The End-Cretaceous Impact Winter Killed Off Slow-Growing Plants The end-Cretaceous mass extinction caused the selective extinction of plant species with slow-growth strategies, consistent with an impact winter hypothesis.
Abstract
The Chicxulub bolide impact caused the end-Cretaceous mass extinction of plants, but the associated selectivity and ecological effects are poorly known. Using a unique set of North Dakota leaf fossil assemblages spanning 2.2 Myr across the event, we show among angiosperms a reduction of ecological strategies and selection for fast-growth strategies consistent with a hypothesized recovery from an impact winter. Leaf mass per area (carbon investment) decreased in both mean and variance, while vein density (carbon assimilation rate) increased in mean, consistent with a shift towards “fast” growth strategies. Plant extinction from the bolide impact resulted in a shift in functional trait space that likely had broad consequences for ecosystem functioning.
Author Summary
Sixty-six million years ago the Chicxulub bolide impacted the Earth, marking the Cretaceous–Paleogene boundary (KPB). This event caused the planet's most recent mass extinction, but the selectivity and functional consequences of the extinction on terrestrial plants has been largely unknown. A key untested hypothesis has been that a subsequent impact winter would have selected against slow-growing evergreen species, a possible cause of the modern dominance of high-productivity deciduous angiosperm forests. We tested this hypothesis using fossil leaf assemblages across a 2-million-year interval spanning the KPB. We assess two key ecological strategy axes—carbon assimilation rate and carbon investment—using leaf minor vein density and leaf mass per area as proxies, respectively. We show that species that survive the KPB have fast-growth ecological strategies corresponding to high assimilation rates and low carbon investment. This finding is consistent with impact winter leading to the nonrandom loss of slow-growing evergreen species. Our study reveals a dramatic example of the effect of rapid catastrophic environmental change on biodiversity.
Introduction
The Cretaceous–Paleogene boundary (KPB) is marked by the Chicxulub bolide impact and mass extinction [1]–[3]. In temperate North America, while the impact resulted in the extinction of more than 50% of plant species [4], a major unresolved issue is whether this killing event was also a large-scale selection event [5]. Wolfe [6] originally proposed that the KPB selected against evergreen species. Specifically, competition in the cold and dark climates during the impact winter [7] should have selected for species with ecological strategies [8] associated with deciduousness. Because variation in leaf traits reflect ecological strategies that are coupled to whole-plant carbon and water fluxes, such a strategy shift would also have caused broad shifts in terrestrial ecosystem functioning [9],[10]. Although some fossil data are consistent with the rise of deciduous species near the KPB, inferences on the extinction's selectivity have been based on qualitative proxies or limited occurrence data [11]–[14]. A lack of quantitative trait data has limited our understanding about the selectivity and ecological implications of this important extinction event.
Here we test if the extinction event was selective for functional traits related to plant ecological strategies. We measure functional traits from fossil leaf assemblages spanning a 2.2 Myr interval across the KPB and assess four differing selection scenarios for functional traits: (i) directional selection—a shift in phenotype space, caused by novel postboundary environments replacing preboundary environments; (ii) stabilizing selection—a reduction in phenotype space caused by postboundary conditions making some strategies temporarily nonviable; (iii) diversifying selection—an increase in phenotype space due to a wider range of postboundary conditions; or (iv) a lack of selection, because previous adaptations were unrelated to survival after major catastrophe.
We assess evidence for these selection scenarios using functional traits [15],[16] related to the leaf economics spectrum [17]. This global spectrum details how several traits are linked to variation in plant growth and fitness. It describes a continuum between “slow-return” leaves (low rates of carbon assimilation, long lifespans, and high tissue carbon investment) and “fast-return” leaves (high rates of carbon assimilation, short lifespans, and low tissue carbon investment). In general, evergreen leaves are “slow.” Within angiosperms, this strategy is thought to be selected for when resource availability is less variable [18]. In contrast, “fast” deciduous angiosperm leaves are thought to be selected for when resource availability is more variable [19]. In this updated context, Wolfe's hypothesis [6] proposed that the strong variation in light levels and temperature after the bolide-caused impact winter [1] should have resulted in directional selection for “fast” strategies.
