Abstract
PURPOSE
To use functional magnetic resonance imaging (fMRI) to prospectively examine pre-treatment predictors of post-treatment fatigue and cognitive dysfunction in women treated with adjuvant chemotherapy for breast cancer. Fatigue and cognitive dysfunction often co-occur in women treated for breast cancer. We hypothesized that pre-treatment factors, unrelated to chemotherapy per se, might increase vulnerability to post-treatment fatigue and cognitive dysfunction.
METHODS
Patients treated with (n=28) or without chemotherapy (n=37) and healthy controls (n=32) were scanned coincident with pre- and one-month-post-chemotherapy during a verbal working memory task (VWMT) and assessed for fatigue, worry, and cognitive dysfunction. fMRI activity measures in the frontoparietal executive network were used in multiple linear regression to predict post-treatment fatigue and cognitive function.
RESULTS
The chemotherapy group reported greater pre-treatment fatigue than controls and showed compromised neural response characterized by higher spatial variance in executive network activity than the non-chemotherapy group. Also, the chemotherapy group reported greater post-treatment fatigue than the other groups. Linear regression indicated that pre-treatment spatial variance in executive network activation predicted post-treatment fatigue severity and cognitive complaints, while treatment group, age, hemoglobin, worry, and mean executive network activity levels did not predict these outcomes.
CONCLUSIONS
Pre-treatment neural inefficiency (indexed by high spatial variance) in the executive network, which supports attention and working memory, was a better predictor of post-treatment cognitive and fatigue complaints than exposure to chemotherapy per se. This executive network compromise could be a pre-treatment neuromarker of risk, indicating patients most likely to benefit from early intervention for fatigue and cognitive dysfunction.
Keywords: Breast cancer, functional magnetic resonance imaging (fMRI), fatigue, executive function, cognitive dysfunction, working memory
Introduction
The term “chemobrain” is widely used, especially by the lay public, to describe cognitive dysfunction following chemotherapy, and is frequently associated with breast cancer. Prospective studies with neuropsychological and neuroimaging measures report mixed results[1–8], with about 35% of women treated with adjuvant chemotherapy for breast cancer showing cognitive impairment, although the range varies widely[1, 2]. Varying levels of impairment have been found in concentration, working memory, processing speed, and other higher level cognitive abilities[9, 10]. Importantly, cognitive complaints and deficits are measurable even before adjuvant treatment[3, 6, 9, 11–13]. Furthermore, cognitive symptoms and fatigue have been shown to co-occur and may be cumulative over time, suggesting they may share common pathogenic mechanisms[14, 15]. Together, these findings suggest that ‘chemo brain’ is not a universal experience and that cognitive dysfunction observed in some patients may be improperly attributed to chemotherapy. Indeed, there may be unexplored pre-treatment factors that increase vulnerability to cognitive dysfunction over the course of adjuvant treatment.
Of particular interest in women with breast cancer is the potential relationship between neurocognitive impairment and fatigue, which is implicated in cognitive dysfunction[16]. Notably, fatigue is the most common, distressing side effect of cancer and its treatment[17], with a reported prevalence of 56% to 95% during and following breast cancer treatment[18].Both fatigue and cognitive dysfunction have been observed as post-treatment outcomes regardless of treatment type (surgery, chemotherapy, endocrine and/or radiotherapy)[19–21]. From a clinical perspective, fatigue and cognitive symptoms can have a detrimental effect on quality of life, disrupting vocational activities and family, social, and interpersonal relationships. Critically, the association of the two kinds of symptoms from pre- to post-adjuvant treatment has not been studied, nor is it known whether early neural vulnerability may affect fatigue and cognitive outcomes over time.
The current longitudinal study characterizes post-treatment fatigue and cognitive dysfunction and examines functional magnetic resonance imaging (fMRI)-based neuromarkers in comparison groups of patients treated with and without chemotherapy for breast cancer and healthy controls without cancer. (Online Resource 1 provides an fMRI overview.) We evaluated two potential neuromarkers within the frontoparietal executive network, a brain system subserving attention and working memory previously implicated in studies of ‘chemo brain’[11, 22–25]. The first was mean activation amplitude,[26–28], referring to the magnitude of fMRI activation averaged across all of the points (voxels) within a particular brain region. The second was spatial variance in activation, referring to the inconsistency of these magnitude values across all voxels within that region. Spatial variance may reflect neural inefficiency and can be more predictive of group differences than mean amplitude[11, 29]. We hypothesized that factors measured prior to treatment (including subsequent treatment type, psychological distress, and executive network neuromarkers) might indicate increased risk of post-treatment fatigue and cognitive dysfunction.
