Abstract
The TNF-family cytokine TL1A (TNFSF15) costimulates T cells and promotes diverse T-cell dependent models of autoimmune disease through its receptor DR3. TL1A polymorphisms also confer susceptibility to inflammatory bowel disease. Here we find that allergic pathology driven by constitutive TL1A expression depends on IL-13, but not T, NKT, mast cells or commensal intestinal flora. Group 2 innate lymphoid cells (ILC2) express surface DR3 and produce IL-13 and other type 2 cytokines in response to TL1A. DR3 is required for ILC2 expansion and function in the setting of T cell dependent and independent models of allergic disease. By contrast, DR3 deficient ILC2 can still differentiate, expand and produce IL-13 when stimulated by IL-25 or IL-33, and mediate expulsion of intestinal helminths. These data identify costimulation of ILC2 as a novel function of TL1A important for allergic lung disease, and suggest that TL1A may be a therapeutic target in these settings.
INTRODUCTION
The tumor necrosis factor (TNF) superfamily of cytokines and receptors function to regulate specific aspects of both innate and adaptive immunity. TL1A (TNFSF15), together with a number of other TNF-family cytokines including LIGHT, OX40L, CD30L, 4-1BBL, and TNF, functions to co-stimulate T-lymphocytes (1). TL1A signals via DR3 (TNFRSF25), which is constitutively expressed on T cells and upregulated upon T cell activation (2). TL1A expression is tightly controlled, and is normally undetectable unless induced in myeloid and endothelial cells by pro-inflammatory stimuli signaling through Toll-like receptors (TLRs) and Fc-receptors (2, 3). TL1A can also be induced in T cells by stimulation through the T cell receptor (TCR), enabling the possibility of autocrine TL1A-DR3 signaling in T cells (4). TL1A, acting through DR3, activates NF-κB and MAP kinase signaling pathways and enhances proliferation and cytokine production in activated CD4+ T lymphocytes, particularly when other costimulatory signals such as those delivered through CD28 are not present (5). Perhaps because of the restricted nature of TL1A expression, compared to other TNF family members, the effects of TL1A on T cells in vivo are mainly apparent at the site of tissue inflammation. DR3-deficient T cells expand normally during primary immune responses, but are defective in expansion and cytokine production in response to antigens presented in the context of inflamed tissue. TL1A-DR3 interactions are essential for the development of disease in T-cell dependent animal models of multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and allergic lung disease (4, 6–8). A role for TL1A in host defense against infection has thus far been limited to controlling T cell responses to Salmonella and selected viral infections (9, 10). These observations, coupled with linkage of polymorphisms in the TNFSF15 locus encoding TL1A to inflammatory bowel disease and detection of elevated levels of TL1A in affected tissue from rheumatoid arthritis and inflammatory bowel disease patients (11–14) have suggested that TL1A may be a pathogenic cytokine in a number of autoimmune diseases.
Another line of evidence suggesting a specific role for TL1A-DR3 interactions in promoting allergic type 2 inflammation has emerged from studies of mice expressing TL1A constitutively. Transgenic mice expressing TL1A on either T cells or dendritic cells spontaneously develop small intestinal pathology characterized by muscular layer and goblet cell hyperplasia, mast cell infiltration and increased mucous production. In mice expressing higher levels of TL1A, an immune cell infiltrate enriched in CD4+ T cells also appears (15–17). Despite abundant levels of IL-13 and IL-5 expression, insufficient T helper (Th) 2 T cells were found in the intestine to explain the elevation of these cytokines; in fact a greater fraction of T cells in the lamina propria or mesenteric lymph node expressed IL-17 than IL-13 or IL-4 (15, 16). In addition, allergic pathology was preserved in TL1A transgenic mice crossed to OT-II TCR transgenic Recombination Activating Gene (RAG) deficient background, which have a monoclonal naïve T cell repertoire. These data raised the possibility that cell types other than T cells may respond to TL1A to produce type 2 cytokines and promote allergic pathology in TL1A transgenic mice. Recent studies with DR3-deficient mice have also suggested roles for DR3 beyond T cell costimulation, implicating DR3 in diverse processes such as macrophage and osteoclast differentiation and corticostriatal innervation in the brain (7, 18, 19).
Recently, distinct populations of lymphocytes lacking clonotypic antigen receptors, T, B or NK cell surface markers were identified in tissues such as the intestine, mesenteric fat, and lung. These cells, termed innate lymphoid cells (ILC), make up only a small proportion of tissue resident lymphocytes, but secrete large amounts of effector cytokines and have been shown to be essential components of a number of different immune pathologies and allergic responses (20, 21). ILCs arise from a common lymphoid progenitor and require signaling through cytokines activating the common gamma chain and the transcription factors TCF-1, RORα or RORγt for their development (22–24). Innate lymphocytes can be divided into three broad groups based on their cytokine secretion patterns. Group 2 ILC (ILC2) secrete large amounts of IL-5 and IL-13 and can be critical for host defense against intestinal parasites and also contribute to allergic lung pathology together with other lymphocyte subtypes such as NKT cells (25–27).
We hypothesized that in addition to its effects on T cells, TL1A may costimulate innate lymphoid cells, particularly ILC2, accounting at least in part for the T-cell independent allergic intestinal pathology found in mice constitutively expressing TL1A. We found that ILC2 expressed surface DR3, and could be directly stimulated by TL1A to produce IL-13 and other type-2 immune cytokines. DR3 was required for the expansion of ILC2 in two models of allergic lung disease. However, ILC2 expansion and host defense against the parasite Nippostrongylyus brasiliensis, which depends on IL-25 and IL-33 was intact in DR3-deficient mice. These data establish a novel role for the TNF superfamily cytokine TL1A as an activator of innate lymphoid cells.