Alternatively, longer-term temperature change may also have selected for certain traits across the KPB. Multiple proxies show a brief warming during the latest Cretaceous, starting approximately 300,000 y before the boundary near the base of Chron 29r, followed by a rapid return to cooler temperatures approximately 50,000 y before the boundary, which were then maintained through the earliest Paleogene [7],[20],[21]. This longer-term temperature change could have counteracted the hypothesized bolide-caused selection against traits that characterize “slow” strategies. Recent trait-climate theory suggests that cooling should lead to reduced evapotranspiration demand, directionally selecting for “slow” strategies (assuming no change in atmospheric [CO2]) [22],[23]. This prediction appears opposite to the predictions of Wolfe's deciduousness hypothesis, but the relative strengths of both predictions have been unknown.
We evaluate the causes and consequences of ecological change across the KPB by producing records of two central leaf functional traits that reflect variation in ecological strategies: leaf mass per area [LMA, g dry leaf mass/m2 leaf area (LA)], and leaf minor vein density (VD, mm vein/mm2 LA). Variation in these traits reflects a tradeoff between the transport of water and carbon [24] and the carbon construction cost of the leaf [23]. LMA is a metric of leaf carbon investment and correlates negatively with deciduousness and leaf lifespan [19],[25]. VD is a metric of the hydraulic and photosynthetic capacity of a leaf. Leaves with higher VD reflect “fast” species with higher rates of water flux and carbon assimilation [23],[24] and are more likely to be found in warm environments [22],[26]. Thus, by examining each trait's pre- and postboundary distribution, we can assess if the KPB extinction event was selective and which of the differing selection scenarios best matches temporal trends in leaf traits.
Results
We measured VD and LMA for leaf fossil assemblages spanning the last ca. 1.4 Myr of the Cretaceous (K) and the first ca. 0.8 Myr of the Paleogene (P) (Figure 1). Floras come from the Hell Creek and Fort Union formations in southwestern North Dakota, United States (paleolatitude 49°N) [27]. Of the known leaf macrofossil assemblages spanning the KPB, these are currently the best preserved and most species-rich.
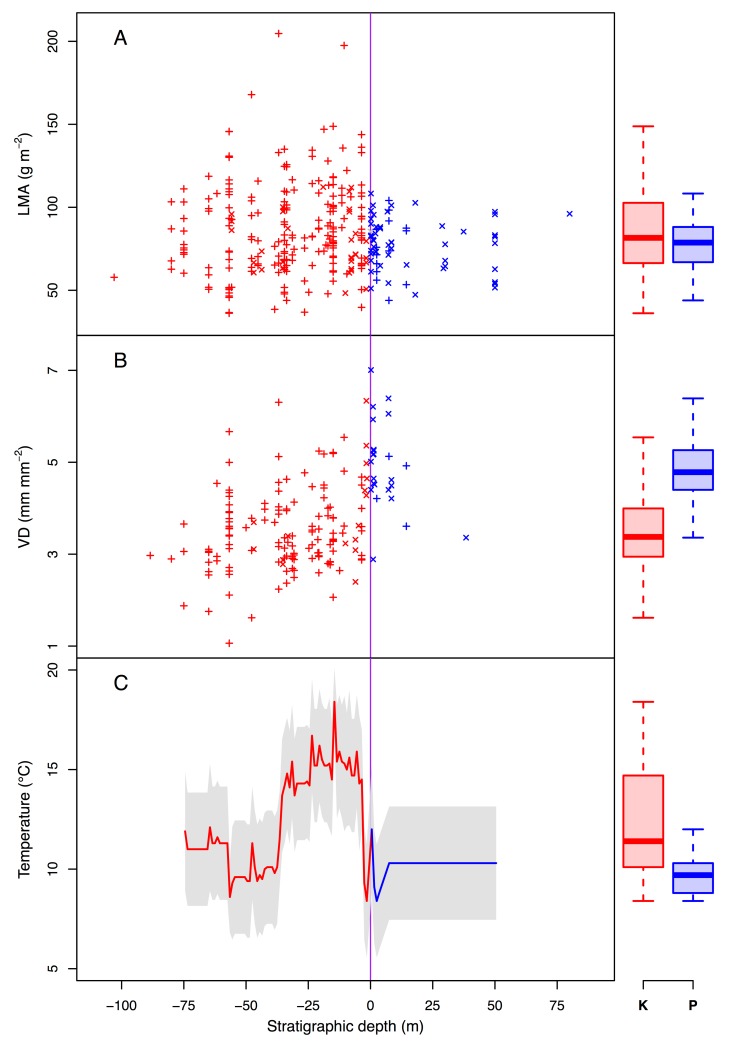
Figure 1. Visual representations of trait changes across the KPB.