Method
Participants
One-hundred sixteen right-handed women were recruited from the University of Michigan Comprehensive Cancer Center. Exclusion criteria included secondary diagnosis of neurological or psychiatric disorder, clinical depression (Patient Health Questionnaire; PHQ-8[30]), and cognitive impairment (Mini Mental Status Examination[31]). We enrolled two groups of patients with localized (Stage 0 to IIIa) breast cancer who had completed primary surgical treatment: 1) those who were to receive adjuvant chemotherapy (n=36) and those who were not (“non-chemotherapy,” n=41). Women in the non-chemotherapy group were to receive radiotherapy, either to complete breast-preserving therapy or to the chest wall after mastectomy (Table 1). Women with negative mammograms volunteered as healthy controls (n=39). Nine participants were excluded due to claustrophobia or inability to lie in the scanner. Five participants did not return for post-treatment assessment due to unstable medical condition, moving to a distant location, or acquisition of MRI contraindication. Five participants (4 control; 1 chemotherapy) completed study procedures, but were excluded from analyses. Of these, one control and the chemotherapy participant were excluded for pre-treatment VWMT accuracy 3.5 standard deviations below their respective group means, and three controls were excluded due to technical issues in the imaging session or extraordinary life stressors. The final sample included 28 chemotherapy patients, 37 non-chemotherapy patients, and 32 healthy controls. A subset of patients (25 chemotherapy and 25 non-chemotherapy) was included in a previous manuscript on pre-treatment worry[32]. Participants provided informed written consent approved by the University of Michigan Institutional Review Board.
Table 1.
Sample Characteristics.
| Characteristics | Chemotherapy group (n = 28) | Non-chemotherapy group (n = 37) | Control group (n = 32) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| no. | Mean | SD | no. | Mean | SD | no. | Mean | SD | |
| Age at Pre-treatment baseline, years | 50 | 10 | 53 | 9 | 50 | 9 | |||
| Education years* | 15 | 2 | 15 | 2 | 17 | 2 | |||
| MMSE | 29 | 1 | 29 | 1 | 30 | 1 | |||
| PHQ-8 | 4 | 4 | 4 | 4 | 3 | 3 | |||
| Days from Surgery to Pre-treatment assessment* | 24 | 9 | 36 | 17 | |||||
| Days from Pre- to Post-treatment assessment | 169 | 41 | 168 | 35 | 174 | 41 | |||
| Race | |||||||||
| White | 22 | 34 | 28 | ||||||
| Non-white | 6 | 3 | 4 | ||||||
| Cancer stage* | |||||||||
| 0 (DCIS) | 0 | 11 | - | ||||||
| I | 5 | 20 | - | ||||||
| II | 16 | 6 | - | ||||||
| IIIa | 7 | 0 | - | ||||||
| Type of surgery* | |||||||||
| Lumpectomy | 15 | 35 | - | ||||||
| Mastectomy | 13 | 2 | - | ||||||
| Lymph node dissection* | 16 | 2 | |||||||
| Received psychiatric medication | |||||||||
| Pre-treatment | 5 | 5 | 7 | ||||||
| Post-treatment | 5 | 7 | 7 | ||||||
| Chemotherapy regimen | |||||||||
| Doxorubicin/cyclophosphamide | 1 | - | - | ||||||
| Doxorubicin/cyclophosphamide/paclitaxel | 22 | ||||||||
| Docetaxel/cyclophosphamide | 5 | - | - | ||||||
| Received radiotherapy (Post-treatment)* | 16 | 37 | - | ||||||
| Received endocrine therapy (Post-treatment)* | 8 | 32 | - | ||||||
| Menstrual status at baseline | |||||||||
| Pre | 14 | 12 | 10 | ||||||
| Peri | 2 | 5 | 7 | ||||||
| Post | 12 | 20 | 15 | ||||||
| Hemoglobin (g/dl) | |||||||||
| Pre-treatment* | 12.8 | 0.8 | 13.7 | 0.7 | 13.3 | 0.9 | |||
| Post-treatment* | 12.3 | 1.1 | 13.4 | 0.8 | 13.2 | 0.8 | |||
Abbreviations: MMSE=Mini Mental State Exam; PHQ=Patient Health Questionnaire; DCIS=ductal carcinoma in situ; g/dl=grams/deciliter.
p < .05 for significant between-group difference in a one-way ANOVA for continuous variables, and in a chi-square test for categorical variables.
One chemotherapy participant, 1 non-chemotherapy participant, and 3 control participants were missing hemoglobin values at pre-treatment assessment. Two chemotherapy participants, 1 non-chemotherapy participant, and 2 control participants were missing hemoglobin values at post-treatment assessment.
Materials and procedures
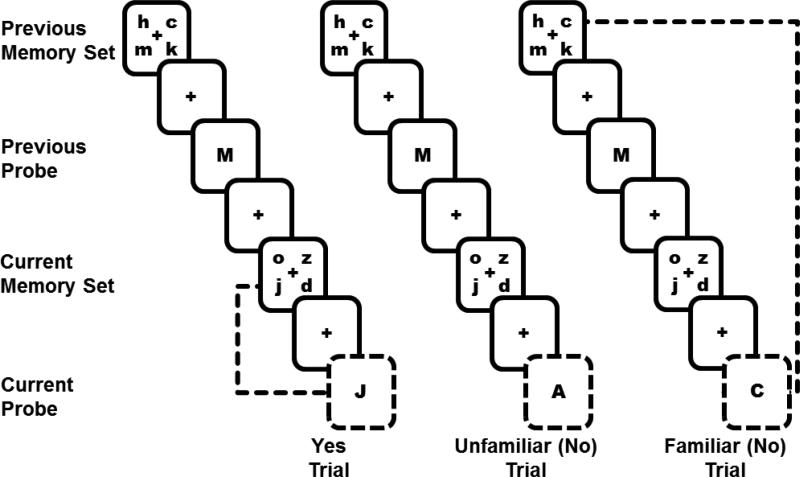
Participants underwent fMRI testing and completed self-report measures at two timepoints. Pre-treatment (baseline) measures in the patient groups were obtained on average 24 to 36 days after surgery, before adjuvant chemotherapy or radiation therapy, but after receiving the diagnosis and treatment plan. Post-treatment measures were obtained approximately five months later, at least one month post-chemotherapy with yoked intervals for the other groups. Assessments included self-reported fatigue (FACIT-F[33]), cognitive functioning (Attentional Function Index[34]) and worry (TIWI[35]), and fMRI during a Verbal Working Memory Task (VWMT; See Figure 1). Online Resources 2 details self-report measures.
