Abstract
Background
Deep brain stimulation provides significant symptomatic benefit for people with advanced Parkinson's disease whose symptoms are no longer adequately controlled with medication. Preliminary evidence suggests that subthalamic nucleus stimulation may also be efficacious in early Parkinson's disease, and results of animal studies suggest that it may spare dopaminergic neurons in the substantia nigra.
Objective
We report the methodology and design of a novel Phase I clinical trial testing the safety and tolerability of deep brain stimulation in early Parkinson's disease and discuss previous failed attempts at neuroprotection.
Methods
We recently conducted a prospective, randomized, parallel-group, single-blind pilot clinical trial of deep brain stimulation in early Parkinson's disease. Subjects were randomized to receive either optimal drug therapy or deep brain stimulation plus optimal drug therapy. Follow-up visits occurred every six months for a period of two years and included week-long therapy washouts.
Results
Thirty subjects with Hoehn & Yahr Stage II idiopathic Parkinson's disease were enrolled over a period of 32 months. Twenty-nine subjects completed all follow-up visits; one patient in the optimal drug therapy group withdrew from the study after baseline. Baseline characteristics for all thirty patients were not significantly different.
Conclusions
This study demonstrates that it is possible to recruit and retain subjects in a clinical trial testing deep brain stimulation in early Parkinson's disease. The results of this trial will be used to support the design of a Phase III, multicenter trial investigating the efficacy of deep brain stimulation in early Parkinson's disease.
Keywords: Parkinson's disease, deep brain stimulation, subthalamic nucleus, research design
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder affecting millions of people with a vast majority of those affected older than 60 years old [1]. Patients experience relentlessly progressive disability from a combination of motor symptoms as well as a profuse collection of poorly treatable nonmotor symptoms [2-4]. Medical management provides significant symptomatic control of the motor symptoms in early PD, but in later stages the benefits are less robust. Although many clinical trials have sought to prove a disease modifying effect, no therapeutic intervention (oral medications, medical devices, or ablative procedures) has been conclusively proven to halt or even slow the progression of the disease [5, 6].
The single most important confounding factor to effectively designing a trial positioned to conclusively prove that the experimental treatment is slowing disease progression or imparting a neuroprotective effect is the lack of a biomarker that can be repeatedly measured and reliably mark disease progression without being subject to corruption by ongoing standard-of-care therapies. Clinical assessments, while not perfect, are in many ways the best measures of disease progression currently available. A host of study designs have been implemented to attempt to prove neuroprotection, including prolonged washout periods or an expanded focus on the time elapsed before experiencing a predefined clinical event, clinical worsening or the need for a new medication. While there is no perfect study design, the following trials have made valiant efforts to test therapies' potential for imparting neuroprotection.
The DATATOP (Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism) study was a placebo-controlled clinical trial attempting to demonstrate neuroprotection based on a delayed need for levodopa and was the first major attempt at neuroprotection [7-9]. However, it was later determined that the symptomatic effect of the treatment was responsible for the observed improvement in motor symptoms [9].
The ELLDOPA (Earlier Versus Later Levodopa) trial utilized a two week medication washout in an attempt to control for symptomatic effects [10]. Although the treatment group had superior UPDRS scores compared to the placebo group, the long half-lives of PD medications, many of which take weeks to fully wash out, made it difficult to separate the symptomatic effect of the treatment from its effect on the underlying disease. Furthermore, the clinical results did not correspond with the neuroimaging results [11].
Several studies, particularly CALM-PD-CIT (Comparison of the Agonist Pramipexole versus Levodopa on Motor Complications of Parkinson's Disease) and REAL-PET (Requip as EArly therapy versus L-dopa-PET), have used neuroimaging to measure dopamine levels as a surrogate for disease progression [9, 12, 13]. These studies ultimately failed to conclusively document disease progression because varying degeneration in dopaminergic neurons did not coincide with clinical changes and benefit. Although imaging studies would theoretically be superior to clinical measures because they are relatively unaffected by subjective factors, no imaging study has been shown to reliably track disease progression.
