Abstract
Previous work has shown that vitamin E δ-tocotrienol (VEDT) prolongs survival and delays progression of pancreatic cancer in the LSL-KrasG12D/+;Pdx-1-Cre mouse model of pancreatic cancer. However, the effect of VEDT alone or in combination with gemcitabine in the more aggressive LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mouse model is unknown. Here, we studied the effects of VEDT and the combination of VEDT and gemcitabine in the KPC mice. KPC mice were randomized into 4 groups: 1) vehicle (olive oil, 1.0 mL/kg PO twice/day and PBS 1.0 mL/kg IP twice/week), 2) gemcitabine (100 mg/kg IP twice/week), 3) VEDT (200 mg/kg PO twice/day), and 4) gemcitabine + VEDT. Mice received treatment until they displayed symptoms of impending death from pancreatic cancer, at which point animals were euthanized. At 16 weeks, survival was 10% in the vehicle group, 30% in the gemcitabine group, 70% in the VEDT group (P<0.01), and 90% in the VEDT combined with gemcitabine group (P<0.05). VEDT alone and combined with gemcitabine resulted in reversal of epithelial-to-mesenchymal transition in tumors. Biomarkers of apoptosis (plasma CK18), PARP1 cleavage, and Bax expression were more greatly induced in tumors subjected to combined treatment versus individual treatment. Combined treatment induced cell cycle inhibitors (p27Kip1 and p21Cip1) and inhibited VEGF, vascularity (CD31), and oncogenic signaling (pAKT, pMEK, and pERK) greater than individual drugs. No significant differences in body weight gain between drug treatment and control mice were observed. These results strongly support further investigation of VEDT alone and in combination with gemcitabine for pancreatic cancer prevention and treatment.
Keywords: δ-tocotrienol, gemcitabine, pancreatic cancer, KrasG12D-p53R172H mouse model, survival
Introduction
Advanced pancreatic ductal adenocarcinoma is a lethal disease with approximately 6 months median survival (1). Gemcitabine is the only approved single agent, with a median survival of 5.7 months and 20% 1-year survival (2, 3). After many phase III trials exploring gemcitabine-based combinations failed to improve overall survival in patients, two recent trials with erlotinib and nab-paclitaxel (Abraxane) showed modest but significant improvements in survival (4, 5). Erlotinib improved median survival to 6.24 months, whereas nab-paclitaxel improved median survival to 8.5 months. At the end of 1 year, 35% of patients who received nab-paclitaxel with gemcitabine were alive, compared with 22% of patients who received only gemcitabine. Because of the moderate activity of the present gemcitabine-based regimens, improved prevention and therapeutic options for pancreatic cancer are a high priority.
There has been intense interest in the role of natural nutritional/dietary factors in the prevention and treatment of pancreatic cancer (6, 7). Several reports have shown that increasing vegetable, fruit, and cereal consumption may impact protection against the development of pancreatic cancer (8, 9). One of the most compelling groups of anti-tumor bioactive compounds in cereal grains are vitamin E tocotrienols (10). Tocotrienols are unsaturated, naturally occurring vitamin E compounds, which exist as four isoforms: α-, β-, δ-, and γ-tocotrienol (11). We have shown that, for pancreatic cancer, vitamin E δ-tocotrienol (VEDT) is the most potent anticancer agent among the four isomers, both in vitro and in vivo (12). We have also shown that oral administration of 100 mg/kg/day of VEDT to mice resulted in satisfactory bioavailability in mouse pancreas tissue with no significant toxicity (13). In a recent study, we also demonstrated that VEDT, when administered for almost 1 year, prolonged the survival and delayed pancreatic intraepithelial neoplasia lesions in the LSL-KRASG12D/PDX-1-Cre genetic mouse model of pancreatic cancer (14).
Activating mutations in the Kras oncogene are found in over 90% of human pancreatic cancers (15). Conditionally targeting an activating point mutation in Kras (G12D) with a Pdx-1 pancreas-specific promoter resulted in pancreatic lesions that display a full spectrum of pancreatic intraepithelial neoplasias in mice (16). These lesions progress to fully invasive and metastatic adenocarcinomas; thus, the LSL-KrasG12D; Pdx-1-Cre transgenic mouse model mimics both the genetic and histologic changes observed in human pancreatic cancer. When a point mutation (R172H) in the p53 tumor suppressor gene, which is mutated in 75% of human pancreatic tumors, was introduced into the pancreas of mice with a KrasG12D/+ mutation, these triple transgenic LSL-KrasG12D;LSL-Trp53R127H; Pdx-1-Cre (KPC) mice developed pancreatic cancer more rapidly than the mice with just the Kras mutation (17). The clinical symptoms, including cachexia and abdominal distension, the defined histopathologic progression, and the genomic instability found in human pancreatic cancer are all replicated in the KPC transgenic mice, making it one of the most relevant animal models for preclinical evaluation of new drugs for pancreatic cancer (18). We hypothesized that VEDT, alone and in combination with gemcitabine, could significantly delay tumor growth and prolong survival in the KPC mice. Based on the previous tumor effects of tocotrienols observed by us and others, we hypothesized that VEDT would inhibit tumor angiogenesis, induce apoptosis, and alter oncogenic signaling.
MATERIALS AND METHODS
Reagents and animals
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. VEDT was obtained from Davos Life Ltd (Helios, Singapore). Gemcitabine-HCl was purchased from Eli Lilly and Company. Pairs of male and female LSL-KRASG12D, LSL-Trp53R127H, and PDX-1-Cre mice were obtained from the National Cancer Institute Mouse Models of Human Cancers Consortium (Frederick, MD). The animal protocol used in the study was approved by our Institutional Animal Care and Use Committee.
