Abstract
Background
Influenza vaccination is widely recommended every year to protect individuals against influenza virus infection and illness. There are few published estimates of influenza vaccine effectiveness against hospitalization in children or from subtropical regions.
Methods
We conducted a test-negative year-round study between October 2009 and September 2013, recruiting children 6 months to 17 years of age admitted to two hospitals in Hong Kong with a febrile acute respiratory infection. Cases were tested for influenza A and B and conditional logistic regression was used to estimate vaccine effectiveness comparing influenza vaccination history of the trivalent influenza vaccine (TIV) among patients testing positive versus negative for influenza, adjusting for age and sex and matching by calendar week of recruitment.
Results
Overall vaccine effectiveness against hospitalization with laboratory-confirmed influenza A and B was estimated to be 61.7% (95% CI: 43.0%, 74.2%). The estimated vaccine effectiveness against A(H3N2) was 36.6% (95% CI: −25.5%, 67.9%) compared to 71.5% (95% CI: 39.4%, 86.6%) for A(H1N1)pdm09 and 68.8% (95% CI: 41.6%, 83.3%) for B.
Conclusions
Vaccine effectiveness against hospitalization in children varied from year to year, but was moderate to high overall even in an area with influenza activity throughout the year.
Keywords: Influenza, Vaccine, Effectiveness, Children, Hospitalization
1. Introduction
Influenza virus causes substantial burden of illness in all age groups during regular epidemics and occasional pandemics [1]. Children often face the highest risk of infections during epidemics, because of higher levels of person-to-person contact and therefore greater exposure [2] as well as lower immunity due to fewer previous infections. Influenza vaccination is effective in reducing the risk of influenza virus infection and illness [3].
In Hong Kong, children between 6 months and 6 years of age as well as those with an underlying condition rendering them at increased risk for complications from influenza virus infection are recommended to receive influenza vaccination each year [4]. The Hong Kong government provides a subsidy for influenza vaccination for children between 6 months and less than 6 years, while children with an underlying condition can receive free influenza vaccination [5]. Each year, advertising is made on television, radio broadcast and posters in public places to bring awareness to the public. The Northern Hemisphere vaccine is used in Hong Kong.
Influenza vaccine effectiveness (VE) can vary from year to year, and it is important to continually monitor VE to confirm that vaccination is providing adequate protection and to identify factors affecting VE. The test negative design is an approach to estimating influenza VE from observational data based on recruitment of cases with acute respiratory infection, and comparison of vaccine coverage among the cases testing positive for influenza virus versus coverage among the cases testing negative for influenza virus [6]. The design has some similarities to a case–control study, but one major difference is the absence of a group of patients prospectively identified and recruited specifically as controls in most such studies. An exception is in test-negative studies conducted retrospectively, when laboratory results can be included in the case definition and a separate group of patients with negative laboratory results for influenza can be recruited [7]. Provided that some assumptions are satisfied [8], [9], the design is thought to allow unbiased estimation of VE. The majority of test-negative studies reported in the literature have been conducted in outpatient settings in temperate locations [10], [11], [12], [13], [14]. We have conducted systematic surveillance of the hospitalization burden of influenza among children in Hong Kong since 1997 [15], [16], [17], and here we use the test-negative approach to estimate influenza VE in this population. This study provided a better estimate of VE against the more severe outcome of influenza.
2. Methods
2.1. Subjects
Our study was based in Queen Mary Hospital and Pamela Youde Nethersole Eastern Hospital, the only 2 public hospitals on Hong Kong Island in Hong Kong with acute paediatric services. Children 6 months to 17 years of age admitted to the general wards of either of these hospitals with a febrile acute respiratory infection, defined as fever measured ≥38 °C with any respiratory symptom such as cough, runny nose, or sore throat were eligible for inclusion in this study. Children with risk factors for potentially severe respiratory infections like prematurity or chronic lung disease were not excluded. Nasopharyngeal aspirates were obtained from all patients and tested for influenza A and B virus by immunofluorescence and culture for seasonal influenza A and B virus, and additional RT-PCR for influenza A(H1N1)pdm09 virus. Influenza vaccination history within 6 months of hospitalization was elicited from the parents or legal guardians of patients using a standardized questionnaire administered by research personnel. The study period was between October 2009 and September 2013.
The study protocol was approved by the Institutional Review Board of the University of Hong Kong which waived the need for written consent since viral investigation was a routine diagnostic test carried out as part of routine care, any patient information was delinked from individual patient identification to maintain patient confidentiality, and participation by responding to the questionnaire was voluntary and indicative of consent.
