Abstract
The DNA content of nuclei is a valuable measure of cell cycle status. Irises is a software tool to facilitate systematic in situ determination of DNA content for cell cycle analysis at single-nucleus resolution within complex tissues. We demonstrate the utility of the tool with analysis of DNA content in germline nuclei of C. elegans. Compared with results obtained by manual analysis, we find the tool greatly facilitates analysis by improving speed at least 5-fold while maintaining accuracy. The source code and instruction manual (including installation for both Mac and PC) are provided.
Keywords: C. elegans, germline, ploidy, cell cycle, image analysis
Introduction
In many areas of cell biological inquiry, knowing the cell cycle status of interphase nuclei in their native position within a tissue is critical. In contrast to fluorescence-activated cell sorting (FACS) where tissues are disrupted and individual cells isolated, in situ analysis is achieved by high-resolution imaging after fixation of the tissue and labeling of DNA with fluorescent dyes such as propidium iodide or 4',6-diamidino-2-phenylindole (DAPI). The relative distribution of fluorescence intensity is then correlated with ploidy (N) to determine which cells are in G1 (2N), S-phase, or G2 (4N) within interphase.1 Despite its wide use in biology, data analysis in the image-based in situ analysis is not as highly automated. Manual analysis is often prohibitive for large numbers of cells.
The C. elegans germ line contains a population of stem/progenitor cells for which cell cycle is an important regulatory target of signaling by the insulin/IGF-like and TOR pathways, among others.2-4 This tissue is not amenable to FACS analysis since each germ cell retains a small opening to a core of shared cytoplasm. In the past, the number, position, and DNA content of interphase nuclei were assessed by quantifying fluorescence from individual planes within a stack of images (pseudo3D by optical sectioning).4-7 This technique requires manual identification and segmentation of each nucleus. While reliable, the process is extremely laborious, limiting the practicality of quantitative analysis.
Results
Here we present Irises, a software tool to facilitate the in situ analysis of cellular DNA content, using the C. elegans germ line as a test case. Irises uses an automated algorithm to segment nuclei from such images and quantify DNA content. Each identified nucleus is modeled as a sphere and the DNA content is estimated by calculating the total pixel intensity of fluorescence within each sphere.8 Meanwhile, it uses supervised data analysis, facilitating user interaction with the data to perform additional analysis such as normalized ploidy in biologically relevant regions.
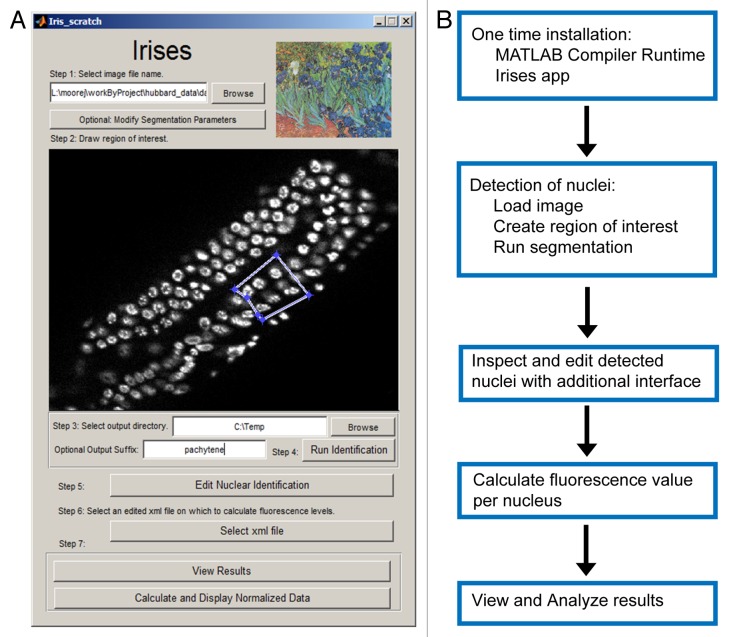
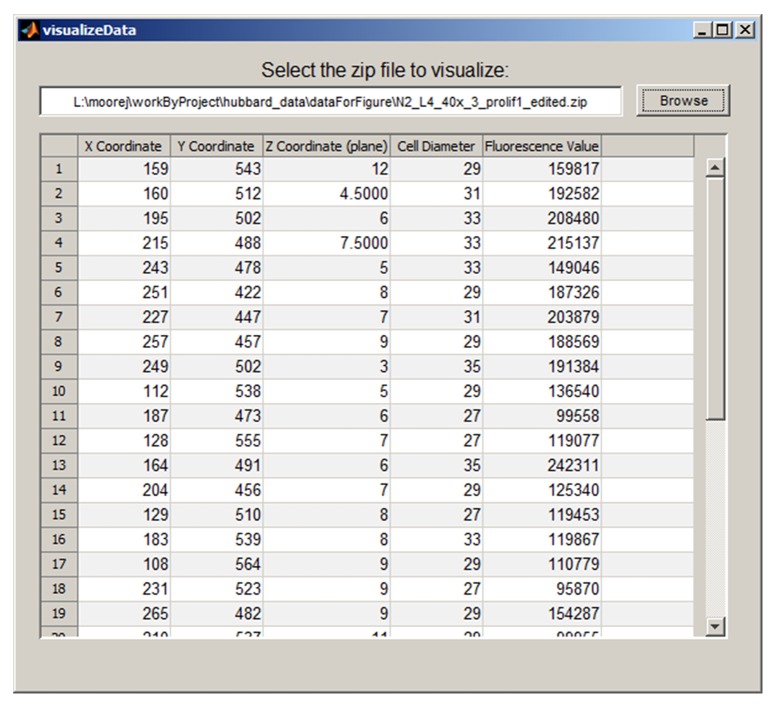
The Irises Graphical User Interface (GUI) is shown in Figure 1A, with a summary of the workflow outlined in Figure 1B. First, given an input image stack, the GUI displays a maximum projection of the stack. Second, it enables the user to define a Region of Interest (ROI) in the input images using mouse clicks. Third, automated segmentation is performed in which the program identifies nuclei within the ROI. Fourth, Irises allows users to conveniently examine and edit the automatically identified nuclei for quality control. Editing is done in an additional GUI in which users can add, remove, or adjust the position and size of each individual nucleus. In addition, nuclei near the top and bottom of the image stack that may be unreliable due to optical aberrations can be removed in bulk by excluding all nuclei from specific image planes.9 Fifth, Irises calculates the fluorescence value per nucleus. For each detected nucleus, the results include its position (the x, y, and z coordinates of its center), size (diameter in pixels), and the fluorescence value (Fig. 2). The results are saved in tabular form, which allows users to export the results and perform additional manipulations using other tools such as Excel.