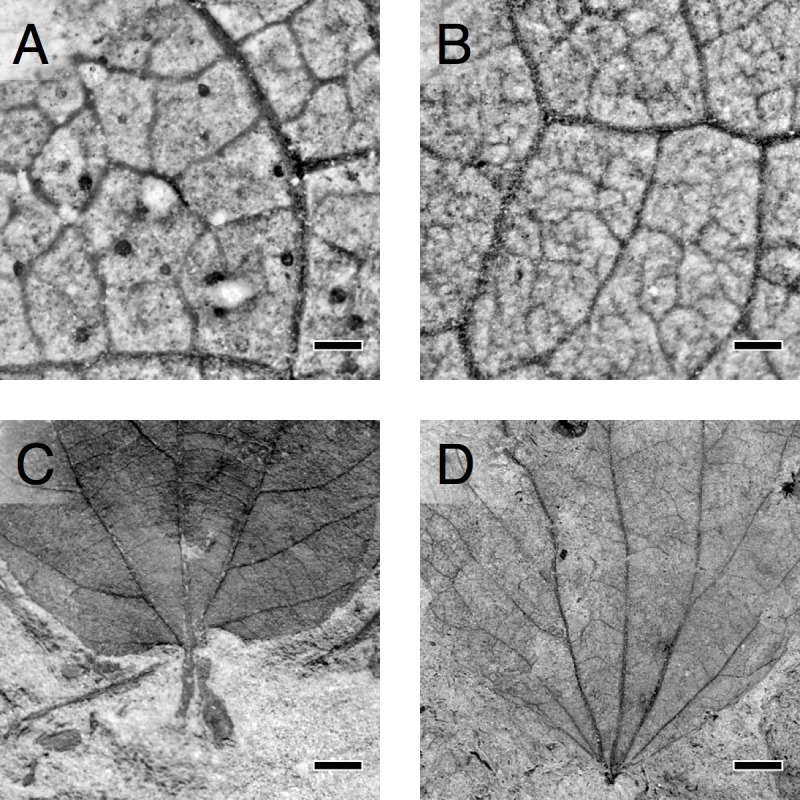
Top row, increase in VD as seen in (A), “Dryophyllum” subfalcatum, −30.7 m stratigraphic depth, VD = 2.5 mm−1 and (B) unknown nonmonocot (morphospecies FU87), 1.275 m depth, VD = 5.3 mm−1. Bottom row, decreases in LMA as seen through decreasing PW for similar LA in (C) “Ficus” planicostata, −3.6 m depth, LMA = 136 g m−2 and (D) “Populus” nebrascensis, 7.2 m depth, LMA = 48 g m−2. Scale bar, (A and B) 500 µm and (C and D), 5 mm.
We found that the mean and variance in LMA decreased across the KPB. Mean values shifted from a Cretaceous value of 86±27 s.d. g m−2 to a Paleogene value of 78±15 s.d. g m−2 (Mann–Whitney location test, p = 0.04; Figures 2A and 3) [17]. This mean shift is small relative to the global range of LMA values (3–2,000 g m−2 [17]) but is of comparable magnitude to shifts across modern ecosystem types (e.g., from tropical rain forest to tropical deciduous forest, 73 versus 83 g m−2, respectively) [28]. Variance in LMA fell by 67% across the KPB (Brown–Forsythe Levene test for homogeneity of variances, p<10−3). These nonparametric tests account for unequal group sample sizes (nK = 256 and nP = 67).
Figure 2. Changes in leaf functional traits and climate across the KPB (purple vertical line).
(A) LMA reflects leaf carbon investment, with lower values associated with deciduous species. (B) VD reflects carbon assimilation capacity, with higher values associated with deciduous species. For (A) and (B), each symbol is a species at site mean: +, channel facies; x, floodplain facies. (C) Changes in temperature reconstructed previously from leaf-margin analysis of the same floras [20]. Color indicates stratigraphic depth, with red for Cretaceous data and blue for Paleogene data. Boxplots of each distribution are shown in the right margin.