2.2. Definition of vaccination status
Vaccinated children were those who had received trivalent inactivated influenza vaccine (TIV) within the 6 months prior to admission in a regimen and dosage appropriate for age and influenza vaccination history according to the recommendations of the Advisory Committee on Immunization Practices, with the last dose more than 2 weeks before hospitalization [18]. Children who should receive 2 doses of TIV but only received 1 dose, or were vaccinated within 2 weeks of hospitalization were categorized as unvaccinated. For the first study year of 1 October 2009 through 30 September 2010, children who had received 1 dose of the monovalent H1N1pdm09 vaccine within 6 months and at least 2 weeks prior to hospitalization were considered vaccinated.
2.3. Outcome definition
NPA specimens were tested for influenza A virus by direct antigen detection by direct immunofluorescence (IF) and virus culture in all recruited patients at the Virology Laboratory at the Queen Mary Hospital, Hong Kong and at the Public Health Laboratory Centre, Hong Kong. The direct immunofluorescence antigen test was carried out as previously described using IMAGENTM respiratory screen and typing reagents (Oxoid Ely Ltd., UK) [19]. All the specimens found positive in the respiratory screen with a pooled IF reagent were further identified using antibody reagents to the individual virus (influenza A or B, respiratory syncytial virus, parainfluenza virus types 1, 2, 3 and adenovirus) using the IMAGEN™ typing kit. To culture the respiratory viruses, MDCK, LLC-MK2, HEp-2 C and RD cell monolayers in culture tubes were inoculated with 150 μl of the nasopharyngeal aspirate-virus transport medium suspension and the virus isolation was carried out as previously described [20].
In addition, during the pandemic wave in 2009, the NPA samples were tested for influenza A(H1N1)pdm09 by RT-PCR. Total nucleic acid from 250 μl NPA was extracted by using NucliSens easyMAG instrument (bioMerieux, Netherlands) according to the manufacturer's instruction and previously described. Nucleic acid was recovered in 55 μl elution buffer and was kept at −80 °C until use. The diagnosis of influenza A virus was performed by RT-PCR targeting the M gene as previously described [19], [21], [22]. Real-time one-step RT-PCR assays were used for the detection of pandemic A(H1N1)pdm09 virus using Invitrogen SuperScript III Platinum One-Step Quantitative Kit in a 7500 Sequence Detection System (Applied Biosystem, Foster City, CA, USA) [23]. Briefly, 5 μl of eluted RNA was amplified in a 25 μl reaction containing 0.5 μl Superscript III Reverse Transcriptase/Platinum Taq DNA polymerase (Invitrogen), 0.05 μl ROX reference dye (25 μM), 12.5 μl of 2× reaction buffer, 800 nmol/l each of forward primer (5′-CCAAAGCTCAGCAAATCCTACAT-3′), reverse primer (5′-GATGGTGAATGCCCCATAGC-3′), and probe 200 nmol/l (Fam-TGATAAAGGGAAAGAAGTCCT-MGB). Reactions were first incubated at 50 °C for 30 min, followed by 95 °C for 2 min, and were then thermal cycled for 50 cycles (95 °C for 15 s, 55 °C for 30 s).
2.4. Statistical analysis
Patients who met the inclusion criteria and consented to participate were included in our study. We used conditional logistic regression models [7], [24], [25], matching on calendar week to account for potential bias due to changes in vaccine coverage over time, and adjusting for age, age-squared (to allow for non-linear effect of age) and sex [26]. VE was estimated as 1 minus the adjusted conditional odds ratio. VE was estimated against influenza A and B combined, and for specific types and subtypes (excluding patients positive for the other types/subtypes of influenza from the control group). VE was estimated overall, and for each of the four study years, and for various age strata. The overall estimates and the 2009–2010 estimates of VE for seasonal vaccination were adjusted for receipt of monovalent A(H1N1)pdm09 vaccine. In sensitivity analyses we examined VE with alternative comparison groups based on detection or non-detection of other respiratory viruses in patients testing negative for influenza.