RESULTS
TL1A-induced intestinal pathology is dependent on IL-13 but not T cells, mast cells or commensal microbiota
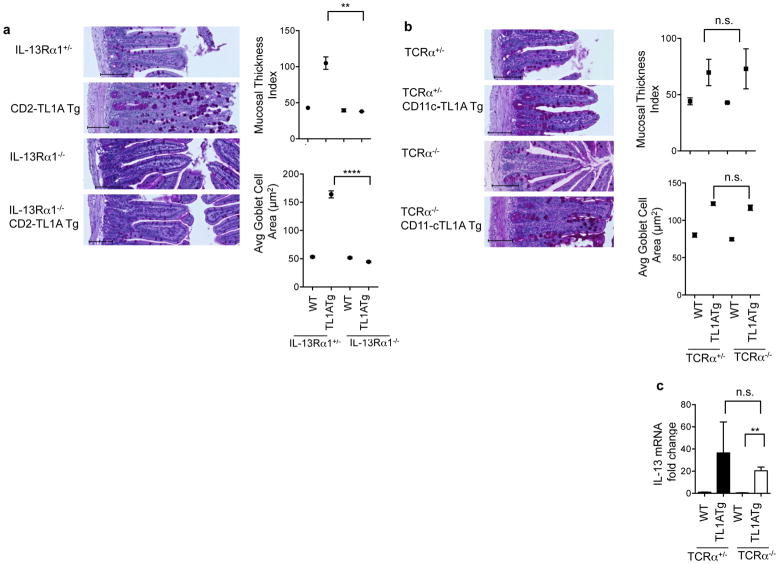
Constitutive expression of TL1A either in T cells or dendritic cells results in hyperplasia and inflammatory changes in the small intestine including macroscopic lengthening, thickening of the muscularis layer with mast cell infiltration, and goblet cell hyperplasia. These pathological changes were associated with marked induction of IL-13 and IL-5 in the small intestine and mesenteric lymph nodes, and elevated levels of circulating IL-13 (15, 16). To confirm that IL-13 signaling is required for the immunopathology induced by TL1A, we crossed TL1A transgenic mice to mice deficient in the α1 chain of the IL-13 receptor, which is required for IL-13 signaling in both immune and non-immune cells. The intestines of TL1A transgenic mice lacking IL-13Rα1 were grossly normal and had normal mucosal thickness and goblet cell size (Figure 1a) showing that chronic TL1A expression induces pathology through the IL-13 signaling pathway.
Figure 1. Small bowel pathology in TL1A transgenic mice is IL-13 dependent but does not require αβ T-cells.
(a) CD2-TL1A transgenic mice were crossed to IL13Rα1 deficient mice. Representative transverse histological ileum sections stained with Periodic acid Schiff (PAS) for goblet cells from 20-week-old mice of the indicated genotypes are shown. Scale bars = 100μm. Mucosal thickness index and goblet cell area for three mice per group are shown with each dot representing the average within a group (mean +/− SEM) with significance from the Mann-Whitney test (**p<0.01, ***p<0.0001). (b) CD11c-TL1A transgenic mice were crossed to TCRα−/− mice. Representative transverse histological ileum sections stained with PAS from 15 week-old mice from the indicated genotypes are shown. Scale bars = 100μm. Mucosal thickness and goblet cell area from three mice per group are shown at right with each dot representing the average within a group. (c) IL-13 mRNA from ileal tissue was measured by qRT-PCR, normalized to β2m expression and shown relative to expression levels in WT mice. Data shown are the mean ± s.e.m. analyzed, with significance shown from the Mann-Whitney test. See also Figure S1.
To determine the relative contribution of T cells to TL1A-induced intestinal pathology, we used CD11c-TL1A transgenic mice expressing TL1A in dendritic cells, which develop small intestinal pathology similar to CD2-TL1A transgenic mice (15, 16). These mice were crossed onto a TCRα-deficient background. Surprisingly, small intestinal pathology developed in TL1A transgenic mice despite the lack of αβ T cells in these mice, with comparable increases in mucosal thickness and goblet cell area in the ileum with or without αβT cells (Figure 1b). IL-13 expression in TCRα-deficient TL1A transgenic mice was still significantly elevated (Figure 1c). Although γδ TCR expressing T cells still develop in TCRα deficient mice, goblet cell hyperplasia and IL-13 production in the small intestine is also preserved in TL1A transgenic mice on a Recombination Activating Gene-1 (RAG-1)-deficient background which prevents γδ T cell development (16). Thus, it is unlikely that either αβ or γδ T cells are required for IL-13 production and intestinal pathology in response to TL1A.
Other cell types besides T cells may be the source of IL-13 induced by constitutive TL1A. Invariant NKT cells producing IL-13 have been implicated in some mouse models of colitis, but are relatively depleted in TL1A transgenic mice (16). Complete deletion of iNKT cells by backcrossing to Jα18 deficient mice or all NKT cells in CD1d deficient mice did not ameliorate small intestinal pathology or IL-13 production in TL1A transgenic mice (Figure S1). Mast cells accumulate in the submucosa of TL1A transgenic mice and can also produce IL-13 and IL-5, but depletion of mast cells on the W/Wv background was ineffective at reducing small intestinal hyperplastic changes in TL1A transgenic mice and only marginally effective in reducing IL-13 production (Figure S1). Taken together, these data suggest that neither NKT nor mast cells are the major sources of IL-13 responsible for intestinal pathology in TL1A transgenic mice.