Fig. 1.
Verbal Working Memory Task (VWMT). This figure represents a 2-trial series in each of three conditions (Yes, Unfamiliar, Familiar) of the task. In all conditions, during each trial, a 4-letter lowercase memory set appeared on the screen (1500 ms) followed by a delay (3000 ms) and a single uppercase probe letter (1500 ms). Participants indicated whether the single probe letter (outlined in dashed lines here) was part of the 4-letter memory set they had just seen (current memory set). Half of the time, the answer was “Yes” (e.g., “J” was a part of the current memory set “o z j d”). The other half of the time the answer was “No.” In the present analysis, we included two types of “No” trials: 1) Unfamiliar trials – The probe letter (A) was not present in the current memory set and had not been present in at least the last 3 trials. 2) Familiar trials – The probe letter had been present in the previous memory set (e.g., Though “C” was not part of the current memory set “o j z d”, it was a part of the previous memory set “h c m k”). Responding “No” to such familiar trials is more difficult than to unfamiliar trials, because familiar trials require knowing exactly when a specific letter was seen. Participants were not instructed about these different types of “No” trials
Verbal Working Memory Task (VWMT)[32, 36]
During fMRI scanning, participants performed 192 trials of a VWMT in four, 48-trial runs, each about 7 minutes (Figure 1). Ten practice trials preceded scanning. Inter-trial Intervals (ITIs) ranged between 1500 and 9000 milliseconds (ms). Each trial began with a 4-letter memory set appearing on the screen for 1500 ms, followed by a 3000 ms delay. Upon presentation of a probe letter (1500 ms), participants indicated whether it had appeared in the current memory set. Half of the probes were positive, requiring a “yes” response, and half were negative, requiring a “no” response. One-fourth of the negative probes were unfamiliar, meaning that the probe letter had not appeared in at least 3 trials, thus these probes were easy to reject (low demand). Half of the negative probes were familiar, because the probe letter appeared in the previous set, making the probe more difficult to reject (high demand). Another one-fourth of negative probes (on which the probe had appeared as a positive probe in the previous trial), all of the positive trials, and incorrect trials were modeled separately but not analyzed.
Generation of fMRI networks of interest
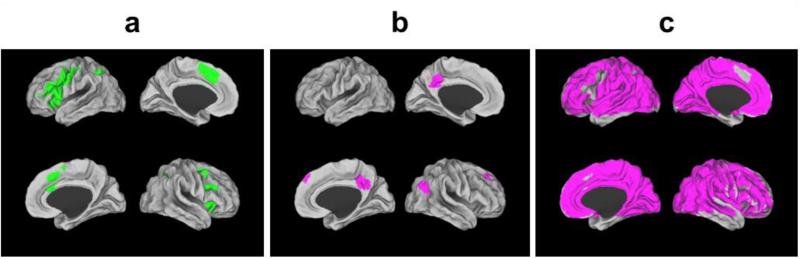
The “Executive Network” used for fMRI analyses constituted a brain system responsive to working memory demand in this task and was defined as all voxels active (Familiar or high demand minus Unfamiliar or low demand) in any group at any timepoint (Figure 2a). (Online Resources 3 provides full details.) We extracted mean and spatial variance (Unfamiliar and Familiar trials) across this network for each participant at each timepoint for analysis. We also extracted comparable values from sub-regions of this executive network (left frontal, right frontal, anterior cingulate, left parietal, right parietal). To ensure our findings were specific to the executive network, mean and variance values were extracted from two additional networks: a default mode network (deactivated regions; Figure 2b) and a null network (all regions not included in the executive network; Figure 2c).
Fig. 2.