3. Results
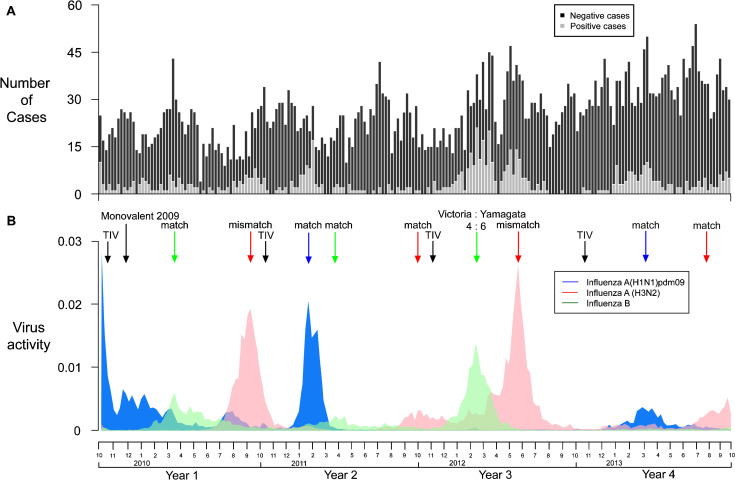
Between 1 October 2009 and 30 September 2013, we recruited 5399 children of whom 451 (8.4%) tested positive for influenza A virus and 211 (3.9%) tested positive for influenza B virus. The timeline of recruitment of patients is shown in Fig. 1 . As previously shown [15], [16], [17], influenza activity and pediatric hospitalization are not limited to the winter peak in Hong Kong. The majority (80.8%) of children were 5 years or younger of age, and there were more males than females although there was no significant gender difference between those who tested positive or negative for influenza (Table 1 ). 9.0% of test-negative patients reported receipt of influenza vaccination in the preceding 6 months, compared to 4.8% of the patients that tested positive for influenza A or B virus (p < 0.001). Among patients between 6 months and 2 years of age, 6.9% reported receipt of influenza vaccination in the preceding 6 months, compared to 12.3% among children 3–5 years and 10.8% among children 6–17 years. Parents were unable to recall the exact dates of vaccination but the history of vaccination given showed that children received TIV well into January. This appears to be a common practice in Hong Kong that is different from places like the US where the main annual influenza vaccination happens over a couple of months in October and November in the fall.
Fig. 1.
(A) Timeline of recruitment of patients testing positive or negative for influenza. (B) Local influenza virus activity obtained by multiplying together local surveillance data on consultation rates of influenza like illnesses with rates of laboratory detections of influenza by type/subtype [43].
Table 1.
Comparison of patients testing positive for influenza A and B and test-negative patients.
| Characteristic | Test-positive (n = 662) N (%) |
Test-negative (n = 4737) N (%) |
p-valuea |
|---|---|---|---|
| Age group | |||
| ≤2 years | 264 (39.9%) | 2617 (55.2%) | <0.001 |
| 3–5 years | 212 (32.0%) | 1269 (26.8%) | |
| 6–17 years | 186 (28.1%) | 851 (18.0%) | |
| Male | 378 (57.1%) | 2749 (58.0%) | 0.679 |
| Receipt of TIV in the preceding 6 months | |||
| Yes | 32 (4.8%) | 428 (9.0%) | <0.001 |
| No | 630 (95.2%) | 4309 (91.0%) | |
| Receipt of monovalent A(H1N1)pdm09 vaccine in the preceding 6 months | |||
| Yes (2009–2010 only) | 3 (2.3%) | 36 (4.1%) | 0.465 |
| No (2009–2010 only) | 130 (97.7%) | 850 (95.9%) | |
p-values estimated by chi-squared tests (sex) and Fisher's exact test (age group and vaccination history).
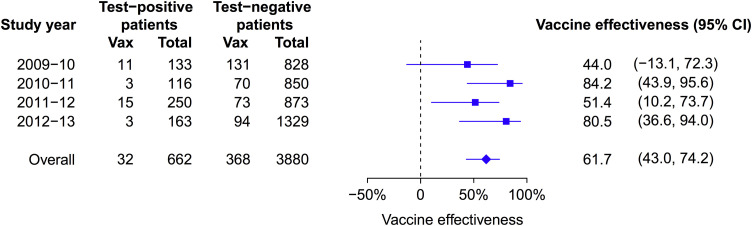
Of the 5399 patients, 857 (15.9%) tested negative for influenza and were recruited in calendar weeks when there were no test-positive patients (Fig. 1). These patients were excluded from our estimation of VE, and 4542 patients remained for analysis of VE. The overall VE was 61.7% (95% confidence interval, CI: 43.0%, 74.2%) against influenza A and B combined, based on reported receipt of seasonal influenza vaccination in the preceding 6 months comparing test-positive to test-negative patients (Table 2 ). Estimated VE was 56.9% (95% CI: 29.2%, 73.8%) for influenza A and 68.8% (95% CI: 41.6%, 83.3%) for influenza B. We examined VE for the four separate study years and found generally similar estimates, with the highest point estimates of VE in the 80% range in 2010–2011 and 2012–2013 (Fig. 2 ). Both years had circulation of all 3 influenza types and subtypes that largely matched the vaccine viruses. There was a suggestion of lower VE in 2011–2012. This year included a major influenza A H3N2 season with the circulating virus antigenically distinct from the 2011–2012 vaccine virus A/Perth/16/2009 (Fig. 1). This was also a year with high activity of influenza viruses throughout most of the year. In 2009–2010 the seasonal TIV did not include the new pandemic virus and a separate monovalent H1N1pdm09 vaccine was used with low uptake in the community. In that year, the estimated VE of the monovalent vaccine against influenza A was 48.1% (95% CI: −344.1%, 93.9%).