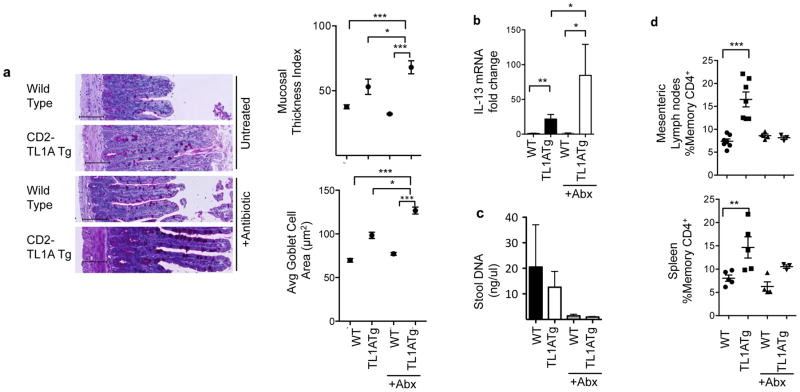
Intestinal microbiota can profoundly influence the development and cytokine secretion potential of T cells and innate immune cells in the gut (28). We previously found that restricting the T cell repertoire with the OT-II TCR transgene did not reduce the intestinal hyperplasia in TL1A transgenic mice (16), suggesting that T cell antigens derived from intestinal microbiota were not required. To determine whether innate immune IL-13 producing cells induced by TL1A require the presence of commensal intestinal flora, we treated CD2-TL1A transgenic mice with oral antibiotics from pregnancy or from birth until three months, an age at which significant intestinal pathology has developed in most TL1A transgenic mice. Antibiotic treatment did not prevent development of goblet cell and intestinal hyperplasia in the presence of TL1A; in fact both parameters were significantly increased in antibiotic-treated compared to age-matched control TL1A transgenic mice (Figure 2a). TL1A transgenic mice depleted of commensal flora also had even more elevated levels of IL-13 mRNA (Figure 2b). Microbial depletion was adequate in these mice, as assessed by reduction in stool DNA concentration (Figure 2c). The effects of microbial depletion could also be seen through the reduction in percentages of memory T cells in antibiotic treated mice, particularly in the mesenteric lymph nodes (Figure 2d). These results strongly suggest that chronically expressed TL1A drives IL-13 production and intestinal pathology in the absence of immune stimuli derived from commensal microbial flora.
Figure 2. Commensal bacteria are not required for TL1A-driven small intestinal pathology.
(a) CD2-TL1A transgenic mice were treated with a quadruple antibiotic regimen for three months. Representative transverse histological ileum sections stained with PAS from 12 weeks old mice. Scale bars = 100μm. Mucosal thickness and goblet cell area are shown at right for 3–6 mice per group with each dot representing the average within a group (mean +/− SEM) with significance from the Mann-Whitney test (**p<0.01, ***p<0.001). (b) IL-13 mRNA from ileal tissue was quantitated by qRT-PCR, normalized to β2m expression and shown relative to expression levels in untreated WT mice. Data represent mean +/− s.e.m. with significance from the unpaired t-test (*p<0.05, **p<0.01, ***p<0.001). (c) Total DNA isolated from mouse fecal matter. (d) The percentage of memory CD4+ cells (CD44hiCD62Llo) in the spleen and mesenteric lymph nodes measured by flow cytometry. Data shown are the mean ± s.e.m. for 3–6 mice per group.
TL1A stimulates ILC2 to produce IL-13 and other type 2 immune cytokines
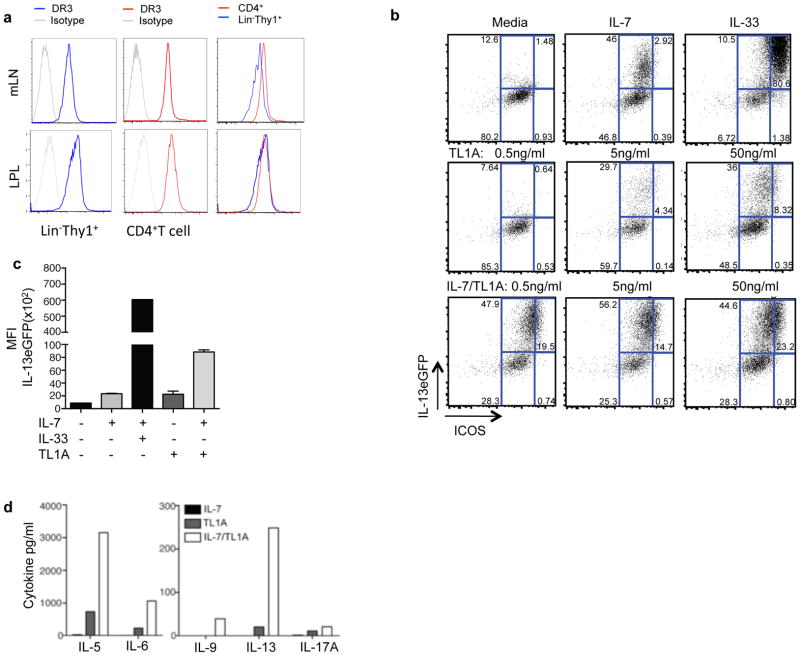
Group 2 innate lymphoid cells (ILC2) lack T, B or NK cell lineage markers, and express the transcription factor GATA-3 and the surface proteins Thy-1, ICOS and KLRG1 (21, 29). ILC2 produce large quantities of IL-13 and IL-5 (but little or no IL-4) in response to the cytokines IL-25 or IL-33. ILC2 expansion and cytokine secretion can also be triggered in the setting of gastrointestinal parasitic infection and allergic lung disease (21, 25–27, 30–32). However, whether these cells express TNF-family receptors or receive costimulatory signals through these receptors is not known. To determine whether TL1A might act directly on ILC through its receptor DR3, we measured surface expression of DR3 on lymphocytes from mesenteric lymph nodes and found that Lin− Thy1+ cells constitutively express DR3 at high levels, comparable to that on CD4+T cells (Figure 3a). More detailed analysis of lineage negative lymphocytes using markers more specific for different groups of ILC (21, 29) revealed that ILC2 (NK1.1−, KLRG1+ or GATA3+) and ILC3 (RORγt+, CD127+) expressed higher levels of DR3 than ILC1 (NK1.1+KLRG1+) (Figures S2a and S2b). All three of these ILC subsets appeared to be present in normal numbers in DR3-deficient mice (Figure S2c), indicating that DR3 was not required for differentiation or homeostasis of ILC.
