Abstract
Background
Dry eye is a common, complex condition that can reduce ocular comfort and visual performance. The impact on quality of life has been rated as similar to the effect of moderate angina and, in more severe cases, dialysis and severe angina. This study aimed to use meta-analysis to compare omega-3 fatty acid and placebo fatty acid in the management of dry eye syndrome.
Material/Methods
Comparative studies published until 1 June 2014 were searched through a comprehensive search of the Medline, Embase, Web of Science, and the Cochrane Library electronic databases. A systematic review and cumulative analysis of comparative studies reporting the effect of omega-3 fatty acid on dry eye syndrome was conducted. All analyses were performed using the Review Manager (RevMan) v.5 software (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
The trials involved a total of 790 participants in 7 independent studies. All the studies are published between 2007 and 2013. Meta-analysis of the 5 studies that reported data in mean SD values revealed that the tear break-up time (TBUT) was significantly greater by 1.58 s (WMD=1.58, 95% CI=0.60 to 2.55; P=0.007). Combination of all the Schirmer’s test data showed that omega-3 fatty acid supplementation could significantly improve the Schirmer’s test (WMD=0.74, 95% CI=0.29 to 1.19; P=0.001). However, the combination of all the OSDI test data showed that omega-3 fatty acid supplementation did not significantly improve the OSDI test results (WMD=−4.54, 95% CI=−9.85 to 0.78; P=0.09).
Conclusions
Based on the data included in our meta-analysis, omega-3 fatty acid was associated with better TBUT and Schirmer’s. No significant differences were detected in OSDI test results. Consequently, our findings suggest that omega-3 fatty acid offers is an effective therapy for dry eye syndrome.
MeSH Keywords: Dry Eye Syndromes, Fatty Acids, Meta-Analysis
Background
Dry eye is a common, complex condition that can reduce ocular comfort and visual performance. The impact on quality of life has been rated as similar to the effect of moderate angina and, in more severe cases, dialysis and severe angina [1]. Dry eye is reported to be a complex condition involving the lacrimal glands, eyelids, and tear film, as well as a variety of ocular surface tissues, including epithelial, inflammatory, immune, and goblet cells [2]. Dry eyes usually affect people aged over 65 years [3]; moreover, dry eyes affect women selectively, as emphasized by several large studies [4,5], with an estimated 3.23 million American women suffering from dry eyes. There are 2 types of dry eye, although clinically they are frequently encountered together: 1) aqueous insufficiency, in which the aqueous secretion from the lacrimal glands is reduced; and 2) evaporative dry eye, in which a deficient lipid layer results in an unstable tear film [6]. The term “dry eye disorder” (DED) has recently been introduced to better define the ocular surface dysfunction that leads to tear film impairment and dry eye.
Omega-3 essential fatty acids have been reported to be associated with several kinds of diseases, such as cancers, cardiovascular diseases, and autoimmune disease [7–9]. In animal models, daily supplementation with omega-3 fatty acid has shown great potential to produce significant therapeutic effectiveness in dry eye treatment [10]. Another study was conducted to investigate the efficacy of the topical application of omega-3 essential fatty acids and hyaluronic acid mixtures in a mouse model of experimental dry eye. The results showed that a mixture of topical omega-3 essential fatty acids and hyaluronic acid may have a greater therapeutic effect on clinical signs and inflammation of dry eye compared with hyaluronic acid mixture artificial tears [11]. Observational studies have reported that consumption of fish oil, which is known to be rich in omega-3, is positively associated with reduced risk of dry eye [12]. The Women’s Health Study (WHS), which included 39876 female health professionals, reported that a higher ratio of omega-3 to omega-6 fatty acid consumption was associated with a significantly reduced risk of dry eye. In addition, tuna consumption was inversely associated with dry eye [13]. However, such observational studies are unable to establish causality because of the difficulty in adjusting for complex confounding factors that also influence the development of dry eye. For this reason, randomized controlled trials (RCTs) are necessary to determine whether an omega-3 fatty acid supplementation is effective in treatment of dry eye. Such trials are considered important because omega-3 fatty acid supplementation may be a low-risk and cost-effective strategy to treat dry eye syndromes. RCTs of omega-3 fatty acid are increasingly being reported, with varying results, and quite discordant conclusions were reported. Accordingly, a comprehensive systematic review of RCTs conducted according to the Cochrane handbook is required to reach a credible conclusion on the effect of omega-3 fatty acid therapy for dry eye.