Table 2.
Estimates of TIV effectiveness against influenza A and B viruses, 2009–2013.
| Vaccine effectivenessa (95% confidence interval) |
|||
|---|---|---|---|
| Test-positive versus all test-negative patients | Test-positive versus pan-negative patientsb | Test-positive versus non-influenza virus positive patientsc | |
| Influenza A or B | 61.7% (43.0%, 74.2%) | 64.4% (46.4%, 76.3%) | 62.5% (38.5%, 77.1%) |
| Influenza A | 56.9% (29.2%, 73.8%) | 59.5% (32.4%, 75.7%) | 63.3% (33.1%, 79.8%) |
| Influenza A(H1N1)pdm09 | 71.5% (39.4%, 86.6%) | 73.5% (42.7%, 87.8%) | 74.9% (40.9%, 89.3%) |
| Influenza A(H3N2) | 36.6% (−25.5%, 67.9%) | 37.9% (−24.8%, 69.1%) | 45.4% (−24.9%, 76.2%) |
| Influenza B | 68.8% (41.6%, 83.3%) | 72.1% (47.0%, 85.3%) | 63.8% (19.2%, 83.8%) |
Effectiveness of TIV in the past 6 months based on conditional logistic regression, adjusted for age, age squared, sex, and receipt of monovalent H1N1pdm09 vaccine in 2009–2010, and matched by calendar week.
Pan-negative patients included the patients who tested negative for influenza virus and were also negative for other respiratory viruses including respiratory syncytial virus, parainfluenza virus types 1, 2, 3 and adenovirus using the IMAGENTM typing kit.
Non-influenza virus positive patients included the patients who tested negative for influenza virus but were positive for other respiratory viruses including respiratory syncytial virus, parainfluenza virus types 1, 2, 3 or adenovirus using the IMAGENTM typing kit.
Fig. 2.
Estimated effectiveness of trivalent inactivated influenza vaccination against influenza A or B combined in each study year and overall. Estimates were adjusted for age, age squared, and sex, and matched by calendar week. Estimates in 2009–2010 and overall were also adjusted for receipt of monovalent A(H1N1)pdm09 vaccine.
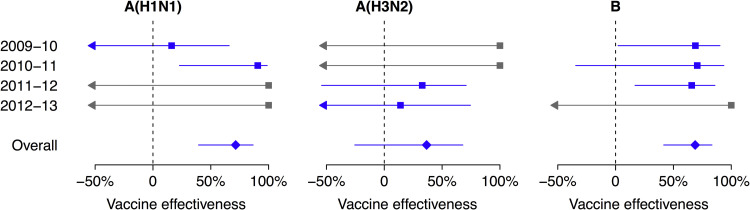
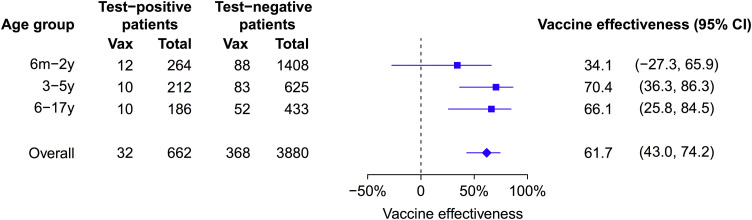
Type/subtype-specific VE estimates are shown in Fig. 3 . The point estimate of VE was lower for H3N2 (36.6%; 95% CI: −25.5%, 67.9%) compared to H1N1pdm (71.5%; 95% CI: 39.4%, 86.6%) and B (68.8%; 95% CI: 41.6%, 83.3%). In some years for some subtypes we estimated VE of 100% with very wide confidence intervals because none of the admitted children testing positive for that influenza type/subtype reported receipt of TIV (Fig. 3). Age-specific VE estimates are shown in Fig. 4 . The point estimate of VE was lower for children 6 months to 2 years of age compared to older children but the difference was not statistically significant.
