Abstract
Over-expression of ornithine decarboxylase (ODC) is known to be involved in the epidermal carcinogenesis. However, the mechanism by which it enhances skin carcinogenesis remains undefined. Recently, role of stem cells localized in various epidermal compartments has been shown in the pathogenesis of skin cancer. To direct ODC expression in distinct epidermal compartments, we have developed keratin 6 (K6)- DC/SKH-1 and keratin 14 (K14)-ODC/SKH-1 mice and employed them to investigate the role of ODC directed to these epidermal compartments on UVB-induced carcinogenesis. K6-driven ODC over-expression directed to outer root sheath (ORS) of hair follicle was more effective in augmenting tumorigenesis as compared to mice where K14-driven ODC expression was directed to inter-follicular epidermal keratinocytes. Chronically UVB-irradiated K6-ODC/SKH-1 developed 15±2.5 tumors/mouse whereas K14-ODC/SKH-1 developed only 6.8±1.5 tumors/mouse. K6-ODC/SKH-1 showed augmented UVB-induced proliferation and much higher pro-inflammatory responses than K14-ODC/SKH-1 mice. Tumors induced in K6-ODC/SKH-1 were rapidly growing, invasive and ulcerative squamous cell carcinoma (SCC) showing decreased expression of epidermal polarity marker E-cadherin and enhanced mesenchymal marker, fibronectin. Interestingly, the number of CD34/CK15/p63 positive stem-like cells was significantly higher in chronically UVB-irradiated K6-ODC/SKH-1 as compared to K14-ODC/SKH-1 mice. Reduced Notch1 expression was correlated with the expansion of stem cell compartment in these animals. However, other signaling pathways such as DNA damage response or mTOR signaling pathways were not significantly different in tumors induced in these two murine models suggesting the specificity of Notch pathway in this regard. These data provide a novel role of ODC in augmenting tumorigenesis via negatively regulated Notch-mediated expansion of stem cell compartment.
Keywords: ODC, UVB, Hair follicle, Stem cell, EMT, Notch
INTRODUCTION
Polyamines are small organic cations that are essential for normal cell growth, cell survival and skin homeostasis in eukaryotes. Dysregulated polyamine metabolism may be involved in the pathogenesis of skin cancer [1, 2]. Ornithine decarboxylase (ODC) is the rate-limiting enzyme which catalyzes the most committed step in the polyamine biosynthetic pathway [3, 4]. Under normal conditions ODC enzyme activity is tightly regulated. However, its expression and activity are induced in response to various external stimuli including tumor promoting agents [4, 5]. ODC is over-expressed in human non-melanoma skin cancers (NMSCs), including squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs), which are the most common human neoplasm [6]. Solar ultraviolet B (UVB), the major etiologic factor for NMSCs in humans induces ODC in the skin. Over-expression of ODC has been demonstrated as an early event in the neoplastic transformation both in murine and human skin keratinocytes [4]. In this regard, employing various animal models of skin carcinogenesis and other epithelial tumors a direct link between increased ODC activity and promotion of neoplastic growth has also been established [7,8].
Keratin 14 (K14) is an intermediate filament produced in squamous epithelia. K14 promoter has been extensively used to direct the expression of various transgenes to the proliferating mouse skin keratinocytes [9]. For example, K14-MEK mice overexpressing MEK protein manifested increased expression of ODC in spontaneously developed skin tumors [10]. Over-expression of ODC in the outer root sheath (ORS) of the hair follicle using both keratin 5 (K5) and keratin 6 (K6) promoters was shown to stimulate skin tumor promotion in transgenic animal models [11-13]. The sub-threshold doses of carcinogens topically applied to these animals lead to squamous papillomas in the absence of further treatments with a tumor promoter suggesting that ODC over-expressing animals are pre-promoted [11]. We showed that K6-ODC over-expressing Ptch+/− mice manifest augmented development of both BCCs and SCCs upon chronic UVB irradiation [14]. These studies unambiguously demonstrate the ability of ODC to augment proliferation of initiated skin keratinocytes contributing to the pathogenesis of NMSCs.
