Abstract
Although diet is believed to influence colorectal cancer risk, the long-term effects of a diet with a high glycemic load are unclear. The growing recognition that colorectal cancer may be promoted by hyperinsulinemia and insulin resistance suggests that a diet inducing high blood glucose levels and an elevated insulin response may contribute to a metabolic environment conducive to tumor growth. We prospectively followed a cohort of 38 451 women for an average of 7.9 years and identified 174 with incident colorectal cancer. We used baseline dietary intake measurements, assessed with a semiquantitative food-frequency questionnaire, to examine the associations of dietary glycemic load, overall dietary glycemic index, carbohydrate, fiber, nonfiber carbohydrate, sucrose, and fructose with the subsequent development of colorectal cancer. Cox proportional hazards models were used to estimate relative risks (RRs). Dietary glycemic load was statistically significantly associated with an increased risk of colorectal cancer (adjusted RR = 2.85, 95% confidence interval [CI] = 1.40 to 5.80, comparing extreme quintiles of dietary glycemic load; Ptrend = .004) and was associated, although not statistically significantly, with overall glycemic index (corresponding RR = 1.71, 95% CI = 0.98 to 2.98; Ptrend = .04). Total carbohydrate (adjusted RR = 2.41, 95% CI = 1.10 to 5.27, comparing extreme quintiles of carbohydrate; Ptrend = .02), nonfiber carbohydrate (corresponding RR = 2.60, 95% CI = 1.22 to 5.54; Ptrend = .02), and fructose (corresponding RR = 2.09, 95% CI = 1.13 to 3.87; Ptrend = .08) were also statistically significantly associated with increased risk. Thus, our data indicate that a diet with a high dietary glycemic load may increase the risk of colorectal cancer in women.
The growing recognition that colorectal cancer may be promoted by hyperinsulinemia and insulin resistance suggests that a diet inducing high blood glucose levels and an elevated insulin response may contribute to a metabolic environment conducive to tumor growth. Dietary and lifestyle risk factors for developing insulin resistance, such as physical inactivity, obesity, and positive energy balance, also increase the risk of developing colorectal cancer and other cancers (1,2). Insulin stimulates pathways that increase levels of insulin-like growth factor, and both insulin and insulin-like growth factor promote mitosis and cell proliferation but inhibit apoptosis in normal and cancer cells of the colonic epithelium (3,4). Foods rapidly digested and absorbed can cause sudden increases in blood glucose and corresponding increases in insulin response (5). The glycemic index is used to rank foods containing a fixed amount of carbohydrate (generally, 50 g) by their effects on blood glucose (6). A food’s glycemic load is determined from its glycemic index and the carbohydrate content of a standard serving (7). Although diet is believed to influence colorectal cancer risk, the long-term effects of a diet with a high glycemic load are unclear. We investigated whether dietary glycemic load was associated with the risk of colorectal cancer by using data from the Women’s Health Study.
The Women’s Health Study is composed of 39 876 health professionals who were 45 years old or older at baseline from April 1993 to January 1996. It was originally designed as a randomized trial of aspirin, vitamin E, and β-carotene for prevention of cardiovascular disease and cancer, although the β-carotene treatment was terminated in January 1996. This study has been conducted according to the ethical guidelines of Brigham and Women’s Hospital. Written informed consent was obtained from all participants.
A 131-item semiquantitative food-frequency questionnaire was administered at baseline to assess average dietary intake during the previous year (8). Women with more than 70 blanks in the dietary questionnaire or with a daily total energy intake of less than 2514 kJ or more than 14 665 kJ were excluded from these analyses, leaving a cohort of 38 451. Participants completed risk factor questionnaires at baseline and annually thereafter. Glycemic index values were obtained from published tables (9) and from The Nutrition Center of the University of Toronto. We used the mean when multiple glycemic index test values were reported. For mixed dishes, we used a weighted average of the glycemic index of each component food. The glycemic load for each food item was calculated by multiplying the food’s glycemic index by the number of carbohydrate grams in a serving. Dietary glycemic load for each participant was estimated by multiplying the glycemic load for each food by the participant’s frequency of consumption and then summing over all foods (8). The overall glycemic index for each participant represents the average glycemic index of carbohydrate in the diet and was obtained by dividing the participant’s dietary glycemic load by total carbohydrate intake. Glucose was used as the standard in calculating glycemic index and glycemic load values.