Figure 1. Irises Interface and Workflow. (A) Screen capture of the Irises application. A region of interest containing nuclei in the pachytene stage is defined and is ready to be segmented. (B) Diagram of Irises workflow.
Figure 2. The View Results window in Irises provides a tabular view of computed nuclear positions, sizes, and fluorescence for the region of interest.
Finally, Irises performs additional analysis to automatically create histograms of raw fluorescence values or normalized N-values (ploidy) by comparing with nuclei of known DNA content. Irises allows the user to sequentially define and analyze multiple ROIs from the same image stack. Figure 3 provides an example analysis of two ROIs in an image stack of the C. elegans germ line. The ROI in Figure 3A (see also Fig. 1A) contains nuclei in the pachytene stage of prophase of meiosis I that are known to possess 4N content. The ROI defined in Figure 3B contains proliferative zone nuclei of unknown DNA content, which can be normalized to the data collected from the first ROI to infer the ploidy of each nucleus.

Figure 3. Ploidy analysis in the C. elegans germ line (A and B) Regions of interests specifying nuclei in the pachytene (A) and proliferative (B) zone. The average nuclear intensity at the pachytene stage, which has known 4N content can be used normalize florescence values at the proliferative stage. (C) Normalized histograms. Normalized florescence values can be used to compare the distribution of DNA content within the proliferative region in WT and mutant worms. The rightward shift in the daf-2 distribution indicates an increased proportion of nuclei in late-S and G2.
To test Irises, we compared its output with manual DNA quantification. We generated and analyzed images from 4th larval stage (L4) wild-type and daf-2(e1370) mutant animals. DAF-2 is the sole insulin/IGF-like receptor in C. elegans. Among many biological roles for this receptor, it is required in the larval germ line to promote the accumulation of proliferative germ cells.3 The Irises-generated distribution of DNA content among nuclei in the proliferative zone (the distal-most 13 cell diameters, normalized to 4N pachytene nuclei in the same image) is shown from one individual gonad arm from each genotype (Fig. 3C). For the wild-type, we also determined that morphologically metaphase and prophase nuclei contained a G2 DNA content (≥ 4N) while each half of an anaphase figure yielded a G1 or early S phase DNA content (≤ 2.7N; data not shown). The Irises analysis indicates a shift in the daf-2(e1370) mutant to a greater proportion of nuclei in late-S and G2 (≥ 3.3N, Fig. 3C). These results are fully consistent with our previously published manually collected data.7 In addition, analysis with Irises is at least five times faster per sample, reducing the analysis from approximately 4 h to 45 min per L4 gonad arm.
The source code, a workflow diagram, and detailed instructions for Irises are provided in Supplementary Material, as well as methods for image collection used in this study.