Fig. 3.
Estimated effectiveness of trivalent inactivated influenza vaccination against influenza A(H1N1), A(H3N2) and B viruses in each study year and overall. Estimates were adjusted for age, age squared, and sex, and matched by calendar week. Estimates in 2009–2010 and overall were also adjusted for receipt of monovalent H1N1pdm09 vaccine. Estimates of 100% VE with wide 95% confidence intervals for influenza A(H1N1) in 2011–2012 and 2012–2013, influenza A(H3N2) in 2009–2010 and 2010–2011, and influenza B in 2012–2013 are shown in grey because there were no vaccinated children in the test-positive group.
Fig. 4.
Estimated effectiveness of trivalent inactivated influenza vaccination against influenza A and B combined by age groups and overall. Estimates were adjusted for age, age squared, and sex, and matched by calendar week. Estimates in 2009–2010 and overall were also adjusted for receipt of monovalent A(H1N1)pdm09 vaccine.
In our primary analysis, we assessed VE comparing test-positive patients to all test-negative patients. In sensitivity analyses, VE was similar when comparing only to the test-negative patients that were also negative for other respiratory viruses, or when comparing only to the test-negative patients that were positive for non-influenza respiratory viruses (Table 2).
4. Discussion
Our 4-year study covered at least 11 periods of influenza activity in Hong Kong, including the emergence of A(H1N1)pdm09 (Fig. 1). The overall VE of the TIV against influenza hospitalization was 61.7% (95% CI: 43.0%, 74.2%). A recently published study from Australia of children presenting to a tertiary pediatric hospital conducted between 2008 and 2012 also showed a VE of 64.7% (95% CI: 33.7%, 81.2%) [27]. However, most of the subjects were recruited in the Emergency Department with a smaller proportion from inpatients. They also excluded subjects enrolled in 2009 from VE calculation. The overall VE for the monovalent A(H1N1)pdm09 vaccine in our study was 48.1% (95% CI: −344.1%, 93.9%), which was somewhat lower than reported elsewhere [28]. The time interval between receipt of the monovalent vaccine and hospitalization in children who tested positive during the first pandemic season may not be adequate for mounting an immunologic response. In contrast, in 2010–2011 when A(H1N1)pdm09 was included in the TIV, VE was very high: 84.2% (95% CI: 43.9%, 95.6%).
The VE against H3N2 appeared to be low in this study. This could be due to the vaccine mismatches in the years 2009–2010 [29] and 2011–2012 in this study. Studies from several countries also showed low to moderate VE for the 2011–2012 season for H3N2 in adults and children when there was also antigenic mismatch with the vaccine [13], [14], [30], [31]. However, the circulating H3N2 strain in 2012–2013 in Hong Kong was matched to the vaccine while VE was still poor. One possible explanation was that the epidemic in 2012–2013 was very late, peaking in September (Fig. 1) and therefore VE may have been low due to waning post-vaccination immunity [11], [31]. A study from the UK showed that the VE for H3N2 in 2011/12 showed waning intra-seasonal protection [32]. VE was moderate in the first three months of the season but reduced in the second three months even when there was antigenic match. We only captured vaccination history within 6 months but did not have information on vaccination within 3 months, while influenza vaccines are typically administered between October and January prior to the winter influenza season in Hong Kong. The H3N2 epidemics in this 4-year study were in the summer and fall and largely mismatched with the vaccine strains. Our finding of VE against H3N2 may not be generalized.
VE against influenza B hospitalization was 68.8% overall (95% CI: 41.6%, 83.3%). This was surprisingly good considering the circulating influenza B lineages of Victoria:Yamagata was at a 4:6 ratio in the largest influenza B epidemic in this study in 2012, with the TIV including a Victoria lineage virus. Specifically, the VE against influenza B in 2012 in a 60% mismatch season was 65.8% (95% CI: 16.8%, 85.9%). The VE seen appears to be higher than that of 38% (95% CI: 4, 60) for a poor match season according to a meta-analysis of controlled trials of TIV in children and non-elderly adults [33]. Other studies have also shown suboptimal VE in the situation of a mismatch season [34], [35]. One explanation of the difference seen between ours and the previous studies may be that since the prevalence of cross-protective antibody is a function of previous priming by antigenically related strains or subtypes, children in Hong Kong with high influenza activity are more likely to have been primed by both influenza B lineages. A study from Canada showed an adjusted VE of 55% (95% CI: 32%, 70%) against a mismatched influenza B for 2007–2008 in adults and children which also demonstrated some cross-protection [36]. The quadrivalent influenza vaccine that contains both B lineages has been licensed and recommended for use in the US for the 2013–2014 season [18]. A multinational VE study of the quadrivalent influenza vaccine against ‘moderate-to-severe’ PCR-confirmed influenza was recently published [37]. Surprisingly, the VE against moderate to severe influenza B disease, defined as a body temperature higher than 39 °C, otitis media, lower respiratory tract illness, or myositis, encephalitis, seizure or myocarditis, was only 46.5 (95% CI: 34.1, 78.7) in a season with almost exclusively the Victoria lineage. Further vaccine effectiveness studies comparing the quadrivalent and trivalent vaccines will have to be conducted to determine whether the theoretical advantage of inclusion of strains from both B lineages could be translated to a real improvement in VE.