Generation of fMRI networks of interest. (A) Executive Network. The brain areas shown in green are composed of voxels that were significantly active (familiar minus unfamiliar) for any group at each time point (see Online Resources 3 for generation of executive network map). This executive network supports the executive processes required to perform the Verbal Working Memory Task. Mean and spatial variance measures were calculated across this network for all reported analyses. The executive network covers parts of bilateral inferior and middle frontal gyri, anterior cingulate cortex, and bilateral intraparietal sulcus. (B) Default Mode Network. This network is known to be more active during periods of rest than during externally-directed tasks. Suppression of this network is often required to successfully perform cognitively-challenging tasks with greater suppression required for more demanding task conditions. The brain regions shown in purple represent the default mode network across all groups at all timepoints (defined as unfamiliar minus familiar) and cover parts of posterior cingulate/precuneus and superior frontal gyrus. (D) Null Network. The brain regions shown in purple are all of the fMRI voxels that were not included in the executive network coverage (A) but were included in functional slice coverage
Statistical analyses
Multiple linear regression analysis was implemented in SAS (SAS Institute, 2006) to determine predictors of three outcome variables: post-treatment fatigue (FACIT-F), perceived cognitive function (AFI), and VWMT error rate. Though predictor variables were considered separately in the model-building process, all of the independent variables were included in the final model simultaneously. The final model included treatment group (chemotherapy, non-chemotherapy, healthy control) as the only categorical variable. The other independent variables were continuous : 1) pre-treatment measures of FACIT-F, AFI, and VWMT error rate; 2) pre- and post-treatment spatial variance in the executive network; 3) pre-and post-treatment spatial mean in the executive network; 4) age given known associations with cognitive functioning; 5) pre- and post-treatment hemoglobin to account for possible differential effects of surgery and illness severity; and 6) pre- and post-treatment self-reported worry based on previous findings implicating pre-treatment worry in co-occurring cognitive dysfunction[32]. There were no group differences in outcome variables by education, thus it was excluded.
Additional descriptive analyses included between-group and within-group comparisons between timepoints, using t-tests and Analysis of Variance (ANOVA), and Pearson correlation.
Results
Demographic results
Table 1 lists demographic and clinical characteristics. Participants were on average middle aged, relatively well-educated, and white. Whereas groups did not differ in age or menopausal status, healthy controls had higher average education (M = 17 years) than patients (M = 15 years). Groups did not differ on MMSE (p= .52) and PHQ8 scores (p=.53), indicating intact cognitive function and no significant depressive symptomatology respectively. As expected given typical treatment approaches, more chemotherapy patients than non-chemotherapy patients were diagnosed with higher stage disease (II or IIIa) and treated with mastectomy. The chemotherapy group received three standard drug regimens with 79% receiving a combination of doxorubicin, cyclophosphamide, and paclitaxel over a four-month interval. Baseline hemoglobin (Hb) differed (p<.001), with the pre-chemotherapy group having lower levels than the non-chemotherapy (p<.001) and healthy control (p=.11) groups. Also across combined patient groups, baseline hemoglobin levels were lower in patients with higher disease stage (II & IIIa vs. 0 & I; Hb g/dl M+SD: 13.0 + 0.8 vs. 13.58 + 0.86, p=.02), and in those who underwent mastectomy (vs. breast conservation; Hb g/dl M+SD: 12.78 + 0.79 vs. 13.51 + 0.85, p=.004).
Pre-treatment indicators of vulnerability
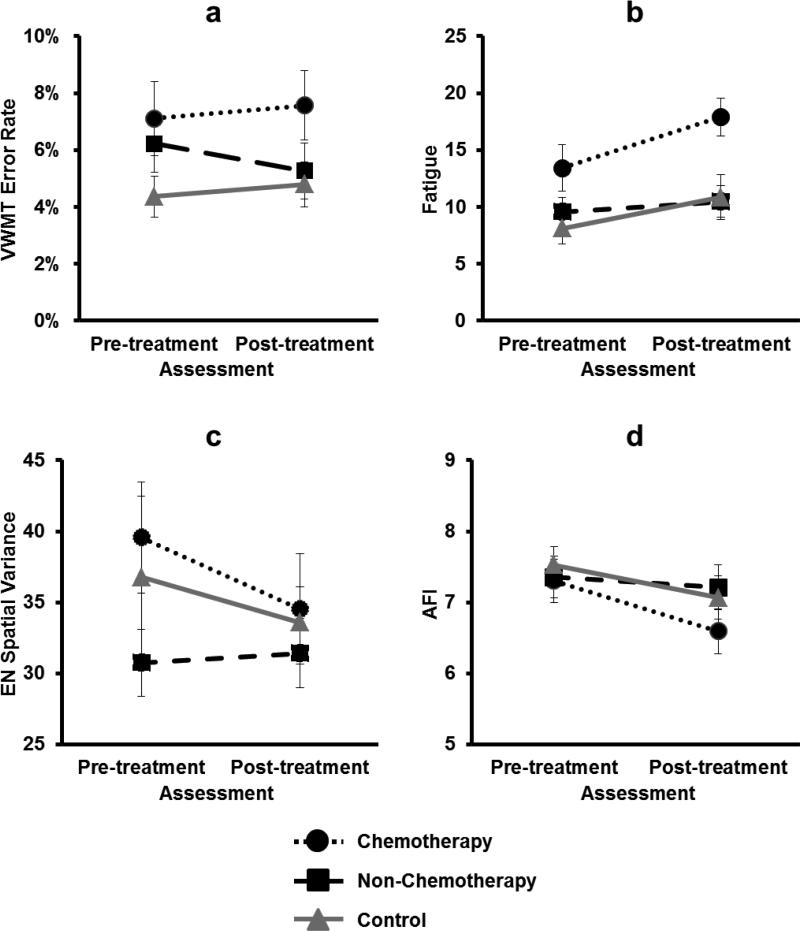
Patients awaiting adjuvant chemotherapy showed indications of compromised neurocognitive function even before adjuvant treatment. One-way ANOVAs comparing all three groups revealed no significant main effects of group on VWMT error rate (p=.08), fatigue (p=.11), or spatial variance (p=.66). T-test comparisons between specific groups, however, revealed differences. Compared to controls, pre-chemotherapy patients showed a trend toward more errors on the VWMT prior to adjuvant treatment (p=.06; Figure 3a; Online Resources 4). Baseline fatigue was also greater for pre-chemotherapy patients than for controls (p=.04), but not non-chemotherapy patients (p=.12; Figure 3b); nevertheless, 32% of women awaiting chemotherapy reported clinically significant fatigue [37] compared with less than 15% in the other groups (p=.10). Fatigue and hemoglobin were correlated at baseline (r=.25, p=.02). Spatial variance across the executive network was greater in pre-chemotherapy patients than in non-chemotherapy patients (p=.05; Figure 3c; Online Resources 4), whereas mean activation within this network did not differ across groups (p=.45).