Figure 3. TL1A is a costimulatory cytokine for ILC2.
(a) Surface expression of DR3 was measured by flow cytometry in Lin− Thy1+ lymphocytes vs. CD4+ T cells in mesenteric lymph nodes and the intestinal lamina propria. (b) Lineage negative/ICOS positive cells were purified from IL-25-treated Il13+/eGFP mice and treated with the indicated cytokines (IL-7 at 10 ng/ml, IL-33 at 10 ng/ml and the indicated concentration of TL1A) for 72 hrs and then assayed for expression of the IL-13eGFP reporter by flow cytometry. Dot plots are gated on lineage negative cells. (c) Mean fluorescence intensity from data presented in (b). (d) Lineage (CD3CD4CD8CD19CD11bCD11cFcεR1Gr1) negative ICOS positive ILCs were purified from IL-25-treated Il13+/eGFP mice, and treated with 10 ng/ml of the indicated cytokines for 72 hours before collection of supernatants for cytokine measurements. See also Figure S2.
To determine whether TL1A could directly induce IL-13 production by ILC2, we isolated lineage (lin)−ICOS+ ILC2 from the mesenteric lymph nodes and spleen of Il13eGFP/+ reporter mice treated with IL-25 to expand numbers of these cells. TL1A induced expression of the IL13-GFP reporter in a dose-dependent fashion, which was enhanced by the addition of IL-7, which is known to promote ILC2 survival and cytokine secretion (Figures 3b and 3c). Analysis of supernatants from stimulated ILC2 showed that TL1A was effective in inducing an ILC2 ‘cytokine signature’ in vitro, including IL-5, IL-6 and IL-13, with much smaller quantities of IL-9 or IL-17A (Figure 3d). Addition of IL-7 enhanced secretion of these cytokines. These data show that TL1A can directly stimulate ILC2 to enhance secretion of type 2 cytokines.
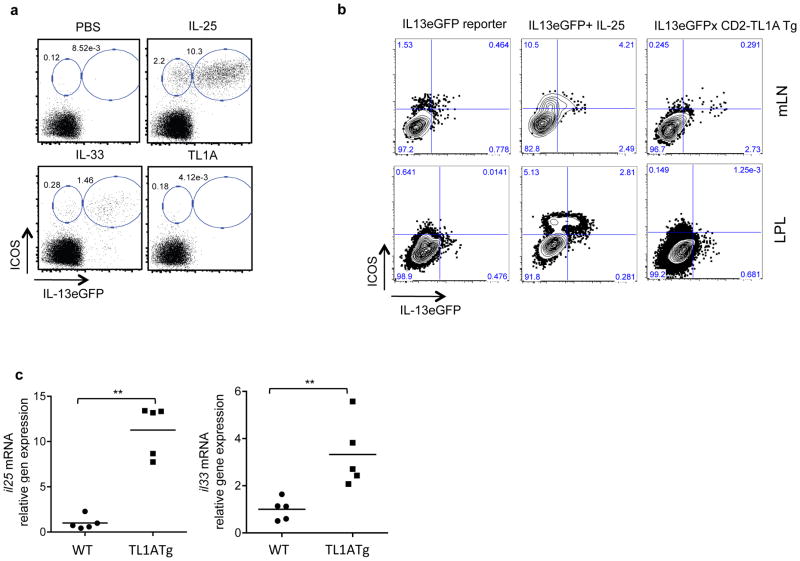
In addition to enhancing the function of ILC2, IL-25 and IL-33 injected in vivo can induce rapid expansion of ILC2 (Figure 4a). However, injection of up to 5μg of recombinant TL1A for three consecutive days failed to expand ILC2 or induce expression of the IL-13eGFP reporter in Lin−ICOS+ cells (Figure 4a). In CD2-TL1A transgenic mice, chronic TL1A expression did not expand ILC2 in the mesenteric lymph nodes and small intestinal lamina propria as occurs with IL-25 treatment (Figure 4b). However, small numbers of IL13-eGFP reporter expressing cells were found in TL1A transgenic mice, with cells in the mesenteric lymph node expressing high levels of the eGFP reporter (Figure 4b). To test the effects of TL1A in an alternate reporter system in which both endogenous alleles of IL-13 remain intact, we used BAC transgenic reporter mice with a DsRED reporter replacing the IL-13 coding region and an AmCyan reporter replacing the IL-4 coding region (4C13R mice) (33). The TL1A transgene induced small numbers of Lin− ICOS+ cells in the mesenteric lymph nodes of 4C13R mice to produce the IL-13 reporter (Figure S3a), comparable to the sub-optimal dose of IL-25 used in these experiments. Again, as in IL13-eGFP reporter mice, there was no expansion of the Lin− ICOS+ population in TL1A transgenic mice. Induction of cells expressing the IL-4 reporter was much weaker than IL-13, consistent with the poor ability of ILC2 to produce IL-4 (Figure S3b). To determine if chronically expressed TL1A may induce IL-25 and/or IL-33, which may serve to sustain IL-13 production by ILC2, we measured expression of these two cytokines in the small intestine, and found that indeed expression of IL-25, and to a lesser extent, IL-33, was elevated in CD2-TL1A transgenic mice (Figure 4c). Taken together these data show that transgene-driven expression of TL1A can induce intestinal ILC2 to spontaneously produce IL-13 without causing the massive expansion of this subset that can be seen with extrinsic administration of IL-25 or IL-33.