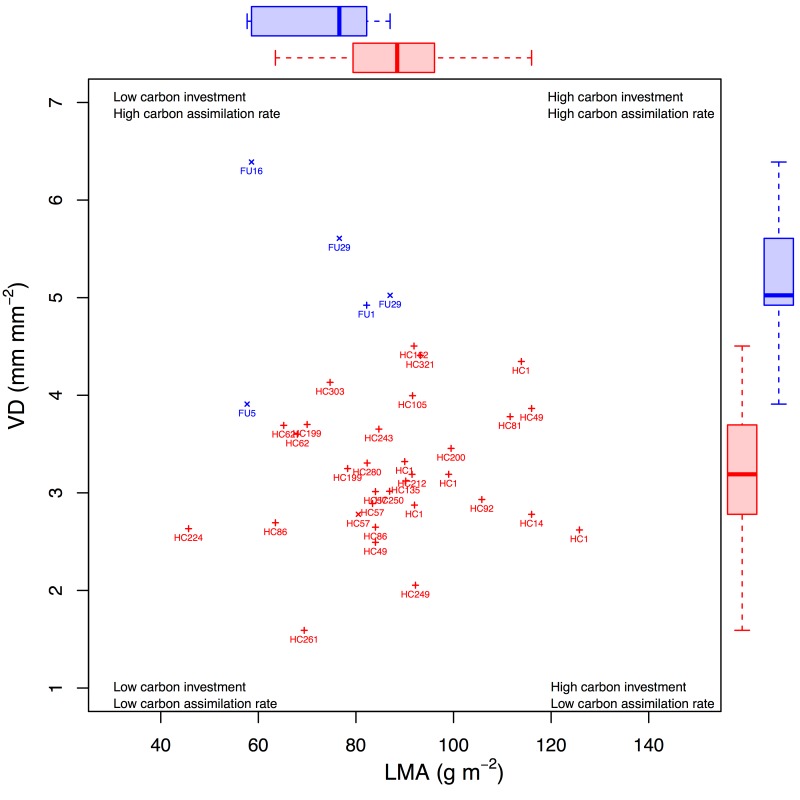
Figure 3. Ecological strategy dynamics across the KPB, as seen in a bivariate trait space of VD and LMA.
Points represent individual specimens for which both VD and LMA measurements were available (labeled by morphospecies). Boxplots of each distribution are shown in the margins. Colors and symbols are as in Figure 2.
We also found that the mean of VD increased across the KPB. Mean values shifted from a Cretaceous value of 3.5±0.6 s.d. to a Paleogene value of 4.6±0.7 s.d. mm−1 (p<10−3; Figures 2B and 3). This shift is comparable to the average difference between extant nonangiosperms and eudicots (1.8 versus 8.0 mm−1, respectively) [29]. Variance in VD did not change across the KPB (p = 0.66). Note that group sizes here were also unequal (nK = 146 and nP = 24).
The trends towards higher VD and reduced ranges of LMA reflect large physiological and biological shifts in plant functioning. The constriction of LMA space was caused primarily by the loss of high-LMA species, many of which were abundant in the latest Cretaceous (Figures 2A and S3). The shift in VD space was caused mostly by the loss of Cretaceous-only species with low VD relative both to species that survive the KPB and to Paleogene-only species (regression; p<10−9, r 2 = 0.22; Figure 2B).
Several of these patterns may be driven by sampling biases—for example, temporal variation in facies preservation [30]. In this dataset, Paleogene sites come primarily from channel facies, whereas Cretaceous sites usually come from floodplain facies. To assess the potential impact of differential preservation, we repeated analyses after subsetting by each facies type for each trait. For channel VD, the mean effect remained significant (p = 0.03; nK = 130 and nP = 4), as it did for floodplain VD (p = 0.004; nK = 16 and nP = 20). For channel LMA, there was no longer a significant shift in mean or variance (both p>0.11; nK = 222 and nP = 15). Similarly no effect in mean or variance was found for floodplain LMA (both p>0.29; nK = 34 and nP = 52). Low Paleogene sample sizes and unavoidable facies shifts prevent further inferences. Nonetheless, together these results indicate a strong evidence for a shift in the mean of the VD distribution but yet weaker evidence for shift in the variance of the LMA distribution. Moreover, because channel (riparian) environments typically support more “fast-return” specialists than distal floodplain environments owing to their higher rates of physical disturbance and greater volatility in nutrient availability [31], our facies effect should have had the opposite effect on LMA and VD than what we observed.
Other data quality issues may also bias our results. First, estimates of VD may be underestimates of true values because of incomplete fossil preservation. Although this is an unavoidable problem when estimating VD for any leaf fossil, our protocol did exclude all but the best-preserved specimens (Materials and Methods). Reported values are consistent with other estimates from late-Cretaceous fossils [32]. Second, estimates of LMA may be down-biased if some species were incorrectly expert-determined to be herbaceous rather than woody. We therefore repeated all LMA analyses across and within facies, with herbaceous taxa removed (omitting n = 21 specimens). We found no qualitative changes in conclusions.