The physiologic relevance and validity of dietary glycemic load, as assessed by food-frequency questionnaires, are supported by several studies. In a cross-sectional study of healthy postmenopausal women (8), dietary glycemic load was positively associated with plasma triacylglycerol concentrations and negatively associated with plasma high-density lipoprotein cholesterol concentrations. In another study (10), it was positively associated with high-sensitivity C-reactive protein, a marker of systemic inflammation and a risk factor for ischemic heart disease. In prospective cohort studies (7,11,12), dietary glycemic load has been positively associated with increased risk of coronary heart disease and diabetes mellitus.
We categorized dietary exposures into quintiles of intake and, after determining that the data met the assumptions for using Cox proportional hazards modeling, we used this method to estimate hazard ratios. We used baseline dietary intake measurements, assessed with a semiquantitative food-frequency questionnaire, to examine the associations of dietary glycemic load, overall glycemic index, carbohydrate, fiber, nonfiber carbohydrate, sucrose, and fructose with colorectal cancer risk. To test for trend, we assigned the quintile median value to each subject in that quintile. We report 95% confidence intervals (CIs) and P values from two-sided statistical tests. All dietary variables were adjusted for total energy intake with the residual method (13). Follow-up time was calculated from baseline through the date of diagnosis of colorectal cancer, death, drop-out, loss to follow-up, or the end of the follow-up period.
Average follow-up was 7.9 years, during which we identified 174 patients with incident colorectal cancer (148 of the colon and 26 of the rectum). The mean dietary glycemic load for the cohort was 117, and the mean overall glycemic index was 53 (Table 1). In age-adjusted models, we observed statistically significant positive associations of both dietary glycemic load and overall glycemic index with colorectal cancer (Table 2). In multivariable analyses that included total energy intake and nutrient risk factors (fat, fiber, folate, calcium, and vitamin D) in the models, colorectal cancer risk estimates for dietary glycemic load increased (adjusted relative risk [RR] = 2.85, 95% CI = 1.40 to 5.80, comparing extreme quintiles of dietary glycemic load; Ptrend = .004), but overall glycemic index risk estimates were essentially unchanged (adjusted RR = 1.71, 95% CI = 0.98 to 2.98; Ptrend = .04, comparing extreme quintiles of overall glycemic index). Including fruit and vegetable, red meat, and whole grain intake in place of the nutrient risk factors resulted in risk estimates similar to those in the age-adjusted models, although confidence intervals were wider and crossed the null (data not shown). Risk estimates for total carbohydrate (adjusted RR = 2.41, 95% CI = 1.10 to 5.27, comparing extreme quintiles of carbohydrate; Ptrend = .02), nonfiber carbohydrate (corresponding RR = 2.60, 95% CI = 1.22 to 5.54; Ptrend = .02), sucrose (corresponding RR = 1.51, 95% CI = 0.90 to 2.54; Ptrend = .06), and fructose (corresponding RR = 2.09, 95% CI = 1.13 to 3.87; Ptrend = .08) were consistent with but lower than the dietary glycemic load findings. Fiber intake was inversely associated with risk, although estimates crossed the null, and there was no evidence of a linear trend (adjusted RR = 0.79, 95% CI = 0.45 to 1.38, comparing extreme quintiles of fiber; Ptrend = .50).
Table 1.