The ADAGIO (Attenuation of Disease Progression with Azilect Given Once Daily) trial is the most recent attempt to document neuroprotection and is arguably the most rigorous and carefully designed study to date [14]. Utilizing a delayed-start design, this double-blind, placebo-controlled, dual dose clinical trial made considerable efforts to circumvent many of the problems associated with previous trials and originally showed promise due to the preliminary results of the TEMPO (Rasagiline (TVP-1012) in Early Monotherapy for Parkinson's Disease Outpatients) trial [15]. However, on October 17, 2011, the FDA advisory committee voted unanimously to withhold the proposed expanded indication of rasagiline as a disease-modifying agent, citing inconsistent and uncompelling evidence. Although the delayed-start design is advantageous because it separates the disease-modifying effect of the tested therapy from its symptomatic effect, it is inherently flawed in that the second phase is open-label and effectively unblind [9]. The FDA's recent rejection of the ADAGIO trial necessitates the design of more clinical trials seeking disease modification using the best methods currently available. Any treatment proven to slow disease progression will be a landmark achievement for people with PD [16].
We recently conducted the first prospective, randomized, single-blind, pilot clinical trial of deep brain stimulation (DBS) in early PD. DBS of the subthalamic nucleus (STN) was FDA approved for PD in 2002 and is a safe and effective adjunctive treatment for treating advancing symptoms that are not adequately controlled with medication [17]. The surgery improves quality of life and markedly improves motor symptoms while reducing motor fluctuations and medication needs [18-22]. Despite its proven efficacy in advanced PD, DBS has not been studied in the very early stages of PD given the good symptomatic control of motor symptoms with oral medications. Nonetheless, there are isolated case reports and trials that have shown positive results in both unilateral and bilateral STN DBS performed in mid-stage PD [23, 24]. None yet have looked at very early stage PD patients [25].
Some assert that earlier use of STN DBS in PD may provide an even greater impact on patient care and quality of life [26-28]. Limited studies of human PD patients implanted with STN DBS for prolonged periods suggest slowing of the clinical progression of PD in some patients, which is inconsistent with the known natural disease course and indirectly suggests a disease-modifying effect [27, 29-32]. Pre-clinical animal studies involving DBS and STN lesions also suggest a potential neuroprotective effect [33-37], although the mechanism of action remains unclear.
Materials and Methods
The U.S. Food and Drug Administration provided an investigational device exemption (IDE) (G050016) allowing 30 subjects with Hoehn and Yahr (H&Y) Stage II PD off medication to participate in this trial, which received Vanderbilt University Institutional Review Board approval (040797). Subjects were randomized to receive either optimal drug therapy (ODT) alone or ODT plus bilateral STN DBS. There were two primary aims of the study: (1) to compare safety and tolerability of bilateral STN DBS plus ODT to ODT alone in early stage PD; and (2) to demonstrate that it is possible to recruit, provide meaningful informed consent, and retain subjects in a long-term clinical trial of bilateral STN DBS for the treatment of early PD. Secondary aims included: (1) to estimate the minimum length of time a subject must remain off both medication and stimulation (aka the wash-out period) to practically measure untreated PD; (2) to provide preliminary data to estimate sample size for a Phase III trial; (3) to test the data management procedures; and (4) to test the viability and functionality of specific safety and patient outcome measures.
Subjects of any gender, race or socioeconomic status, aged 50 to 75, H&Y Stage II off medication, free of motor fluctuations or dyskinesias, exhibiting a stable response to levodopa, and on dopaminergic therapy longer than six months but less than four years were eligible to enroll in this trial. Because unique ethical concerns are involved in offering surgery to early PD patients, a multiphase informed consent process originally developed by biomedical ethicists (MB, SF) was implemented prior to any study procedures. This involved: (1) distribution of the informed consent document and educational materials to interested parties; (2) three informational follow-up visits; and (3) a formal visit to provide informed consent no earlier than seventy-two hours after the last informational session. During informational visits, each potential subject met individually with the study principal investigator, neurosurgeon, and the biomedical ethicist for an hour each. The biomedical ethicist provided subjects a series of questions to further consider before planning the informed consent visit. The formal consent visit was attended by the study coordinator, a neurologist (TD) other than the principal investigator, and the biomedical ethicist to answer any final questions. After providing consent, subjects underwent a detailed screening evaluation to ensure that they met the enrollment criteria.