Material and Methods
Study selection
We systematically searched the following electronic databases: Medline, Embase, Web of Science, and Cochrane Library, for articles addressing the effect omega-3 fatty acid on the treatment of dry eye. The following key words were used: “ophthalmoxerosis”, “xerophthalmia”“dry eye”, “xeroma”, “dry eye syndrome”, “keratoconjunctivitis sicca”, and combined with “fatty acid”, “omega-3”, and “n-3”. In addition, reference lists were scanned to identify any additional studies. No language restrictions were set in the literature search. All the reports were published before 1 June 2014. We sought randomized controlled trials (RCTs) involving the effect of omega-3 on dry eye. Letters, review articles, animal or laboratory studies, and conference abstracts were not included.
Data extraction
Two reviewers (AL and JJ) independently extracted the following data from each study: first author, year of publication, study population characteristics, study design, content of case and control groups, and the tear film break up time (TBUT), Schirmer’s test result, and Ocular Surface Disease Index (OSDI) change in both groups.
Inclusion criteria
To be included, studies had to:
Report the effect of omega-3 fatty acid supplementation on the management of dry eye.
Compare omega-3 and placebo drugs.
Report on at least 1 of the outcome measures mentioned (TBUT, Schirmer’s test, and OSDI).
Contain a previously unreported patient group (if patient material was reported more than once, we chose the most informative and recent article).
When 2 studies were reported by the same institution, our analysis included either the one of better quality, or the most recent publication.
Exclusion criteria
The following criteria were used to exclude studies from our analysis:
Studies in which the outcomes of interest (mentioned below) were not reported for omega-3 fatty acid or it was impossible to calculate these from the published results.
Only oral omega-3 fatty acid was considered in this study and other routes of administration were excluded.
Studies in which the standard deviation of the mean for continuous outcomes of interest (length of stay and operative time) were not reported.
Quality of the comparative studies
Assessment of quality characteristics used the following criteria: 1) Random sequence generation; 2) Allocation concealment; 3) Blinding of participants; 4) Blinding of outcome assessment; 5) Follow-up ≥80%; 6) Free of selective reporting; and 7) Free of other bias. The adequate (low risk of bias); unclear (unknown risk of bias) and inadequate (high risk of bias) items were marked for each study. More adequate item demonstrated better quality of the included studies.
Statistical analyses
Continuous parameters were analyzed by using the estimated weighted mean differences. However, different outcomes were reported in different studies and the number of included studies for each parameter was different. All the data were measured as the change from baseline and required calculation was conducted. Interstudy heterogeneity was measured using the Q-test. Heterogeneity was also quantified with the I2 metric, which is independent of the number of studies included in the cumulative analysis. The scale of I2 values ranges between 0% and 100%, with higher values denoting a greater degree of heterogeneity. Data were pooled using fixed-effects or random-effects models according to the heterogeneity. The random-effects model incorporates an estimate of the interstudy variance and tends to provide wider CIs; it was used when heterogeneity was present. The Begg funnel plot and Egger’s test were conducted to identify potential publication bias. The significance of the intercept was determined by the t test.. All analyses were performed using the Review Manager (RevMan) v.5 software package (Nordic Cochrane Centre, Copenhagen, Denmark; http://www.cc-ims.net/revman/download). All p values were calculated using the 2-tailed t test, and p values were considered statistically significant at p<0.05.