To study the effects of VEDT and gemcitabine on pancreatic tumor development, the KPC mouse model from Hingorani et al. (17) was used. LSL-KRASG12D, LSL-Trp53R127H, and PDX-1-Cre mice were maintained as heterozygous lines. They were crossed and bred in the vivarium of our center. Tail snips were harvested from offspring of LSL-KRASG12D, LSL-Trp53R127H, and PDX-1-Cre mice and allowed to digested overnight; genomic DNA was then extracted and estimated using the DNAeasy Blood & Tissue (250) kit from Qiagen Inc, as per the manufacturer’s instructions.
Genotyping analysis
PCR primer sequences used to detect PDX-1-Cre, LSL-KRASG12D, and LSL-Trp53R127H were as follows: 5′-CTGGACTACATCTTGAGTTGC-3′ and 5′-GGTGTACGGTCAGTAAATTTG-3′ (PDX-1-Cre forward and reverse); 5′-AGGTAGCCACCATGGCTTGAGTAAGTCTGCA-3′ and 5′-CCTTTACAAGCGCACGCAGACTGTAGA-3′ (LSL-KRAS612D forward and reverse); and 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′ and 5′-CTTGGAGACATAGCCACACTG-3′ (LSL-Trp53R127H forward and reverse) (all from IDT Technologies). The PCR reaction of 25 μL was composed of buffer, 2.0 mM MgCl2, 0.2 mM dNTP mix, 0.025 U/μL DNA Taq polymerase, 0.5 μM primers, and 50 ng DNA. The reaction was carried out in the PTC-200 thermal cycler programmed as preheat at 94°C for 3 minutes and then 35 cycles of (30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C) followed by 72°C for 3 minutes. PCR products were mixed with 5 μL of loading dye and separated on a 2% agarose gel containing ethidium bromide. The electrophoresis was run for 1 hour at 100 volts using Tris base, acetic acid, and EDTA buffer. A 650-bp product (PDX), a 550-bp product (KRAS), and 270-bp product (p53) were identified using DNA ladder, and the bands were imaged using AlphaImage analysis.
Drug treatments
Offspring of LSL-KRASG12D, LSL-Trp53R127H, and PDX-1-Cre mice (triple gene positive), as shown in Figure 1A, were randomized into four groups: 1) vehicle (ethanol extracted olive oil, 1.0 ml/kg twice/day by oral gavage) (n = 10), 2) gemcitabine (100 mg/kg ip twice/week) (n = 10), 3) VEDT (200 mg/kg twice/day by oral gavage) (n = 10), and 4) gemcitabine + VEDT (n = 10). The treatment was started at the age of 4 weeks and continued for 12 weeks, as shown in Figure 1B. The body weights of the mice were recorded twice weekly, mortality was noted, and survival curves were plotted (see Figure 1C). When the mice displayed symptoms of impending death such as cachexia, abdominal distension, rapid weight loss, or labored breathing, animals were euthanized. Blood was collected in heparinized tubes, and the entire tumor tissues were harvested and weighed. Half of the tissues were fixed in buffered formalin for histological analysis, with the remainder snap frozen in liquid nitrogen and kept at −80°C for protein extraction and Western blot analysis.
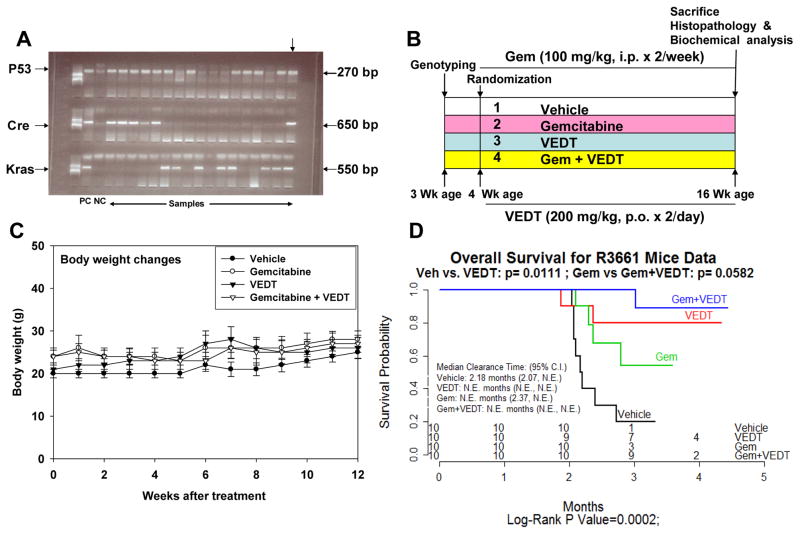
Fig. 1. Vitamin E δ-tocotrienol (VEDT), alone and in combination with gemcitabine, increases median survival of LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) mice.
A, Genotyping of Pdx-1-Cre + KrasG12D and Trp53R172H offspring by PCR. Top arrow indicates triple-positive gene. B, Experimental design. C, Body weights over 12 weeks by treatment group. Body weight gain was not significantly altered by VEDT treatment (by ANOVA), shown as means and SE (bars) (n = 3–10 mice). D, Kaplan-Meier survival curves. Survival curves were significantly different among the 4 groups by log-rank test (P = 0.0002). CI, confidence interval. Pairwise comparison with Bonferroni correction of P value showed significant survival increase by VEDT treatment versus vehicle treatment (P = 0.01) and gemcitabine treatment versus gemcitabine + VEDT treatment (P = 0.05). NE, not evaluable.