Figure 4. Chronic, but not acute exposure to TL1A can induce IL-13 expression in ILC.
(a) ICOS and IL-13eGFP expressing cells were quantitated in mesenteric lymph node following four consecutive doses over four days of either PBS, IL-25 (2 μg/dose), IL-33 (0.5 μg/dose), or TL1A (5 μg/dose) given to Il13+/eGFP reporter mice. Dot plots are gated on lineage (CD3CD4CD8CD19CD11bCD11cFcεR1) negative cells. Examples are representative of 3–5 mice per group. (b) ICOS and IL-13eGFP expressing cells were quantitated in mesenteric lymph node and lamina propria from Il13+/eGFP reporter mice untreated, given 2 μg of IL-25 for four consecutive days, or crossed to CD2-TL1A transgenic mice. (c) il25 and il33 mRNA were measured by qRT-PCR in the ileum of CD2-TL1A transgenic mice and normalized to the average levels found in wild-type age-matched controls and analyzed with Mann Whitney (**p<0.01). See also Figure S3
Expansion of ILC2 in allergic lung disease but not parasitic infection depends on TL1A-DR3 interactions
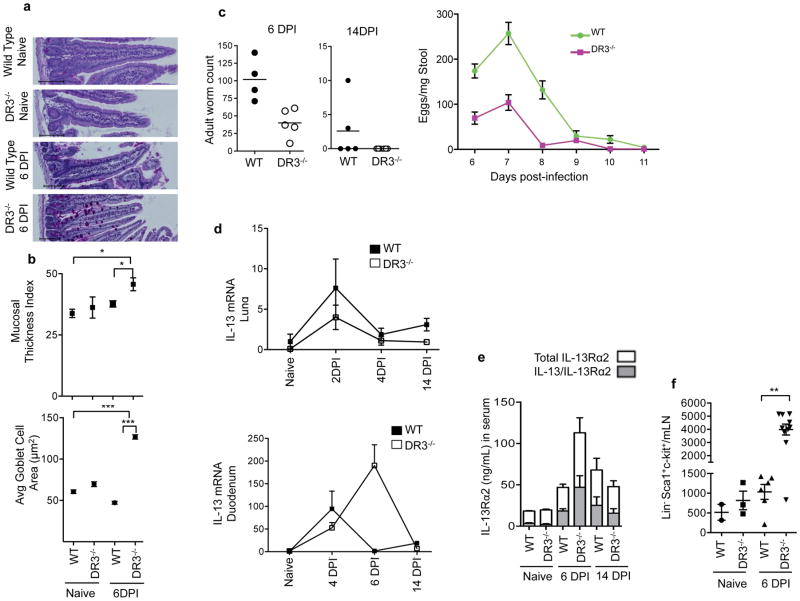
A number of features of the intestinal pathology in TL1A transgenic mice resemble those that occur in the small intestine during responses to intestinal parasites, such as Nippostrongylus brasiliensis. IL-25, IL-33 and ILC2-derived IL-13 are known to play key roles in immunity to N. brasiliensis (34, 35). These findings raised the possibility that TL1A may promote host defense against N. brasiliensis by stimulating ILC2 to produce IL-13. To test this hypothesis, we infected wild-type and DR3-deficient mice with N. brasiliensis and measured the ability of DR3-deficient mice to mount an anti-parasitic immune response, and clear the parasite efficiently from the intestines, as most wild-type mice do by 14 days post infection (DPI). Contrary to this notion, DR3-deficient mice mounted a robust response to infection, with significantly more goblet cell hyperplasia and mucosal thickening in the duodenum, the most affected portion of the small intestine, than wild-type controls (Figures 5a and 5b). Numbers of adult worms in the small intestine of DR3-deficient mice were lower at both the peak of infection at day 6 or at day 14 compared to wild-type mice, with corresponding reductions in the number of eggs in the stool (Figure 5c), indicating highly effective immunity to N. brasiliensis in the absence of DR3.
Figure 5. DR3 is not required for IL-13 production and clearance of parasites in Nippostrongylus brasiliensis infection.
(a) Representative PAS-stained duodenum sections from wild-type or DR3−/− mice without or 6 days post infection (DPI) with Nippostrongylus brasiliensis. Scale bar=100μm. (b) Mucosal thickness and goblet cell area in these samples are shown for 4–9 mice per group with each dot representing the average within a group. Data represent mean ± SEM, analyzed with Mann Whitney (*p<0.05, ***p<0.001). (c) Adult worm counts from the intestine at 6 and 14 days post infection, and counts of eggs in the stool over the course of infection. Data represent mean ± SEM. (d) IL-13 mRNA expression in the duodenum over the course of infection normalized to β2m expression and shown relative to expression levels in untreated WT mice with mean ± SEM. (e) Serum levels of IL-13Rα2 (white bars) and IL-13 bound to IL-13Rα2 (grey bars) measured in serum at the indicated times after Nippostrongylus infection in the indicated mice. (f) ILC2 quantitated in mesenteric lymph nodes of uninfected and 6DPI Nippostrongylus infected mice using Lin−(CD3CD4CD8CD11bCD19MHCIIFcεRI) ckit+ Sca1+ as markers of ILC2. See also Figures S4 and S5.
