Abstract
Clostridium difficile is the most common cause of nosocomial infectious diarrhea. The incidence of C difficile infection (CDI) is increasing in both inpatients and outpatients, and outbreaks caused by a hypervirulent strain of C difficile are resulting in more severe disease. Moreover, community-associated CDI is occurring in persons who lack the traditional risk factors, which include antibiotic use, advanced age, and severe underlying disease. The clinical severity of CDI ranges from a mild, self-limited diarrheal illness to a fulminant, life-threatening colitis. Enzyme-linked immunosorbent assay is the most common laboratory method used for detection of C difficile toxins and can confirm the diagnosis within several hours. The choice of treatment should be based on disease severity. Oral metronidazole is generally regarded as the treatment of choice for mild to moderate CDI, while oral vancomycin is recommended for severe disease. Timely surgical intervention is important in patients who have severe complicated CDI.
Keywords: Clostridium difficile, Pseudomembranous colitis
Clostridium difficile is a spore-forming, anaerobic, gram-positive bacillus. Although the bacterium was discovered in 1935,1 it was not associated with pseudomembranous colitis until 1977.2, 3 C difficile is now recognized as the most common cause of nosocomial infectious diarrhea.4 It is responsible for up to 25% of cases of antibiotic-associated diarrhea,5 up to 75% of cases of antibiotic-associated colitis, and greater than 90% of cases of antibiotic-associated pseudomembranous colitis.6
This review provides an overview of C difficile infection (CDI), including a discussion of epidemiology, risk factors, clinical presentation, diagnosis, treatment, and preventative measures.
EPIDEMIOLOGY
C difficile is primarily a nosocomial pathogen. The prevalence of asymptomatic colonization in healthy adults is only 3%7; however, the prevalence in long-term care facilities has been reported to be up to 50%.8, 9 Colonized persons can serve as a reservoir for infection by contaminating the environment with C difficile spores.10, 11 The organism can be isolated from patient rooms several months after an infected patient has been discharged.12 Health care workers are thought to be the primary source of transmission in health care facilities, spreading the organism on their hands or medical equipment after coming into contact with patients with symptomatic infection. 11
The risk of becoming colonized with C difficile is directly proportional to the length of hospitalization,10 with a median time from admission to acquisition of about 2 weeks. Length of stay is likely to be a surrogate marker for the risk of exposure to other patients with C difficile infection (CDI). 13, 14
The occurrence of symptomatic disease from C. difficile varies widely between hospitals, but the overall incidence is estimated to be approximately 1% of all inpatients in acute care facilites.15 Although numerous strains exist within a single center, outbreaks typically are linked to a single strain.15 The risk of symptomatic disease is higher in newly exposed and infected patients, possibly because those who are already colonized have pre-existing immunity to C difficile toxins.16 After infection, the patient’s immune response to C difficile toxins appears to play an important role in determining whether the person becomes asymptomatically colonized or whether disease develops. Persons who mount high antibody titers to toxin A are less likely to have diarrhea than are patients with a poor antibody response.17
The incidence and severity of CDI among hospitalized patients are increasing worldwide. The number of patients discharged from US hospitals with diagnosed CDI nearly doubled from 31 per 100,000 population in 1996 to 61 per 100,000 population in 2003.18 Since 2000, many CDI epidemics in the United States, Canada, United Kingdom, and Europe have been associated with the North American pulsed-field gel electrophoresis type 1 (NAP1) strain of C difficile.15, 19, 20 CDI outbreaks associated with the NAP1 strain are more severe, leading to more colectomies21 and an attributable mortality of 16.7%.22
A deletion in the negative regulator of toxin production, tcdC, is thought to be the reason that the NAP1 strain produces greater than 15 times more toxin A and B than previously identified strains.20 This strain is highly resistant to fluoroquinolones, and outbreaks attributed to this strain are strongly associated with the use of fluoroquinolones, but other antibiotics are also implicated.21,22
No national surveillance system is in place in the United States for tracking community-associated CDI; however, the incidence is estimated to be 8 to 12 cases per 100,000 population. Surveillance data from Connecticut and Philadelphia show incidences of 6.9 and 7.6 community-associated CDI cases per 100,000 population, respectively.23, 24 In Philadelphia this corresponded to one case of community-associated CDI per 5549 outpatient antibiotic prescriptions, although only 76% of these patients had received antibiotics in the preceding 3 months. 23 Furthermore, severe cases of community-associated CDI have occurred in relatively young and healthy persons who lack the traditional risk factors for CDI.23
This increase in community-associated CDI has raised the concern that C difficile may be a zoonotic or food-borne pathogen. Although a definitive link between the food supply and CDI remains unproven, the spore form of C difficile is capable of surviving the standard cooking process and could potentially infect humans if consumed during a meal.25 CDI has been described in many animals,26–29 including house pets,30 and C difficile or its toxins have been isolated from the stool of livestock and poultry,31–33 causing concerns about the contamination of meat and dairy products. Added support for a possible food-borne association comes from a study in which C difficile was cultured from 12 of 60 (20%) retail ground beef samples during a 10-month period in Canada.34 One quarter of the isolates were identical to non-NAP1 isolates that were also found in humans with CDI.
Further support of a possible zoonotic link comes from the recent outbreaks of severe CDI in the Netherlands. The predominant strain of C. difficile infecting pigs and cattle in this area is a ribotype 078 that contains a toxin pattern similar to that of the NAP1(ribotype 027) strain. The number of infections in humans secondary to the ribotype 078 strain has been increasing rapidly in the Netherlands, from 3% in 2005 to 13% in 2008.35 Patients infected with this strain of C. difficile were more likely to be younger and have community-acquired disease. Severity of the illness in patients infected with ribotype 078 is equivalent to that in patients infected with the NAP1 strain. Analysis of these strains isolated from both pigs and humans revealed overlapping antimicrobial susceptibilities and numerous other similarities indicating a very close genetic relationship.35, 36
PATHOGENESIS
C difficile causes symptoms by producing exotoxins in the intestinal tract. Toxin A causes mucosal damage via an intense neutrophilic infiltrate that leads to inflammatory diarrhea. Toxin B is a very potent cytotoxin but appears to be less enterotoxic than toxin A.37 Direct interaction between the toxins and surface receptors in the colonic mucosa result in actin filament degradation that causes necrosis and sloughing of cellular debris into the colonic lumen. Both toxins and other surface proteins further induce an inflammatory response by triggering cytokine release from monocytes and dendritic cells.