The bulge stem cells are known to play major role in maintaining the skin homeostasis and tumorigenesis [15]. The origin of SCCs may occur from the slow proliferating stem cell populations located either in inter-follicular epidermis or in the bulge region of hair follicle [16]. Oncogenic mutations or mutational inactivation of tumor suppressor genes in the epidermal keratinocytes are known to drive the pathogenesis of skin cancers. A recent study using ODC-ER transgenic has shown that augmented epidermal ODC activity in the hair follicle bulge stem cells promotes skin chemical carcinogenesis [17].
In the epidermis, Notch and its ligands are abundantly expressed which play an essential role in postnatal hair follicle differentiation and homeostasis. In addition, activated Notch signaling pathway controls stem cell self-renewal [18]. Conditional deletion of Notch1 resulted in development of spontaneous BCC-like lesions in newborn mice [19]. Notch1 expression in non-melanoma skin cancer varies differentially depending on the anatomical site and the tumor histotype. Inhibition of Notch in primary human keratinocytes expressing activated ras gene leads to formation of SCCs [20]. Notch1 is down-regulated in UVB-induced invasive SCC, possibly as a consequence of mutational inactivation of p53 [21].
We have generated two novel murine models over-expressing ODC driven by K14 and K6 promoters in SKH-1 genetic background. These promoters respectively target gene expression to inter-follicular epidermis and ORS of hair follicles [7, 17]. Chronic UVB-irradiation of these animals showed significant differences in the tumor phenotype, tumor numbers and tumor volume. These differences in K6-ODC/SKH-1 mice and K14-ODC/SKH-1 mice were correlated with the ability of ODC in various epidermal compartments to differentially expand stem cell populations. We also show that Notch which plays a key role in stem cell renewal was down-regulated more effectively in K6-ODC/SKH-1 than in K14-ODC/SKH-1 mice. These data indicate that ODC over-expression regulates stem cell compartment by negatively regulating Notch leading to significant alterations in UVB-induced tumorigenesis.
MATERIALS & METHODS
Animals
K6-ODC/SKH-1 mice were generated by breeding male (6–7 weeks old) hemizygous ODC transgenic B6.Cg-Tg (K6-Odc) 55Tgo strain (Taconic, Germantown, NY, USA) with female SKH-1 (Jackson Laboratory, Bar Harbor, ME, USA). These mice were backcrossed to SKH-1 for nine generations. K14-ODC/SKH-1 mice were generated by microinjecting the ODC gene carrying a K14 promoter into SKH-1 zygotes with support obtained from the transgenic core facility at University of Alabama at Birmingham. These mice were then crossed with SKH-1 to develop a suitable size colony. Tail biopsies obtained at day 11 were used for genotyping using K6-ODC and K14-ODC primers (Supplementary Table S1). The animal experiments were conducted using protocols approved by the IACUC of the University of Alabama at Birmingham.
UVB irradiation protocol
Both K6-ODC/SKH-1 and K14-ODC/SKH-1 mice were divided into two groups consisting of non-UVB-irradiated age-matched control and UVB-irradiated experimental animals (n=10). These animals were chronically exposed to UVB radiation (180mJ/cm2) three times/week for 29 weeks using UV irradiation Unit (Daavlin Co., Bryan, OH, USA). Tumors were counted and measured at every two week intervals. At the end of the experiment, tumors and skin were harvested and randomly selected for the immunohistochemical and western blot analysis.
Histology, immunohistochemical or immunoflurorescence staining of tissue sections
Histology, immunohistochemical or immunofluorescence staining of skin and tumor sections were performed according to the standard protocol as described earlier [22].
Western Blot analysis
Tissue lysates were prepared in ice-cold lysis buffer containing 50 mmol/L Tris pH, 1% Triton X 100, 0.25% NaF, 10 mmol/L -glycerophosphate, 1 mmol/L EDTA, 5 mmol/L sodium pyrophosphate, 0.5 mmol/L Na3VO4, 10 mmol/L dithiothreitol, 1% phenylmethylsulfonylfluoride, and protease inhibitors cocktail. For Western blot analysis, proteins (60–80 μg) were resolved on 4%, 10% and 15% Tris-glycine gel based on their molecular weights and transferred onto a nitrocellulose membrane (Bio-Rad) as described previously [22]. For sequential antibody reprobing, blots were stripped using Restore western blot stripping buffer (Pierce Biotechnology, Rockford, IL, USA) according to manufacturer's instructions. List of antibodies is summarized in Supplementary Table S2.