We previously reported an increased risk of non-influenza respiratory virus infections among children 6–17 years of age who received influenza vaccination [38], implying that the estimates of VE from test-negative studies might vary depending on the choice of comparison group [39]. We therefore examined the difference in VE estimates using alternative comparison groups but did not find any major differences in this study of hospitalization (Table 2), in which the majority of patients were 5 years of age or younger. A similar observation was also reported in the US in children below 5 years of age seeking ambulatory care or admitted to hospital [40].
This is the first study of VE in a geographic site outside areas with sharp peaks of influenza season in the winter. Unlike most influenza VE studies, our study was conducted year round. This allowed us to capture all influenza hospitalization. This is particularly relevant for areas with prolonged or multiple influenza A seasons, as well as for influenza B epidemics that can occur any time of the year. Another strength of our study includes matching test-positive patients with test-negative patients recruited in the same week which may be particularly important when influenza activity is not concentrated in a few weeks. Furthermore, we have shown that our results are robust even when using different criteria to define the “test negative” group (Table 2), viz. influenza negative, negative for all viruses tested or those positive for other viruses.
One limitation of this analysis was the use of immunofluorescence and virus culture to identify other respiratory viruses leading to a lack of sensitivity to identify some rhinoviruses and some other non-influenza viruses (e.g. human coronaviruses, human metapneumovirus). The direct immunofluorescence antigen tests for respiratory viruses only identified 7 respiratory viruses including influenza A, influenza A, RSV, parainfluenza 1, parainfluenza 2, parainfluenza 3 and adenovirus [19]. To culture respiratory viruses, it is able to isolate more respiratory viruses but lack of sensitivity to certain viruses such as human metapneumovirus [compared to RT-PCR for HMPV, sensitivity of culture is 13.6% (unpublished data)], or unable to recover such as rhinovirus type C [41] or unable to isolate human coronaviruses [42].
Furthermore, our reliance on virus culture and immunofluorescence assays rather than RT-PCR may have led to some under-detection of influenza-positive patients and consequent underestimation of VE. A second limitation is that vaccination history in the preceding 6 months was self-reported and there may have been some misclassification bias leading to underestimation or overestimation of VE overall, while as mentioned above we did not collect data on exact date of vaccination. We also did not have information on comorbidity. In addition, our definition of the vaccination status to be those receiving vaccine within 6 months of hospitalization may falsely bias toward higher VE in a setting where the influenza season lasts for many months. Given that it may not be feasible to vaccinate more than once each year, true VE late in the influenza season may be lower than our estimates for children vaccinated in the November–December period as recommended in Hong Kong. Finally, our definition of the vaccination status to be those who had received vaccine within 6 months of hospitalization precluded assessment of VE for the whole year in line with the practice of annual influenza vaccination.
In conclusion, we found that influenza vaccination was effective in preventing hospitalizations associated with confirmed influenza A and B virus infections in children. Vaccine effectiveness varied by year correlating with vaccine match although the impact of a mismatched B lineage is less clear. Protection against hospitalization also appeared to decrease when an epidemic happens late in the year. In our analyses, we chose to match test-positive and test-negative patients by calendar week to allow fairer estimation of VE given changing vaccine coverage during the course of the year. This approach may be particularly valuable in subtropical and tropical settings with prolonged influenza activity.
Acknowledgments
We thank the nurses of ward B6 of Pamela Youde Nethersole Eastern Hospital and ward K7 N of Queen Mary Hospital for research support.
Funding: This study was supported by the Research Fund for the Control of Infectious Diseases (Project No. CHP-CE-06), the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06). BJC is supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Conflicts of interest: JSMP receives research funding from Crucell NV and serves as an ad hoc consultant for GlaxoSmithKline and Sanofi Pasteur. BJC has received research funding from MedImmune Inc. and Sanofi Pasteur, and consults for Crucell NV. The authors report no other potential conflicts of interest.