Baseline distributions of nutrients and colorectal cancer risk factors by quintile of energy-adjusted dietary glycemic load
| Cohort mean |
Quintile |
P value |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Glycemic load and overall glycemic index medians | |||||||
| Dietary glycemic load | 117 | 92 | 106 | 117 | 127 | 143 | |
| Overall glycemic index | 53 | 49 | 51 | 53 | 54 | 57 | |
| Energy-adjusted glycemic load* | |||||||
| No. of women in quintile | 7690 | 7690 | 7691 | 7690 | 7690 | ||
| Energy, kJ/d | 7222 | 7103 | 7341 | 7359 | 7270 | 7031 | .009† |
| Fat, g/d | 58 | 69 | 63 | 58 | 54 | 46 | <.001† |
| Protein, g/d | 81 | 89 | 85 | 82 | 79 | 71 | <.001† |
| Carbohydrate, g/d | 222 | 177 | 205 | 222 | 237 | 267 | <.001† |
| Fiber, g/d | 19 | 16 | 18 | 19 | 20 | 22 | <.001† |
| Nonfiber carbohydrate, g/d | 203 | 161 | 187 | 202 | 217 | 245 | <.001† |
| Fructose,‡ g/d | 42 | 31 | 38 | 42 | 46 | 56 | <.001† |
| Sucrose, g/d | 41 | 31 | 37 | 40 | 44 | 51 | <.001† |
| Calcium, mg/d | 1015 | 971 | 1022 | 1037 | 1038 | 1005 | <.001† |
| Folate, µg/d | 428 | 383 | 410 | 432 | 449 | 467 | <.001† |
| Vitamin D, IU/d | 352 | 349 | 354 | 359 | 357 | 339 | .030† |
| Age, y | 53.9 | 53.5 | 53.6 | 53.9 | 54.2 | 54.4 | <.001† |
| Body mass index, kg/m2 | 26.0 | 26.6 | 26.3 | 26.1 | 25.7 | 25.2 | <.001† |
| Physical activity, kJ/wk | 4056 | 3485 | 3804 | 4102 | 4319 | 4562 | <.001† |
| Alcohol use rarely/never, % | 32.8 | 40.1 | 43.1 | 48.4 | 59.0 | <.001§ | |
| Never smoker, % | 39.2 | 48.3 | 53.0 | 56.3 | 58.2 | <.001§ | |
| History of oral contraceptive use, % | 72.2 | 70.5 | 69.0 | 69.0 | 66.9 | <.001§ | |
| Hormone replacement therapy use, % | 40.2 | 41.6 | 42.2 | 42.9 | 42.5 | <.001§ | |
| Nonsteroidal anti-inflammatory drug use >1 time per wk, % | 21.2 | 20.2 | 20.6 | 19.5 | 18.4 | <.001§ | |
| Colon polyp history, % | 2.8 | 2.4 | 2.4 | 2.4 | 2.5 | .40§ | |
| Family history of colorectal cancer, % | 10.2 | 10.2 | 11.3 | 10.8 | 9.2 | <.001§ | |
| History of diabetes at baseline, % | 3.8 | 2.7 | 2.5 | 2.0 | 1.6 | <.001§ | |
All values except age have been standardized according to the age distribution of the cohort. Within quintiles of glycemic load, continuous variables are reported as means and categorical variables are reported as percentages. Nutrient variables are energy-adjusted.
P for a test for linear trend.
Fructose includes the fructose component of sucrose.
P for a test of independence.
Table 2.