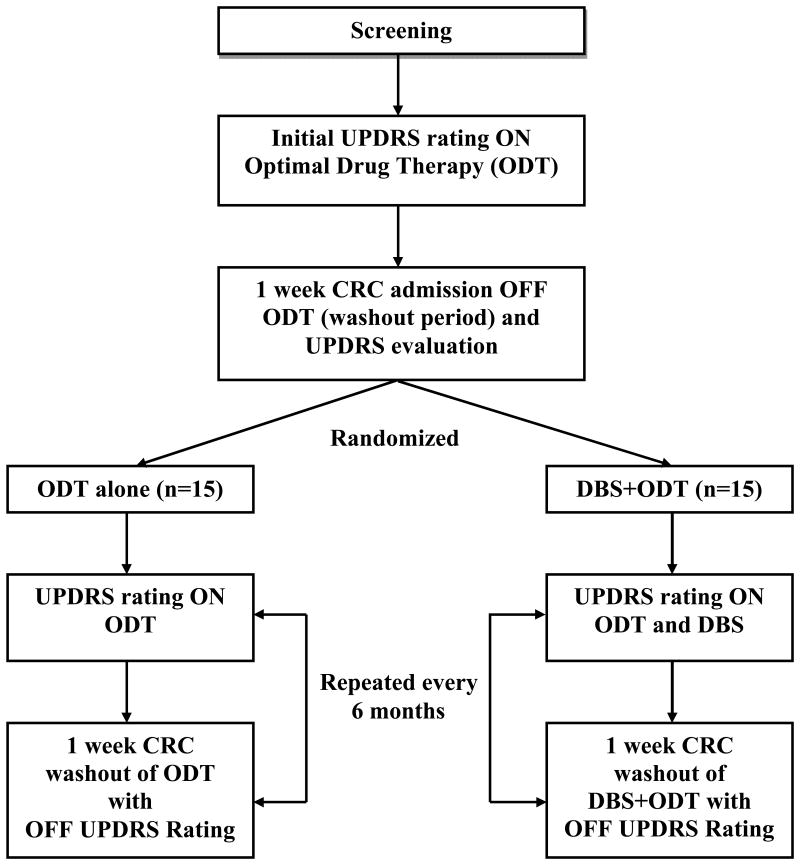
Screening consisted of a history and physical examination by the primary investigator (DC), a complete psychiatric examination (RS), a neuropsychological assessment, and a MRI of the brain with 2.5 mm slices, which was reviewed by the neurosurgeon (PK). Subjects had to have a clinical diagnosis of PD and be deemed medically and neurologically suitable for brain surgery to pass screening. PD was diagnosed by Vanderbilt University Movement Disorders Specialists (DC, TD, PH, FP) based on the presence of three out of four clinical features (resting tremor, bradykinesia, rigidity, asymmetric onset) and an absence of features suggestive of an alternative diagnosis. H&Y and Unified Parkinson's Disease Rating Scale (UPDRS-III) scores were obtained 36 hours off medications. Medication response was documented by at least a 30% reduction in score on the motor subsection of the (UPDRS-III) from the “36-hour off medication” to “on medication” state. Complete inclusion/exclusion criteria are presented in Figure 1 and study design is presented in Figure 2.
Figure 1. Inclusion/Exclusion Criteria.
Detailed screening measures were employed in order to ensure patient appropriateness for the trial. Subjects not meeting the inclusion/exclusion criteria were not eligible to participate in the trial.
Figure 2. Study Design.
This trial was a prospective, randomized, single-blind clinical trial comparing the safety and tolerability of DBS+ODT to ODT alone. Detailed inpatient assessments were performed every 6 months for a total of 2 years to monitor safety.
After completing the screening assessment, subjects were admitted to Vanderbilt University's General Clinical Research Center (GCRC) for an initial baseline “Core Assessment” to characterize the severity of their PD. This consisted of an eight-day inpatient evaluation and medication washout during which subjects were assessed “ON” (the condition when both the subject and physician agreed that the patient experienced maximal therapeutic benefit from medication alone or from medication and stimulation (if randomized to the treatment group) in future visits) and “OFF” (the condition when a subject had received no antiparkinsonian medications and the DBS stimulation was off (if randomized to the treatment group) in future visits).
