0.28 | 3/3 | ||
| ICR | P77 | 4.98 | 0.24 | 4/4 | |
| P181 | 4.70 | 0.72 | 4/4 | ||
|
| |||||
| P90 | CB6F1 | P90 | 2.00 | 0.00 | 0/4 |
| P181 | nd | ||||
| C3H | P90 | 5.00 | 0.60 | 4/4 | |
| P181 | 4.86* | ||||
| ICR | P90 | 2.98 | 0.81 | 2/4 | |
| P181 | 2.33 | 0.46 | 0/4 | ||
| GMT titers of immune sera determined as last dilution with OD higher than OD control serum + 3 SD. Responder animal is arbitrarily considered positive if GMT value is ≥ 3. | |||||
| *pool of 4 sera | |||||
| Table 4b: Immunogenicity of P181 in mice immunized with different adjuvants and serum cross-reactivity. | |||||
|---|---|---|---|---|---|
| Adjuvant | Mice | Antigen | GMT | SD | Responders |
| GLA-SE | C3H | P181 | 5.00 | 0.55 | 3/3 |
| P90 | 2.28 | 0.55 | 0/3 | ||
| P27 | 3.89 | 0.48 | 3/3 | ||
| P77 | 4.68 | 0.55 | 3/3 | ||
| CB6F1 | P181 | 3.77 | 0.46 | 4/4 | |
| P90 | 2.00 | 0.00 | 0/4 | ||
| P27 | 2.86 | 0.87 | 2/4 | ||
| P77 | 2.11 | 0.24 | 0/4 | ||
| ICR | P181 | 4.45 | 0.67 | 9/9 | |
| P90 | 2.18 | 0.42 | 0/9 | ||
| P27 | 4.00 | 0.84 | 8/9 | ||
| P77 | 3.38 | 0.62 | 5/8 | ||
|
| |||||
| Alum | C3H | P181 | 5.34 | 0.00 | 4/4 |
| P90 | 5.10 | 0.28 | 4/4 | ||
| P27 | 4.97 | 0.46 | 4/4 | ||
| P77 | 5.34 | 0.00 | 4/4 | ||
| CB6F1 | P181 | 4.15 | 0.72 | 5/5 | |
| P90 | 2.09 | 0.21 | 0/5 | ||
| P27 | 3.14 | 0.26 | 2/5 | ||
| P77 | 3.50 | 1.37 | 3/5 | ||
| ICR | P181 | 4.21 | 1.14 | 6/7 | |
| P90 | 2.43 | 0.55 | 1/7 | ||
| P27 | 3.57 | 1.03 | 5/7 | ||
| P77 | 3.96 | 1.06 | 6/7 | ||
GMT titers of immune sera determined as last dilution with OD higher than OD control serum + 3 SD. Responder animal is arbitrarily considered positive if GMT value is ≥ 3.
All mouse sera recognized parasite structures in malaria-infected erythrocytes by IFA (Fig. 3b) and in lysates in Western blots (Fig. 4b). PF08_0048 has a predicted size of 247 KDa, thus the full length protein was not detected on Western Blots.
ADCI and association with protection
For ADCI experiments, only those experiments in which an optimal growth rate of the parasite was obtained (e.g. >6-fold increase per 48 h), were kept for analysis. Table 5 shows the SGI values obtained as compared to the positive control, the pool of Ivory Coast adult immunoglobulins previously employed in passive transfer experiments in humans. As the ADCI assay is not a quantitative assay and because there is a dose dependent increase in ADCI activity only at very low antibody concentrations [19], whereas, at higher concentration, there is a plateau of activity, all tested antibodies were assessed at a single antibody concentration of 15 μg/ml.
To assess the association with protection longitudinal follow-up studies were performed among inhabitants of Dielmo, Senegal. The first study involved 45 individuals among whom 22 had no malaria attack during a 3-year follow-up period and 23 had one or more malaria attacks during the same period. Both have the same age-distribution. These results were also confirmed among 102 Ndiop inhabitants while also adjusting for age and occurrence of malaria attacks.