Relative risk of colorectal cancer by quintiles of energy-adjusted dietary glycemic load, overall glycemic index, carbohydrate, fiber, nonfiber carbohydrate, sucrose, and fructose
| Quintile of intake | |||||||
|---|---|---|---|---|---|---|---|
| Lowest | 2 | 3 | 4 | Highest | Ptrend* | Continuous† | |
| Dietary glycemic load | |||||||
| No. of colorectal cancer cases‡ | 26 | 30 | 37 | 32 | 49 | ||
| Total No. of person-years of follow-up | 61 084 | 61 213 | 61 190 | 60 976 | 60 872 | ||
| Age-adjusted relative risk (95% CI) | 1.00 (referent) | 1.13 (0.67 to 1.91) | 1.34 (0.81 to 2.22) | 1.14 (0.68 to 1.90) | 1.68 (1.04 to 2.71) | .03 | 1.08 (1.00 to 1.15) |
| P = .04 | |||||||
| Multivariable-adjusted relative risk (95% CI)§ | 1.00 (referent) | 1.34 (0.76 to 2.34) | 1.81 (1.02 to 3.21) | 1.63 (0.86 to 3.09) | 2.85 (1.40 to 5.80) | .004 | 1.18 (1.04 to 1.33) |
| P = .007 | |||||||
| Overall glycemic index | |||||||
| No. of colorectal cancer cases‡ | 24 | 37 | 31 | 43 | 39 | ||
| Total No. of person-years of follow-up | 61 153 | 60 879 | 61 132 | 61 183 | 60 988 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 1.58 (0.94 to 2.63) | 1.31 (0.77 to 2.24) | 1.86 (1.13 to 3.07) | 1.73 (1.04 to 2.87) | .03 | 1.06 (1.01 to 1.11) |
| P = .02 | |||||||
| Multivariable-adjusted risk (95% CI)§ | 1.00 (referent) | 1.59 (0.95 to 2.67) | 1.24 (0.71 to 2.16) | 1.93 (1.15 to 3.24) | 1.71 (0.98 to 2.98) | .04 | 1.05 (1.00 to 1.11) |
| P = .05 | |||||||
| Carbohydrate | |||||||
| No. of colorectal cancer cases‡ | 30 | 25 | 37 | 36 | 46 | ||
| Total No. of person-years of follow-up | 61 100 | 61 241 | 61 159 | 61 079 | 60 756 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 0.80 (0.47 to 1.37) | 1.14 (0.71 to 1.85) | 1.07 (0.66 to 1.75) | 1.31 (0.83 to 2.08) | .12 | 1.03 (0.99 to 1.08) |
| P = .15 | |||||||
| Multivariable-adjusted risk§(95% CI) | 1.00 (referent) | 1.04 (0.59 to 1.85) | 1.58 (0.88 to 2.83) | 1.71 (0.89 to 3.29) | 2.41 (1.10 to 5.27) | .02 | 1.11 (1.02 to 1.21) |
| P = .02 | |||||||
| Fiber | |||||||
| No. of colorectal cancer cases‡ | 35 | 36 | 22 | 41 | 40 | ||
| Total No. of person-years of follow-up | 60 976 | 61 242 | 61 052 | 61 074 | 60 990 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 0.93 (0.58 to 1.49) | 0.53 (0.31 to 0.91) | 0.91 (0.58 to 1.44) | 0.80 (0.50 to 1.27) | .49 | 0.89 (0.68 to 1.15) |
| P = .35 | |||||||
| Multivariable-adjusted risk (95% CI)§ | 1.00 (referent) | 0.99 (0.61 to 1.62) | 0.57 (0.32 to 1.00) | 0.96 (0.58 to 1.59) | 0.79 (0.45 to 1.38) | .50 | 0.83 (0.61 to 1.14) |
| P = .25 | |||||||
| Nonfiber carbohydrate | |||||||