Induction of IL-13 expression in the lung, which the larvae pass through before reaching the intestine, was similar in wild-type and DR3 deficient mice, indicating that the reduced parasitemia in the intestine is not simply the result of lower overall infection efficiency in DR3-deficient mice. IL-13 induction was also robust in the duodenum of DR3-deficient mice exposed to N. brasiliensis, with more sustained elevation in IL-13 mRNA (Figure 5d). Furthermore, systemic levels of IL-13 protein and its secreted receptor, IL-13Rα2, a marker of IL-13 bioactivity, also increased in infected DR3 deficient mice with similar kinetics and with equal saturation of IL-13Rα2 by IL-13 (Figure 5e). ILC2 also expanded robustly in DR3-deficient mice infected with N. brasiliensis, (Figure 5f) suggesting that helminth-driven expansion of ILC2 is independent of DR3. IL-33 and IL-25 are necessary promote expansion of ILC2 during Nippostrongylus infection and sufficient to stimulate ILC2 expansion on their own (34, 35). To test whether DR3 is required for expansion of ILC2 by these cytokines, we treated DR3-deficient and control wild-type mice with IL-25 and IL-33. ILC2 expanded normally in response to either IL-25 or IL-33 in DR3-deficient mice (Figure S4) showing that ILC expansion in this setting is largely independent of DR3. Taken together, these data show that despite the similarity in pathology between TL1A transgenic mice and helminth-infected mice, DR3 is not required to expand ILC2 or mount a protective immune response to N. brasiliensis.
Other parasitic infections may require DR3 to induce a type 2 immune response. To test this, we infected wild-type and DR3-deficient mice with Schistosoma mansoni. During schistosomal infection, host IL-13 drives the granulomatous response that contains parasite eggs in the liver, but also promotes hepatic fibrosis (36, 37). In response to S. mansoni infection, DR3-deficient mice produced at least as much IL-13, IL-10 and IFN-γ as wild-type C57BL/6 control mice (Figures S5a and S5b). The hepatic response to S. mansoni also appeared to be normal in DR3-deficient mice, with comparable size and percentage of eosinophils in liver granulomas, and liver weight, a measure of hepatomegaly (Figure S5c). DR3-deficient mice and controls also had comparable worm and egg counts (Figure S5d). The only defect seen in DR3-deficient mice was lower leukocyte cell yields, consistent with the known ability of DR3 to costimulate T cell expansion (Figure S5e). Hepatic fibrosis was similarly proportional to the burden of infection in wild-type and DR3 deficient mice (Figure S5f). These data show that the immune responses to the helminth parasites S. mansoni and N. brasiliensis, develop independently of costimulation through DR3.
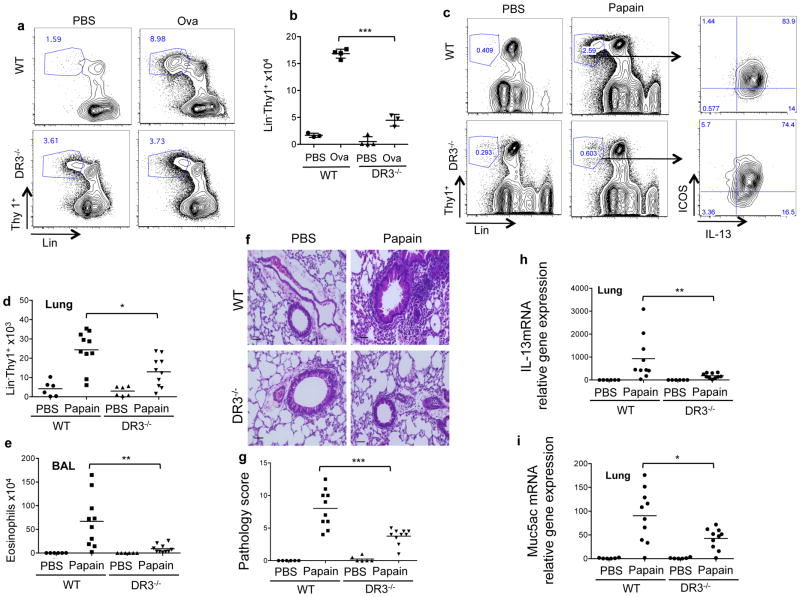
To determine the physiological role of DR3 on ILC2 in the context of allergic disease, we measured ILC numbers in the lungs of mice induced to develop hypersensitivity pneumonitis to ovalbumin, a mouse model for allergic asthma. Using this model system, we have previously shown that DR3-deficient mice have reduced pathology and IL-13 production in the lung despite a normal systemic Th2 response to ovalbumin (4). However, the role of ILC in this defect has not been previously investigated. In contrast to the exaggerated expansion of ILC2 in the setting of Nippostrongylus infection, expansion of Lin−Thy1+ lymphocytes was curtailed in the lungs of DR3-deficient mice, with approximately 2.5-fold fewer of cells with this phenotype in the lungs of DR3-deficient mice challenged with ovalbumin compared to wild-type mice (Figures 6a and 6b).