In multivariate analysis, when both age and the time spent in the village were systematically controlled in each statistical test, antibodies to P27 were high when the number of malaria attacks was low after 1 and 3 years of active and daily clinical follow-up of the villagers (after 1-year F ratio = 8.92 and p = 0.0047, after 3 years F ratio = 11.74; p = 0.0012; Table 6). For P181, after 1 year of follow-up, the F ratio was 11.43; p = 0.0016 whereas after 3 years of study, the F ratio was 15.48; p = 0.0003). For a given age and a determined period of presence in the village, a two-fold increase in anti-P27 antibody response was potentially associated with a 1.5-fold decrease in the number of malaria attacks expected to occur after 3 years of follow-up. The mean age of the cohort studied was 16.1 ± 11.1 years. The mean period of time spent in the village was 319 ± 85 days during the first year of follow-up (i.e. people remained continuously in the village during 87.4% of the year) and 862 ± 282 days during the 3 years of follow-up (people remained permanently in the village during 78.6% of the 3 year period). A two-fold increase in anti-P181 antibody responses was potentially linked with a 1.6 fold reduction in the number of malaria attacks expected during the same period of study. The marked association between protection and anti-P181 responses reflects the contribution of the results obtained when testing anti-P27 antibody responses; the representation of P27 epitopes within the multi-epitope construct also suggests that anti-P27 constitute a substantial part of Abs to P181.
Discussion
As a follow-up to our previous studies4,23 in which several novel B cell epitopes were identified as possible targets of a protective immune response against P. falciparum, we describe here the evaluation of a poly-epitope constructed by selecting 3 promising fragments, P90, P77 and P27 derived from 3 novel proteins PFD0520c, PF08_0048, PFF0165c. By combining fragments with specific immunological properties (e.g high antigenicity, high immunogenicity, association with protection in humans, high activity of specific antibodies in ADCI assay), we attempted to integrate these features in a single construct and, thus, increase the capacity of the poly-subunit antigen to induce effective immune responses in a genetically heterogeneous human populations since each fragment can act as a helper T-cell epitope. Use of hybrid recombinant constructs as vaccine candidates has already been applied for malaria, influenza, HIV, tuberculosis and allergy.24-28
With regard to the structural characteristics of P181, the data obtained by CD and analytical ultracentrifugation measurements indicate that the poly-epitope has a high content of α-helical structure, and is a dimer in equilibrium with its monomer, thus, suggesting a two-strand α-helical coiled coil conformation (Fig. 2). Indeed, recombinant protein PFD0520c, from which P90 is derived, has also been determined to be a dimer with high α-helical structure content (manuscript in preparation), and P27 is predicted to fold as a dimer. The linking together of several epitopes stabilises the α-helical structure of individual components as indicated by the CD data. Changing the order of peptides in the poly-epitope does not seem to substantially change the physico-chemical and immunological properties of the new construct (data not shown).
The resemblance of the poly-epitope structure to that assumed by the individual fragments within the corresponding proteins has been the basis of our initial epitope selection, and is of paramount importance to the identification and development of an erythrocytic malaria vaccine where protection is mediated by antibodies.
With regards to the antigenic properties of P181, the poly-peptide was better recognized by antibodies (similar or higher prevalence and higher average OD) and by PBMC from Nigerian donors in terms of prevalence and SI. Similarly, when considering immunogenicity in mice, the poly-epitope was generally more immunogenic in terms of antibody titers than the individual fragments. In addition, it provided T-cell help to those individual antigens, which were not immunogenic by themselves in certain strains (see P90, for example; table 4a). This concept is important when considering the use of P181 as vaccine candidate in humans with respect to a mixture of single components.
Affinity-purified human antibodies and mouse sera specific for P181 and its components recognized parasitic structures in IFA and highlighted a limited number of proteins at MW consistent with the size of the corresponding parasitic proteins (24, 240 and 160 kD) or their fragments, except for anti-P90 antibodies which consistently failed to bind proteins of the 25 kD range (manuscript in preparation). Human specific antibodies to the 4 constructs are all active in ADCI, consistent with our previous observation for the constituent polypeptides.4 Of note however is the fact that the observed ADCI activity of P181 is lower than that observed for P27, but higher than that seen for P77 and P90. Thus, it reflects the mean ADCI activity of the individual components. Alternatively, this might reflect experimental variation in the ADCI assay, which is only a semi-quantitative assay, or a competition of different antibodies with FcγRII and FcγRIII receptors present on human monocytes.17 More work is needed to clarify this point. Finally, the association of the antibody response to protection from malaria indicate a stronger relationship with P181 than for its single components. We would like to think that this is due to the better structural similarity of the poly-epitope to the native structure of the individual components.