Qualitative and quantitative polymerase chain reaction (PCR)
RNA was extracted using Trizol, and reverse transcribed using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, NY, USA). Quantitative PCR was performed using Taqman Fast Advanced Master Mix Product Insert (Applied Biosystems, NY, USA). Relative quantification of the steady-state target mRNA levels was done after normalization of total amount of cDNA to GAPDH (glyceraldehyde-3-phosphate dehydrogenase), an endogenous reference. The list of primers used in this study is described in Supplementary Table S1.
Statistical Analysis
Tumor numbers and volumes are expressed as mean ± SE. Statistical analysis was performed using Microsoft Excel software 2007. The significance between two test groups was determined using the Student t test. A value of P<0.05 was considered to be significant.
RESULTS
To address the tissue context-dependent role of ODC over-expression, we developed K6-ODC/SKH-1 and K14-ODC/SKH-1 hairless mice in SKH-1 genetic background. SKH-1 is a highly susceptible genetic background for UVB-induced carcinogenesis. These animals carry homozygous mutations in hairless gene which is required for the normal development of hair follicles. However, as a result of mutant hairless gene, the growth of hair follicles is disrupted leading to a hairless phenotype [23].
Phenotypic and genotypic differences between K6-ODC/SKH-1 and K14-ODC/SKH-1 mice
ODC over-expression was driven to hair follicular and inter-follicular regions of epidermis in the skin using K6 & K14 promoters respectively in K6-ODC/SKH-1 and K14-ODC/SKH-1 mice. These animals generated in SKH-1 genetic background are described for the first time to the best of our knowledge. We confirmed that both K6-ODC/SKH-1 and K14-ODC/SKH-1 mice manifest increased levels of ODC mRNA and protein expression compared to their respective littermates without ODC transgene as shown in Supplementary Figure 1. K6-ODC/SKH-1 and K14-ODC/SKH-1 mice manifest apparent differences in their phenotypic appearance. K6-ODC/SKH-1 mice developed thicker skin which was distinct from week 10 and continued to grow thereafter. At week 30, a dramatic increase in skin mass was noticed resulting in extreme wrinkling and folding. However, this phenotype was substantially less severe in K14-ODC/SKH-1 mice (Supplementary Figure 1). Histological examinations of the skin revealed considerable enlargement of dermal cysts filled with amorphous masses of cornified debris in K6- ODC/SKH-1 mice as also reported earlier for K6-ODC mice in C57BL/6 genetic background [24]. In K14-ODC/SKH-1 mice these cysts were also present but were significantly smaller in size (Figure 1B). Further analyses also revealed a disruption in hair follicle and bulb regions leading to follicular degeneration in both the mouse models. However, the follicular degeneration in these animals may be due to homozygous mutation in hairless gene which is associated with SKH-1 genetic background. Moreover, the ODC over-expression in the skin is also known to disrupt the development of hair follicles, but this could not be distinguished in these animals at least up to the age of 6 weeks. None-the-less, rudimentary hair-follicles could be seen in both of these mice. However, no significant differences could be seen in the epidermis of untreated control or chronically UVB-irradiated animals.
Figure 1. ODC over-expression augments UVB-induced skin carcinogenesis.
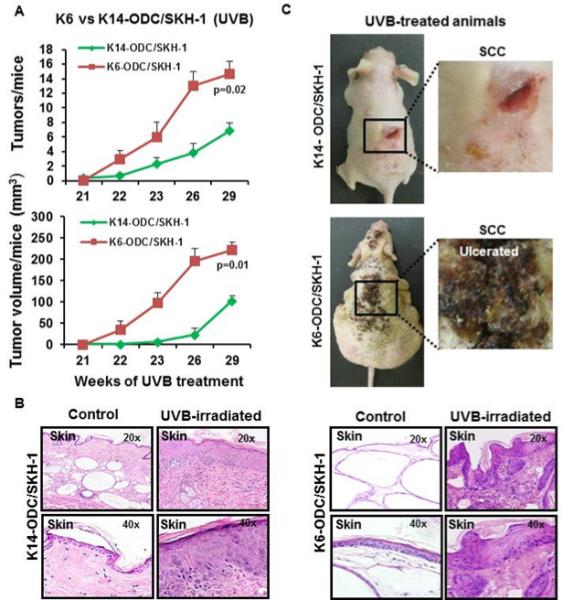
(A) Tumor growth in K6-ODC/SKH-1 and K14-ODC/SKH-1 mice; (B) Illustrative pictures of tumor-bearing K6-ODC/SKH-1 & K14-ODC/SKH-1 mice chronically irradiated with UVB for 29 weeks; (C) Histology of skin excised from UVB-irradiated K6-ODC/SKH-1 & K14-ODC/SKH-1 mice.