Histologic evaluation
Formalin-fixed, paraffin-embedded tissues were sectioned (4 μm) and stained with hematoxylin-eosin. Immunohistochemistry was performed using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) per manufacturer’s protocol with proprietary reagents. Briefly, slides were deparaffinized on the automated system with EZ Prep solution. Sections were heated for antigen retrieval. For immunohistochemistry, tissue sections were incubated with anti-caspase 3, Ki-67, and CD31 at 1:4000 dilutions for 60 minutes. Detection was performed using the Ventana OmniMap kit.
Assessment of immunohistochemical expression
All stained tissues were examined by one independent observer (BAC). Caspase 3 and Ki-67 stained tissues were assessed for expression in neoplastic and non-neoplastic areas. Percent expression was recorded for each area and then averaged for each mouse. For CD31, sections were examined at low power to identify cancers and associated hot spots. The vessels per x400 field were counted manually. Single cells and groups expressing CD31 were counted as vessels in addition to groups with lumens. Sections not showing a cancer were assessed for hot spots at low power.
Apoptosis marker CK18 ELISA
Heparinized blood from mice was centrifuged at 5000 rpm for 5 minutes, and plasma was carefully isolated and stored at −80°C until analysis. The apoptosis marker cytokeratin-18 (CK18) was assayed using the M30-Apoptosense ELISA kit (PEVIVA, Bromma, Sweden).
Western blot analysis
Proteins were extracted from pancreatic tumor tissues using RIPA lysis buffer containing protease inhibitors (Thermo Scientific, Rockford, IL). Extracted proteins (40 μg) were resolved on 12.5% SDS-polyacrylamide gel (SDS-PAGE) running gel and a 5% stacking gel. Proteins were then electrotransferred onto nitrocellulose membranes. After blocking in 5% nonfat powdered milk for 1 hour, membranes were washed and then treated with antibodies to PARP-1, E-cadherin, vimentin, pAKT, pMEK, pERK, VEGF, Bax, p21Cip1, p27kip-1, and β-actin (1:1000) overnight at 4°C (Santa Cruz Biotechnology, Santa Cruz, CA; Cell Signaling, Danvers, MA). After blots were washed, they were incubated with horseradish peroxidase-conjugated secondary antibody IgG (1:5000) for 1 hour at room temperature. The washed blot was then treated with SuperSignal West Pico chemiluminescent substrate (Pierce) for positive antibody reaction. Membranes were exposed to X-ray film (KODAK) for visualization and densitometric quantization of protein bands using AlphaEaseFC software (Alpha Innotech).
Statistical analysis
Data are expressed as means ± SEM, analyzed statistically using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests using SAS statistical software for comparison between different treatment groups. Significance was set at P < 0.05.
RESULTS
Effects of VEDT alone and in combination with gemcitabine on survival and body weight in KPC mice
To assess whether VEDT alone and in combination with gemcitabine affected survival of our mice, we analyzed the effects of the drugs using Kaplan-Meier survival curves. We found that the overall survival of vehicle-treated mice from the onset of treatment was 2.18 months (Figure 1D), which is in agreement with earlier reports of survival/death of KPC mice (17, 19). Only 10% of the vehicle-treated mice were alive at 4 months of age. VEDT treatment significantly increased the percentage of mice that were alive at 4 months to 70% (P<0.01); 30% of gemcitabine-treated mice were alive at 4 months of age. However, VEDT treatment combined with gemcitabine resulted in 90% (P<0.05) of mice being alive at 4 months of age. It is of great interest that, at 5 months of age, 40% of VEDT-treated mice and 20% of VEDT combined with gemcitabine-treated mice were alive without symptoms of impending death, whereas none of the vehicle or gemcitabine-treated mice were alive (Figure 1D). There was no significant difference in food intake and body weight gain between drug treatment groups and the control vehicle treatment group during the study period (Figure 1C). Our data indicated no obvious toxicity or side effects of the drugs alone and in combination after chronic dosing for 12 weeks in mice with pancreatic cancer, indicating that symptoms of impending death were entirely related to tumor burden. This finding was confirmed at autopsy in all of the mice.
Effects of VEDT alone and in combination with gemcitabine on tumor weight, EMT, and proliferation index in KPC mice
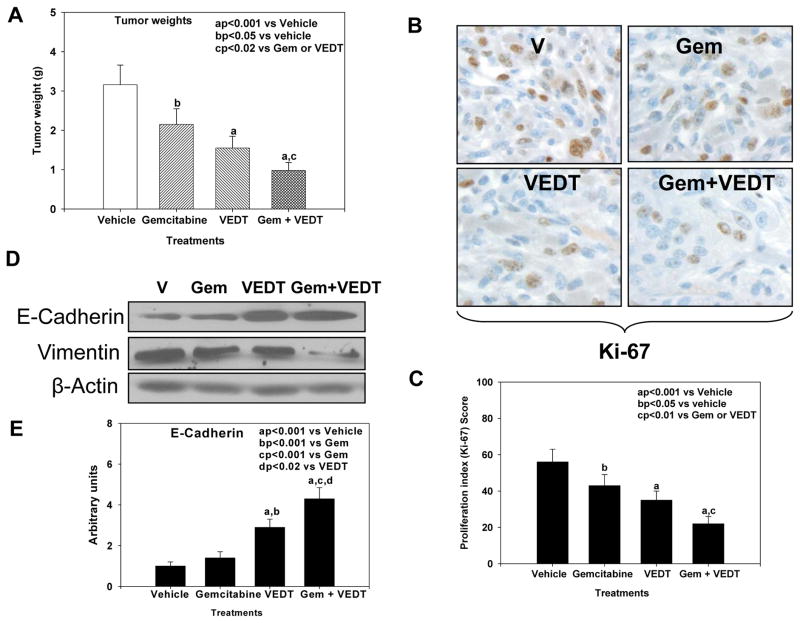
Mice treated with gemcitabine and VEDT alone and in combination had significantly reduced tumor weights of 32% (P<0.05), 51% (P<0.001), and 69% (P<0.001), respectively, versus vehicle (Figure 2A). The combination resulted in a significant reduction of tumor weight compared to either drug alone (P<0.02). Interestingly, VEDT treatment resulted in a greater decrease in tumor weight than gemcitabine. Gemcitabine alone, VEDT alone, and combined gemcitabine and VEDT significantly decreased Ki-67 staining (a marker of proliferation index) in tumors at 27% (P<0.05), 39% (P<0.001), and 61% (P<0.001), respectively, compared with vehicle (Figure 2B and C). Interestingly the combination of the two drugs resulted in a greater inhibition of proliferation than either drug alone. Because recent studies revealed that tocotrienols inhibit cancer cell invasion through reversal of epithelial-to-mesenchymal transition (EMT), we evaluated the effects of VEDT on EMT in the mouse tumors (20, 21). E-cadherin, a marker of epithelial phenotype, slightly increased after gemcitabine treatment, whereas with VEDT alone and in combination with gemcitabine it was profoundly increased (Figure 2D and E). In contrast, vimentin, a marker of mesenchymal phenotype, decreased after treatment with gemcitabine or VEDT alone; however, when the two drugs were combined, expression was almost completely abolished (Figure 2D). These finding clearly support the reversal of EMT by VEDT in the pancreatic tumors.