References
- 1.Cromer D., van Hoek A.J., Jit M., Edmunds W.J., Fleming D., Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68:363–371. doi: 10.1016/j.jinf.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uyeki T.M. Preventing and controlling influenza with available interventions. N Engl J Med. 2014;370:789–791. doi: 10.1056/NEJMp1400034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Health Protection Scientific Committee on Vaccine Preventable Diseases . 2013. Recommendation on seasonal influenza vaccine for the 2013/14 season. [Google Scholar]
- 5.Center for Health Protection . 2013. Protect your child from seasonal influenza. [Google Scholar]
- 6.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Cheng A.C., Kotsimbos T., Kelly H.A., Irving L.B., Bowler S.D., Brown S.G. Effectiveness of H1N1/09 monovalent and trivalent influenza vaccines against hospitalization with laboratory-confirmed H1N1/09 influenza in Australia: a test-negative case–control study. Vaccine. 2011;29:7320–7325. doi: 10.1016/j.vaccine.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 8.Orenstein E.W., De Serres G., Haber M.J., Shay D.K., Bridges C.B., Gargiullo P. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–631. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 9.Foppa I.M., Haber M., Ferdinands J.M., Shay D.K. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–3109. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan S.G., Komadina N., Grant K., Jelley L., Papadakis G., Kelly H. Influenza vaccine effectiveness during the 2012 influenza season in Victoria, Australia: influences of waning immunity and vaccine match. J Med Virol. 2013 doi: 10.1002/jmv.23847. [DOI] [PubMed] [Google Scholar]
- 12.Kafatos G., Pebody R., Andrews N., Durnall H., Barley M., Fleming D. Effectiveness of seasonal influenza vaccine in preventing medically attended influenza infection in England and Wales during the 2010/2011 season: a primary care-based cohort study. Influenza Other Respir Viruses. 2013;7:1175–1180. doi: 10.1111/irv.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohmit S.E., Thompson M.G., Petrie J.G., Thaker S.N., Jackson M.L., Belongia E.A. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–327. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skowronski D.M., Janjua N.Z., Sabaiduc S., De Serres G., Winter A.L., Gubbay J.B. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–12 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014 doi: 10.1093/infdis/jiu048. [DOI] [PubMed] [Google Scholar]
- 15.Chiu S.S., Lau Y.L., Chan K.H., Wong W.H., Peiris J.S. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 16.Chiu S.S., Chan K.H., Chen H., Young B.W., Lim W., Wong W.H. Virologically confirmed population-based burden of hospitalization caused by influenza A and B among children in Hong Kong. Clin Infect Dis. 2009;49:1016–1021. doi: 10.1086/605570. [DOI] [PubMed] [Google Scholar]
- 17.Chiu S.S., Chan K.H., So L.Y., Chen R., Chan E.L., Peiris J.S. The population based socioeconomic burden of pediatric influenza-associated hospitalization in Hong Kong. Vaccine. 2012;30:1895–1900. doi: 10.1016/j.vaccine.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . 2013. Summary recommendations: prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – (ACIP) – United States, 2013–14. [Google Scholar]
- 19.Chan K.H., Peiris J.S.M., Lim W., Nicholls J.M., Chiu S.S. Comparison of nasopharyngeal flocked swabs and aspirates from pediatric patients for rapid diagnosis of respiratory viruses. J Clin Virol. 2008;42:65–69. doi: 10.1016/j.jcv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Lo J.Y. Respiratory Infections during SARS Outbreak, Hong Kong, 2003. Emerg Infect Dis. 2005;11:1738–1741. doi: 10.3201/eid1111.050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan K.H., Yam W.C., Pang C.M., Chan K.M., Lam S.Y., Lo K.F. Comparison of the NucliSens easyMAG and Qiagen BioRobot 9604 nucleic acid extraction systems for detection of RNA and DNA respiratory viruses in nasopharyngeal aspirate samples. J Clin Microbiol. 2008;46:2195–2199. doi: 10.1128/JCM.00315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . 2007. CDC realtime RT-PCR (rRTPCR) protocol for detection and characterization of influenza (version 2007) CDC REF. # I-007-05. [Google Scholar]