Effects of directing ODC expression to different epidermal compartments on UVB-induced skin carcinogenesis
Upon chronic UVB irradiation, K6-ODC/SKH-1 mice showed increase in epidermal hyperplasia, invasive epithelial lesions and enlargement of intradermal cysts with increased thickness of the squamous epithelium surrounding the intradermal cysts. Similar changes were also noticed in K14-ODC/SKH-1 mice but the magnitude of these alterations was less severe. K6-ODC/SKH-1 mice developed significantly more ulcerative tumors (p=0.02) with larger tumor volumes (p=0.01) as compared to K14-ODC/SKH-1 mice (Figure 1A & 1C). None of these murine models developed spontaneous tumors (Supplementary Figure 1D).
UVB-induced inflammatory responses in the skin differed based on the differential expression of ODC
Myeloid-derived suppressor cells (MDSC) (CD11b+Gr-1+) are known to be induced by pro-inflammatory cytokines and their presence in the tumor microenvironment play a significant role in angiogenesis and tumorigenesis [25]. Interestingly, we observed differences in UVB-induced inflammatory responses in K6-ODC/SKH-1 and K14-ODC/SKH-1 mice. K6-ODC/SKH-1 mice showed increased signatures of inflammation and abrasion as compared to K14-ODC/SKH-1 mice. The number of MDSCs and F4/80 positive cells in tumor-adjacent skin and SCCs were higher in K6-ODC/SKH-1 mice as compared to K14-ODC/SKH-1 mice (Figure 2A & B). Expression of pro-inflammatory cytokines such as IL-6, TGF1, IFN and TNF was also significantly increased (p=0.05) in UVB-irradiated skin of K6-ODC/SKH-1 mice as compared to K14-ODC/SKH-1 mice (Figure 2C).
Figure 2. K6-ODC/SKH-1 & K14-ODC/SKH-1 mice show alterations in UVB-induced inflammatory responses.
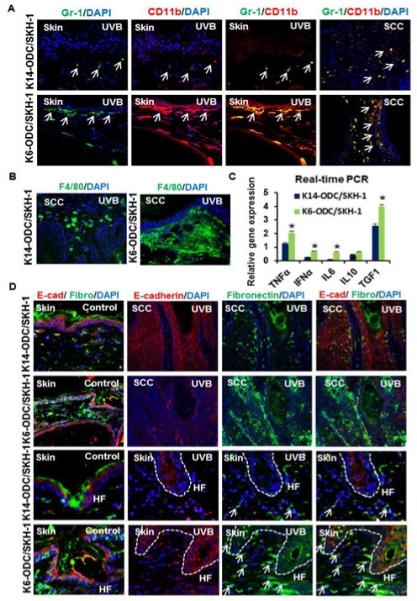
(A) Immunofluorescence staining of MDSCs (CD11b & Gr-1) in UVB-irradiated skin & tumors excised from K6-ODC/SKH-1 & K14-ODC/SKH-1 mice; (B) F4/80 positive cells (macrophage surface marker) in UVB-irradiated K6-ODC/SKH-1 compared to K14-ODC/SKH-1 mice; (C) Relative gene expression of pro-inflammatory cytokines in UVB-irradiated skin of K6-ODC/SKH-1 & K14-ODC/SKH-1 mice; (D) Coimmunostaining showing expression of EMT markers, E-cadherin and fibronectin in control & UVB-induced skin and tumors. Hair follicular regions in control and UVB-irradiated skin are denoted as ‘HF’ in represented images.