Shifts in both functional traits were associated with ongoing climate change. The bin-mean VD and temperature were negatively correlated (r 2 = 0.15, p = 0.01), whereas bin-mean LMA and temperature were positively correlated (r 2 = 0.19, p = 0.005). Similarly at the specimen level, VD and LMA were not correlated with each other across (p = 0.53) or within facies (both p>0.45), but this null result is likely due to the low number of available samples (channel, nK = 1 and nP = 4; floodplain, nK = 31 and nP = 1). Overall, these results indicate a systematic shift in trait space across the KPB whether examined at the bin-mean or specimen level.
Discussion
The Chicxulub bolide impact appears to have led to the selective extinction of plant species with “slow” leaf ecological strategies. Consistent with Wolfe's hypothesis, this mass extinction was characterized by directional selection away from evergreen species [6], as seen through both VD and LMA, as well as stabilizing selection, as seen through LMA. Our study therefore provides strong evidence that the KPB mass extinction was functionally selective for plants.
The increase in VD from the late Cretaceous to the early Paleogene in our data parallels the increases in VD seen across angiosperm taxa in the early-mid Cretaceous and then again across the KPB [32]. The increase reported by Feild et al. (2011) occurred globally over an ∼70 Myr time span, with most of this increase occurring conclusively before the KPB. In contrast, our findings show a VD increase in a single region, over a much shorter time period (∼2 Myr), with the majority of the increase occurring at or after the KPB. Nevertheless, it is possible that similar atmospheric changes drove both trends. Higher VD in angiosperms and increasing angiosperm dominance across the Cretaceous could be driven by declining atmospheric [CO2], because lower carbon dioxide availability could select for higher hydraulic capacity to maintain productivity [33]. Trait-climate theory also predicts a negative correlation between temperature and VD under low [CO2] [22], consistent with observed data for the KPB. A hypothetical rapid [CO2] decrease across the KPB therefore could be a key driver to our findings of an observed shift in VD. One hypothetical driver of lower [CO2] could be enhanced chemical weathering, via late-Cretaceous Deccan volcanism [34]. However, extant KPB proxies do not yet have sufficient age control [35] or temporal resolution [35],[36] to accurately reconstruct finer scale [CO2] dynamics within the time interval of interest. More detailed assessments of atmospheric composition and temperature across the KPB would be needed before this prediction could be tested.
There is an important criticism of the proposed mechanism coupling between VD and [CO2]. Although selection against low hydraulic capacity in low [CO2] environments should occur for all plants, for nonangiosperms and shade species, low values of VD appear to have successfully persisted in the fossil record across the Cretaceous [37], suggesting that low values were not necessarily selected against as originally hypothesized [33]. We therefore suggest that the increase in VD seen across the KPB is more likely to be a direct consequence of the bolide impact selecting for specific leaf economic strategies rather than of ongoing longer-term climate change. Nevertheless, the increasing dominance of deciduous angiosperms by the early Paleogene appears to have been reinforced by both bolide impact and longer-term climate change.
Observed trait dynamics across the KPB likely ramify to influence ecosystem functioning. Because of the close linkage between leaf economic traits and ecosystem resource fluxes [9],[10], selection at the KPB should have also strongly modulated net primary productivity in terrestrial systems [28], as well as regional hydrological cycles [38]. Our results therefore suggest that there were associated functional changes in terrestrial ecosystems in the aftermath of the Chicxulub impact. Functional trait dynamics are of wide interest when studying succession, invasion, and other dynamical questions, but contemporary time-series data are very rare (e.g., [39]) Our study thus highlights the power of paleoecological functional trait data to integrate information on climate change, extinction, and species performance across time.
Materials and Methods
Data Sources
We analyzed fossils of nonaquatic nonmonocot angiosperm taxa from sites previously collected from the Hell Creek and Fort Union Formations, now located in southwestern North Dakota. The stratigraphy of these sites and the identification of specimens has been described previously [40]. Each specimen was previously assigned to one of 312 morphotypes and one of 208 sites corresponding to a known stratigraphic position and sedimentary facies.