Results
Results of the literature search
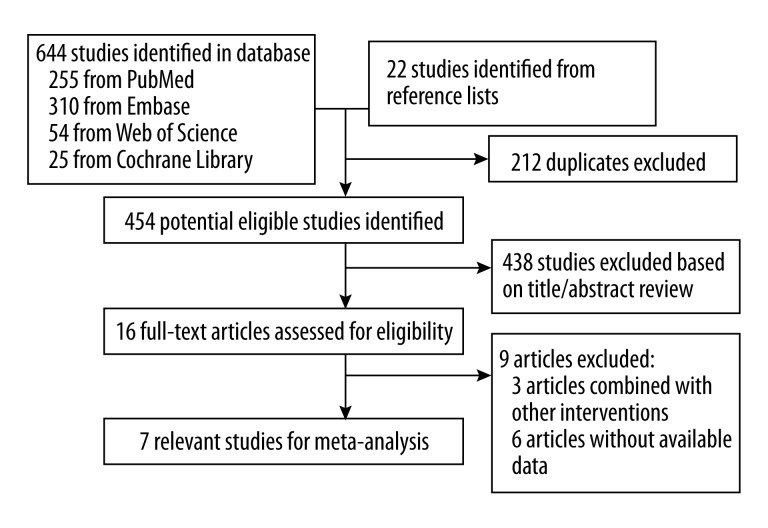
The method used to select the studies is shown in Figure 1. The initial search identified 666 reports (255 from Medline, 310 from Embase, 54 from Web of Science, 25 from Cochrane Library and 22 studies identified from reference lists), of which 212 duplicates were excluded. The title and abstract of the remaining 454 reported were identified and 438 studies were excluded. A total of 16 full-text articles were then assessed for eligibility. The studies were excluded in the full-text assessment and in which 3 articles combined omega-3 fatty acid with other interventions and 5 articles without available data. Finally, a total of 8 relevant randomized controlled studies were included in this study [14–21].
Figure 1.
Flow chart of the literature search. The literature search was conducted in Medline, EMBASE, Web of Science, and Cochrane Library. The reference lists of the relevant studies were reviewed as well.
Study characteristics
The trials involved a total of 790 participants in 7 independent studies. All the studies are published between 2007 and 2013. The data are from the USA, Italy, Brazil, Iran, Spain, and Japan. In most studies, both females and males are included in the analyses. Most studies were based on a relatively older group (50 years and older). The sources of omega-3 fatty acid were fish oil, flax seed, and synthetic. In the control group, the placebo group was wheat germ oil or other placebo oral agents. The follow-up durations of all the included studies are from 1 month to 6 month and the most frequent duration was 3 months in 4 studies.
Methods of included trials
All trials reported a randomized design. Two trials generated the randomization sequence with colored marbles to represent trial groups and 5 did not clearly report how the randomization sequence was generated. Concealed allocation was performed in 3 studies. Blinding of participants was undertaken in all the included studies and the blinding of outcome assessment in 4 studies. All the studies had a follow-up rate of over 80%. A total of 3 studies were free of selective reporting and 5 studies were free of other bias.
Quantitative analyses
TBUT
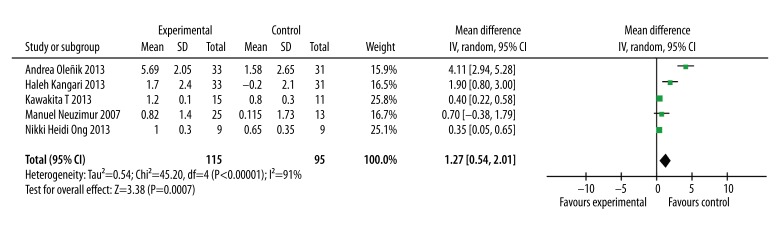
Five of the included studies reported the TBUT in omega-3 and placebo groups. Moreover, meta-analysis of the 5 studies that reported data in mean SD values revealed that the TBUT was significantly greater by 1.58 s (WMD=1.58, 95% CI=0.60 to 2.55; P=0.007, Figure 2)
Figure 2.
Forest plot of the tear film break-up time for omega-3 fatty acid on dry eye syndrome. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate pooled ORs. A random-effects model was obtained.