Exudation of inflammatory cells and proteins from the resulting ulcers causes the visible yellow plaques that form the characteristic pseudomembrane. The number of lesions appears to be dose-dependent, with greater amounts of toxin resulting in a more confluent pseudomembrane.37
RISK FACTORS
The development of CDI depends on both an interruption in the usual host flora and acquisition of the organism. Risk factors most consistently identified in the literature include antibiotic use, advanced age, and severe underlying disease.38 The most important risk factor in hospitalized patients is antibiotic exposure.39 A history of antibiotic exposure is found in more than 90% of inpatients with CDI.7 Clindamycin, ampicillin, and cephalosporins were most frequently implicated before the NAP1 epidemic.39, 40 Fluoroquinolones were implicated in the most recent epidemic of CDI in hospitalized patients.22 Longer duration of antimicrobial administration and the use of multiple antimicrobials also have been associated with an increased risk.22
Aging is a risk factor for CDI. Incidence increases with each decade of life, with the greatest increase seen in patients older than 65 years.38 Colonization rates in the elderly are up to 100 times greater than rates in young adults and adolescents, and disease rates are 20-fold higher.15, 41 Institutionalization, longer hospital stays, and comorbid conditions and infections that necessitate frequent treatment with antibiotics are contributing factors.
Immunosuppression from HIV infection or from chemotherapy or transplants also puts patients at higher risk for CDI. A retrospective analysis of 44,778 patients over 10 years revealed that CDI was the cause of diarrhea in more than 5% of HIV-positive patients.42 The incidence in solid organ and hematopoietic stem cell transplant recipients ranged from 1% to 31%.43–45
Gastric acid suppression also may increase the risk of CDI. The acidic environment of the stomach has been shown to be fatal to vegetative C difficile45; however this effect is nullified once gastric pH reaches 5.46 The increasing use of proton-pump inhibitors (PPIs) has also been correlated with the increasing incidence of CDI.47 Several studies have shown that CDI is more than twice as likely to develop in hospitalized patients prescribed PPIs than in other patients.14, 15, 48 The use of PPIs also has been shown to alter the normal flora, which may enhance the ability of C difficile to colonize the GI tract.49
Enteral feeding has been shown to increase the risk of C difficile acquisition from 8% to 20% and the risk of CDI from 1% to 9%.50 Several factors associated with tube feeding are thought to increase the risk of infection, including contamination of the formula during preparation or the equipment by handling or alteration of the normal colonic flora.51, 52 Although any feeding tube appears to increase the incidence of CDI, post-pyloric tubes confer the greatest risk.50
CLINICAL MANIFESTATIONS
The clinical severity of CDI ranges from a mild, self-limited diarrheal illness to a fulminant, life-threatening colitis. Symptoms can develop within the first several days after antibacterial treatment is initiated or be delayed until up to 10 weeks after cessation of antibiotics.53, 54 Low-grade fever and cramping abdominal pain often accompany the diarrhea of CDI. The disease progresses to fulminant colitis in 1% to 8% of patients.55
Mild to moderate CDI consists of diarrhea with abdominal cramping, but not systemic symptoms. Severe disease involves profuse diarrhea, abdominal pain, abdominal distention, leukocytosis, and systemic symptoms such as fever. Severe disease with complications includes paralytic ileus, colonic dilitation with systemic toxicity (toxic megacolon), or other immediate life-threatening conditions. In severe CDI, ileus or toxic megacolon may cause a paradoxical decrease in the volume of diarrhea.53
CDI may also have a variety of unusual or unexpected presentations. If patients have ileus but not the diarrhea typically associated with severe CDI, clinicians will need to rely on supportive examination and laboratory findings. Striking leukocytosis, often greater than 30,000 cells/uL, is a common supportive laboratory finding in patients with CDI that frequently precedes organ dysfunction.56, 57 Other unusual manifestations of CDI may include perforation of the small or large bowel,58 protein-losing enteropathy,59 and a variety of extracolonic manifestations such as visceral abscesses, reactive arthritis, and post-traumatic soft tissue infections.60 These otherwise unexplained findings should prompt the clinician to consider CDI to prevent the high morbidity and mortality that results from undiagnosed disease.
The differential diagnosis of CDI includes both non-infectious and other infectious causes of antibiotic-associated diarrhea. Antibiotics may directly cause diarrhea through osmotic factors or stimulation of intestinal motility. Many other medications that are commonly co-administered with antibiotics in hospitalized patients, such PPIs, should also be considered as potential causes of diarrhea.61 Other causes of non-infectious colitis may be confused with CDI, especially in patients with inflammatory bowel disease since there is an increase in C. difficile colonization in this group that may result in misleading diagnostic study results.62 Other less common infectious causes of antibiotic-associated diarrhea include Stapylococcus aureus, especially if methicillin-resistant, and antibiotic-resistant gram negative bacilli, such as Klebsiella oxytoca.63, 64
DIAGNOSIS
The diagnosis of CDI is most frequently established by confirming the presence of C. difficile or one of its toxins in the stool of a symptomatic patient. The clinical laboratory gold standard is the cytotoxicity cell assay,65 but cost and the 48-hour delay in results have caused this test to be replaced by the enzyme-linked immunosorbent assay (ELISA) in most US laboratories.66 ELISA is the most common diagnostic laboratory method used in the United States for detection of C difficile toxins. Growth in culture does not differentiate toxigenic from non-toxigenic strains of C difficile. This is important because only toxigenic strains of C. difficile can cause CDI, and therefore stool culture false-positive rate can exceed 10%.65
Toxin detecting ELISA can confirm the diagnosis within several hours and is relatively inexpensive. The major disadvantage is that its sensitivity is 70% to 90%, whereas the sensitivity of the cytotoxicity cell assay is greater the 90%.67, 68 Most currently available ELISAs detect both toxin A and toxin B; however older assays that only detect toxin A have a higher false-negative rate. There appears to be little value in repeating the ELISA after an initial negative test as few convert to positive69 and there is a precipitous decline in the positive predictive value of the test.