Discussion
Irises is a useful software tool for image analysis to measure DNA content from 3D fluorescence images in a systematic and objective fashion. It is worth noting that proper setup for image acquisition is a vital step to obtain the appropriate data for informative measurements. In particular, one should consider the appropriate spacing between z planes given different types of microscopy. Detailed considerations and guidelines can be found in a recent review.10 Two features in Irises help users to evaluate potential over- or under-sampling. First, the tabular output (Fig. 2) contains the diameter of each detected nucleus. This allows the users to estimate the number of z planes that are sampled in individual nuclei. More importantly, it allows users to examine potential shape and size changes of nuclei in mutants, which could affect the number of z planes sampled per nucleus and the apparent total fluorescent signal due to changed sampling rate. Another common issue in 3D imaging is the reduction of the apparent fluorescent intensity at the deep end of a specimen due to optical aberrations. The tabular output contains the z position and total fluorescent signal per nucleus. A simple plot in Excel using the tabular output would allow users to examine the correlation between the two variables and determine the meaningful z range to be used. As mentioned, Irises allows users to remove nuclei in bulk by excluding all nuclei from specific image planes. Finally, when imaging a complex tissue, it would not be practical to obtain the ideal image of each nucleus. Therefore, it is critical to sample a sufficient number of nuclei to achieve meaningful statistics. In this regard, the automated and objective measurements provided by Irises is particularly valuable.
Irises is useful for studies in which the relative position of nuclei together with their DNA content is of interest and/or for situations where FACS analysis is not feasible. In principle, any tissue where nuclei can be imaged with consistent fluorescence levels between planes can be analyzed using Irises and simple DNA stains. The underlying algorithm sums up the per pixel fluorescence intensity within an identified nucleus, regardless of the fluorophore used for labeling. More importantly, Irises allows specific cells to be included or excluded from the analysis based on position or other information (e.g., additional markers). For example, in the developing brain and retina, stereotypical movements are associated with cell cycle under normal conditions.11,12 The cell cycle status may be more easily tracked under a variety of conditions and in conjunction with other markers using this approach. In addition, the Irises approach could potentially be useful in analysis of samples in which changes in ploidy beyond 4N are linked to development or pathology.13 In some experimental systems this is currently achieved with methods such as fluorescence in situ hybridization FISH.14 Finally, we envisage that this tool could also be used to track subtle changes in other fluorescent markers such as label dilution experiments.15
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health (GM061706, GM102254, AG042551 to Hubbard EJA, and GM097576, HD075602 to Bao Z).
Acknowledgments
We are grateful to Jeremy Nance for suggesting the possibility that modifications of the StarryNite software could facilitate DNA content analysis, to Fiona Doetsch for suggesting additional applications of the approach and to David Lu for help with testing and troubleshooting the program.
References
- 1.Darzynkiewicz Z, Huang X. Analysis of DNA content by flow cytometry. In: Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, eds. Current protocols in Immunology: Wiley 2004. [Google Scholar]
- 2.Hubbard EJA. Caenorhabditis elegans germ line: a model for stem cell biology. Dev Dyn. 2007;236:3343–57. doi: 10.1002/dvdy.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubbard EJA. Insulin and Germline Proliferation in Caenorhabditis elegans. Elsevier Inc., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubbard EJ, Korta DZ, Dalfó D. Physiological control of germline development. Adv Exp Med Biol. 2013;757:101–31. doi: 10.1007/978-1-4614-4015-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng H, Zhong W, Punkosdy G, Gu S, Zhou L, Seabolt EK, Kipreos ET. CUL-2 is required for the G1-to-S-phase transition and mitotic chromosome condensation in Caenorhabditis elegans. Nat Cell Biol. 1999;1:486–92. doi: 10.1038/70272. [DOI] [PubMed] [Google Scholar]
- 6.Fox PM, Vought VE, Hanazawa M, Lee M-H, Maine EM, Schedl T. Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development. 2011;138:2223–34. doi: 10.1242/dev.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaelson D, Korta DZ, Capua Y, Hubbard EJA. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–80. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santella A, Du Z, Nowotschin S, Hadjantonakis AK, Bao Z. A hybrid blob-slice model for accurate and efficient detection of fluorescence labeled nuclei in 3D. BMC Bioinformatics. 2010;11:580. doi: 10.1186/1471-2105-11-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle TJ, Bao Z, Murray JI, Araya CL, Waterston RH. AceTree: a tool for visual analysis of Caenorhabditis elegans embryogenesis. BMC Bioinformatics. 2006;7:275. doi: 10.1186/1471-2105-7-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Heuvel S, Kipreos ET. C. elegans cell cycle analysis. Methods Cell Biol. 2012;107:265–94. doi: 10.1016/B978-0-12-394620-1.00009-6. [DOI] [PubMed] [Google Scholar]
- 11.Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–42. doi: 10.1038/35072555. [DOI] [PubMed] [Google Scholar]
- 12.Hayes NL, Nowakowski RS. Exploiting the dynamics of S-phase tracers in developing brain: interkinetic nuclear migration for cells entering versus leaving the S-phase. Dev Neurosci. 2000;22:44–55. doi: 10.1159/000017426. [DOI] [PubMed] [Google Scholar]
- 13.Hedgecock EM, Culotti JG, Thomson JN, Perkins LA. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol. 1985;111:158–70. doi: 10.1016/0012-1606(85)90443-9. [DOI] [PubMed] [Google Scholar]
- 14.Prieto P, Moore G, Shaw P. Fluorescence in situ hybridization on vibratome sections of plant tissues. Nat Protoc. 2007;2:1831–8. doi: 10.1038/nprot.2007.265. [DOI] [PubMed] [Google Scholar]
- 15.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.