Figure 6. DR3 is required for ILC2 expansion and lung pathology during allergic lung disease.
(a) Percentage of Lin− (CD3,CD4,CD8,CD11b,CD11c,B220,GR1,Ter119,NK1.1) Thy1+ ILC gated in CD45+ population in the lung of mice of the indicated genotype immunized and challenged with ovalbumin or PBS control. (b) Quantitation of Lin− Thy1+ cells from the lungs of mice immunized and challenged as in (a). Each point represents one mouse with mean ± SEM, with significance from the unpaired t-test (***p<0.001). (c) Percentage of Lin− (CD3,CD4,CD8,CD11b,CD11c,B220,GR1,Ter119,NK1.1) Thy1+ ILC gated in CD45+ population in the lung of mice of the indicated genotype intranasally challenged with papain or control (PBS). (d) Quantitation of Lin− Thy1+ cells from the lungs of mice immunized and challenged as in (c). (e) Numbers of eosinophils in the BAL of these mice, calculated from yield and flow cytometric assessment of BAL as described in the methods. (f) Representative histological sections from the lungs of mice challenged with Papain as in (c). H&E staining, scale bar = 50 μm Quantitation of immunopathology in lung histological sections examined from groups of mice treated as in (c) is shown in (g). Scoring was performed as described in the methods. Each symbol represents the score of one mouse. Expression of il13 (h) and muc5ac (i) and mRNA in lung tissue isolated from the mice treated as in (c) measured by qRT-PCR and normalized to the average levels found in wild-type age-matched controls. Data were analyzed with Mann Whitney test (*p<0.05, **p<0.01, ***p<0.001).
To study the requirement for DR3 on ILC2 in an allergic response in which innate immune cells play a more central role, we exposed wild-type and DR3-deficient mice to inhaled papain, an environmental plant allergen whose cysteine protease activity triggers an acute allergic response through direct activation of innate immune cells including basophils and inflammatory dendritic cells (38, 39). Papain-induced allergic lung pathology is only partially dependent on the presence of T cells with T-cell dependence not observed until at least two weeks of exposure to papain (31, 40). In response to exposure to four doses of inhaled papain over 6 days, Lin− ILCs expanded poorly in DR3-deficient mice compared to wild-type C57Bl/6 controls (Figure 6c). Restimulation of isolated cells with PMA and Ionomycin revealed that almost all Lin−Thy1+ in papain-treated mice were able to produce IL-13. However, significantly fewer Lin−Thy1+ cells were found in the lungs of papain-challenged DR3-deficient mice compared to wild-type controls (Figure 6d). These defects had significant consequences for airway inflammation, as DR3-deficient mice challenged with papain had dramatically reduced airway eosinophila and lung pathology, including greatly diminished peribronchiolar infiltration and goblet cell hyperplasia (Figure 6e–g). Expression of IL-13 and Mucin 5ac, which are associated with goblet cell metaplasia and increased mucous production, were also significantly reduced in the lungs of papain challenged DR3 deficient mice (Figure 6h, i) Taken together, these data show that TL1A-DR3 interactions are important for the innate lymphocyte response and disease severity in two distinct models of allergic lung disease, the latter primarily dependent on ILC2.
DISCUSSION
While transcription factors such as TCF-1 and RORα have been identified as being important for the development of ILC2 from lymphoid precursors (23, 24), the signals that promote ILC2 to secrete type 2 cytokines have not been as well defined. Here we uncover costimulation of ILC2 as a new function for TL1A. TL1A induces expression of IL-13 and other type 2 cytokines in ILC2, likely contributing to the allergic intestinal phenotype seen in mice constitutively expressing TL1A. This effect is direct, in that TL1A can induce IL-13 expression in purified ILC. However, in vivo, unlike IL-25 or IL-33, TL1A does not expand ILC2 numbers or acutely induce IL-13 expression in these cells by itself. Over time, likely due to the presence of low levels of endogenous stimulators, chronic TL1A expression in TL1A transgenic mice induces IL-13 production by ILC2 without expanding them. The fact that TL1A transgenic mice treated with antibiotics developed more severe allergic intestinal pathology suggest that commensal gut flora do not provide this endogenous stimulation, and may actually provide inhibitory signals that constrain the effects of TL1A on ILC2. Low levels of IL-33 secreted during epithelial cell turnover in the intestine may be one possible source of a primary signal for ILC2 in this setting, as it was recently reported that IL-33 is constitutively expressed by epithelial cells from barrier tissues (41). We propose that TL1A should be thought of as a costimulator or ‘signal 2’ which fully activates ILC function, analogous to its costimulatory role in T cell biology. Whether or not other TNF-family cytokines which act on lymphocytes can also costimulate innate lymphoid cells will be an important topic for future study.
These findings reveal a novel role for TL1A in the biology of innate lymphoid cells, and prompt re-examination of findings in DR3-deficient mice to consider effects on ILC in addition to T cells. The defective expansion of ILC2 in the lung during ovalbumin and induced lung hypersensitivity suggests that the function of ILC2 during allergic lung disease also depends on TL1A-DR3 interactions. In this model the defect in ILC2 expansion may be secondary to the defective expansion of DR3-deficient T cells after ova challenge in the lung (4). In acute papain-induced allergic lung disease, ILC2 can expand and produce IL-13 independently of T cells (24, 42), suggesting that defective ILC2 expansion in response to papain in DR3-deficient mice is due to a cell-intrinsic requirement for TL1A costimulation by these cells.