ELISAs that detect C difficile glutamate dehydrogenase (GDH) are widely available and inexpensive.70 The sensitivity of the assay exceeds 95% with a negative predictive value of over 99%;71 however the positive predictive value is only about 50%.70 GDH is constitutively produced by all strains of C difficile, so this test cannot differentiate between toxigenic and non-toxigenic strains.72 To improve the specificity of the GDH assay, a second confirmatory test that identifies C difficile toxins must also be used. A 2-step algorithm consisting of the GDH assay followed by a confirmatory cytotoxicity assay on positive samples has superior sensitivity and specificity to toxin detecting ELISAs, but increases turnaround time for positive results by several days.70
Polymerase chain reaction (PCR) shows promise as a sensitive and rapid method for diagnosing CDI.73, 74 Real-time PCR detection of the toxin B gene also has proved promising. In a study of 1368 stool specimens, the sensitivity was 93% compared with 78% for the cytotoxicity cell assay and 73% for a toxin-detecting EIA. 75 CT scanning may reveal patterns that suggest CDI and support the diagnosis in uncertain cases. The most common finding is diffuse or segmental thickening of the bowel wall more than 4 mm.76 Other supportive findings include colonic distention, pericolonic stranding, colonic fold effacement, and nodular fold thickening.76, 77 The sensitivity of CT is approximately 50%.
Endoscopy is generally not necessary to establish the diagnosis and may increase the incidence of perforation in patients with severe disease.78 Sigmoidoscopy or colonoscopy may be used if the ELISA finding is negative but clinical suspicion remains high or the diagnosis cannot be delayed.78
TREATMENT
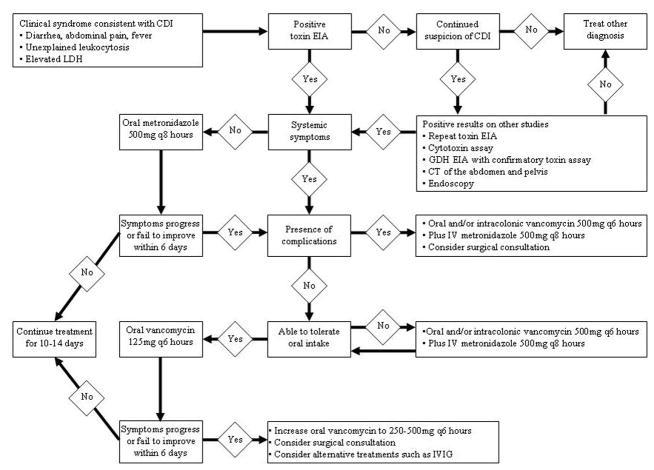
The choice of treatment should be based on disease severity (Figure). Cessation of the causative antibiotic is important, although this may not always be feasible. If the patient has a concomitant infection that requires antibiotic therapy, it is reasonable to change the antibiotic to a more narrow-spectrum alternative or use antimicrobials that are less often associated with CDI.
Figure 1.
Diagnosis and Treatment of CDI
Oral metronidazole, 500 mg tid or 250 mg qid for 10 to 14 days, is generally regarded as the drug of choice for mild to moderate CDI. This recommendation is based on the significant cost savings and comparable efficacy compared with oral vancomycin fro mild to moderate CDI.79–81 Oral vancomycin, 125 mg qid for 10 to 14 days, is becoming the preferred first-line agent for patients with severe CDI, based on studies that showed significantly improved cure rates compared with metronidazole in these patients.81, 82
When severe CDI is complicated by ileus, the efficacy of oral or nasogastric vancomycin may be compromised by an inability of the drug to reach the site of infection. Although this approach has not been studied, increasing the vancomycin dose to 250–500mg every six hours may theoretically improve the chance that adequate drug concentrations are achieved in the colon. Intracolonic administration of vancomycin is another possible strategy in the management of patients with ileus and the route is supported by several case reports when used in conjunction with other treatments.83–86
Timely surgical intervention is important in patients with severe complicated CDI. Even before the emergence of the NAP1 strain of C difficile, it was noted that mortality was reduced if surgery was done within 48 hours of a failure to respond to medical therapy.87 Because rapid progression to death is associated with the hypervirulent strain of C difficile, surgical consultation is especially urgent in these patients.
Patients who are elderly or immunocompetent or have leukocytosis or elevated lactate levels benefit most by emergency colectomy.88 Postoperative mortality is increased in patients with very severe underlying illness, mental status changes, protracted poor response to medical treatment, or hypotension requiring treatment with vasopressors before colectomy.89
In addition to vancomycin and metronidazole, possible antibiotic therapy includes bacitracin, rifampin, rifaximin, nitazoxanide, fusidic acid, and teicoplanin. Symptomatic cure rates with bacitracin are similar to those of vancomycin, but bacteriological cure rates are inferior.90 The efficacy of rifaximin, nitazoxanide, and fusidic acid appears to be identical to that of metronidazole and vancomycin.90
Teicoplanin was found to be superior to vancomycin and metronidazole at reducing toxin levels in the stool, but was not statistically superior in reducing symptoms or relapse rates.90 Although teicoplanin has shown some potential benefit in comparison with vancomycin, it is not available in the United States and is much more expensive than other commonly used medications. As a result of the equal efficacy in clinical cure, limited availability, and greater experience with metronidazole and vancomycin, these other antibiotics are rarely used.