Schirmer’s test
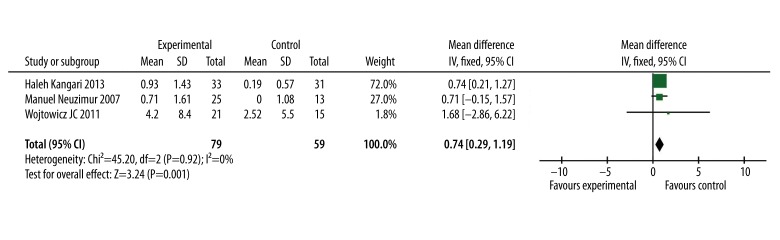
In 3 of all the included studies, the results of Schirmer’s test were reported. Through the meta-analysis, the combination of all the Schirmer’s test data showed that omega-3 fatty acid supplementation significantly improved the Schirmer’s test result (WMD=0.74, 95% CI=0.29 to 1.19; P=0.001, Figure 3)
Figure 3.
Forest plot of the Schirmer’s test results for omega-3 fatty acid effect on dry eye syndrome. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled ORs. A fixed-effects model was obtained.
OSDI
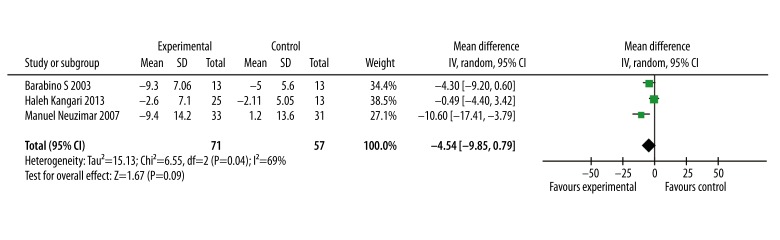
In 3 of the included studies, the OSDI test results were reported. Through the meta-analysis, the combination of all the OSDI test data showed that omega-3 fatty acid supplementation did not significantly improve the OSDI test result (WMD=−4.54, 95% CI=−9.85 to 0.78; P=0.09, Figure 4)
Figure 4.
Forest plot of the Ocular Surface Disease Index for the effect of omega-3 fatty acid on dry eye syndrome. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate pooled ORs. A random-effects model was obtained.
Heterogeneity and sensitivity analysis
In the TBUT test, a significant heterogeneity was detected (heterogeneity: P<0.0001); I2=91%). To find the source of heterogeneity, the included studies were excluded one by one and we found no significant changes in the heterogeneity or the results. The sensitivity analysis showed that no significant change was detected after excluding the studies with lower methodological quality.
Publication bias
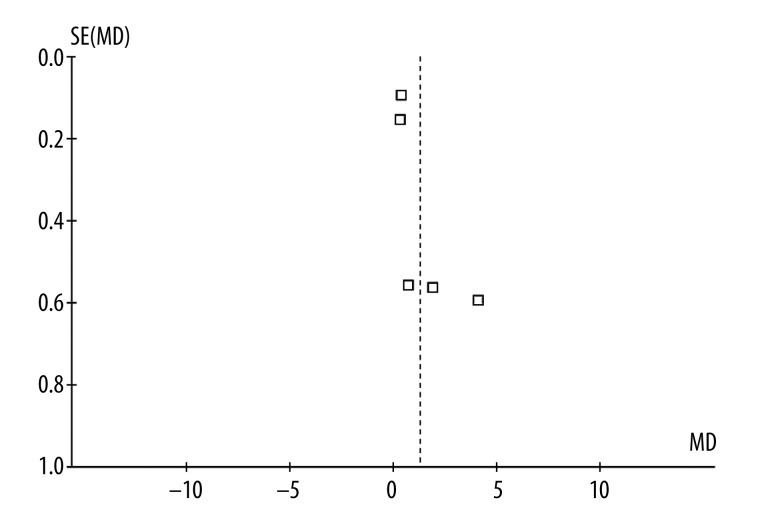
Funnel plots (Figure 5) and Egger’s regression asymmetry test results of the included studies suggested no significant publication bias (Egger’s test P=0.513) over the effects of omega-3 fatty acid supplementation on dry eye syndrome.
Figure 5.
Funnel plot of all the included studies.
Discussion
In this study, by pooling the results of the available randomized controlled trials, we found that omega-3 fatty acid supplementation improved the TBUT and Schirmer’s test results of the patients with dry eye syndrome. Previous studies indicated that omega-3 fatty intake was associated with a reduced risk of dry eye, and the results of our study demonstrated that oral omega-3 fatty acid supplementation improved TBUT and Schirmer’s test results, indicating that it may be an important mechanism underlying the therapeutic effect of omega-3 fatty acid. However, omega-3 fatty acid did not improve the OSDI scale results.