Fig. 2. VEDT, alone and in combination with gemcitabine (Gem), inhibits tumor growth and epithelial-to-mesenchymal transition (EMT) in KPC mice.
A, Mean pancreatic tumor weight changes in mice after drug treatment. Gemcitabine, VEDT, and gemcitabine + VEDT treatment significantly inhibited tumor growth by 32% (bP<0.05), 51% (aP<0.001), and 69% (aP<0.001) compared to vehicle treatment. Bars indicate SE (n=5). B, Proliferation index (Ki-67) immunostaining in pancreatic tumor after drug treatment. C, Semiquantitative analysis (histogram) shows that gemcitabine (bP<0.05), VEDT (aP<0.001), and gemcitabine + VEDT (aP<0.001) treatment significantly inhibited tumor cell proliferation compared to vehicle treatment. Gemcitabine + VEDT significantly inhibited tumor cell proliferation (cP<0.01) compared to gemcitabine or VEDT alone. Results are mean and SE (bars; n=5). D, Western blot of E-cadherin and vimentin in tumor tissues of KPC mice (n = 5). E-cadherin expression in the tumors was inversely related to vimentin expression, and EMT was profoundly inhibited when gemcitabine was combined with VEDT. E, Semiquantitative analysis (histogram) shows that VEDT (aP<0.001) and gemcitabine + VEDT (aP<0.001) treatment significantly increased E-cadherin expression compared to vehicle treatment. Gemcitabine + VEDT significantly increased E-cadherin expression compared to Gemcitabine (cP<0.001) and VEDT (dP<0.02). Results are mean and SE (bars; n=5). All statistical analyses were performed using ANOVA with Duncan test.
Effect of VEDT alone and in combination with gemcitabine on VEGF expression and tumor angiogenesis in KPC mice
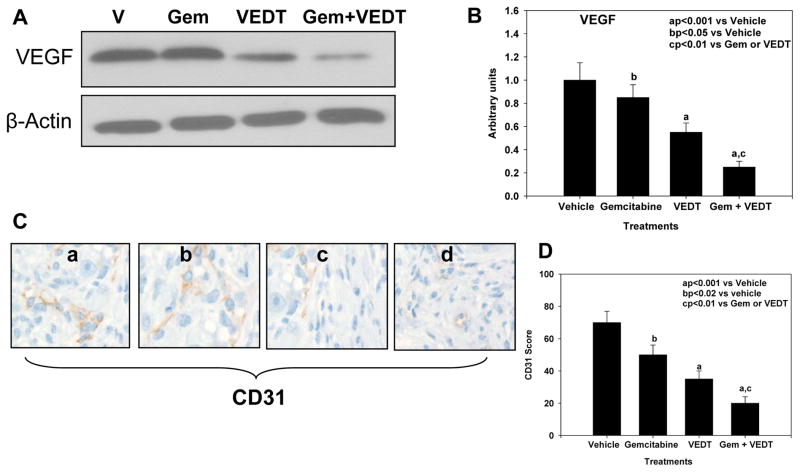
The antiangiogenic properties of tocotrienols have been demonstrated in several studies (22–25). Specifically, VEGF has been implicated as a major target of tocotrienol antitumor activity. Since the effect of VEDT on VEGF expression and angiogenesis in pancreatic cancer is unknown, we examined the effect of VEDT on VEGF expression and angiogenesis in our mouse tumors. Mice treated with gemcitabine alone, VEDT alone, and the combination showed significantly reduced VEGF expression: 21% (P<0.05), 55% (P<0.001), and 78% (P<0.001), respectively, compared with vehicle (Figure 3B). The tumor antiangiogenic effects of VEDT are far greater than gemcitabine. On the other hand, when the two drugs are combined, there is a greater inhibition of VEGF expression than with either drug alone. The tumor blood vessel counts (CD31 immunoreactivity) were significantly decreased with gemcitabine alone (29%, P<0.02), VEDT alone (57%, P<0.001), and gemcitabine + VEDT combined (97%, P<0.001) compared to vehicle (Figure 3D). The combination of the two drugs resulted in greater inhibition of CD31 immunostaining than either drug alone.