Probiotics such Saccharomyces or Lactobacillus tablets have been studied as adjunctive therapy because they theoretically restore nonpathogenic flora to the GI tract, inhibit C difficile toxin production, and stimulate the host immune system. Several randomized controlled trials have been conducted but none have shown that the addition of a probiotic to vancomycin or metronidazole therapy improved the course of disease.91–94 Adverse effects of probiotics are rare in immunocompetent patients; however, probiotics should be avoided in patients who are immunocompromised or critically ill or have central venous catheters, because organisms in the probiotic preparation may cause bloodstream infections.95, 96
Cholestyramine and colestipol bind the toxins of C difficile in vitro; however, the efficacy of these agents in acute disease has not been demonstrated.97–99 Furthermore, because these agents bind vancomycin, concomitant administration of these drugs may result in subtherapeutic fecal concentrations of vancomycin.100
Several case studies and series suggest that toxin-binding resins may help prevent relapses, but further study is needed before such medications can be recommended in the management of CDI.39, 101, 102 Avoidance of medications that may exacerbate CDI is also important. Although the evidence is anecdotal, antiperistaltic agents, including narcotics, may contribute to the development of toxic megacolon and, thus, generally should not be administered to patients with CDI.103
Treatment failure and relapse
Factors associated with metronidazole failure include a serum albumin level of 2.5 g/dL or less, previous or current stay in the ICU, and continuation of the causative antibiotic.104, 105 Although antibiotic resistance does not appear to be a significant cause of metronidazole failure, switching to oral vancomycin is a common practice and may provide symptomatic relief.106, 107
CDI recurs in up to 35% of patients who respond to therapy with metronidazole or vancomycin,108, 109 and more half of these patients will have additional recurrences.110 Associated risk factors are similar to those for disease acquisition and include antibiotic use, older age, gastric acid-suppressive therapy, prolonged hospital stay, nursing home residence, and the presence of comorbidities.6 More than half of relapses appear to be the result of reinfection with a different strain of C difficile rather than reactivation of spores remaining in the colon.108
The first relapse should be treated similarly to the initial episode, with severity of the disease guiding the choice of medication.108 Management of multiple relapses has not been definitively studied, but prolonged tapering and pulsed dosing of oral vancomycin have been used successfully (Table).111, 112
Table 1.
Possible Treatment Options for Multiple Recurrences of CDI
| Treatment Phase | Tapering Phase | |||||
|---|---|---|---|---|---|---|
| Week # | 1 | 2 | 3 | 4 | 5 | 6 |
| Vancomycin Taper | ||||||
| Vancomycin Dose |
125mg q6 hours |
125mg q6 hours |
125mg q8 hours |
125mg q12 hours |
125mg q24 hours |
125mg q48 hours |
| Vancomycin Pulse | ||||||
| Vancomycin Dose |
125mg q6 hours |
125mg q6 hours |
125mg qMWF |
125mg qMWF |
125mg qMWF |
125mg qMWF |
| Rifaximin Chaser | ||||||
| Vancomycin Dose |
125mg q6 hours |
125mg q6 hours |
--- | --- | --- | --- |
| Rifaximin Dose |
--- | --- | 200–400mg Q12 |
200–400mg Q12 |
--- | --- |
Several studies suggest that rifaximin, which has in vitro activity against C difficile, may be effective in treating recurrent disease.113–115 In a case series of 8 women who each had at least 4 previous episodes of CDI and who received a 2-week course of rifaximin after vancomycin therapy, 7 remained symptom-free after a mean follow-up of 233 days.115 After receiving a second course of rifaximin, the eighth patient had no further relapses. Similar results were generated in another case series involving 6 symptomatic patients who had at least 1 previous episode of CDI.113 Five patients had no recurrence after a mean follow-up of 310 days. Interestingly, the patient that continued to have recurrent disease was culture-negative for C difficile. The emergence of rifaximin resistance during therapy has been noted in several studies and may be a potential deterrent to routine use.113, 115
PREVENTION
The prevention of CDI depends both on eliminating the spread of the organism and reducing the risk of infection in individual patients. Reducing C difficile transmission within institutions entails strict hand hygiene and appropriate contact precautions, such wearing a gown and gloves when entering an infected patient’s room.116–119 C difficile spores are resistant to standard disinfectants and can contaminate dry surfaces for months.13 Bleach diluted 1:10 with water is sporicidal in 10 minutes. 120 It is not clear whether routine environmental decontamination with a sporicidal agent is necessary, although it may be of benefit during disease outbreaks.121, 122
Antimicrobial stewardship programs and formulary restrictions are important in reducing the patient’s risk of infection after exposure to C difficile. A 54% reduction in antibiotic use after introduction of an antimicrobial stewardship program during the nosocomial CDI outbreak of the NAP1 strain in Quebec was associated with a 60% decrease in the incidence of CDI.123 Similar results were reported with an antimicrobial stewardship program that reduced use of broad-spectrum antibiotics while leaving overall antibiotic use unchanged.124
Specific restrictions of cephalosporins and clindamycin have led to statistically significant decreases in the incidence of CDI at many centers.125–128 Because CDI outbreaks caused by the NAP1 strain are increasingly being associated with fluoroquinolones, restriction on fluoroquinolones use may prove beneficial.
SUMMARY
The incidence and severity of CDI in both the inpatient and outpatient settings are increasing worldwide. Although traditional risk factors for CDI continue to apply in nosocomial disease, severe community-associated CDI has begun to appear in previously healthy persons. The continued search for a deeper understanding of the epidemiology of CDI has become even more important now that a hypervirulent strain of C difficile—NAP1—has emerged.
Treatment recommendations are evolving. Vancomycin is now the drug of choice for treatment of severe disease. Additional therapies, however, are needed to stem the increasing morbidity and mortality associated with CDI. Attention to infection control practices, including hand hygiene and contact precautions, in combination with antimicrobial control programs, have proved beneficial in controlling nosocomial outbreaks and should be used to reduce the rising incidence of CDI.