Fig. 3.
Group differences before and after treatment. (A) Overall error rate on the Verbal Working Memory Task by group at pre-treatment and post-treatment assessments. (B) Self-reported fatigue by group at pre-treatment and post-treatment assessments. (C) Executive Network (EN) spatial variance by group at pre-treatment and post-treatment assessments. (D) Attentional Function Index (AFI) scores by group at pre-treatment and post-treatment assessments.
Post-treatment impairment
Women who had received chemotherapy reported greater fatigue and displayed worse objectively-measured cognitive functioning than women in the other two groups. Repeated measures ANOVA on fatigue across pre- and post-treatment assessments showed main group (p=.004) and time (p=.01) effects but no group by time interaction (p=.48). A three-group, one-way ANOVA showed a post-treatment group difference (p=.004), with the chemotherapy group reporting higher levels of post-treatment fatigue than both the non-chemotherapy (p=.001) and control (p=.009) groups. Only the chemotherapy group showed a significant increase in fatigue from pre- to post-treatment (p=.03; Figure 3b). Fifty-two percent of chemotherapy patients reported clinically significant levels of fatigue following treatment compared with less than 20% in the other groups (p=.005).
Notably, there was no group by time interaction for VWMT (p=.55) as mean error rate was higher in the chemotherapy group both pre- and post-treatment. One-way ANOVAs comparing the three groups post-treatment revealed no significant difference in error rate (p=.24); however, women who received chemotherapy made more post-treatment errors on the VWMT than women in the other two groups combined (p=.05; Figure 3a; Online Resources 4). Although the chemotherapy group reported increased cognitive complaints (AFI) between pre-and post-treatment assessments (p=.01), there was no group by time interaction (p=.84) or main effect of group on post-treatment cognitive complaints (p=.37; Figure 3d). Importantly, differences specific to chemotherapy in both objective and subjective measures of cognitive function were modest, apparent only in comparisons between specific groups, suggesting a potential role for other indicators of vulnerability to post-treatment cognitive dysfunction.
Spatial variance in executive network activation predicts post-treatment fatigue and cognitive dysfunction
Previously-described multiple linear regression models were employed to investigate which pre-treatment factors might predict post-treatment fatigue and cognitive dysfunction, thereby serving as potential markers of vulnerability to adverse psychological outcomes. Importantly, greater pre-treatment spatial variance in activation of the executive network, but not treatment with chemotherapy per se, was predictive of worse complaints about fatigue and cognitive function following treatment.
Predicting Post-Treatment Fatigue
In the model predicting post-treatment fatigue (Table 2), higher pre-treatment fatigue was a strong predictor of higher post-treatment fatigue (p<.0001). Greater spatial variance within the executive network prior to treatment was also a significant predictor of greater post-treatment fatigue (p=.002). Less post-treatment spatial variance was significantly associated with greater fatigue (p=.04), however a Pearson correlation between post-treatment variance and post-treatment fatigue did not indicate this negative relationship (p=.57) suggesting the apparent negative relationship may be due to controlling other factors in the model. Importantly, a three-group (chemotherapy, non-chemotherapy, control) contrast within this model did not predict post-treatment fatigue (p=.21). These results demonstrate that greater pre-treatment fatigue and spatially variant executive network activity are likely more indicative of risk for post-treatment fatigue than is treatment group.
Table 2.
Multiple Linear Regression Models Predicting Post-treatment Fatigue.
| Independent Variable | Parameter Estimate | Standard Error | P |
|---|---|---|---|
| Chemotherapy vs. Non-chemotherapy | 3.78 | 2.30 | .11 |
| Healthy control vs. Non-chemotherapy | 0.13 | 2.01 | .95 |
| Age | 0.14 | 0.10 | .14 |
| Pre-treatment Hemoglobin | 1.33 | 1.24 | .29 |
| Post-treatment Hemoglobin | −2.23 | 1.17 | .06 |
| Pre-treatment Fatigue | 0.48 | 0.11 | <0001* |
| Pre-treatment Worry | −1.67 | 1.49 | .27 |
| Post-treatment Worry | 2.49 | 1.31 | .06 |
| Pre-treatment EN mean | 0.008 | 0.33 | .98 |
| Post-treatment EN mean | 0.24 | 0.32 | .47 |
| Pre-treatment EN spatial variance | 0.15 | 0.05 | .002* |
| Post-treatment EN spatial variance | −0.13 | 0.06 | .04* |
Note. Model F value = 6.38 (p<.0001); R-Square=0.50. EN=Executive Network.