The Women’s Health Study is a randomized, double-blind, placebo-controlled trial among 39 876 female health professionals to assess the benefits and risks of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. The baseline and follow-up data showed that for the highest vs. the lowest fifth of omega-3 fatty acid intake, the odds ratio was 0.83 with a CI=0.70 to 0.98 [22]. A series of randomized controlled studies showed that omega-3 fatty helped in the treatment of dry eye syndrome. In this study, the combination of 7 studies showed that omega-3 fatty acid improved TBUT and Schirmer’s test results but not OSDI test results. In general, omega-3 fatty acid helps in tear secretion and tear film stability. However, omega-3 fatty acid does not improve the degree of comfort of patients with dry eye syndrome. This is particularly relevant in the context of intervention decisions for therapeutic strategies, thus more advanced studies are required to detect whether the intervention should be used for the primary prevention of dry eye.
In a mouse model, topical application of the n-3 fatty acid linoleic acid (18: 3n-3; ALA) produces a significant decrease in epithelial damage, expression of inflammatory cytokines, and macrophage infiltration [23]. The beneficial effects could be due to the action of ALA, to the elongation and desaturation products, EPA and DHA, or to new derivatives of these fatty acids, such as resolvins and neuroprotectins. Several studies showed omega-3 fatty acids and their derivatives, resolvins and neuroprotectins, decrease inflammation and increase tear production, and the combination of NGF and PEDF with DHA improves nerve regeneration after injury [24]. Although DHA is a minute component of lipids in the cornea, this tissue can synthesize NPD1 when treated with a combination of DHA and PEDF. Therefore, this docosanoid could have therapeutic value in preventing serious consequences of nerve damage, such as DE, epithelial erosions, and corneal ulcerations [25].
Inflammation is now understood to be a key process in development of dry eye syndrome. A previous study showed that a significant decrease in the levels of IL-1β, -17, and IP-10 were observed in the 0.2% essential fatty acids mixture-treated group compared with the other groups. In the mice treated with the mixture containing 0.2% omega-3 fatty acids, the concentration of 4-hydroxynonenal was also lower than in the other groups. Although the 0.2% omega-3 essential fatty acids alone group also had significant improvement in corneal irregularity scores and IL-17, IL-10, and 4-hydroxynonenal levels compared with the other groups, the efficacy was lower than in the 0.2% omega-3 mixture group [11]. Thus, we suspect that the therapeutic effect of omega-3 fatty on dry eye syndrome might be through suppressing inflammatory reactions in ocular tissues.
In this study, we only evaluated the effect of oral omega-3 fatty acid on the treatment of dry eye syndrome. However, eye drops containing omega-3 fatty acid might be an option for the management of dry eye. Omega-3 fatty acid can also be combined with other anti-inflammatory agents during treatment. When combined with an anti-inflammatory agent, cyclosporine A, omega-3 fatty acid preferably improves the TBUT compared with the omega-3 fatty acid supplement only group [23].
As previously reported, meta-analysis has been used in the past to assess the treatment of dry eye. Ng et al. studies the effect of omega-3 and omega-6 polyunsaturated fatty acids for dry eye syndrome, using a combination of different kinds of polyunsaturated fatty acids. However, the combination of different fatty acids might be able to demonstrate the effect of omega-3 fatty acid. This study has investigated the possible benefits of omega-3 fatty acid in the management of dry eye syndrome. The strengths of this study are that, to the best of our knowledge, this research is the first meta-analysis detecting the effect of omega-3 fatty acid in the treatment of dry eye, encompassing a total of 7 studies, and that no publication bias was detected by Begg’s funnel plot or Egger’s tests. The overall results did not change remarkably after sensitivity analyses were performed. Our analysis combined the data from all studies that passed our predefined criteria; therefore, we are confident of the validity of our findings. It is important, however, to address the limitations of the meta-analysis, which are as follows: First, the longest follow-up duration of the included studies was 6 months. Considering that omega-3 fatty acid is an essential nutrient, longer duration of treatment and follow-up are required. Second, the data of the included studies were insufficient to conduct a dose-response meta-analysis. There points all indicate the need for additional well-designed studies in the future. Third, it is important to bear in mind non-publication bias, particularly in meta-analytic research based on published studies.