Fig. 3. VEDT, alone and in combination with gemcitabine (Gem), inhibits tumor angiogenesis in KPC mice.
A, Western blot of VEGF in tumors of KPC mice treated with drugs and vehicle (V) over 12 weeks (n = 5). Gemcitabine and VEDT alone and in combination decreased VEGF expression. B, Semiquantitative analysis shows significant reduction of VEGF expression of 21% (bP<0.05), 55% (aP<0.001), and 78% (aP<0.001) with gemcitabine, VEDT alone, and gemcitabine + VEDT, respectively, compared with vehicle. C, Effect of vehicle (a), gemcitabine (b), VEDT (c), and gemcitabine + VEDT (d) on CD31 immunostaining in tumors of KPC mice. D, Semiquantitative analysis shows that gemcitabine, VEDT, and gemcitabine + VEDT significantly decreased CD31 immunostaining by 29% (bP<0.02), 57% (aP<0.001), and 97% (aP<0.001), respectively, compared to vehicle. Results are means and SE (n=5). All statistical analyses were performed using ANOVA with Duncan test.
Effect of VEDT alone and in combination with gemcitabine on induction of apoptosis in pancreatic tumor tissues of KPC mice
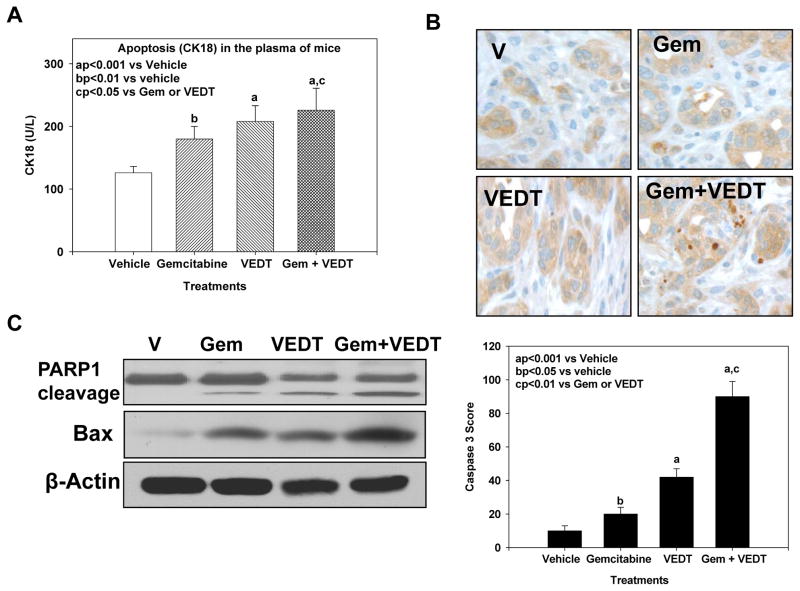
Earlier studies have shown in vitro using pancreatic cancer cell lines and in vivo using a xenograft mouse model and a conditional KrasG12D mouse model that VEDT induces apoptosis and inhibits cell survival (12, 26–28). Therefore, we evaluated the effect VEDT on apoptosis in the KPC mouse tumors. As shown in Figure 4A, gemcitabine, VEDT, and the combination of the two drugs significantly elicited apoptotic cell death in the circulating tumor cells (CK18) in the blood: 45% (P<0.01), 71% (P<0.001), and 84% (P<0.001), respectively, compared to vehicle, suggesting that loss of viable cells is due to the induction of the cell death pathway. We confirmed this by more intense and significant immunostaining of cleaved caspase 3 in gemcitabine (P<0.05), VEDT (P<0.001), and gemcitabine + VEDT (P<0.001) groups, respectively, compared with the vehicle group (Figure 4B). Further Western immunoblotting in the pancreatic tumor tissues of mice showed enhanced protein expression of the proapoptotic protein Bax in gemcitabine, VEDT, and gemcitabine + VEDT-treated groups compared to control (Figure 4C). The combination of the two drugs resulted in more than additive effect on Bax expression when compared with sum of Bax expression induced by either drug alone. PARP-1, a 116-kDa nuclear poly(ADP-ribose) polymerase 1, is one of the main cleavage targets of caspase 3 in vivo, serving as a marker of cells undergoing apoptosis (29). Our Western blot results clearly showed the PARP1 cleavage in pancreatic cancer tissues of mice treated with gemcitabine, VEDT, and gemcitabine + VEDT compared to control. VEDT cleaved PARP1 greater than gemcitabine, but the combination had more than additive effects on apoptosis compared with sum of apoptosis induced by either drug alone.
Fig. 4. VEDT, alone and in combination with gemcitabine (Gem), induces apoptosis in plasma and tumors of KPC mice.
A, Gemcitabine, VEDT, and combination of the 2 drugs significantly induced apoptotic tumor cells (CK18) in the plasma by 45% (bP<0.01), 71% (aP<0.001), and 84% (aP<0.001), respectively, compared to vehicle. Results are mean and SE (n=5). B, Cleaved caspase 3 immunostaining in pancreatic tumors after drug treatment (top). Semiquantitative analysis (histogram at bottom) shows that gemcitabine (bP<0.05), VEDT (aP<0.001), and gemcitabine + VEDT (aP<0.001) treatment significantly increased immunostaining of cleaved caspase 3 versus vehicle. Results are means and SE (n=5). C, Western blot of PARP1 cleavage and pro-apoptotic protein Bax expression in tumor tissues of drug-treated KPC mice. Gemcitabine, VEDT, and gemcitabine + VEDT increased apoptosis marker PARP1 cleavage and Bax expression compared to vehicle (n =5). All statistical analyses were performed using ANOVA with Duncan test.