Contributor Information
David J. Riddle, Dr, Divisions of internal medicine and pediatric infectious diseases, Washington University School of Medicine in St. Louis.
Erik R. Dubberke, Dr, Division of infectious diseases, Washington University School of Medicine in St. Louis.
References
- 1.Hall IC, O’Toole E. Intestinal flora in ew-born infants with a description of a new pathogenic anaerobe, Bacillus difficilis. American Journal of Diseases in Children. 1935;49:390–402. [Google Scholar]
- 2.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 4.Gursoy S, Guven K, Arikan T, et al. Clostridium difficile infection frequency in patients with nosocomial infections or using antibiotics. Hepatogastroenterology. 2007 Sep;54(78):1720–1724. [PubMed] [Google Scholar]
- 5.Barbut F, Corthier G, Charpak Y, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996 Jul 8;156(13):1449–1454. [PubMed] [Google Scholar]
- 6.Aslam S, Musher DM. An update on diagnosis, treatment, and prevention of Clostridium difficile-associated disease. Gastroenterol Clin North Am. 2006 Jun;35(2):315–335. doi: 10.1016/j.gtc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Barbut F, Petit JC. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect. 2001 Aug;7(8):405–410. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 8.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007 Oct 15;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 9.Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol. 2002 Nov;23(11):696–703. doi: 10.1086/501997. [DOI] [PubMed] [Google Scholar]
- 10.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992 Sep;166(3):561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981 Jan;143(1):42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Dubberke ER, Reske KA, Noble-Wang J, et al. Prevalence of Clostridium difficile environmental contamination and strain variability in multiple health care facilities. Am J Infect Control. 2007 Jun;35(5):315–318. doi: 10.1016/j.ajic.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007 Dec 15;45(12):1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 15.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005 Dec 8;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 16.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998 Feb 28;351(9103):633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 17.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000 Feb 10;342(6):390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 18.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006 Mar;12(3):409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005 Dec 8;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 20.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005 Sep 24–30;366(9491):1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 21.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005 Mar;26(3):273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 22.Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005 Nov 1;41(9):1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 23.Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005 Dec 2;54(47):1201–1205. [PubMed] [Google Scholar]
- 24.Surveillance for community-associated Clostridium difficile--Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008 Apr 4;57(13):340–343. [PubMed] [Google Scholar]
- 25.Rodriguez-Palacios A, Stampfli HR, Duffield T, et al. Clostridium difficile PCR ribotypes in calves, Canada. Emerg Infect Dis. 2006 Nov;12(11):1730–1736. doi: 10.3201/eid1211.051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arroyo LG, Staempfli H, Weese JS. Molecular analysis of Clostridium difficile isolates recovered from horses with diarrhea. Vet Microbiol. 2007 Feb 25;120(1–2):179–183. doi: 10.1016/j.vetmic.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Bojesen AM, Olsen KE, Bertelsen MF. Fatal enterocolitis in Asian elephants (Elephas maximus) caused by Clostridium difficile. Vet Microbiol. 2006 Sep 10;116(4):329–335. doi: 10.1016/j.vetmic.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Perkins SE, Fox JG, Taylor NS, Green DL, Lipman NS. Detection of Clostridium difficile toxins from the small intestine and cecum of rabbits with naturally acquired enterotoxemia. Lab Anim Sci. 1995 Aug;45(4):379–384. [PubMed] [Google Scholar]
- 29.Songer JG, Anderson MA. Clostridium difficile: an important pathogen of food animals. Anaerobe. 2006 Feb;12(1):1–4. doi: 10.1016/j.anaerobe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Clooten J, Kruth S, Arroyo L, Weese JS. Prevalence and risk factors for Clostridium difficile colonization in dogs and cats hospitalized in an intensive care unit. Vet Microbiol. 2008 May 25;129(1–2):209–214. doi: 10.1016/j.vetmic.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Keel K, Brazier JS, Post KW, Weese S, Songer JG. Prevalence of PCR ribotypes among Clostridium difficile isolates from pigs, calves, and other species. J Clin Microbiol. 2007 Jun;45(6):1963–1964. doi: 10.1128/JCM.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirs T, Ocepek M, Rupnik M. Isolation of Clostridium difficile from food animals in Slovenia. J Med Microbiol. 2008 Jun;57(Pt 6):790–792. doi: 10.1099/jmm.0.47669-0. [DOI] [PubMed] [Google Scholar]
- 33.Simango C, Mwakurudza S. Clostridium difficile in broiler chickens sold at market places in Zimbabwe and their antimicrobial susceptibility. Int J Food Microbiol. 2008 Jun 10;124(3):268–270. doi: 10.1016/j.ijfoodmicro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Palacios A, Staempfli HR, Duffield T, Weese JS. Clostridium difficile in retail ground meat, Canada. Emerg Infect Dis. 2007 Mar;13(3):485–487. doi: 10.3201/eid1303.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goorhuis A, Debast SB, van Leengoed LA, et al. Clostridium difficile PCR ribotype 078: an emerging strain in humans and in pigs? J Clin Microbiol. 2008 Mar;46(3):1157. doi: 10.1128/JCM.01536-07. author reply 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debast SB, van Leengoed LA, Goorhuis A, Harmanus C, Kuijper EJ, Bergwerff AA. Clostridium difficile PCR ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ Microbiol. 2009 Feb;11(2):505–511. doi: 10.1111/j.1462-2920.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 37.Riegler M, Sedivy R, Pothoulakis C, et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995 May;95(5):2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006 Feb;73(2):187–197. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 39.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998 Sep;40(1):1–15. doi: 10.1016/s0195-6701(98)90019-6. [DOI] [PubMed] [Google Scholar]