| No. of colorectal cancer cases‡ | 27 | 29 | 40 | 32 | 46 | ||
| Total No. of person-years of follow-up | 61 129 | 61 198 | 61 131 | 61 069 | 60 809 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 1.01 (0.60 to 1.72) | 1.42 (0.87 to 2.30) | 1.09 (0.65 to 1.82) | 1.52 (0.94 to 2.45) | .08 | 1.04 (1.00 to 1.09) |
| P = .08 | |||||||
| Multivariable-adjusted risk (95% CI)§ | 1.00 (referent) | 1.30 (0.73 to 2.29) | 1.97 (1.11 to 3.52) | 1.62 (0.83 to 3.13) | 2.60 (1.22 to 5.54) | .02 | 1.11 (1.02 to 1.21) |
| P = .02 | |||||||
| Sucrose | |||||||
| No. of colorectal cancer cases‡ | 28 | 29 | 33 | 39 | 45 | ||
| Total No. of person-years of follow-up | 60 883 | 60 871 | 61 169 | 61 126 | 61 286 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 1.00 (0.60 to 1.69) | 1.11 (0.67 to 1.84) | 1.27 (0.78 to 2.07) | 1.41 (0.88 to 2.27) | .08 | 1.07 (0.96 to 1.19) |
| P = .21 | |||||||
| Multivariable-adjusted risk (95% CI)§ | 1.00 (referent) | 1.05 (0.62 to 1.80) | 1.19 (0.70 to 2.02) | 1.41 (0.84 to 2.35) | 1.51 (0.90 to 2.54) | .06 | 1.08 (0.96 to 1.21) |
| P = .19 | |||||||
| Fructose‖ | |||||||
| No. of colorectal cancer cases‡ | 21 | 38 | 38 | 34 | 43 | ||
| Total No. of person-years of follow-up | 61 002 | 61 117 | 61 065 | 61 107 | 61 044 | ||
| Age-adjusted risk (95% CI) | 1.00 (referent) | 1.66 (0.97 to 2.83) | 1.59 (0.93 to 2.71) | 1.36 (0.79 to 2.35) | 1.69 (1.00 to 2.85) | .18 | 1.02 (0.92 to 1.14) |
| P = .73 | |||||||
| Multivariable-adjusted risk (95% CI)§ | 1.00 (referent) | 1.87 (1.07 to 3.28) | 1.88 (1.06 to 3.33) | 1.68 (0.93 to 3.06) | 2.09 (1.13 to 3.87) | .08 | 1.04 (0.91 to 1.18) |
| P = .60 | |||||||
P values were obtained from two-sided Wald tests.
Continuous variables are 10 U/day, except for overall glycemic index, which is 1 U/day. CI = confidence interval.
The 174 cases of colorectal cancer and the number of person-years are shown for age-adjusted models; five cases were dropped from the multivariable models (n = 169) because of missing covariate information.
Multivariable model was adjusted for age (y), body mass index (kg/m2), history of oral contraceptive use (yes, no), postmenopausal hormone use (never, past, current), family history of colorectal cancer (yes, no), smoking (never, past, current), alcohol use (never/rarely, 1–3 drinks per mo, 1–6 drinks per wk, ≥1 drink per day), physical activity (tertiles of kJ expended weekly in recreational activity and stair climbing), nonsteroidal anti-inflammatory use (never/rarely, >1 time per wk), total energy intake (natural logarithm-transformed kJ), energy-adjusted total fiber (g) (except for fiber model), energy-adjusted total fat (g), energy-adjusted folate (µg), energy-adjusted calcium (mg), and energy-adjusted vitamin D (mg).