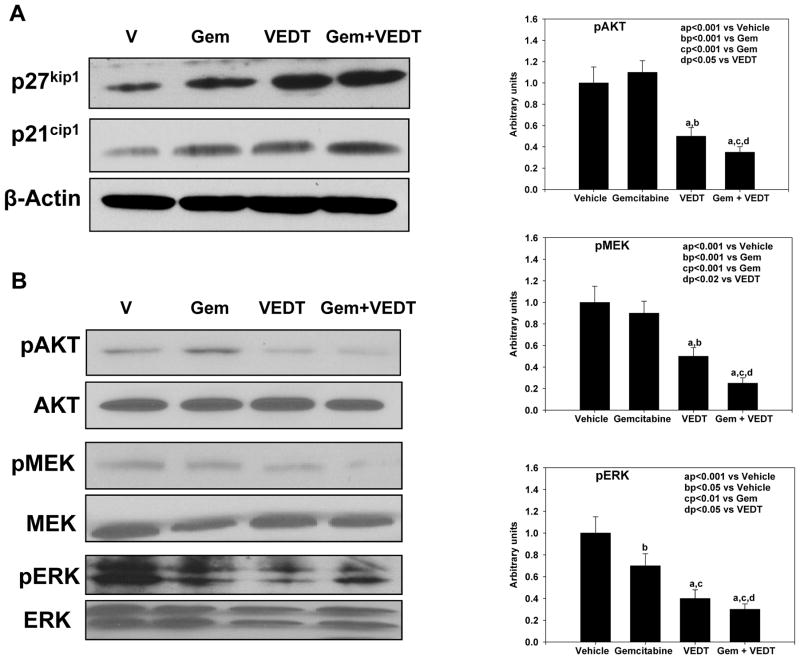
Effect of VEDT alone and in combination with gemcitabine on inhibition of cell cycle and survival pathways in pancreatic tumor tissues of KPC mice
Earlier studies have shown in vitro using pancreatic cancer cell lines and in vivo using a xenograft mouse model and conditional KrasG12D mouse model that VEDT inhibits cell survival, including oncogenic Kras signaling (12, 14, 26–28). Here, we investigated the effect of VEDT on Kras signaling and cell cycle proteins. As shown in Figure 5A, gemcitabine, VEDT, and gemcitabine + VEDT significantly induced cell cycle inhibitor proteins p21Cip1and p27kip-1 in pancreatic tumor tissues, suggesting a potential cell cycle arrest. However, the induction of both cell cycle inhibitors was greater with the combination than with either drug alone. Results further demonstrate an inhibition of pMEK and pERK expression in pancreatic tumor tissues of mice treated with gemcitabine, VEDT, and gemcitabine + VEDT compared to control (Figure 5B). However, the inhibition was more significant with the combination than with either drug alone. Interestingly, gemcitabine was unable to inhibit pAKT in the pancreatic tumors, although its expression was slightly increased (Figure 5B). On the other hand, VEDT inhibited pAKT expression, but the combination of the two drugs was more effective in inhibition of the pAKT signaling pathway.
Fig. 5. VEDT, alone and in combination with gemcitabine (Gem), induces cell cycle arrest and inhibits survival pathway in tumors of KPC mice.
A, Western blot of p27Kip1 and p21Cip1 in tumors of drug-treated KPC mice. Gemcitabine, VEDT, and gemcitabine + VEDT treatment induced cell cycle inhibitor proteins p21Cip1and p27kip-1 in pancreatic tumor tissues (n = 5). B, Western blots of pAKT, pMEK, and pERK in tumors of KPC mice (n = 5). A significant inhibition of pAKT and pMEK (aP<0.001) was noted in VEDT- and gemcitabine + VEDT-treated mice compared to vehicle treatment. Gemcitabine (bP < 0.05), VEDT (aP < 0.001), and gemcitabine + VEDT (aP < 0.001) significantly inhibited pERK expression in tumor tissues of KPC mice (right). All statistical analyses were performed using ANOVA with Duncan test.
Discussion
The goal of this study was to evaluate the preclinical efficacy of VEDT, a naturally occurring dietary product, alone and in combination with gemcitabine, a commonly used chemotherapeutic agent against pancreatic cancer, in a highly relevant and aggressive model of pancreatic cancer (KPC mouse model). We recently reported in our chemoprevention study that VEDT prevented pancreatic intraepithelial neoplasia lesions and increased survival in the conditional Kras mouse model of pancreatic cancer (14). In the present study, we found that VEDT augmented gemcitabine inhibition of tumor growth (tumor weight and proliferation index) and increased survival in the KPC mouse model.
The mechanisms for the chemopreventive effects of VEDT include a protective role against oxidative stress-induced DNA damage (11), enhancement of the immune response (30), and elimination of cancer stem cells (31). One intriguing finding that has been demonstrated consistently by us and others is the selective killing effect of VEDT against cancer cells (12, 14, 32, 33). We have also demonstrated selective induction of apoptosis by VEDT of pancreatic transformed and malignant epithelial cells but not normal immortalized human pancreatic ductal epithelial cells (12, 32). Other anticancer effects of tocotrienol include the inhibition of cell migration and invasion and the inhibition of angiogenesis (21, 24, 34). Our present data also confirm that VEDT decreased VEGF expression in the tumors and inhibited angiogenesis in KPC mice. We found that gemcitabine was unable to inhibit pancreatic tumor angiogenesis significantly in this metastatic model of pancreatic cancer; however, VEDT significantly inhibited tumor angiogenesis. Earlier studies have also reported a mild anti-angiogenic response of gemcitabine in orthotopic pancreatic tumors (35, 36). A tocotrienol-rich fraction, including γ- and δ-tocotrienols, has been shown to suppress mouse mammary tumor cell and colorectal adenocarcinoma cell angiogenesis (34, 37). Augmentation of gemcitabine antitumor activity by VEDT is associated with inhibition of angiogenic factor VEGF protein expression and endothelial factor CD31 immunostaining in the tumor blood vessels. However, to our knowledge, this is the first report showing in vivo that the combination of gemcitabine and VEDT significantly inhibited pancreatic tumor angiogenesis in this aggressive genetic mouse model of pancreatic cancer. Recent studies revealed that VEDT inhibited cancer cell invasion through reversal of EMT (20, 21, 24). We also found that VEDT influenced EMT in the KPC mice.