- 40.Gurwith MJ, Rabin HR, Love K. Diarrhea associated with clindamycin and ampicillin therapy: preliminary results of a cooperative study. J Infect Dis. 1977 Mar;135( Suppl):S104–110. doi: 10.1093/infdis/135.supplement.s104. [DOI] [PubMed] [Google Scholar]
- 41.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Cmaj. 2004 Aug 31;171(5):466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992–2002. Clin Infect Dis. 2005 Dec 1;41(11):1621–1627. doi: 10.1086/498027. [DOI] [PubMed] [Google Scholar]
- 43.Dettenkofer M, Ebner W, Bertz H, et al. Surveillance of nosocomial infections in adult recipients of allogeneic and autologous bone marrow and peripheral blood stem-cell transplantation. Bone Marrow Transplant. 2003 May;31(9):795–801. doi: 10.1038/sj.bmt.1703920. [DOI] [PubMed] [Google Scholar]
- 44.Gunderson CC, Gupta MR, Lopez F, et al. Clostridium difficile colitis in lung transplantation. Transpl Infect Dis. 2008 Feb 29; doi: 10.1111/j.1399-3062.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 45.Stelzmueller I, Goegele H, Biebl M, et al. Clostridium difficile colitis in solid organ transplantation--a single-center experience. Dig Dis Sci. 2007 Nov;52(11):3231–3236. doi: 10.1007/s10620-007-9770-z. [DOI] [PubMed] [Google Scholar]
- 46.Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother. 2007 Aug;51(8):2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayatilaka S, Shakov R, Eddi R, Bakaj G, Baddoura WJ, DeBari VA. Clostridium difficile infection in an urban medical center: five-year analysis of infection rates among adult admissions and association with the use of proton pump inhibitors. Ann Clin Lab Sci. 2007 Summer;37(3):241–247. [PubMed] [Google Scholar]
- 48.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003 Jul;54(3):243–245. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 49.Thorens J, Froehlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996 Jul;39(1):54–59. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998 Dec 15;129(12):1012–1019. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 51.Rolfe RD. Role of volatile fatty acids in colonization resistance to Clostridium difficile. Infect Immun. 1984 Jul;45(1):185–191. doi: 10.1128/iai.45.1.185-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurn J, Crossley K, Gerdts A, Maki M, Johnson J. Enteral hyperalimentation as a source of nosocomial infection. J Hosp Infect. 1990 Apr;15(3):203–217. doi: 10.1016/0195-6701(90)90028-m. [DOI] [PubMed] [Google Scholar]
- 53.Hurley BW, Nguyen CC. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch Intern Med. 2002 Oct 28;162(19):2177–2184. doi: 10.1001/archinte.162.19.2177. [DOI] [PubMed] [Google Scholar]
- 54.Tedesco FJ. Pseudomembranous colitis: pathogenesis and therapy. Med Clin North Am. 1982 May;66(3):655–664. doi: 10.1016/s0025-7125(16)31413-4. [DOI] [PubMed] [Google Scholar]
- 55.Kyne L, Merry C, O’Connell B, Kelly A, Keane C, O’Neill D. Factors associated with prolonged symptoms and severe disease due to Clostridium difficile. Age Ageing. 1999 Mar;28(2):107–113. doi: 10.1093/ageing/28.2.107. [DOI] [PubMed] [Google Scholar]
- 56.Peled N, Pitlik S, Samra Z, Kazakov A, Bloch Y, Bishara J. Predicting Clostridium difficile toxin in hospitalized patients with antibiotic-associated diarrhea. Infect Control Hosp Epidemiol. 2007 Apr;28(4):377–381. doi: 10.1086/513723. [DOI] [PubMed] [Google Scholar]
- 57.Wanahita A, Goldsmith EA, Marino BJ, Musher DM. Clostridium difficile infection in patients with unexplained leukocytosis. Am J Med. 2003 Nov;115(7):543–546. doi: 10.1016/s0002-9343(03)00420-0. [DOI] [PubMed] [Google Scholar]
- 58.Hayetian FD, Read TE, Brozovich M, Garvin RP, Caushaj PF. Ileal perforation secondary to Clostridium difficile enteritis: report of 2 cases. Arch Surg. 2006 Jan;141(1):97–99. doi: 10.1001/archsurg.141.1.97. [DOI] [PubMed] [Google Scholar]
- 59.Rybolt AH, Bennett RG, Laughon BE, Thomas DR, Greenough WB, 3rd, Bartlett JG. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet. 1989 Jun 17;1(8651):1353–1355. doi: 10.1016/s0140-6736(89)92803-1. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2001 Mar;80(2):88–101. doi: 10.1097/00005792-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Pilotto A, Franceschi M, Vitale D, et al. The prevalence of diarrhea and its association with drug use in elderly outpatients: a multicenter study. Am J Gastroenterol. 2008 Nov;103(11):2816–2823. doi: 10.1111/j.1572-0241.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 62.Clayton EM, Rea MC, Shanahan F, et al. The Vexed Relationship Between Clostridium Difficile and Inflammatory Bowel Disease: An Assessment of Carriage in an Outpatient Setting Among Patients in Remission. Am J Gastroenterol. 2009 Mar 24; doi: 10.1038/ajg.2009.4. [DOI] [PubMed] [Google Scholar]
- 63.Lo TS, Borchardt SM. Antibiotic-associated diarrhea due to methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2009 Apr;63(4):388–389. doi: 10.1016/j.diagmicrobio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Zollner-Schwetz I, Hogenauer C, Joainig M, et al. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis. 2008 Nov 1;47(9):e74–78. doi: 10.1086/592074. [DOI] [PubMed] [Google Scholar]
- 65.Peterson LR, Kelly PJ. The role of the clinical microbiology laboratory in the management of Clostridium difficile-associated diarrhea. Infect Dis Clin North Am. 1993 Jun;7(2):277–293. [PubMed] [Google Scholar]