Indicates p<.05.
To test the specificity of spatial variance within the executive network as a predictor of post-treatment fatigue, parallel multiple linear regression models were run with pre- and post-treatment mean activation and spatial variance calculated for 1) individual clusters within the executive network (anterior cingulate, left frontal, right frontal, left parietal, and right parietal), 2) a default mode network, and 3) a null network excluding the executive network clusters. (See Online Resources 5 for descriptive statistics.) Pre-treatment spatial variance, but not mean, activation was significantly associated with post-treatment fatigue for all executive network regions except the right parietal cluster (Online Resources 6). By contrast, post-treatment fatigue was not predicted by spatial variance in pre-treatment activation in the default mode or null networks (Online Resources 7). Together, these results suggest that spatial variance specifically in the executive network, signifying compromised function under working memory load, may be a neuromarker indicating greater vulnerability to fatigue following treatment.
Predicting Cognitive Complaints
In the model predicting cognitive complaints (AFI; Table 3), worse pre-treatment cognitive complaints (p<.0001) and higher pre-treatment spatial variance in the executive network (p<.04) predicted worse post-treatment subjective cognitive functioning. No other factors, including treatment group (p=0.66), were significantly associated with post-treatment subjective cognitive functioning. Interestingly, including post-treatment fatigue in this model eliminated the association between pre-treatment variance and post-treatment cognitive complaints (Online Resources 8). These results suggest that similar mechanisms may underlie negative post-treatment outcomes in both fatigue and cognitive complaints.
Table 3.
Multiple Linear Regression Models Predicting Post-treatment Cognitive Functioning.
| Post-treatment AFI | Post-treatment VWMT Error Rate | |||||
|---|---|---|---|---|---|---|
| Independent Variable | Parameter Estimate | Standard Error | P | Parameter Estimate | Standard Error | P |
| Pre-treatment AFI | 0.66 | 0.11 | <.0001* | |||
| Pre-treatment VWMT Error Rate | 0.44 | 0.10 | <.0001* | |||
| Chemotherapy vs. Non-chemotherapy | −0.38 | 0.43 | 0.38 | 0.03 | 0.02 | .07 |
| Healthy control vs. Non-chemotherapy | −0.06 | 0.37 | 0.87 | 0.005 | 0.01 | .69 |
| Age | 0.02 | 0.02 | .20 | 0.001 | 0.001 | .24 |
| Pre-treatment Hemoglobin | 0.16 | 0.23 | .49 | 0.006 | 0.008 | .51 |
| Post-treatment Hemoglobin | −0.15 | 0.22 | .49 | −0.01 | 0.008 | .12 |
| Pre-treatment Worry | 0.29 | 0.28 | .30 | 0.002 | 0.01 | .85 |
| Post-treatment Worry | −0.31 | 0.24 | −0.007 | 0.009 | .41 | |
| Pre-treatment EN mean | −0.04 | 0.06 | .48 | 0.002 | 0.002 | .31 |
| Post-treatment EN network mean | −0.03 | 0.06 | .67 | 0.002 | 0.002 | .46 |
| Pre-treatment EN spatial variance | −0.02 | 0.008 | .04* | <.0001 | <.0001 | .77 |
| Post-treatment EN spatial variance | 0.02 | 0.01 | .12 | <.0001 | <.0001 | .41 |
Note. AFI Model: F value = 6.16 (p<.0001); R-Square=0.49. VWMT Error Rate Model: F value = 3.03 (p=.002); R-Square=0.32. EN=Executive Network.
Indicates p<.05
Predicting Objective Performance on the VWMT
Unlike with fatigue and cognitive complaints, spatial variance in the executive network was not a good indicator of post-treatment objective cognitive performance (Table 3). Only pre-treatment error rate was a significant predictor (p<.0001) of post-treatment error rate. Inclusion of pre- and post-treatment fatigue in this model did not significantly change these relationships (Online Resources 8). These results suggest a distinction between subjective reports of fatigue and cognitive functioning in daily life and objective measures of cognitive functioning. Our current cognitive testing conditions may not be as sensitive to decline as self-assessed performance on daily activities, though both rely on similar underlying neural resources.
Other Influences on Spatial Variance
Though spatial variance has previously been associated with poor function[29], we also considered other salient factors that might influence spatial variance including inability to perform the task and non-neural sources (e.g., scanner signal noise and motion). In particular, task error rate was not correlated with spatial variance either pre-(r=.00, p=.99) or post-treatment (r=.03, p =.75). Also in the null network (areas of the brain outside the executive network) which would be susceptible to the same non-neural noise, spatial variance was not predictive of fatigue or cognitive complaints (Online Resources 7 and 9), and motion (maximum total displacement) was equally correlated with spatial variance in both the null (r=.20, p = .05) and executive (r=.19, p =.05) networks. Thus, these data argued against these influences driving the relationships between spatial variance and fatigue and cognitive complaints.