VEDT antitumor action involves multiple signaling pathways. The NF-kB transcription factor functions as a crucial regulator of cell survival and chemoresistance in pancreatic cancer (11, 38). We have shown in vitro as well as in vivo studies that VEDT targets the transcription factor NF-kB signaling, a pro survival pathway (12, 14). Inhibition of constitutively active NF-kB resulted in the depletion of NF-kB–regulated gene products such as cell survival anti-apoptotic proteins (BCl-XL, XIAP, and cFLIP) favoring the induction of caspases (-8, -9 and -3) leading to both extrinsic and intrinsic pathways of apoptosis. However, gemcitabine antitumor activity was related to inhibition of DNA synthesis and induction of apoptosis through the intrinsic pathway (39). Gemcitabine chemoresistance is directly related to NF-kB and NF-kB-regulated gene products such as anti-apoptotic proteins BCl-XL and cFLIP (38). Inhibition of constitutively active NF-kB resulted in the depletion of NF-kB-regulated gene products for angiogenesis (VEGF) and for metastasis (ICAM-1 and VCAM-1) (11, 38). We have also shown in vitro that VEDT induced p27-dependent G1 cell cycle arrest via an E2F-1-dependent mechanism in pancreatic cancer cells (32). Our present data also confirm that VEDT induced expression of CDK inhibitors (p27 and p21) in the tumors in KPC mice.
Kras mutations are prevalent in human pancreatic cancer, and mutant Kras has been shown to be required for the initiation and maintenance of pancreatic cancer and are associated with poor prognosis and shorter patient survival time. In addition, tumors harboring Kras are resistant to chemotherapy and anti-signaling agents (15). The activated Ras-Raf-MEK-ERK signaling pathway regulates the cell-cycle inhibitor p27Kip1 in pancreatic cancer cells (40). VEDT has been shown to inhibit pAKT, pERK, and NF-kB, which are well-known downstream effectors of oncogenic Kras (12, 14, 32). Furthermore, consistent with inhibition of these effectors, downstream targets of pERK (i.e., p27) and those of NF-kB (i.e., Bax) were significantly induced by VEDT treatment, whereas levels of the prosurvival protein Bcl-xL were decreased (14, 32). Therefore, the augmentation of gemcitabine antitumor activity by VEDT is likely associated with multiple signaling pathways leading to inhibition of tumor growth, cell cycle progress, and angiogenesis and induction of apoptosis in KPC mice.
In summary, this is the first report evaluating the effect of oral feeding of VEDT alone and combined with gemcitabine on the aggressive/metastatic pancreatic tumors in the KPC mouse model. Our results show that the oral intake of VEDT leads to increased survival of KPC mice and potentiates the antitumor activity of gemcitabine. VEDT inhibited tumor proliferation, reversed EMT, reduced angiogenesis, disrupted oncogenic Kras signaling, and induced apoptosis in KPC mouse pancreatic tumors. Our data support the clinical investigation of VEDT alone and in combination with gemcitabine for the prevention and treatment of human pancreatic cancer. Because the toxicity of gemcitabine makes it unsuitable for chemoprevention in healthy subjects, the combination of VEDT and gemcitabine will be most useful in the prevention of metastasis. Patients who have had curative surgery for pancreatic cancer have a >70% risk of relapse with a median survival of 22 months. Currently, gemcitabine is FDA approved as a single agent to be used as adjuvant therapy for these patients with pancreatic cancer to improve their outcomes. Based on our results, the combination of VEDT and gemcitabine should be investigated with the goal of increasing the disease-free interval, thereby prolonging the survival of these patients.
Acknowledgments
Financial support: The study was supported in part by National Cancer Institute/USPHS Grant 1RO1 CA-129227-01A1.