- 66.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008 Jan 15;46( Suppl 1):S12–18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 67.Lyerly DM, Neville LM, Evans DT, et al. Multicenter evaluation of the Clostridium difficile TOX A/B TEST. J Clin Microbiol. 1998 Jan;36(1):184–190. doi: 10.1128/jcm.36.1.184-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musher DM, Manhas A, Jain P, et al. Detection of Clostridium difficile toxin: comparison of enzyme immunoassay results with results obtained by cytotoxicity assay. J Clin Microbiol. 2007 Aug;45(8):2737–2739. doi: 10.1128/JCM.00686-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohan SS, McDermott BP, Parchuri S, Cunha BA. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006 Apr;119(4):356, e357–358. doi: 10.1016/j.amjmed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 70.Gilligan PH. Is a two-step glutamate dehyrogenase antigen-cytotoxicity neutralization assay algorithm superior to the premier toxin A and B enzyme immunoassay for laboratory detection of Clostridium difficile? J Clin Microbiol. 2008 Apr;46(4):1523–1525. doi: 10.1128/JCM.02100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ticehurst JR, Aird DZ, Dam LM, Borek AP, Hargrove JT, Carroll KC. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J Clin Microbiol. 2006 Mar;44(3):1145–1149. doi: 10.1128/JCM.44.3.1145-1149.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkins TD, Lyerly DM. Clostridium difficile testing: after 20 years, still challenging. J Clin Microbiol. 2003 Feb;41(2):531–534. doi: 10.1128/JCM.41.2.531-534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG. Rapid detection of Clostridium difficile in feces by real-time PCR. J Clin Microbiol. 2003 Feb;41(2):730–734. doi: 10.1128/JCM.41.2.730-734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samie A, Obi CL, Franasiak J, et al. PCR detection of Clostridium difficile triose phosphate isomerase (tpi), toxin A (tcdA), toxin B (tcdB), binary toxin (cdtA, cdtB), and tcdC genes in Vhembe District, South Africa. Am J Trop Med Hyg. 2008 Apr;78(4):577–585. [PubMed] [Google Scholar]
- 75.Peterson LR, Manson RU, Paule SM, et al. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin Infect Dis. 2007 Nov 1;45(9):1152–1160. doi: 10.1086/522185. [DOI] [PubMed] [Google Scholar]
- 76.Kirkpatrick ID, Greenberg HM. Evaluating the CT diagnosis of Clostridium difficile colitis: should CT guide therapy? AJR Am J Roentgenol. 2001 Mar;176(3):635–639. doi: 10.2214/ajr.176.3.1760635. [DOI] [PubMed] [Google Scholar]
- 77.Kawamoto S, Horton KM, Fishman EK. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics. 1999 Jul-Aug;19(4):887–897. doi: 10.1148/radiographics.19.4.g99jl07887. [DOI] [PubMed] [Google Scholar]
- 78.Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile--Associated diarrhea: A review. Arch Intern Med. 2001 Feb 26;161(4):525–533. doi: 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
- 79.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983 Nov 5;2(8358):1043–1046. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 80.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996 May;22(5):813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 81.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007 Aug 1;45(3):302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 82.Louie T, Gerson M, Grimard D, et al. Results of a phase III trial comparing tolevamar, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhea (CDAD). Paper presented at: 47th Annual ICAAC; 2007; Chicago. [Google Scholar]
- 83.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002 Sep 15;35(6):690–696. doi: 10.1086/342334. [DOI] [PubMed] [Google Scholar]
- 84.Malnick SD, Zimhony O. Treatment of Clostridium difficile-associated diarrhea. Ann Pharmacother. 2002 Nov;36(11):1767–1775. doi: 10.1345/aph.1A160. [DOI] [PubMed] [Google Scholar]
- 85.Nathanson DR, Sheahan M, Chao L, Wallack MK. Intracolonic use of vancomycin for treatment of clostridium difficile colitis in a patient with a diverted colon: report of a case. Dis Colon Rectum. 2001 Dec;44(12):1871–1872. doi: 10.1007/BF02234471. [DOI] [PubMed] [Google Scholar]
- 86.Pasic M, Carrel T, Opravil M, Mihaljevic T, von Segesser L, Turina M. Systemic absorption after local intracolonic vancomycin in pseudomembranous colitis. Lancet. 1993 Aug 14;342(8868):443. doi: 10.1016/0140-6736(93)92861-m. [DOI] [PubMed] [Google Scholar]
- 87.Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. 2008 Jan;74(1):20–26. doi: 10.1177/000313480807400105. [DOI] [PubMed] [Google Scholar]
- 88.Lamontagne F, Labbe AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007 Feb;245(2):267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008 Feb;143(2):150–154. doi: 10.1001/archsurg.2007.46. discussion 155. [DOI] [PubMed] [Google Scholar]
- 90.Nelson R. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev. 2007;(3):CD004610. doi: 10.1002/14651858.CD004610.pub3. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence SJ, Korzenik JR, Mundy LM. Probiotics for recurrent Clostridium difficile disease. J Med Microbiol. 2005 Sep;54(Pt 9):905–906. doi: 10.1099/jmm.0.46096-0. [DOI] [PubMed] [Google Scholar]
- 92.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. Jama. 1994 Jun 22–29;271(24):1913–1918. [PubMed] [Google Scholar]
- 93.Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000 Oct;31(4):1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 94.Wullt M, Hagslatt ML, Odenholt I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: a double-blind, placebo-controlled trial. Scand J Infect Dis. 2003;35(6–7):365–367. doi: 10.1080/00365540310010985. [DOI] [PubMed] [Google Scholar]
- 95.Cassone M, Serra P, Mondello F, et al. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J Clin Microbiol. 2003 Nov;41(11):5340–5343. doi: 10.1128/JCM.41.11.5340-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lherm T, Monet C, Nougiere B, et al. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002 Jun;28(6):797–801. doi: 10.1007/s00134-002-1267-9. [DOI] [PubMed] [Google Scholar]