Fructose includes the fructose component of sucrose.
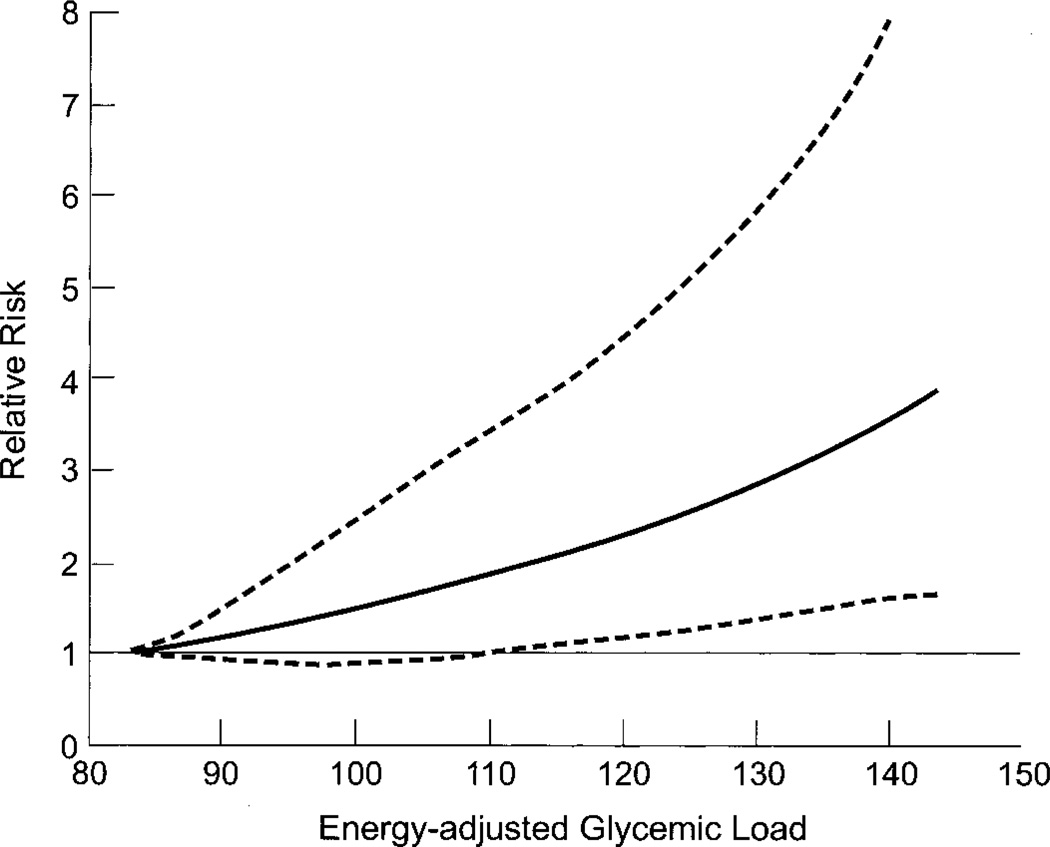
Dietary glycemic load and overall glycemic index risk estimates did not change appreciably in separate analyses when we excluded the first year of follow-up or when we restricted the outcome to colorectal cancer to women who did not report a colon polyp at baseline or to women with no history of diabetes mellitus (data not shown). Although the randomized treatments in this study should not be associated with the exposure measures and, hence, should not be a source of confounding, we checked this possibility by including these variables in a set of models and found that estimates were unchanged. We used restricted cubic spline regression (14) to test dietary glycemic load (Fig. 1), overall glycemic index, and carbohydrate intake for nonlinearity of the dose–response curves; none of the results was statistically significant. Risk was greater for distal colorectal cancer (adjusted RR = 2.87, 95% CI = 0.98 to 8.46, comparing extreme quintiles of dietary glycemic load; Ptrend = .08) than for proximal colorectal cancer (corresponding RR = 1.75, 95% CI = 0.53 to 5.77; Ptrend = .18), although these estimates are based on few cases. We examined the combined effects of dietary glycemic load and body mass index on colorectal cancer risk (adjusted RR = 2.91, 95% CI = 1.26 to 6.75, comparing body mass index ≥25 kg/m2 and highest quintile of glycemic load to body mass index <25 kg/m2 and lowest quintile of glycemic load; Pinteraction = .77) and the combined effects of dietary glycemic load and physical inactivity (adjusted RR = 2.31, 95% CI = 0.85 to 6.23, comparing highest quintile of glycemic load and lowest tertile of physical activity to lowest quintile of glycemic load and highest tertile of physical activity; Pinteraction = .28), but we did not have sufficient statistical power to fully examine this question.
Fig. 1.
Multivariable-adjusted relative risk of colorectal cancer as a function of glycemic load. Data were fit by using a restricted cubic spline Cox proportional hazards model, adjusted for the same covariates as in Table 2. Glycemic load values above the 95th percentile were deleted to make the graph more stable; knots were placed at the 5th, 25th, 75th, and 95th percentiles of the remaining observations. Dotted lines = 95% confidence intervals; solid line = adjusted relative risk of colorectal cancer as a function of glycemic load.