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Footnotes
Disclosure of potential conflicts of interest: Drs. Malafa and Sebti are named as inventors on US Patent “Delta-Tocotrienol Treatment and Prevention of Pancreatic Cancer (June 26, 2007; OTML docket number 06A069) but do not have financial interest in the companies that have licensed this patent.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Vickers MM, Powell ED, Asmis TR, Jonker DJ, Hilton JF, O’Callaghan CJ, et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer - results from NCIC CTG PA. 3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur J Cancer. 2012;48:1434–42. doi: 10.1016/j.ejca.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Arshad A, Al-Leswas D, Al-Taan O, Stephenson J, Metcalfe M, Steward WP, et al. Pooled survival and response data from phase III randomized controlled trials for gemcitabine-based regimes in the treatment of advanced pancreatic cancer. Am J Clin Oncol. 2011 doi: 10.1097/COC.0b013e3182124216. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Drug Combo Effective against Pancreatic Cancer. Cancer Discov. 2013;3:OF8. doi: 10.1158/2159-8290.CD-NB2014-089. [DOI] [PubMed] [Google Scholar]
- 6.Taylor PR, Greenwald P. Nutritional interventions in cancer prevention. J Clin Oncol. 2005;23:333–45. doi: 10.1200/JCO.2005.06.190. [DOI] [PubMed] [Google Scholar]
- 7.Stolzenberg-Solomon RZ, Albanes D, Nieto FJ, Hartman TJ, Tangrea JA, Rautalahti M, et al. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J Natl Cancer Inst. 1999;91:535–41. doi: 10.1093/jnci/91.6.535. [DOI] [PubMed] [Google Scholar]
- 8.Chan JM, Wang F, Holly EA. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol Biomarkers Prev. 2005;14:2093–7. doi: 10.1158/1055-9965.EPI-05-0226. [DOI] [PubMed] [Google Scholar]
- 9.Silverman DT, Swanson CA, Gridley G, Wacholder S, Greenberg RS, Brown LM, et al. Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1998;90:1710–9. doi: 10.1093/jnci/90.22.1710. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal B, Nesaretnam K. Vitamin E tocotrienols: life beyond tocopherols. Genes Nutr. 2012;7:1. doi: 10.1007/s12263-011-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–31. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 2011;10:2363–72. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain K, Francois RA, Hutchinson SZ, Neuger AM, Lush R, Coppola D, et al. Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology. 2009;83:157–63. doi: 10.1159/000190792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, et al. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E delta-tocotrienol. Carcinogenesis. 2013 doi: 10.1093/carcin/bgt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Schutte U, Bisht S, Brossart P, Feldmann G. Recent developments of transgenic and xenograft mouse models of pancreatic cancer for translational research. Expert Opin Drug Discov. 2011;6:33–48. doi: 10.1517/17460441.2011.534453. [DOI] [PubMed] [Google Scholar]
- 19.Liby KT, Royce DB, Risingsong R, Williams CR, Maitra A, Hruban RH, et al. Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 2010;3:1427–34. doi: 10.1158/1940-6207.CAPR-10-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang PN, Yap WN, Lee DT, Ling MT, Wong YC, Yap YL. Evidence of gamma-tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutrition and cancer. 2009;61:357–66. doi: 10.1080/01635580802567166. [DOI] [PubMed] [Google Scholar]
- 21.Yap WN, Chang PN, Han HY, Lee DT, Ling MT, Wong YC, et al. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. British journal of cancer. 2008;99:1832–41. doi: 10.1038/sj.bjc.6604763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M, Miyazawa T. Anti-angiogenic activity of tocotrienol. Bioscience, biotechnology, and biochemistry. 2003;67:1623–7. doi: 10.1271/bbb.67.1623. [DOI] [PubMed] [Google Scholar]
- 23.Wells SR, Jennings MH, Rome C, Hadjivassiliou V, Papas KA, Alexander JS. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. The Journal of nutritional biochemistry. 2010;21:589–97. doi: 10.1016/j.jnutbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Weng-Yew W, Selvaduray KR, Ming CH, Nesaretnam K. Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutrition and cancer. 2009;61:367–73. doi: 10.1080/01635580802582736. [DOI] [PubMed] [Google Scholar]
- 25.Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264. 7 macrophages. Lipids. 2009;44:787–97. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- 26.Shibata A, Nakagawa K, Sookwong P, Tsuzuki T, Oikawa S, Miyazawa T. Tumor anti-angiogenic effect and mechanism of action of delta-tocotrienol. Biochem Pharmacol. 2008;76:330–9. doi: 10.1016/j.bcp.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Hussein D, Mo H. d-delta-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas. 2009;38:e124–36. doi: 10.1097/MPA.0b013e3181a20f9c. [DOI] [PubMed] [Google Scholar]
- 28.Shin-Kang S, Ramsauer VP, Lightner J, Chakraborty K, Stone W, Campbell S, et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med. 2011;51:1164–74. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 30.Hafid SR, Radhakrishnan AK, Nesaretnam K. Tocotrienols are good adjuvants for developing cancer vaccines. BMC Cancer. 2010;10:5. doi: 10.1186/1471-2407-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luk SU, Yap WN, Chiu YT, Lee DT, Ma S, Lee TK, et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int J Cancer. 2011;128:2182–91. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 32.Hodul PJ, Dong Y, Husain K, Pimiento JM, Chen J, Zhang A, et al. Vitamin E delta-tocotrienol induces p27(Kip1)-dependent cell-cycle arrest in pancreatic cancer cells via an E2F-1-dependent mechanism. PloS one. 2013;8:e52526. doi: 10.1371/journal.pone.0052526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346:447–53. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- 34.Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Tomita S, Shirakawa H, et al. Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1alpha. J Nutr. 2008;138:2136–42. doi: 10.3945/jn.108.093237. [DOI] [PubMed] [Google Scholar]
- 35.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int J Cancer. 2012;131:E292–303. doi: 10.1002/ijc.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K, Aiura K, Matsuda S, Itano O, Takeuchi O, Umezawa K, et al. Combined effect of dehydroxymethylepoxyquinomicin and gemcitabine in a mouse model of liver metastasis of pancreatic cancer. Clin Exp Metastasis. 2012 doi: 10.1007/s10585-012-9544-7. [DOI] [PubMed] [Google Scholar]
- 37.Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretnam K. Palm tocotrienols decrease levels of pro-angiogenic markers in human umbilical vein endothelial cells (HUVEC) and murine mammary cancer cells. Genes Nutr. 2012;7:53–61. doi: 10.1007/s12263-011-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70:8695–705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, et al. Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer. 2004;109:182–8. doi: 10.1002/ijc.11679. [DOI] [PubMed] [Google Scholar]
- 40.Gysin S, Lee SH, Dean NM, McMahon M. Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 2005;65:4870–80. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]