LMA Measurements
During the summer of 2013, we examined all appropriate specimens at the Denver Museum of Nature and Science (DMNH) and Yale Peabody Museum (YPM). We digitally photographed specimens with (1) intact petioles at the point of insertion with the leaf blade and (2) LAs that could be directly measured or confidently reconstructed. For each image, leaves were separated from their rock matrix using the lasso tool in Adobe Photoshop; LA and petiole width (PW) were then measured using ImageJ following the protocol of [41]. For woody species, LMA was then calculated using the following empirical scaling function [41]:
| (1) |
and for herbaceous species [42]:
| (2) |
The final dataset comprises 612 specimens representing 135 morphotypes and 102 sites, of which only 21 specimens are designated as herbaceous.
VD Measurements
During June 2012, we examined all appropriate specimens at DMNH, including holomorphotypes on loan from YPM (approximately 6,000 specimens total). We selected specimens that appeared to have complete preservation of the minor venation network in at least one region of the fossil, discarding all others (retaining 1,150 specimens). In specimens containing more than one leaf, we made a measurement for each leaf. We captured a digital image of each leaf fossil centered on the region of interest using a dissecting microscope coupled to a digital camera (T2i, Canon). Illumination was provided by ring-light. Image resolution was 243 pixels per millimeter.
We then estimated VD using a line-counting program [22] developed in MATLAB. To prevent any investigator bias, images were unlabeled and analyzed in random order. We converted each image to grayscale and applied a contrast limited adaptive histogram equalization to improve quality. If vein preservation appeared incomplete (e.g., clear fragmentation of specimen, higher-order veins preserved in one region but not in another, dramatically fewer veins in one of two conspecific specimens), we discarded the specimen at this step. For acceptable specimens, we delineated a polygonal region of interest in each image (corresponding to the area in which veins were potentially preserved). The program then generated a randomly oriented line segment spanning this polygon. We manually counted the number of vein-line intersections and repeated this process for 10 random line segments. We then computed the mean distance between veins as the sum of all line counts divided by the sum of all distances. This distance was then converted to VD via an equation [22] stating that VD (mm−1) and intervein distance (d, mm) are related as:
| (3) |
This procedure yielded a final dataset of 468 specimens from 66 species and 61 sites.
Facies Assignment
Each specimen was originally assigned a facies type by the original collector (table 3 in [40]). Channel facies were defined as those recorded as “aban chan,” “channel,” “channel la,” “channel x,” or “Colgate channel.” Floodplain facies were defined as those originally recorded as “ash bed,” “carb shale,” “carb splay,” “floodplain soil,” “pond,” “pond ls,” “pond vb,” “splay,” “splay/levee,” or “volcanic ash.”
Stratigraphic Binning
We calculated time series of VD and LMA using a binning approach. We first assigned each specimen to a 1-m depth interval defined by integer-rounding the measured stratigraphic depth. Within each bin we calculated species-at-site mean trait values, then used these to compute site means. We used these site-means to infer the distribution (i.e., mean, upper quartile, lower quartile) of trait values within each bin. Because VD and LMA data were not always available within the same bin, we used piecewise linear interpolation to infer LMA values at each bin for which VD values were available (n = 40).
Paleo-Temperature Data
An index of temperature (Tc; °C) was obtained from a leaf margin analysis of the same fossil floras using the range-through mean annual temperature values corresponding to the supplementary table 8 of [20]. These leaf-reconstructed temperatures are supported by carbonate clumped isotope paleothermometry from the same region [21]. Because vein data and margin data were not always available for the same stratigraphic bins, we estimated temperatures with a piecewise linear interpolation of range-through temperature against stratigraphic bin, choosing constant values at endpoints. We modeled the uncertainty in Tc using the error estimates provided in the original source, using the mean plus (minus) one standard deviation as the upper (lower) quartile. When no standard deviations were available, we assigned a standard deviation equivalent to the mean of all other standard deviations (2.8°C).
Supporting Information
VD data, broken out by stratigraphy and facies. Symbols indicate species at-site means. Black points, data for each category; gray points, all other data.
(PDF)
LMA data, broken out by stratigraphy and facies. Symbols indicate species at-site means. Black points, data for each category; gray points, all other data.
(PDF)
LMA of species with different stratigraphic ranges and abundances. High LMA leaves are common in species that are found only in the Cretaceous but drop out at all abundances in species that cross the KPB or are found only in the Paleogene. Each point represents a species. Abundance data come from the quantitative census of Wilf and Johnson [4].
(PDF)
Correlations between traits for time series data shown in Figure 2. Data for VD and LMA have been aggregated into 1 m bin grand means (across species at-site means and across sites). Points are colored as in Figure 2.
(PDF)
VD data for all measured specimens.