Upon admission to the GCRC (Day 0), an admission history and neurological examination, H&Y, Schwab and England (S&E), medications and adverse events were recorded. On Day 1, a complete UPDRS and a videotaped UPDRS-III assessment were completed in the “ON” state. An eight-hour diary while on medication was used to capture the percent “ON” time and “ON” time without dyskinesias. All medications (and DBS if present at later visits) were discontinued at 1600 on Day 1. For the next seven days, UPDRS-III OFF ratings were recorded daily. Abbreviated autonomic testing was also performed to monitor the potential development of a neuroleptic malignant like syndrome resulting from dopaminergic drug withdrawal. Evaluation of neuropsychological function was performed in the “OFF” state. On the seventh day off medication, another videotaped UPDRS-III was completed, and H&Y and S&E ratings were recorded. Medications (and DBS if present at later visits) were restored and optimized prior to patient discharge from the GCRC on Day 8. Subjects could elect to ‘escape’ the washout period at any time if the increasing PD symptoms became intolerable. In this case, the same videotaped assessment planned for Day 8 would be performed and medications (and DBS, if present at later visits) would be restored. No subjects elected this option.
The biostatistician (LW) created a computer generated random number table using the R software (http://www.r-project.org/). This table randomized fifteen subjects into the control group (ODT alone) and fifteen subjects into the treatment group (bilateral STN DBS +ODT). Block randomization ensured equal numbers of subjects in the two groups during the course of the trial. To minimize bias, the randomization scheme was concealed in identical sealed envelopes labeled by subject ID and the investigators were unaware of the size or order of the blocks. After a subject completed the expanded informed consent process, screening, and baseline evaluation; the principal investigator and subject together opened the envelope assigning treatment status.
Subjects randomized to receive DBS were implanted within sixty days. The procedure for implantation of the leads and neurostimulators in this study occurred in three stages, was identical to standard of care for advanced PD, and is well documented in the medical literature and device manufacturer technical manuals. All DBS surgeries were performed by the same surgeon (PK) and interpretation of microelectrode recordings and stimulation effects were completed by the same neurosurgeon, neurophysiologist, and neurologist (PK, CK, and DC). Operative and microelectrode targeting procedures for all of the subjects in this study are described in detail in Kahn et al and Remple et al [38, 39].
Subjects presented for the first programming session four weeks after Stage II and thirty-six hours off medication. The device was initially turned on at a standard frequency of 130 Hz and a pulse width of 60 μs. Each lead contact was individually tested using monopolar stimulation with the contact as the cathode and the case of the neurostimulator as the anode. The voltage was slowly increased while assessing the improvement in parkinsonian motor signs and production of side effects. If voltage adjustment alone did not produce sufficient clinical benefit with an acceptable side effect profile, then various lead combinations (i.e., bipolar stimulation), frequencies, and pulse durations could be used. After conclusion of the initial programming session, stimulators were left on twenty four hours per day in all subjects. Two additional programming sessions were performed to optimize stimulation settings and medications. In an effort to minimize the effect of investigator bias, ongoing medication management was performed by the subjects' treating neurologists (not the principal investigator). Modest stimulation parameters were utilized and adjusted to the subjects' individual symptoms. No attempt was made to aggressively advance stimulation and subjects were encouraged to continue medications and not attempt to be treated with stimulation alone.
Visits identical to the core baseline assessment were performed every six months for two years. The visits encompassed the same eight day inpatient period with washout, daily assessments and videotaped UPDRS-III scores. Neuropsychological function was measured annually. All study procedures performed in the “ON” and “OFF” states in the surgery group included stimulation being on or off for those respective states. All videotaped assessments were randomly ordered and rated by an independent, blinded viewer after the conclusion of the study.