Transition of epithelial cells into mesenchymal phenotype, known as epithelial– mesenchymal transition (EMT), is considered integral to stem cell behavior, and also contributes to malignant cancer progression [26]. Double immunostaining of tissue sections with fibronectin, a mesenchymal marker and E-cadherin, an epithelial marker in K6-ODC/SKH-1 and K14-ODC/SKH-1 mice showed no significant differences in the expression of these proteins in the skin of age-matched control animals. However, in UVB-irradiated animals, the expression of these proteins was distinct in the two murine models. Although, higher expression of E-cadherin was noticed in the follicular keratinocytes of K6-ODC/SKH-1 as compared to K14-ODC/SKH-1 mice but it was more diffused and cytosolic rather than associated with the plasma membrane. Similarly fibronectin expression was also higher in K6-ODC/SKH-1 mice. In K14-ODC/SKH-1 mice, the association of follicular keratinocytes around the hair follicle bulb region with fibronectin positive dermal fibroblasts also seem to be reduced (Figure 2D). Similarly, increased level of EMT in SCCs induced in K6-ODC/SKH-1 mice suggests that these tumors were more invasive as compared to those induced in K14-ODC/SKH-1 mice (Figure 2D). However, it remained to be ascertained whether these observations are related to differential inflammatory responses in the two murine models.
ODC overexpression driven to different epidermal compartments manifests distinct effects on the expansion of stem cell compartments
Double immunostaining for ODC and CK17 (a marker for hair follicle) revealed high expression of ODC in the follicular regions in K6-ODC/SKH-1 which was not clearly apparent in K14-ODC/SKH-1 mice (Figure 3A). We also determined the impact of differential ODC localization on UVB-induced proliferation of skin keratinocytes. K6-ODC/SKH-1 mice manifested increased expression of PCNA, CK14 and CK5 as compared to K14-ODC/SKH-1 mice (Supplementary Figure 2A & B). Then, we investigated whether other known UVB signature signaling pathways such as DNA damage (p- -H2AX, p-ATM, p-chk1, RPA-32), cell-cycle and mTOR signaling proteins are differentially regulated in these two murine models. UVB-induced DNA damage signaling pathway was not significantly different in tumors developed in the two mouse strains (Supplementary Figure 3A & B). In addition, no significant changes in the mRNA and protein expression of cell cycle and mTOR signaling proteins could be discerned between the two murine models (Supplementary Figure 4A, B, C & D).
Figure 3. Staining for stem cell compartment in K6-ODC/SKH-1 & K14-ODC/SKH-1 mice.
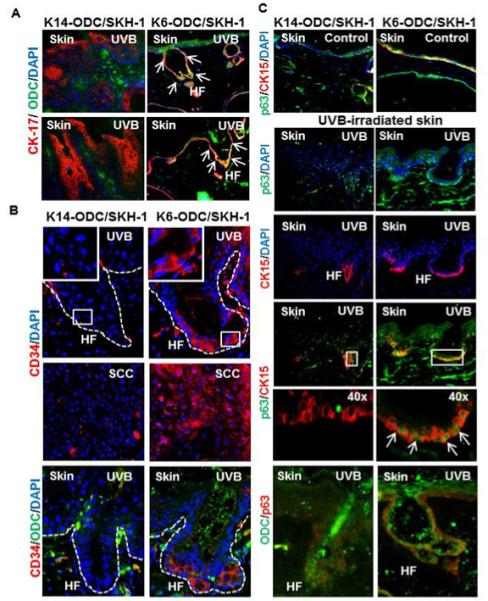
(A) Co-localization of CK-17 (hair follicle marker) & ODC in follicular regions of K6-ODC/SKH-1 and K14-ODC/SKH-1 mice; (B & C) Staining for stem cell markers (p63, CK-15 & CD34) in tumor-adjacent skin and tumors developed in K6-ODC/SKH-1 and K14-ODC/SKH-1 mice. Hair follicular regions in UVB-irradiated skin are denoted as ‘HF’ in represented images.
To determine the effects of over-expression of ODC on the expansion of keratinocyte stem cell compartment, we stained stem cells using various marker proteins such as CK15, CD34 and p63. Interestingly, analyses of tissue sections with UVB-irradiated skin of K6-ODC/SKH-1 mice revealed increased expression for CK15 and p63 as compared to K14-ODC/SKH-1 mice which co-localized in the hair follicular regions (Figure 3C). Another hematopoietic cell surface stem cell marker CD34 may stain cells that reside in the hair follicles. These cells are known to play an important role in skin tumor development [27]. In K6-ODC/SKH-1 mice, UVB irradiation showed enhanced number of CD34+ cells in the follicular regions which often correlated with the augmented ODC expression in these cells. Similar conclusion could be drawn based on the typing of follicular keratinocytes positive for both p63 and ODC (Figure 3B & C). Immunostaining analysis of CD34 in SCCs revealed the presence of enhanced number of CD34+ cells in tumors induced in K6-ODC/SKH-1 as compared to K14-ODC/SKH-1 mice (Figure 3B).