Discussion
This prospective study examined potential contributions of adjuvant chemotherapy, neural functioning, and psychological distress to negative outcomes of fatigue and cognitive dysfunction in women treated for breast cancer. Like other investigators, we found that women treated with chemotherapy reported greater fatigue after treatment than did other women[17–19, 21]. However, women treated with chemotherapy also showed greater pre-treatment fatigue than controls and greater executive network variance than women treated without chemotherapy. One might expect that chemotherapy and non-chemotherapy groups would be equivalent in executive network function prior to adjuvant treatment; however, we have previously reported differences between these groups, related to psychological distress following diagnosis[32]. Notably, our findings indicate that pre-treatment spatial variance of executive network fMRI activity was a better indicator of both fatigue and cognitive complaint outcomes than treatment type per se. This suggests these symptoms may share similar underlying mechanisms, not limited to chemotherapy, and that pre-treatment spatial variance may be an early indicator, or neuromarker, of alterations in important underlying systems.
Variability in neural activation has previously been associated with neurocognitive alterations across a range of subject groups (depression[29], ADHD[38], aging[39], self-control[40]); however, increased variance is not always linked to worse performance. In the present sample, increased variance in the executive network was related to worse post-treatment fatigue and cognitive complaints, arguing that spatial variance in the executive network during the VWMT indicates neural inefficiency that is more likely to lead to future problems. Though the present study does not test a mechanistic hypothesis, pre-treatment neural inefficiency could make everyday cognitive demands more effortful, leading to a feedback loop, whereby effortful cognitive tasks evoke greater fatigue leading to less effective cognitive task performance. Neural inefficiency in this population may be associated with psychological distress of diagnosis or may be a downstream consequence of cancer-related processes and primary treatment (e.g., pro-inflammatory cytokine immune response to cancer)[15]. We did not find that worry, an important indicator of pre-treatment function in our previous work, was a significant predictor of post-treatment cognitive complaints, but this may be due to inclusion of controls (in addition to patients) or the measure used (general worry rather than combined general and cancer-specific worry). We cannot rule out the effects of immune or other biological characteristics not measured here and believe such factors deserve further investigation. However, hemoglobin, an indicator of extent of surgery, treatment, and oxygenation, was only marginally significant as a contributor to post-treatment fatigue and was not associated with cognitive complaints or objective performance.
This study is the first to examine spatial variance in the frontoparietal executive network as a potential neuromarker and to demonstrate its predictive relationship to post-treatment complaints of cognitive dysfunction and fatigue in women with breast cancer. Previous work has focused on voxelwise analysis across the whole brain, which is influenced by both the mean signal within a voxel and the consistency of this signal across voxels to meet cluster/extent thresholds. The present results indicate the importance of explicitly examining spatial variance across the executive network to detect early cognitive problems and extend other evidence for altered frontoparietal activation in women with breast cancer. Ferguson and colleagues[22] demonstrated a broader extent of activation post-chemotherapy in a similar network, along with more cognitive complaints in a breast cancer patient compared to her twin without breast cancer. de Ruiter and colleagues showed reduced prefrontal and parietal activity in survivors 10 years post-chemotherapy and stem cell transplantation than in survivors treated without chemotherapy[23]. Similarly, Deprez and colleagues showed decreased frontoparietal activation during multitasking in women recently post-chemotherapy compared to non-chemotherapy patients and healthy controls and a significant relationship between decreased frontoparietal activation and increased cognitive complaints[41]. Kesler and colleagues showed that patients treated with and without chemotherapy failed to recruit left frontal regions during a memory task[24]. We have previously shown that women awaiting chemotherapy showed compensatory recruitment of frontal executive regions during a VWMT [11]. However, McDonald's group reported significant bilateral frontal activation supporting working memory prior to treatment followed by a decrease in activation one month post-chemotherapy[25]. These wide-ranging findings are complex likely due to variations in tasks, imaging contrasts, and patient groups, but point to a consistent interpretation; namely that the frontoparietal executive network, subserving attention and memory, is affected in women with breast cancer. Signs of compromise within this network are related to common patient complaints.
While one of the largest fMRI studies to examine cognitive dysfunction in breast cancer patients, our small sample size and correlations between some of the predictor variables prevent making strong claims about null results. Clearly, pre-treatment executive network inefficiency signals a trajectory of cumulative fatigue and cognitive complaints over time, and is a stronger predictor of post-treatment cognitive complaints and fatigue than is treatment with chemotherapy per se in this sample. Future research incorporating larger samples, a wider range of disease stages and additional biological covariates (e.g. cytokines) is likely to confirm other important contributing factors. Indeed, accumulating evidence suggests that cognitive problems post-treatment are likely multivariate in nature, influenced by interacting biological and psychological mechanisms related to diagnosis, treatment, and response to illness. Many of the biological mechanisms affecting post-treatment outcomes are currently beyond our control even with pharmaceutical intervention. However, early assessment and interventions to reduce fatigue and improve working memory efficiency prior to treatment are practicable and could ameliorate the severity of fatigue and cognitive complaints following treatment.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health R01 NR01039 (BC) and Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale ™ (DFH).