The primary endpoint to assess safety was the blinded UPDRS-III score in the off-medication (and off-stimulation, if present) state after a seven day washout. The null hypothesis for this pilot trial was that the DBS+ODT group would not worsen faster or more prominently than the ODT group. The time lapsed from a subject's baseline until a four point increase (worsening) in their UPDRS-III motor score assessed in the off condition represented a clinically important event related to disease progression. To preliminarily measure safety, the DBS+ODT group could not progress more rapidly or accrue more events (4 point worsening) when compared to the ODT group utilizing Kaplan-Meier curves. A two-sided log rank test with alpha of 0.05 was used to assess the statistical significance of the difference between the two survival curves. To protect against increasing the false-positive error rate from repeat interim analyses, a truncated O'Brien-Fleming-type boundary was computed with the use of the LAN-DeMets procedure. The estimated sample size of 30 subjects was based on this primary survival analysis and assumed a predicted average annual deterioration in the UPDRS-III score of the control group of 8.91 ± 8.41 based on previously published data in early stage PD [40], a predicted DBS effect size of 20%, and a dropout rate of 20% (3 subjects per group by 24 months). We estimated that a sample size of twelve in each group would have 86% power to detect the difference between a control group portion of 20% at six months and a treatment group portion of 80% at six months. This is based on a 0.05 alpha level, two sided chi-square test (Table 1).
Table 1. Power calculations for difference in proportions of the two groupsa.
It was estimated that 12 subjects in each group would achieve an 86% power calculation and detect the difference between the two groups at six months.
| Control Group (ODT alone) | Treatment Group (ODT plus STN DBS) | Odds Ratio | Power | Statistical N per Group |
|---|---|---|---|---|
| 0/12 | 6/12 | NA | NA | 12 |
| 1/12 | 8/12 | 22 | 87.2% | 12 |
| 2/12 | 9/12 | 15 | 84.3% | 12 |
| 3/12 | 10/12 | 15 | 84.3% | 12 |
| 4/12 | 11/12 | 22 | 87.2% | 12 |
Based on two-tailed chi-square tests and alpha levels of 0.05.
An independent Data Safety Monitoring Board consisting of a neurologist, a neurosurgeon, an internist, and a biostatistician was constituted at study start-up and empowered to recommend early termination of the study or change to the study procedures if it observed a treatment effect or identified safety concerns that exceeded pre-study specified boundaries. The board met biannually to review confidential interim analyses of safety outcomes and tabulation of adverse events. The DSMB never halted or modified the study.
Results
Enrollment opened in August 2006 and was completed in April 2009 with 15 subjects in each arm of the trial. All thirty subjects completed the baseline washout visit without major side effects. With the exception of one subject in the ODT group who withdrew from the study due to a family emergency and financial hardship, the remaining 29 subjects completed all follow-up assessments at 6, 12, 18, and 24 months and elected to join a second study collecting long term follow-up data through 5 years of therapy.
The average duration of anti-Parkinson's medication was approximately two years for each group. The groups were similar based on age and UPDRS-III scores. There was no significant difference between the groups with regard to medication use or total medication dose as measured by levodopa equivalents (Table 2). Baseline characteristics of the patients in this study are presented in detail in Charles et al [41].
Table 2. Baseline Patient Characteristicsa.
At baseline, the mean age of subjects was 60 years old and the mean medication use was just over 2 years in both groups. The mean total UPDRS score in subjects randomized to DBS+ODT was 3 points higher than subjects randomized to ODT; mean UPDRS-III scores were nearly equal.
| Characteristic | ODT (n=15) | ODT+DBS (n=15) |
|---|---|---|
| Gender | ||
| Male | 13 | 14 |
| Female | 2 | 1 |
| Age (yrs) at Enrollment | ||
| Mean | 60 ± 7.0 | 60 ± 6.8 |
| Range | 51 - 69 | 52 - 74 |
| Baseline Medicine Use | ||
| Mean Duration (yrs) | 2.1 ± 1.1 | 2.2 ± 1.4 |
| Mean L-dopa equivalents (mg/day)b | 569 ± 389 | 451 ± 304 |
| Baseline UPDRS Score | ||
| Mean Total | 36 ± 15 | 39 ± 14 |
| Mean UPDRS-III | 15 ± 7.6 | 15 ± 8.5 |
Reprinted from PD Charles, RM Dolhun, CE Gill, TL Davis, MJ Bliton, MG Tramontana, RM Salomon, L Wang, P Hedera, FT Phibbs, JS Neimat, PE Konrad, Deep brain stimulation in early Parkinson's disease: Enrollment experience from a pilot trial, 18 / 3, 268-73, 2011, with permission from Elsevier.