ODC induction correlates with the reduced Notch expression
It is known that Notch signaling pathways play a role in stem cell renewal, differentiation and tumorigenesis in the skin [18, 19]. We therefore decided to investigate whether the observed alterations in stem cell compartment in these animals could be correlated with the Notch expression. Consistently, K6-ODC/SKH-1 mice showed a significant down-regulation in the expression of Notch1, Notch2 and Notch3 in UVB-irradiated tumor-adjacent skin and SCCs as compared to their epidermal expression in K14-ODC/SKH-1 mice (Figure 4A & B). The enhancement of ODC in the skin and SCCs in these animals was negatively correlated with the inhibition in Notch expression (Figure 4B). Similarly, expansion of stem cell compartment characterized by the keratinocytes positive for p63, CK15 and CD34 also negatively correlated with Notch1 expression (Figure 4A).
Figure 4. Reduced Notch expression in K6-ODC/SKH-1 correlates with the ODC over-expression.
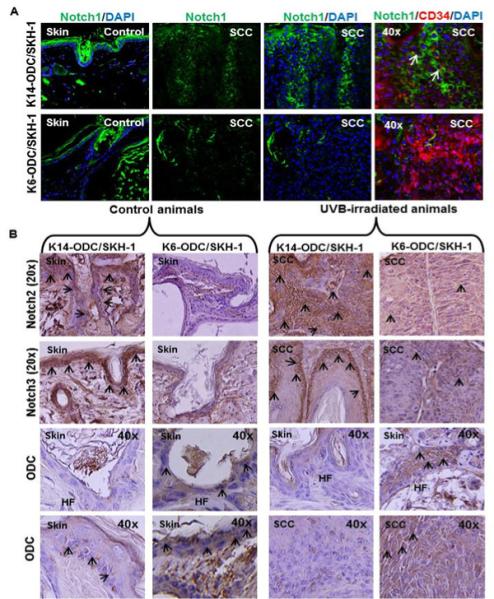
(A) Notch1 expression in control and UVB-induced skin, along with Notch1 and CD34 co-localization in UVB-induced tumors of K6-ODC/SKH-1 and K14/ODC/SKH-1 mice; (B) Immunohistochemical staining for Notch2, 3 and ODC in control and UVB-irradiated skin of K6-ODC/SKH-1 and K14-ODC/SKH-1 mice. Hair follicular regions in control and UVB-irradiated skin are denoted as ‘HF’ in represented images.
DISCUSSION
ODC over-expression catalyzes production of putresine, a precursor of other polyamines, spermidine and spermine. These polyamines are necessary for normal cell metabolism but their overproduction may contribute to uncontrolled cell proliferation [1, 2]. In this study, we describe that levels of ODC over-expression driven by different promoters to distinct cellular compartments of the epidermis are not identical and depends on the potency of promoters besides other external stimuli. This notion is based on the observations in this study that ODC expression is quantitatively higher in the epidermal and hair follicle compartments of K6-ODC/SKH-1 mice as compared to its expression in K14-ODC/SKH-1 mice. This could be particularly distinguished in follicular area characterized by CK17 expression. In this regard, earlier studies also showed that a group of outer root sheet cells in the vicinity of follicle bulge express ODC [28]. Interestingly, we also observed in this study that this region also contained significantly higher number of stem-like p63+ and CK15+ double positive cells suggesting that ODC over-expression plays a role in the expansion of this compartment. Parallel to these observations, earlier studies also showed that spermidine modulates human epithelial stem cell functions [29].
It is believed that invasive SCCs are derived from the tumor initiating cells residing in the bulge area of hair follicle [16]. Hayes et al showed that elevated ODC stimulate the recruitment of bulge stem cells during the pathogenesis of skin cancer [8]. CK15+ and CD34+ cells in hair follicle mark keratinocytes with stem cell characteristics. It has been demonstrated that similar to hair follicles, tumors formed by chemical carcinogenesis protocol contain small population of cells that express CD34 [30]. Consistently, higher number of CD34+ keratinocytes was observed in invasive SCCs developed in K6-ODC/SKH-1 mice than those developed in K14-ODC/SKH-1 suggesting their role in UVB-induced cancer pathogenesis. Our observations that ODC over-expression is high in CD34+ follicular keratinocytes further provide a possibility for a role of ODC in recruiting follicular keratinocytes with some stemness in the pathogenesis of SCCs.