In summary, given the higher prevalence and recognition of P181 by natural antibodies in ELISA, its higher stimulation index in the T-cell proliferation assay, its greater immunogenicity in mice, together with the inhibitory activity of antibodies specific to P181 as assessed by ADCI and with the strong association with protection from clinical malaria as determined in a 3-year longitudinal study in adults, P181 appears to be a stronger vaccine candidate than the individual peptides and warrants a further development as a malaria vaccine.
Finally, since chemical synthesis of larger fragments are now possible,21 it is conceivable to design constructs similar to P181 but with a wider immunological spectrum by including P. falciparum and P. vivax fragments.
Table 5. In vitro inhibition of P. falciparum 3D7 strain by human affinity purified antibodies specific for P181 and its constituent peptides.
| SGI* | |
|---|---|
| P181 | 55% |
| P90 | 43% |
| P77 | 36% |
| P27 | 106% |
SGI- specific growth inhibition of human specific antibodies.
Table 6.
The antibody responses found in Ndiop were tested in multivariate analysis by stepwise regression. The number of malaria attacks identified during the 3 years following the serum sampling was used as a continuous variable. Both age and Log10-transformed antibody responses were simultaneously tested as explanatory variables.
| Anti-peptide IgGs: | F ratios: | p values: |
|---|---|---|
| IgG1-P27 | 11.74 | 0.001 |
| IgG3-P77 | 3.31 | 0.076 |
| IgG1-P90 | 0.88 | 0.354 |
| IgG1-P181 | 15.48 | 0.0003 |
The F ratio, (i.e.. the ratio of the mean square for the effect divided by the mean square of the error) was calculated, and F statistics were used in order to determine that the effect test was null. By testing the hypothesis that the lack of fit was zero, the F test indicated if all parameters of an individual effect were null. The probabilities indicated in the Table correspond to the significance levels determined for the F ratio values (i.e. the probability that given that the null hypothesis is true, an even larger F statistic would occur due to random error). Using Bonferroni correction (i.e. taking into account that 18 different statistical tests were carried out), individual p values ≤0.0028 can still be considered as significant.
Acknowledgments
This work was supported by the Swiss National Science Foundation grant 310000-112244 and by the grants of the Swiss Secretary for Education and Research (No 0536) in the context of Commission of the European Communities, Sixth Framework Programme, contract LSHP-CT-2003-503240, “Mucosal Vaccines for Poverty-Related Diseases” (MUVAPRED) and the Intramural Research Program of the NIBIB, NIH. We would like to thank Drs S. Reed and T. Vedvick for providing advice and GLA-SE whose work was supported by a grant#42387 from the Bill & Melinda Gates Foundation.
References
- 1.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7(6) doi: 10.1371/journal.pmed.1000290. p. 1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10(8):545–55. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 3.Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villard V, Agak GW, Frank G, Jafarshad A, Servis K, Nebie I, et al. Rapid identification of malaria vaccine candidates based on alpha-helical coiled coil protein motif. PLoS One. 2007;2(7):645. doi: 10.1371/journal.pone.0000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262(5138):1401–7. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- 6.Atherton E, Sheppard RC. Solid phase synthesis; a practical approach. IRL Press, Oxford University Press; Oxford: 1989. [Google Scholar]
- 7.Balbo A, Brown PH, Braswell EH, Schuck P. Measuring protein-protein interactions by equilibrium sedimentation. Curr Protoc Immunol. 2007;Chapter 18 doi: 10.1002/0471142735.im1808s79. Unit 18 8. [DOI] [PubMed] [Google Scholar]
- 8.Balbo A, Zhao H, Brown PH, Schuck P. Assembly, loading, and alignment of an analytical ultracentrifuge sample cell. J Vis Exp. 2009;(33) doi: 10.3791/1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PH, Balbo A, Schuck P. Characterizing protein-protein interactions by sedimentation velocity analytical ultracentrifugation. Curr Protoc Immunol. 2008;Chapter 18 doi: 10.1002/0471142735.im1815s81. Unit 18 15. [DOI] [PubMed] [Google Scholar]
- 10.Schuck P. 2010 p. https://sedfitsedphat.nibib.nih.gov/software/default.aspx.