100 mg of Levodopa with a dopa-decarboxlase inhibitor = 130 mg of controlled-release Levodopa reparations = 83 mg of Levodopa with dopa-decarboxylase and COMT inhibitors = 1 mg of pergolide, pramipexole or lisuride = 3 mg of ropinirole [20].
Discussion
Our hypothesis that bilateral STN DBS will have a disease modifying effect if applied in very early PD was not intended to be answered by this pilot clinical trial. The purpose of this trial was to gather preliminary safety and tolerability data necessary for a future large scale trial and prove that STN DBS is a feasible experimental therapy for subjects with early PD. Furthermore, we intended to prove that subjects with very early stage PD could be ethically recruited and would provide informed consent, enroll, and complete a trial testing DBS even though they could otherwise expect years of satisfactory treatment with conventional medications.
This trial confirmed that the consent process and study design utilized in this trial is viable and can be applied to future multicenter endeavors. We were able to easily recruit and retain the maximum number of subjects allowed under the FDA IDE. Subjects remained engaged in the trial and were remarkably compliant with study protocol and follow-up visits. Surgeries, medication adjustments, washout periods, and other study procedures were well tolerated.
Weaknesses of the current study include an incomplete washout period and single-blind design. Use of sham surgery in order to create a double-blind design was not appropriate for the current pilot trial whose purpose was to gather preliminary safety and tolerability data. Recognizing the limitations and flaws of previous clinical trials seeking neuroprotection, a trial employing a washout period, while not ideal, remains the best design option. Nevertheless, a trial based on therapy washout also has inherent difficulties. There is not a predetermined time period for a washout of PD medications and the period tends to vary among medication classes due to different pharmacodynamics [10, 40, 42]. Standard periods for a washout of levodopa vary between one to two weeks and some assert even longer periods for dopamine agonist medications. Clinical trials, however, involve people and compromise must balance the desire for a perfect trial with what is reasonable to request of study participants.
Without a proven biomarker for disease progression that is not influenced by symptomatic therapy, some will conclude that a disease modifying effect could never be conclusively proven and therefore should never be attempted. This argument must be rejected, however, because the pre-clinical evidence for STN DBS potentially providing a neuroprotective effect is simply too strong to ignore [25-28, 31]. Though the discovery of a biomarker may be decades away, it would be unjust to patients suffering from this debilitating disease to wait until one has been identified to continue searching for a therapy that slows the relentless progression of PD. Furthermore, if a therapy is truly neuroprotective, its effect on the underlying disease may be so significant that it could be conclusively demonstrated to modify disease progression even without a biomarker.
We believe that this preliminary study provides the necessary data to launch a Phase III multicenter trial positioned to determine as best as possible whether the early application of DBS modifies disease progression, improves quality of life, and slows the development of disability in people with PD when compared to ODT. This holds inherent value as it allows physicians to limit medications and their systemic side effects. At the very least, because DBS is such a potent therapy in advanced disease, it is likely that the therapy will prove to be an effective adjunctive therapy to medical management in early stage PD as well. The design of a future trial should mimic the same construct with parallel groups but will likely include a vigorous debate on how to best assess comparative efficacy of our treatment groups as well as potential disease modification or neuroprotection.
Acknowledgments
Research reported in this publication was supported by Medtronic, Inc., by Vanderbilt CTSA grant UL1TR000445 from the National Center for Advancing Translational Sciences (NCATS), by NCATS/NIH award UL1TR000011, by NIH R01 EB006136, and by private donations. Medtronic representatives did not take part in data collection, management, analysis, or interpretation of the data or in preparation, review, or approval of the manuscript.
Footnotes
Authors' Contributions: DC made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; obtaining funding; supervision. DC has full access to the study data and takes full responsibility for the integrity and accuracy of data analysis.
CT made substantial contributions to the interpretation of data ; drafting of the manuscript ; critical revision of the manuscript.
TLD made substantial contributions to the conception and design; critical revision of the manuscript; administrative, technical or material support.