Conclusions
Based on the data included in our meta-analysis, omega-3 fatty acid appears to be associated with better TBUT and Schirmer’s test results. No significant differences were detected in the OSDI test. Consequently, our results suggest that omega-3 fatty acid is effective in treatment of dry eye syndrome. Accordingly, oral omega-3 fatty acid might be a potential therapy for patients with dry eye syndrome. Larger, well-designed, multicenter RCTs with more extensive follow-up are needed to confirm our findings.
Table 1.
Study characteristics of included studies.
| Author | Year | Site | Patients | Age | Gender (female/male) | Source | Case group | Control group |
|---|---|---|---|---|---|---|---|---|
| Wojtowicz JC | 2011 | USA | 21/15 | 61 (29–84) | 20/16 | Fish oil | 450 mg of eicosapentaenoic acid, 300 mg of docosahexaenoic acid 1000 mg of flaxseed oil | Wheat germ oil |
| Barabino S | 2003 | Italy | 13/13 | 63.4±8.2/54.3±11.3 | 9:4/8:5 | Drug | LA (28.5 mg) and GLA (15 mg) twice daily | Preservative-free substitute tears |
| Manuel Neuzimar Pinheiro Jr | 2007 | Brazil | 25/13 | NA | Women | Flax seed | Flaxseed oil capsules | Basal oleic acid |
| Haleh Kangari | 2013 | Iran | 33/31 | 61.8±8/60.6±8.8 | 38/26 | Drug | 180 mg of EPA and 120 mg DHA | Medium-chain triglycerides |
| Nikki Heidi Ong | 2013 | USA | 9/9 | 31.1±6.2/32.1±10.6 | 12/6 | Drug | 750 mg of omega-3 EFAs both eicosapentaenoic (EPA) docosahexaenoic acid (DHA), 1000 mg offlaxseed oil about 183 IU of vitamin E per day | Same medical regimen |
| Andrea Oleñik | 2013 | Spain | 33/31 | 58/54 | 22: 9/24: 9 | Drug | DHA 350 mg, EPA 42.5 mg | Placebo oral agent |
| Kawakita T | 2013 | Japan | 15/11 | 52.5±2.5/51.9±2.2 | 15/12 | Fish oil | EPA 1245 mg; DHA 540 mg | Placebo supplement without EPA and DHA |
Table 2.
The study quality of the included studies assessed by the Cochrane collaboration tool.
| Study | Year | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Follow-up ≥80% | Free of selective reporting | Free of other bias |
|---|---|---|---|---|---|---|---|---|
| Wojtowicz JC | 2011 | ? | ? | + | ? | + | + | + |
| Barabino S Manuel Neuzimar | 2003 | + | ? | + | + | + | + | + |
| Pinheiro Jr | 2007 | ? | + | + | ? | + | ? | − |
| Haleh Kangari | 2013 | + | + | + | + | + | + | + |
| Nikki Heidi Ong | 2013 | ? | + | + | ? | + | ? | ? |
| Andrea Oleñik | 2013 | ? | ? | + | + | + | ? | + |
| Kawakita T | 2013 | ? | ? | + | + | + | ? | + |
+, Adequate (low risk of bias); ?, unclear (unknown risk of bias); −, inadequate (high risk of bias).