(CSV)
LMA data for all measured specimens.
(CSV)
Temporally binned VD and LMA values, paired with proxy temperature values.
(CSV)
Acknowledgments
B. Boyle, J. Harte, G. Jordan, P. Wilf, and J. Sperry provided thoughtful feedback on the manuscript.
Abbreviations
- K
Cretaceous
- KBP
Cretaceous–Paleogene boundary
- LA
leaf area
- LMA
leaf mass per unit area
- P
Paleogene
- PW
petiole width
- VD
vein density
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
BB was supported by a Rocky Mountain Biological Laboratory graduate research fellowship, a Geological Society of America student research grant, and a NSF pre-doctoral fellowship. BE was supported by an NSF ATB and Macrosystems award (Grant numbers: Macrosystems - NSF DEB 1065861; ATB - NSF EF 0742800). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schulte P, Alegret L, Arenillas I, Arz JA, Barton PJ, et al. (2010) The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 2. Renne PR, Deino AL, Hilgen FJ, Kuiper KF, Mark DF, et al. (2013) Time scales of critical events around the Cretaceous-Paleogene boundary. Science 339: 684–687. [DOI] [PubMed] [Google Scholar]
- 3. Alvarez LW, Alvarez W, Asaro F, Michel HV (1980) Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science 208: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 4. Wilf P, Johnson KR (2004) Land plant extinction at the end of the Cretaceous: a quantitative analysis of the North Dakota megafloral record. Paleobiology 30: 347–368. [Google Scholar]
- 5.Raup DM (1992) Extinction: bad genes or bad luck? London: W. W. Norton & Company. [PubMed] [Google Scholar]
- 6. Wolfe JA (1987) Late Cretaceous-Cenozoic history of deciduousness and the terminal Cretaceous event. Paleobiology 13: 215–226. [Google Scholar]
- 7. Vellekoop J, Sluijs A, Smit J, Schouten S, Weijers JWH, et al. (2014) Rapid short-term cooling following the Chicxulub impact at the Cretaceous–Paleogene boundary. Proc Natl Acad Sci U S A 111 21: 7537–7541 doi:10.1073/pnas.1319253111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33: 125–159. [Google Scholar]
- 9. Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16: 545–556. [Google Scholar]
- 10. Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, et al. (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 11. Wolfe JA, Upchurch GR (1987) Leaf assemblages across the Cretaceous-Tertiary boundary in the Raton Basin, New Mexico and Colorado. Proc Natl Acad Sci U S A 84: 5096–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Upchurch G, Wolfe J (1987) Extinction patterns in laurales at the Cretaceous-Tertiary boundary, Southern Rocky-Mountains. Amer J Bot 74: 692–693. [Google Scholar]
- 13.McIver EE (1989) Fossil flora of the Paleocene Ravenscrag Formation, southwestern Saskatchewan, Canada. PhD thesis, University of Saskatchewan.
- 14. McIver EE, Sweet AR, Basinger JF (1991) Sixty-five-million-year-old flowers bearing pollen of the extinct triprojectate complex—a Cretaceous-Tertiary boundary survivor. Rev Palaeobot Palyno 70: 77–88. [Google Scholar]
- 15. Westoby M, Wright IJ (2006) Land-plant ecology on the basis of functional traits. Trends Ecol Evol 21: 261–268. [DOI] [PubMed] [Google Scholar]
- 16. McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21: 178–185. [DOI] [PubMed] [Google Scholar]
- 17. Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- 18. Chabot B, Hicks D (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13: 229–259. [Google Scholar]
- 19. Reich P, Walters M, Ellsworth D (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci U S A 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilf P, Johnson KR, Huber BT (2003) Correlated terrestrial and marine evidence for global climate changes before mass extinction at the Cretaceous–Paleogene boundary. Proc Natl Acad Sci U S A 100: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tobin TS, Wilson GP, Eiler JM, Hartman JH (2014) Environmental change across a terrestrial Cretaceous-Paleogene boundary section in eastern Montana, USA, constrained by carbonate clumped isotope paleothermometry. Geology 42 4: 351. [Google Scholar]
- 22. Blonder B, Enquist BJ (2014) Inferring climate from angiosperm leaf venation networks. New Phytol 204 1: 116–26 doi:10.1111/nph.12780 [DOI] [PubMed] [Google Scholar]
- 23. Blonder B, Violle C, Bentley LP, Enquist BJ (2011) Venation networks and the origin of the leaf economics spectrum. Ecol Lett 14: 91–100. [DOI] [PubMed] [Google Scholar]