- 97.Ariano RE, Zhanel GG, Harding GK. The role of anion-exchange resins in the treatment of antibiotic-associated pseudomembranous colitis. Cmaj. 1990 May 15;142(10):1049–1051. [PMC free article] [PubMed] [Google Scholar]
- 98.Keighley MR. Antibiotic-associated pseudomembranous colitis: pathogenesis and management. Drugs. 1980 Jul;20(1):49–56. doi: 10.2165/00003495-198020010-00003. [DOI] [PubMed] [Google Scholar]
- 99.Mogg GA, Burdon DW, Keighley M. Oral metronidazole in Clostridium difficile colitis. Br Med J. 1979 Aug 4;2(6185):335. doi: 10.1136/bmj.2.6185.335-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor NS, Bartlett JG. Binding of Clostridium difficile cytotoxin and vancomycin by anion-exchange resins. J Infect Dis. 1980 Jan;141(1):92–97. doi: 10.1093/infdis/141.1.92. [DOI] [PubMed] [Google Scholar]
- 101.Kunimoto D, Thomson AB. Recurrent Clostridium difficile-associated colitis responding to cholestyramine. Digestion. 1986;33(4):225–228. doi: 10.1159/000199299. [DOI] [PubMed] [Google Scholar]
- 102.Pruksananonda P, Powell KR. Multiple relapses of Clostridium difficile-associated diarrhea responding to an extended course of cholestyramine. Pediatr Infect Dis J. 1989 Mar;8(3):175–178. [PubMed] [Google Scholar]
- 103.Bartlett JG, Perl TM. The new Clostridium difficile--what does it mean? N Engl J Med. 2005 Dec 8;353(23):2503–2505. doi: 10.1056/NEJMe058221. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J Clin Gastroenterol. 2004 May-Jun;38(5):414–418. doi: 10.1097/00004836-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Nair S, Yadav D, Corpuz M, Pitchumoni CS. Clostridium difficile colitis: factors influencing treatment failure and relapse--a prospective evaluation. Am J Gastroenterol. 1998 Oct;93(10):1873–1876. doi: 10.1111/j.1572-0241.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 106.Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008 Jul;52(7):2403–2406. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchez JLGD, Olson MM, Johnson S. Metronidazole susceptibility in Clostridium difficile isolates recovered from cases of C. difficile–associated disease treatment failures and successes. Anaerobe. 1999;5:201–204. [Google Scholar]
- 108.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000 Jun;38(6):2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997 Mar;24(3):324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 110.McFarland LV. Alternative treatments for Clostridium difficile disease: what really works? J Med Microbiol. 2005 Feb;54(Pt 2):101–111. doi: 10.1099/jmm.0.45753-0. [DOI] [PubMed] [Google Scholar]
- 111.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002 Jul;97(7):1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 112.Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1985 Nov;80(11):867–868. [PubMed] [Google Scholar]
- 113.Garey KW, Salazar M, Shah D, Rodrigue R, DuPont HL. Rifamycin antibiotics for treatment of Clostridium difficile-associated diarrhea. Ann Pharmacother. 2008 Jun;42(6):827–835. doi: 10.1345/aph.1K675. [DOI] [PubMed] [Google Scholar]
- 114.Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005 Apr;3(2):201–211. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 115.Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007 Mar 15;44(6):846–848. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 116.Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med. 1990 Feb;88(2):137–140. doi: 10.1016/0002-9343(90)90462-m. [DOI] [PubMed] [Google Scholar]
- 117.Muto CA, Blank MK, Marsh JW, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis. 2007 Nov 15;45(10):1266–1273. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]
- 118.Vonberg RP, Kuijper EJ, Wilcox MH, et al. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008 May;14( Suppl 5):2–20. doi: 10.1111/j.1469-0691.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 119.Zafar AB, Gaydos LA, Furlong WB, Nguyen MH, Mennonna PA. Effectiveness of infection control program in controlling nosocomial Clostridium difficile. Am J Infect Control. 1998 Dec;26(6):588–593. doi: 10.1053/ic.1998.v26.a84773. [DOI] [PubMed] [Google Scholar]
- 120.Perez J, Springthorpe VS, Sattar SA. Activity of selected oxidizing microbicides against the spores of Clostridium difficile: relevance to environmental control. Am J Infect Control. 2005 Aug;33(6):320–325. doi: 10.1016/j.ajic.2005.04.240. [DOI] [PubMed] [Google Scholar]
- 121.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995 Aug;16(8):459–477. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 122.McMullen KM, Zack J, Coopersmith CM, Kollef M, Dubberke E, Warren DK. Use of hypochlorite solution to decrease rates of Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2007 Feb;28(2):205–207. doi: 10.1086/511791. [DOI] [PubMed] [Google Scholar]
- 123.Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis. 2007 Sep 1;45( Suppl 2):S112–121. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 124.Fowler S, Webber A, Cooper BS, et al. Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother. 2007 May;59(5):990–995. doi: 10.1093/jac/dkm014. [DOI] [PubMed] [Google Scholar]
- 125.Climo MW, Israel DS, Wong ES, Williams D, Coudron P, Markowitz SM. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann Intern Med. 1998 Jun 15;128(12 Pt 1):989–995. doi: 10.7326/0003-4819-128-12_part_1-199806150-00005. [DOI] [PubMed] [Google Scholar]
- 126.Davey P, Brown E, Fenelon L, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005;(4):CD003543. doi: 10.1002/14651858.CD003543.pub2. [DOI] [PubMed] [Google Scholar]
- 127.Khan R, Cheesbrough J. Impact of changes in antibiotic policy on Clostridium difficile-associated diarrhoea (CDAD) over a five-year period in a district general hospital. J Hosp Infect. 2003 Jun;54(2):104–108. doi: 10.1016/s0195-6701(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 128.O’Connor KA, Kingston M, O’Donovan M, Cryan B, Twomey C, O’Mahony D. Antibiotic prescribing policy and Clostridium difficile diarrhoea. Qjm. 2004 Jul;97(7):423–429. doi: 10.1093/qjmed/hch076. [DOI] [PubMed] [Google Scholar]