CEG made substantial contributions to the acquisition of data; interpretation of data; drafting of the manuscript; critical revision of the manuscript; administrative or technical support.
ALM made substantial contributions to the acquisition of data; interpretation of data; drafting of the manuscript; critical revision of the manuscript; administrative or technical support.
MJB made substantial contributions to the conception and design; critical revision of the manuscript; administrative, technical or material support.
MGT made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
RMS made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
CK made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
LW made substantial contributions to the the conception and design; interpretation of data; statistical analysis; critical revision of the manuscript.
PH made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support.
FTP made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support
JSN made substantial contributions to the conception and design; critical revision of the manuscript; administrative, technical or material support; supervision.
PEK made substantial contributions to the conception and design; critical revision of the manuscript; administrative, technical or material support; supervision.
Conflicts of Interest: Individual author conflict of interest declarations are as follows. Vanderbilt University has received income in excess of $10,000 from grants or con- tracts with Medtronic, Allergan, Ipsen, Merz, UCB, and Teva for research or educational programs led by Dr. Charles. Dr. Charles has received income in excess of $10,000 from Medtronic, Allergan, and Ipsen for education or consulting services. Dr. Davis has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. He also served as a consultant for Allergan, Novartis, and UCB and has received payment for lectures from Allergan and Teva as well as payment for development of educa- tional presentations from UCB and Teva. Dr. Hedera has received payment for lectures from Lundbeck. Dr. Phibbs has served as a consultant for Boston Scientific and Medtronic and has received payment for educational presentations for Teva. Dr. Neimat has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. Dr. Neimat has also received payment for lectures from Medtronic and FHC, Inc. He has also received reimbursement for travel/accommodations/meeting expenses apart from consultancy. Dr. Konrad has received personal compensation and Vanderbilt University has received grants to support research from Medtronic in excess of $10,000. Dr. Konrad also serves as a consultant for Medtronic and FHC, Inc.
References
- 1.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 2.Lim SY, Lang AE. The nonmotor symptoms of Parkinson's disease--an overview. Mov Disord. 25(Suppl 1):S123–30. doi: 10.1002/mds.22786. [DOI] [PubMed] [Google Scholar]
- 3.Salawu FK, Danburam A, Olokoba AB. Non-motor symptoms of Parkinson's disease: diagnosis and management. Niger J Med. 19:126–31. doi: 10.4314/njm.v19i2.56496. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. J Am Geriatr Soc. 2004;52:784–8. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology. 2009;72:S59–64. doi: 10.1212/WNL.0b013e318199068b. [DOI] [PubMed] [Google Scholar]
- 6.Pavese N, Kiferle L, Piccini P. Neuroprotection and imaging studies in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S33–7. doi: 10.1016/S1353-8020(09)70832-6. [DOI] [PubMed] [Google Scholar]
- 7.Shoulson I. Deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP). Parkinson Study Group. Acta Neurol Scand Suppl. 1989;126:171–5. doi: 10.1111/j.1600-0404.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 8.Effect of deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med. 1989;321:1364–71. doi: 10.1056/NEJM198911163212004. [DOI] [PubMed] [Google Scholar]
- 9.de la Fuente-Fernandez R, Schulzer M, Mak E, Sossi V. Trials of neuroprotective therapies for Parkinson's disease: problems and limitations. Parkinsonism Relat Disord. 16:365–9. doi: 10.1016/j.parkreldis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 12.Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 13.Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 14.Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med. 2009;361:1268–78. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 15.Hauser RA, Lew MF, Hurtig HI, Ondo WG, Wojcieszek J, Fitzer-Attas CJ. Long-term outcome of early versus delayed rasagiline treatment in early Parkinson's disease. Mov Disord. 2009;24:564–73. doi: 10.1002/mds.22402. [DOI] [PubMed] [Google Scholar]