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- AFI
Attentional Function Index
- ANOVA
Analysis of Variance
- DCIS
Ductal Carcinoma In Situ
- EN
Executive Network
- FACIT-F
Functional Assessment of Chronic Illness Therapy-Fatigue
- fMRI
functional Magnetic Resonance Imaging
- g/dl
grams/deciliter
- Hb
Hemoglobin
- MMSE
Mini Mental State Exam
- ms
milliseconds
- PHQ
Patient Health Questionnaire
- TIWI
Three-Item Worry Index
- VWMT
Verbal Working Memory Task
Footnotes
Disclosures
None.
References
- 1.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–75. doi: 10.1007/s11910-012-0264-9. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 2.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8. doi: 10.1016/S1470-2045(10)70294-1. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 3.Ruzich M, Ryan B, Owen C, et al. Prospective evaluation of cognitive function in patients with early breast cancer receiving adjuvant chemotherapy. Asia Pac J Clin Oncol. 2007;3:125–133. [Google Scholar]
- 4.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 5.Hedayati E, Alinaghizadeh H, Schedin A, et al. Effects of adjuvant treatment on cognitive function in women with early breast cancer. Eur J Oncol Nurs. 2012;16:315–22. doi: 10.1016/j.ejon.2011.07.006. doi: 10.1016/j.ejon.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–9. doi: 10.1002/cncr.20272. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 7.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–13. doi: 10.1002/cncr.22610. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 8.Reuter-Lorenz P, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–56. doi: 10.1002/cncr.25098. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 10.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578–87. doi: 10.1200/JCO.2011.39.5640. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–31. doi: 10.1080/13803390903032537. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 12.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–52. doi: 10.1007/s10549-007-9686-5. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherling C, Collins B, Mackenzie J, et al. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: an FMRI study. Front Hum Neurosci. 2011;5:122. doi: 10.3389/fnhum.2011.00122. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–57. doi: 10.1016/j.bbi.2012.06.011. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan S, Berman MG. Directed attention as a common resource for executive functioning and self-regulation. Psychol Sci. 2010;5:43–57. doi: 10.1177/1745691609356784. [DOI] [PubMed] [Google Scholar]
- 17.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–9. doi: 10.1002/cncr.27475. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 18.De Jong N, Candel MJ, Schouten HC, et al. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 19.Noal S, Levy C, Hardouin A, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2011;81:795–803. doi: 10.1016/j.ijrobp.2010.06.037. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2013;2:231–238. doi: 10.1136/bmjspcare-2011-000172. doi: 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehnert A, Scherwath A, Schirmer L, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couseling. 2007;66:108–18. doi: 10.1016/j.pec.2006.11.005. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–70. doi: 10.1200/JCO.2007.10.8639. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68:1447–53. doi: 10.1001/archneurol.2011.245. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald BC, Conroy SK, Ahles TA, et al. Alterations in Brain Activation During Working Memory Processing Associated With Breast Cancer and Treatment: A Prospective Functional Magnetic Resonance Imaging Study. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.38.5674. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–151. doi: 10.1038/nrn730. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- 27.Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Proc Int Sch Magn Reson Brain Funct Front Brain Funct MRI Electrophysiol Methods Proc Int Sch Magn Reson Brain Funct. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. doi: 10.1111/j.1467-8721.2008.00570.x. [Google Scholar]
- 29.Berman MG, Nee DE, Casement M, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11:85–96. doi: 10.3758/s13415-010-0014-x. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Berman MG, Askren MK, Sook Jung M, et al. Pretreatment Worry and Neurocognitive Responses in Women With Breast Cancer. Health Psychol. 2013 doi: 10.1037/a0033425. doi: 10.1037/a0033425. [DOI] [PubMed] [Google Scholar]
- 33.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. doi: 10.1016/S0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 34.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index—a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 35.Kelly WE. A Brief Measure of General Worry: The Three Item Worry Index. North Am J Psychol. 2004;6:219–225. [Google Scholar]
- 36.Nelson JK, Reuter-Lorenz PA, Sylvester C-YC, et al. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proc Natl Acad Sci. 2003;100:11171–11175. doi: 10.1073/pnas.1334125100. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belle S, Paridaens R, Evers G, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13:246–254. doi: 10.1007/s00520-004-0734-y. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 38.Depue BE, Burgess GC, Willcutt EG, et al. Symptom-correlated brain regions in young adults with combined-type ADHD: Their organization, variability, and relation to behavioral performance. Psychiatry Res Neuroimaging. 2010;182:96–102. doi: 10.1016/j.pscychresns.2009.11.011. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The Modulation of BOLD Variability between Cognitive States Varies by Age and Processing Speed. Cereb Cortex. 2013;23:684–693. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman MG, Yourganov G, Askren MK, et al. Dimensionality of brain networks linked to lifelong individual differences in self-control. Nat Commun. 2013;4:1373. doi: 10.1038/ncomms2374. doi: 10.1038/ncomms2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deprez S, Vandenbulcke M, Peeters R, et al. Longitudinal Assessment of Chemotherapy-Induced Alterations in Brain Activation During Multitasking and Its Relation With Cognitive Complaints. J Clin Oncol. 2014;32:2031–2038. doi: 10.1200/JCO.2013.53.6219. doi: 10.1200/JCO.2013.53.6219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.