- 11.Cohn EJ, Edsall JT. Density and apparent specific volume of proteins. In: Cohn EJ, Edsall JT, editors. Proteins, Amino Acids and Peptides. Princeton, NJ: Van Nostrand-Reinhold; 1943. pp. 370–381. [Google Scholar]
- 12.Lepori L, Mollica V. Volumetric properties of dilute aqueous-solutions of poly(ethylene glycols) J Polym Sci B. 1978;16(6):1123–1134. [Google Scholar]
- 13.Schuck P. 2010 https://sedfitsedphat.nibib.nih.gov/software/default.aspx.
- 14.Vistica J, Dam J, Balbo A, Yikilmaz E, Mariuzza RA, Rouault TA, et al. Sedimentation equilibrium analysis of protein interactions with global implicit mass conservation constraints and systematic noise decomposition. Anal Biochem. 2004;326(2):234–56. doi: 10.1016/j.ab.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Olugbile S, Kulangara C, Bang G, Bertholet S, Suzarte E, Villard V, et al. Vaccine potentials of an intrinsically unstructured fragment derived from the blood stage associated Plasmodium falciparum protein PFF0165c. Infect Immun. 2009;77:5701–9. doi: 10.1128/IAI.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers T, Poshusta GR, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75(1):123–32. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton G, Vedvick T, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27(23):3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172(6):1633–41. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafarshad A, Dziegiel MH, Lundquist R, Nielsen LK, Singh S, Druilhe P. A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcgammaRII and FcgammaRIII. J Immunol. 2007;178(5):3099–106. doi: 10.4049/jimmunol.178.5.3099. [DOI] [PubMed] [Google Scholar]
- 20.Rogier C, Trape JF. Study of premunition development in holo- and meso-endemic malaria areas in Dielmo and Ndiop (Senegal): preliminary results, 1990-1994. Med Trop (Mars) 1995;55(4 Suppl):71–6. [PubMed] [Google Scholar]
- 21.Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape JF. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4(11):320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olugbile S, Habel C, Servis C, Spertini F, Verdini A, Corradin G. Malaria vaccines-The long synthetic peptide approach: Technical and conceptual advancements. Curr Opin Mol Ther. 2010;12(1):64–76. [PubMed] [Google Scholar]
- 23.Agak GW, Bejon P, Fegan G, Gicheru N, Villard V, Kajava AV. Longitudinal analyses of immune responses to Plasmodium falciparum derived peptides corresponding to novel blood stage antigens in coastal Kenya. Vaccine. 2008;26(16):1963–71. doi: 10.1016/j.vaccine.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17(2):189–94. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 25.Kallinteris NL, Lu X, Wu S, Hu H, Li Y, Gulfo JV, et al. Ii-Key/MHC class II epitope hybrid peptide vaccines for HIV. Vaccine. 2003;21(27-30):4128–32. doi: 10.1016/s0264-410x(03)00493-6. [DOI] [PubMed] [Google Scholar]
- 26.Kocken CH, Hundt E, Knapp B, Brazel D, Enders B, Narum DL, et al. Immunization of Aotus monkeys with recombinant Plasmodium falciparum hybrid proteins does not reproducibly result in protection from malaria infection. Infect Immun. 1998;66(1):373–5. doi: 10.1128/iai.66.1.373-375.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115(5):1010–6. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 28.Naruse H, Ogasawara K, Takami K, Kajino K, Gotohda T, Itoh Y, et al. Analysis of epitopic residues introduced into the hybrid peptide vaccines prepared according to the cassette theory. Vaccine. 1994;12(9):776–82. doi: 10.1016/0264-410x(94)90285-2. [DOI] [PubMed] [Google Scholar]