Previous studies examining dietary glycemic load and colorectal cancer have yielded mixed results. Three case–control studies (15–17) have reported positive associations, but a large prospective cohort study of Canadian women (18) found no increase in risk (adjusted RR = 1.05, 95% CI = 0.73 to 1.53, comparing extreme quintiles of dietary glycemic load; Ptrend = .94). As in our study, however, an increase in risk was reported for cancer of the distal colon.
The dietary and lifestyle factors that we examined are interrelated and difficult to measure. Our findings may be biased by unmeasured confounders or by residual confounding. In this cohort, women with a high dietary glycemic load intake, compared with women with a low intake, had an otherwise beneficial risk profile. Residual confounding by risk factors such as body mass index, physical inactivity, smoking, alcohol use, and nutrient intake would most likely bias risk estimates toward the null, implying that true risk may be greater than our estimates. When the glycemic index value of a particular food was unavailable, we used the reported value for a similar food. This procedure is a source of possible measurement error because glycemic index values can vary greatly, depending largely on how a food is processed and cooked. A dietary questionnaire designed primarily to measure glycemic load could include different or additional food items and groupings that would allow better discrimination between participants and possibly facilitate comparison between studies. A diet with a high glycemic load may increase the risk of colorectal cancer by affecting insulin and insulin-like growth factors or, as suggested by the cross-sectional association between dietary glycemic load and C-reactive protein (10), by exacerbating proinflammatory responses, either locally or systemically. Further work is needed to elucidate these mechanisms. In conclusion, findings from this prospective cohort study suggest that a diet with a high glycemic load may increase the risk of colorectal cancer in women.
Acknowledgments
Supported by Public Health Service grants CA47988 and T32 CA09142 (National Cancer Institute), HL43851, HL58755 (National Heart, Lung, and Blood Institute), DK02767 (National Institute of Diabetes and Digestive and Kidney Diseases), from the National Institutes of Health, Department of Health and Human Services.
We acknowledge the crucial contributions of the entire staff of the Women’s Health Study (WHS), under the leadership of David Gordon, as well as Susan Burt, Mary Breen, Marilyn Chown, Lisa Fields-Johnson, Georgina Friedenberg, Inge Judge, Jean MacFadyen, Geneva Mc-Nair, David Potter, Claire Ridge, and Harriet Samuelson. We are also indebted to the 39,876 dedicated and committed participants of the WHS.
References
- 1.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 2.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- 3.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999;51(Suppl 3):34–41. doi: 10.1159/000053160. [DOI] [PubMed] [Google Scholar]
- 5.Wolever TM, Miller JB. Sugars and blood glucose control. Am J Clin Nutr. 1995;62(1 Suppl):212S–221S. doi: 10.1093/ajcn/62.1.212S. discussion 221S–227S. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560–566. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 9.Foster-Powell K, Miller JB. International tables of glycemic index. Am J Clin Nutr. 1995;62:871S–890S. doi: 10.1093/ajcn/62.4.871S. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 12.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 13.Willett W. Nutritional epidemiology. 2nd ed. Vol. 30. New York (NY): Oxford University Press; 1998. [Google Scholar]
- 14.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi S, Dal Maso L, Augustin L, Negri E, Parpinel M, Boyle P, et al. Dietary glycemic load and colorectal cancer risk. Ann Oncol. 2001;12:173–178. doi: 10.1023/a:1008304128577. [DOI] [PubMed] [Google Scholar]
- 16.Slattery ML, Benson J, Berry TD, Duncan D, Edwards SL, Caan BJ, et al. Dietary sugar and colon cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:677–685. [PubMed] [Google Scholar]
- 17.Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C. Glycaemic index, breast and colorectal cancer. Ann Oncol. 2002;13:1688–1689. doi: 10.1093/annonc/mdf261. [DOI] [PubMed] [Google Scholar]
- 18.Terry PD, Jain M, Miller AB, Howe GR, Rohan TE. Glycemic load, carbohydrate intake, and risk of colorectal cancer in women: a prospective cohort study. J Natl Cancer Inst. 2003;95:914–916. doi: 10.1093/jnci/95.12.914. [DOI] [PubMed] [Google Scholar]