- 16.Charles PD, Gill CE, Davis TL, Konrad PE, Benabid AL. Is deep brain stimulation neuroprotective if applied early in the course of PD? Nat Clin Pract Neurol. 2008;4:424–6. doi: 10.1038/ncpneuro0848. [DOI] [PubMed] [Google Scholar]
- 17.Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 18.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 19.Charles PD, Padaliya BB, Newman WJ, Gill CE, Covington CD, Fang JY, et al. Deep brain stimulation of the subthalamic nucleus reduces antiparkinsonian medication costs. Parkinsonism Relat Disord. 2004;10:475–9. doi: 10.1016/j.parkreldis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 21.Lozano AM, Mahant N. Deep brain stimulation surgery for Parkinson's disease: mechanisms and consequences. Parkinsonism Relat Disord. 2004;10(Suppl 1):S49–57. doi: 10.1016/j.parkreldis.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schupbach WM, Maltete D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–71. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 24.Shichi T, Okiyama R, Yokochi F, Taniguchi M, Takahashi H, Hamada I. Unilateral subthalamic stimulation for early-stage Parkinson's disease. No To Shinkei. 2005;57:495–8. [PubMed] [Google Scholar]
- 25.Mesnage V, Houeto JL, Welter ML, Agid Y, Pidoux B, Dormont D, et al. Parkinson's disease: neurosurgery at an earlier stage? J Neurol Neurosurg Psychiatry. 2002;73:778–9. doi: 10.1136/jnnp.73.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espay AJ, Vaughan JE, Marras C, Fowler R, Eckman MH. Early versus delayed bilateral subthalamic deep brain stimulation for parkinson's disease: a decision analysis. Mov Disord. 25:1456–63. doi: 10.1002/mds.23111. [DOI] [PubMed] [Google Scholar]
- 27.Ostergaard K, Aa Sunde N. Evolution of Parkinson's disease during 4 years of bilateral deep brain stimulation of the subthalamic nucleus. Mov Disord. 2006;21:624–31. doi: 10.1002/mds.20776. [DOI] [PubMed] [Google Scholar]
- 28.Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–95. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–9. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 30.Tagliati M, Martin C, Alterman R. Lack of motor symptoms progression in Parkinson's disease patients with long-term bilateral subthalamic deep brain stimulation. Int J Neurosci. 120:717–23. doi: 10.3109/00207454.2010.518777. [DOI] [PubMed] [Google Scholar]
- 31.Visser-Vandewalle V, van der Linden C, Temel Y, Celik H, Ackermans L, Spincemaille G, et al. Long-term effects of bilateral subthalamic nucleus stimulation in advanced Parkinson disease: a four year follow-up study. Parkinsonism Relat Disord. 2005;11:157–65. doi: 10.1016/j.parkreldis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: a target for neuroprotection. Ann Neurol. 1998;44:S175–88. doi: 10.1002/ana.410440726. [DOI] [PubMed] [Google Scholar]
- 33.Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg. 2004;100:679–87. doi: 10.3171/jns.2004.100.4.0679. [DOI] [PubMed] [Google Scholar]
- 34.Nakao N, Nakai E, Nakai K, Itakura T. Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced by the mitochondrial toxin 3-nitropropionic acid. Ann Neurol. 1999;45:640–51. [PubMed] [Google Scholar]
- 35.Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408–14. doi: 10.1111/j.1460-9568.1996.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 36.Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100–5. doi: 10.1016/j.brainres.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 37.Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- 38.Kahn E, D'Haese PF, Dawant B, Allen L, Kao C, Charles PD, et al. Deep brain stimulation in early stage Parkinson's disease: operative experience from a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry. 83:164–70. doi: 10.1136/jnnp-2011-300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remple MS, Bradenham CH, Kao CC, Charles PD, Neimat JS, Konrad PE. Subthalamic nucleus neuronal firing rate increases with Parkinson's disease progression. Mov Disord. 26:1657–62. doi: 10.1002/mds.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med. 1993;328:176–83. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 41.Charles PD, Dolhun RM, Gill CE, Davis TL, Bliton MJ, Tramontana MG, et al. Deep brain stimulation in early Parkinson's disease: Enrollment experience from a pilot trial. Parkinsonism Relat Disord. doi: 10.1016/j.parkreldis.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser RA, Koller WC, Hubble JP, Malapira T, Busenbark K, Olanow CW. Time course of loss of clinical benefit following withdrawal of levodopa/carbidopa and bromocriptine in early Parkinson' s disease. Mov Disord. 2000;15:485–9. [PubMed] [Google Scholar]
- 43.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]