Another interesting observation in these studies is that CD34+ keratinocytes in SCCs show significantly reduced staining for Notch1. Notch signaling plays diverse roles in tissue homeostasis and carcinogenesis in a tissue context-dependent manner. In the skin, it mainly acts as a tumor suppressor. Inhibition of Notch activity has been linked to maintenance of immature keratinocyte stem cell by p63 [31], which is consistent with our observations that CD34 expressing cells which are low in Notch1 show high staining for p63. Ablation of Notch1 expression in the skin has been shown to induce epidermal hyperplasia followed by tumorigenesis [19]. These studies suggest that ODC over-expression may be involved in the proliferation of stem cell-like cancer initiating cells by down-regulating Notch1 expression. Mechanistic studies are needed to understand the cascade whereby ODC alters Notch1 expression either directly or indirectly.
Our observations that ODC over-expression in K6-ODC/SKH-1 mice not only enhances the mesenchymal characteristics of epidermal keratinocytes but also affects association of dermal fibroblasts to follicular keratinocytes. At this stage, implications of such interactions in terms of UVB-induced skin cancer are not clear but it is known that ODC contributes to extracellular matrix-dependent epithelial cell differentiation [32].
In summary, these data reveals an important role of ODC in terms of expansion of stem cell compartment in the epidermis and SCCs and its potential to induce highly invasive skin neoplasms which seems to be dependent on the tissue/cellular levels of ODC.
Supplementary Material
Targeting ODC to hair follicle augments skin carcinogenesis and invasive SCCs
Hair follicle ODC expands stem cell compartment carrying CD34+/K15+/p63+ keratinocytes
Negatively regulated Notch1 is associated with expansion of stem cell compartment
ACKNOWLEDGMENT
This work was supported by NIH grant R01 CA138998.
We would also like to thank Dr. Bob Kesterson, UAB transgenic & genetically engineered model core facility for his help in generating the K14-ODC/SKH-1 mice.
Abbreviations
- NMSC
non-melanoma skin cancer
- SCC
squamous cell carcinoma
- BCC
basal cell carcinoma
- ODC
ornithine decarboxylase
- UVB
ultraviolet B
- K6
keratin 6
- K14
keratin 14
- EMT
epithelial-mesenchymal transition
- CK15
cytokeratin 15
- CK5
cytokeratin
- mTOR
mammalian target of rapamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gilmour SK. Polyamines and nonmelanoma skin cancer. Toxicol. Appl. Pharmacol. 2007;224:249–256. doi: 10.1016/j.taap.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 3.Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, Cleveland JL. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;19:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad N, Gilliam AC, Katiyar SK, O'Brien TG, Mukhtar H. A definitive role of ornithine decarboxylase in photocarcinogenesis. Am. J. Pathol. 2001;159:885–892. doi: 10.1016/S0002-9440(10)61764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–223. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 6.Nowotarski SL, Shantz LM. Cytoplasmic Accumulation of the RNA-binding Protein HuR Stabilizes the Ornithine Decarboxylase Transcript in a Murine Nonmelanoma Skin Cancer Model. J. Biol. Chem. 2010;285:31885–31894. doi: 10.1074/jbc.M110.148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feith DJ, Origanti S, Shoop PL, Sass-Kuhn S, Shantz LM. Tumor suppressor activity of ODC antizyme in MEK-driven skin tumorigenesis. Carcinogenesis. 2006;27:1090–1098. doi: 10.1093/carcin/bgi343. [DOI] [PubMed] [Google Scholar]
- 8.Hayes CS, DeFeo K, Dang H, Trempus CS, Morris RJ, Gilmour SK. A prolonged and exaggerated wound response with elevated ODC activity mimics early tumor development. Carcinogenesis. 2011;32:1340–1348. doi: 10.1093/carcin/bgr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staggers WR, Paterson AJ, Kudlow JE. Sequence of the functional human keratin K14 promoter. Gene. 1995;153:297–298. doi: 10.1016/0378-1119(94)00731-7. [DOI] [PubMed] [Google Scholar]