Footnotes
Source of support: The study was funded by Tianjin Municipal Education Committee Foundation (Project No. 20110132)
References
- 1.Fiscella RG. Understanding dry eye disease: a managed care perspective. Am J Manag Care. 2011;17(Suppl 16):S432–39. [PubMed] [Google Scholar]
- 2.Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol. 2013;131:1331–38. doi: 10.1001/jamaophthalmol.2013.4503. [DOI] [PubMed] [Google Scholar]
- 3.Doughty MJ. Rose bengal staining as an assessment of ocular surface damage and recovery in dry eye disease-a review. Cont Lens Anterior Eye. 2013;36:272–80. doi: 10.1016/j.clae.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763–68. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 6.Dana MR, Sullivan DA, Parke AL. Toward optimal health: the experts discuss dry eye syndrome. Interview by Jodi Godfrey Meisler. J Womens Health Gend Based Med. 2001;10:725–29. doi: 10.1089/15246090152636460. [DOI] [PubMed] [Google Scholar]
- 7.Eynard AR, Navarro A. Crosstalk among dietary polyunsaturated fatty acids, urolithiasis, chronic inflammation, and urinary tract tumor risk. Nutrition. 2013;29:930–938. doi: 10.1016/j.nut.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Culic V. n-3 fatty acids in patients with cardiac risk factors. N Engl J Med. 2013;369:781. doi: 10.1056/NEJMc1308780. [DOI] [PubMed] [Google Scholar]
- 9.Maclean CH, Issa AM, Newberry SJ, et al. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid Rep Technol Assess (Summ) 2005;(114):1–3. [PMC free article] [PubMed] [Google Scholar]
- 10.Harauma A, Saito J, Watanabe Y, Moriguchi T. Potential for daily supplementation of n-3 fatty acids to reverse symptoms of dry eye in mice. Prostaglandins Leukot Essent Fatty Acids. 2014;90:207–213. doi: 10.1016/j.plefa.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Choi JH, Oh HJ, et al. Effects of Eye Drops Containing a Mixture of Omega-3 Essential Fatty Acids and Hyaluronic Acid on the Ocular Surface in Desiccating Stress-induced Murine Dry Eye. Curr Eye Res, Curr Eye Res. 2014;39(9):871–78. doi: 10.3109/02713683.2014.884595. [DOI] [PubMed] [Google Scholar]
- 12.Cakiner-Egilmez T. Omega 3 fatty acids and the eye. Insight. 2008;33:20–25. quiz 26–27. [PubMed] [Google Scholar]
- 13.Miljanovic B, Trivedi KA, Dana MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–93. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojtowicz JC, Butovich I, Uchiyama E, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–14. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 15.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro MN, Jr, dos Santos PM, dos Santos RC, et al. Oral flaxseed oil (Linum usitatissimum) in the treatment for dry-eye Sjogren’s syndrome patients. Arq Bras Oftalmol. 2007;70:649–55. doi: 10.1590/s0004-27492007000400016. [in Portuguese] [DOI] [PubMed] [Google Scholar]
- 17.Kangari H, Eftekhari MH, Sardari S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120:2191–96. doi: 10.1016/j.ophtha.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Ong NH, Purcell TL, Roch-Levecq AC, et al. Epithelial healing and visual outcomes of patients using omega-3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea. 2013;32:761–65. doi: 10.1097/ICO.0b013e31826905b3. [DOI] [PubMed] [Google Scholar]
- 19.Olenik A, Jimenez-Alfaro I, Alejandre-Alba N, Mahillo-Fernandez I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–38. doi: 10.2147/CIA.S48955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakita T, Kawabata F, Tsuji T, et al. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomed Res. 2013;34:215–20. doi: 10.2220/biomedres.34.215. [DOI] [PubMed] [Google Scholar]
- 21.Bhargava R, Kumar P, Kumar M, et al. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6:811–16. doi: 10.3980/j.issn.2222-3959.2013.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iribarren C, Markovitz JH, Jacobs DR, Jr, et al. Dietary intake of n-3, n-6 fatty acids and fish: relationship with hostility in young adults – the CARDIA study. Eur J Clin Nutr. 2004;58:24–31. doi: 10.1038/sj.ejcn.1601739. [DOI] [PubMed] [Google Scholar]
- 23.Rashid S, Jin Y, Ecoiffier T, et al. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–25. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 24.Brandt D, Volkmann X, Anstatt M, et al. Serum biomarkers of cell death for monitoring therapy response of gastrointestinal carcinomas. Eur J Cancer. 2010;46:1464–73. doi: 10.1016/j.ejca.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Bazan HE, Bazan NG. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea. Comparison of epithelium, stroma and endothelium. Curr Eye Res. 1984;3:1313–19. doi: 10.3109/02713688409007418. [DOI] [PubMed] [Google Scholar]