- 24. Brodribb T, Feild T, Jordan G (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144: 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cornwell WK, Westoby M, Falster DS, FitzJohn RG, O'Meara BC, et al. (2014) Functional distinctiveness of major plant lineages. J Ecol 102: 345–356. [Google Scholar]
- 26. Sack L, Scoffoni C (2013) Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol 198: 983–1000. [DOI] [PubMed] [Google Scholar]
- 27. Hay WW, DeConto RM, Wold CN, Wilson KM, Voigt S, et al. (1999) Alternative global Cretaceous paleogeography. Geol Soc Am Spec Pap 332: 1–47. [Google Scholar]
- 28. Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182: 565–588. [DOI] [PubMed] [Google Scholar]
- 29. Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA (2009) Angiosperm leaf vein evolution was physiologically and environmentally transformative. P Roy Soc B 276: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ellis B, Johnson KR (2013) Comparison of leaf samples from mapped tropical and temperate forests: implications for interpretations of the diversity of fossil assemblages. Palaios 28: 163–177. [Google Scholar]
- 31.Grime J (2001) Plant strategies, vegetation processes, and ecosystem properties. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 32. Feild TS, Brodribb TJ, Iglesias A, Chatelet DS, Baresch A, et al. (2011) Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc Natl Acad Sci U S A 108: 8363–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brodribb TJ, Feild TS (2010) Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett 13: 175–183. [DOI] [PubMed] [Google Scholar]
- 34. Robinson N, Ravizza G, Coccioni R, Peucker-Ehrenbrink B, Norris R (2009) A high-resolution marine 187-Os/188-Os record for the late Maastrichtian: distinguishing the chemical fingerprints of Deccan volcanism and the KP impact event. Earth Planet Sc Lett 281: 159–168. [Google Scholar]
- 35. Nordt L, Atchley S, Dworkin SI (2002) Paleosol barometer indicates extreme fluctuations in atmospheric CO2 across the Cretaceous-Tertiary boundary. Geology 30: 703–706. [Google Scholar]
- 36. Beerling DJ, Lomax B, Royer D, Upchurch G, Kump L (2002) An atmospheric pCO2 reconstruction across the Cretaceous-Tertiary boundary from leaf megafossils. Proc Natl Acad Sci U S A 99: 7836–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyce CK, Zwieniecki MA (2012) Leaf fossil record suggests limited influence of atmospheric CO2 on terrestrial productivity prior to angiosperm evolution. Proc Natl Acad Sci U S A 109: 10403–10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosch JM, Hewlett J (1982) A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J Hydrol 55: 3–23. [Google Scholar]
- 39. Kahmen S, Poschlod P (2004) Plant functional trait responses to grassland succession over 25 years. J Veg Sci 15: 21–32. [Google Scholar]
- 40. Johnson KR (2002) Megaflora of the Hell Creek and lower Fort Union Formations in the western Dakotas: vegetational response to climate change, the Cretaceous-Tertiary boundary event, and rapid marine transgression. Geol Soc Am Spec Pap 361: 329–391. [Google Scholar]
- 41. Royer DL, Sack L, Wilf P, Lusk CH, Jordan GJ, et al. (2007) Fossil leaf economics quantified: calibration, Eocene case study, and implications. Paleobiology 33: 574. [Google Scholar]
- 42. Royer DL, Miller IM, Peppe DJ, Hickey LJ (2010) Leaf economic traits from fossils support a weedy habit for early angiosperms. Amer J Bot 97: 438–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VD data, broken out by stratigraphy and facies. Symbols indicate species at-site means. Black points, data for each category; gray points, all other data.
(PDF)
LMA data, broken out by stratigraphy and facies. Symbols indicate species at-site means. Black points, data for each category; gray points, all other data.
(PDF)
LMA of species with different stratigraphic ranges and abundances. High LMA leaves are common in species that are found only in the Cretaceous but drop out at all abundances in species that cross the KPB or are found only in the Paleogene. Each point represents a species. Abundance data come from the quantitative census of Wilf and Johnson [4].
(PDF)
Correlations between traits for time series data shown in Figure 2. Data for VD and LMA have been aggregated into 1 m bin grand means (across species at-site means and across sites). Points are colored as in Figure 2.
(PDF)
VD data for all measured specimens.
(CSV)
LMA data for all measured specimens.
(CSV)
Temporally binned VD and LMA values, paired with proxy temperature values.
(CSV)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.