- 10.Feith DJ, Bol DK, Carbini JM, Lynch MJ, Sass-Kuhn S, Shoop PL, Shantz LM. Induction of ornithine decarboxylase activity is a necessary step for mitogen-activated protein kinase kinase-induced skin tumorigenesis. Cancer Res. 2005;65:572–578. [PubMed] [Google Scholar]
- 11.O'Brien TG, Megosh LC, Gilliard G, Peralta-Soler A. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- 12.Peralta-Soler A, Gilliard G, Megosh L, George K, O'Brien TG. Polyamines regulate expression of the neoplastic phenotype in mouse skin. Cancer Res. 1998;58:1654–1659. [PubMed] [Google Scholar]
- 13.Lan L, Trempus C, Gilmour SK. Inhibition of ornithine decarboxylase (ODC) decreases tumor vascularization and reverses spontaneous tumors in ODC/Ras transgenic mice. Cancer Res. 2000;60:5696–5703. [PubMed] [Google Scholar]
- 14.Tang X, Kim AL, Feith DJ, Pegg AE, Russo J, Zhang H, Aszterbaum M, Kopelovich L, Epstein EH, Jr., Bickers DR, Athar M. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/– mice. J. Clin. Invest. 2004;113:867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. U S A. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes CH, DeFeo-Mattox K, Woster PM, Gilmour SK. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids. 2014;46:543–552. doi: 10.1007/s00726-013-1559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 19.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 20.Lefort K, Dotto GP. Notch signaling in the integrated control of keratinocyte growth/differentiation and tumor suppression. Semin. Cancer Biol. 2004;14:374–386. doi: 10.1016/j.semcancer.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Panelos J, Tarantini F, Paglierani M, Di Serio C, Maio V, Pellerito S. Photoexposition discriminates Notch 1 expression in human cutaneous squamous cell carcinoma. Mod. Pathol. 2008;23:316–325. doi: 10.1038/modpathol.3801007. [DOI] [PubMed] [Google Scholar]
- 22.Arumugam A, Weng Z, Talwelkar SS, Chaudhary SC, Kopelovich L, Elmets CA, Afaq F, Athar M. Inhibiting cycloxygenase and ornithine decarboxylase by diclofenac and alpha difluoromethylornithine blocks cutaneous SCCs by targeting Akt-ERK axis. PLoS One. 2013;8:e80076. doi: 10.1371/journal.pone.0080076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Casta A, Tang X, Luke CT, Kim AL, Bickers DR, Athar M, Christiano AM. Loss of hairless confers susceptibility to UVB-induced tumorigenesis via disruption of NF-kappaB signaling. PLoS One. 2012;7:e39691. doi: 10.1371/journal.pone.0039691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panteleyev AA, Christiano AM, O'Brien TG, Sundberg JP. Ornithine decarboxylase transgenic mice as a model for human atrichia with papular lesions. Exp. Dermatol. 2000;9:146–151. doi: 10.1034/j.1600-0625.2000.009002146.x. [DOI] [PubMed] [Google Scholar]
- 25.Wesolowski R, Markowitz J, Carson WE. Myeloid derived suppressor cells – a new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin. Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M, Cotsarelis G, Nijhof JG, Peckham J, Flagler N, Kissling G, Humble MM, King LC, Adams LD, Desai D, Amin S, Tennant RW. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nancarrow MJ, Nesci A, Hynd PI, Powell BC. Dynamic expression of ornithine decarboxylase in hair growth. Mech. Dev. 1999;84:161–164. doi: 10.1016/s0925-4773(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 29.Ramot Y, Tiede S, Bíró T, Abu Bakar MH, Sugawara K, Philpott MP, Harrison W, Pietilä M, Paus R. Spermidine promotes human hair growth and is a novel modulator of human epithelial stem cell functions. PLoS One. 2011;6:e22564. doi: 10.1371/journal.pone.0022564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signaling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 31.Bach-Cuc N, Karine L, Anna M, Dario A, Vikram D, et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–1042. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam K, Zhang L, Bewick M, Lafrenie RM. HSG cells differentiated by culture on extracellular matrix involves induction of S-adenosylmethione decarboxylase and ornithine decarboxylase. J. Cell Physiol. 2005;203:353–361. doi: 10.1002/jcp.20247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