- 23.To K.K., Wong S.S., Li I.W., Hung I.F., Tse H., Woo P.C. Concurrent comparison of epidemiology, clinical presentation and outcome between adult patients suffering from the pandemic influenza A (H1N1) 2009 virus and the seasonal influenza A virus infection. Postgrad Med J. 2010;86:515–521. doi: 10.1136/pgmj.2009.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng A.C., Holmes M., Irving L.B., Brown S.G., Waterer G.W., Korman T.M. Influenza vaccine effectiveness against hospitalisation with confirmed influenza in the 2010–11 seasons: a test-negative observational study. PLoS ONE. 2013;8:e68760. doi: 10.1371/journal.pone.0068760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg K.W., Szilagyi P.G., Fairbrother G., Griffin M.R., Staat M., Shone L.P. Vaccine effectiveness against laboratory-confirmed influenza in children 6 to 59 months of age during the 2003–2004 and 2004–2005 influenza seasons. Pediatrics. 2008;122:911–919. doi: 10.1542/peds.2007-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pebody R., Andrews N., Waight P., Malkani R., McCartney C., Ellis J. No effect of 2008/09 seasonal influenza vaccination on the risk of pandemic H1N1 2009 influenza infection in England. Vaccine. 2011;29:2613–2618. doi: 10.1016/j.vaccine.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 27.Blyth C.C., Jacoby P., Effler P.V., Kelly H., Smith D.W., Robins C. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics. 2014 doi: 10.1542/peds.2013-3707. [DOI] [PubMed] [Google Scholar]
- 28.Yin J.K., Khandaker G., Rashid H., Heron L., Ridda I., Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta-analysis. Influenza Other Respir Viruses. 2011;5:299–305. doi: 10.1111/j.1750-2659.2011.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowling B.J., Chan K.H., Fang V.J., Lau L.L.H., So H.C., Fung R.O.P. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–2184. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kissling E., Valenciano M., Larrauri A., Oroszi B., Cohen J.M., Nunes B. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case–control study. Euro Surveill. 2013;18 doi: 10.2807/ese.18.05.20390-en. pii: 20390. [DOI] [PubMed] [Google Scholar]
- 31.Castilla J., Martinez-Baz I., Martinez-Artola V., Reina G., Pozo F., Garcia Cenoz M. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18 doi: 10.2807/ese.18.05.20388-en. pii: 20388. [DOI] [PubMed] [Google Scholar]
- 32.Pebody R., Andrews N., McMenamin J., Durnall H., Ellis J., Thompson C.I. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill. 2013;18 doi: 10.2807/ese.18.05.20389-en. pii: 20389. [DOI] [PubMed] [Google Scholar]
- 33.DiazGranados C.A., Denis M., Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31:49–57. doi: 10.1016/j.vaccine.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 34.Skowronski D.M., De Serres G., Dickinson J., Petric M., Mak A., Fonseca K. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis. 2009;199:168–179. doi: 10.1086/595862. [DOI] [PubMed] [Google Scholar]
- 35.Lo Y.C., Chuang J.H., Kuo H.W., Huang W.T., Hsu Y.F., Liu M.T. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–12 season. PLoS ONE. 2013;8:e58222. doi: 10.1371/journal.pone.0058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janjua N.Z., Skowronski D.M., De Serres G., Dickinson J., Crowcroft N.S., Taylor M. Estimates of influenza vaccine effectiveness for 2007–2008 from Canada's sentinel surveillance system: cross-protection against major and minor variants. J Infect Dis. 2012;205:1858–1868. doi: 10.1093/infdis/jis283. [DOI] [PubMed] [Google Scholar]
- 37.Jain V.K., Rivera L., Zaman K., Espos R.A., Jr., Sirivichayakul C., Quiambao B.P. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369:2481–2491. doi: 10.1056/NEJMoa1215817. [DOI] [PubMed] [Google Scholar]
- 38.Cowling B.J., Fang V.J., Nishiura H., Chan K.H., Ng S., Ip D.K. Increased risk of non-influenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54:1778–1783. doi: 10.1093/cid/cis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowling B.J., Nishiura H. Virus interference and estimates of influenza vaccine effectiveness from test-negative studies. Epidemiology. 2012;23:930–931. doi: 10.1097/EDE.0b013e31826b300e. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram M.E., McClure D.L., VanWormer J.J., Friedrich T.C., Meece J.K., Belongia E.A. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis. 2013;57:789–793. doi: 10.1093/cid/cit379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau S.K., Yip C.C., Tsoi H.W., Lee R.A., So L.Y., Lau Y.L. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong J.Y., Wu P., Nishiura H., Goldstein E., Lau E.H., Yang L. Infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013;177:834–840. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]