Remarkably, although TL1A-DR3 interactions are required for the ILC2 cell number expansion and functional activation in sterile allergic responses, host defense against the parasites N. Brasiliensis and S. Mansonii is not compromised. This divergence is particularly interesting given that allergens and parasites share many common pathways for activating a type-2 immune response, including induction of the ILC-expanding cytokines IL-25 and IL-33 (43). One possibility is that quantities of IL-25 and/or IL-33 induced by N. Brasiliensis parasites are sufficient to bypass the need for costimulation of ILC2 through DR3. Alternatively, a cytokine or cytokines induced in the setting of parasitic infection, but not allergic disease may substitute for TL1A in costimulating ILC2. The exaggerated intestinal pathology, ILC expansion and more rapid clearance of N. Brasiliensis in DR3-deficient mice suggests that the absence of ‘signal 2’ delivered through DR3 may sensitize ILC2 to cytokines induced during parasite infections that promote the host protective response. Group 2 ILC promote pathological allergic responses but provide benefit to the host through defense against intestinal parasites and tissue repair after influenza infection in the lung (44). Human counterparts of ILC2 have been found in the nasal passages of patients with allergic rhinitis (45) and in the lung (44). Our findings suggest blocking TL1A costimulation may reduce the contribution of ILC2-derived cytokines to allergic diseases while preserving the ability of these cells to function in host defense, an important goal in immunotherapy for allergic disease.
MATERIALS AND METHODS
Mice
C57BL/6 mice were obtained from Taconic. Tnfrsf25−/− mice were generated as previously described (46), and were back-crossed to the C57BL/6 background for at least ten generations. TL1A Tg mice were produced as previously described (16) and were back-crossed to the following mouse strains: Balbc.IL13Rα1−/−, TCRα−/− (Taconic), KitW-sH/W-sH, Jα18−/−, CD1d−/−, Il13eGFP (26), 4C13R reporter mice(33).
Histological Quantification
Cross sectional intestinal tissues were stained with Periodic Acid Shiff (PAS) or hematoxylin and eosin (H&E). Images were acquired with a BZ-9000 microscope and analyzed with the BZ-II Analyzer software package. The mucosal thickness index was calculated by dividing the cross sectional area of the muscularis and submucosa by the circumference of the center line of those tissues. Three sections were quantified per mouse. Average goblet cell area was calculated by measuring the average area of all goblet cells in five protruding villi in each mouse.
Antibiotic Treatment and Fecal DNA Quantification
The antibiotics ampicillin (1 g/l), vancomycin (500 mg/l), neomycin trisulfate (1 g/l), and metronidazole (1 g/l) were added to the drinking water of cages housing mice from pregnancy or from birth. To harvest stool for DNA quantitation, the hindquarters and tails of mice were wiped with 70% ethanol and then held such that they defecated into sterile 2mL microcentrifuge tubes. Two pellets from each mouse were then processed with the QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturers protocol and total DNA concentrations were quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop).
Cytokine measurement and analysis
IL-13 and Muc5ac mRNA were measured by following the manufacturer’s protocol with 100ng of total RNA, IL-25 and IL-33 mRNA were measured with 200ng of total RNA using the ABI Taqman primer probe sets Mm00434204_m1, Mm01276718, Mm00499822_m1 and Mm00505403_m1 respectively using BioRad iScript One Step RT-PCR reagents. Samples were run on a CFX96 RT-PCR detection system (BioRad). For cytokine measurements by ex-vivo stimulated ILC, cells were cultured at 40,000 cells per well for 72 hours and cytokine levels measured with the Millipore Magpix system.
IL-25, IL-33 and TL1A administration
0.4 μg–2 μg per dose of recombinant mouse IL-25, 0.5 μg of recombinant mouse IL-33 or 5 μg of TL1A (R&D Systems) in PBS was administered daily for 3 or 4 days intraperitoneally. Mice were sacrificed 24 hours later and tissues harvested for analysis. Control animals received PBS only.
Mesenteric lymph nodes and LPL isolation
Cells from spleen and mesenteric lymph nodes were prepared by smashing the tissue through 40 um cell strainer. Lamina propria lymphocyte (LPL) were extracted from the small intestine. In brief, the small intestine was collected and the Peyer’s patches removed. Intestines were opened longitudinally and cut into small pieces (<5 mm). Intraepithelial cells were removed by washing with HBSS and incubating with 5 mM EDTA for 20 min at 37°C. The intestinal pieces were washed with RPMI, and LPLs were isolated by digestion with 1 μg/ml DNase (Sigma-Aldrich) and 500 μg/ml Collagenase D (Roche) for 30 min at 37°C.
Parasite Infection
Mouse-adapted Nippostrongylus brasiliensis were provided by Dr. Joseph Urban (U.S. Dept. of Agriculture, Beltsville, MD) and their life cycle maintained as described (47). Mice were infected with 300–500 L3 stage larvae by subcutaneous injection. Serum, perfused lungs, mesenteric lymph nodes, and rinsed intestines were collected at euthanasia. For histology, duodenum sections were slit lengthwise, rolled, and fixed in Bouin’s Hollande. Adult worms were recovered from the entire small intestine by washing and counted using a dissection microscope. Tissue samples for RNA were collected from matched lung lobes or 2–4 equivalent samples from a grossly inflamed section of duodenum. For live egg counts, stool samples were collected daily, weighed, and dissolved in aqueous saturated NaCl. Buoyant eggs were counted using a McMaster slide (Chalex). Schistosoma mansoni were provided by the Biomedical Research Institute (Rockville, MD). Mice were infected with 35 cercariae by tail immersion as described (48) and analyzed at 9 weeks. Adult worms were perfused from the mesenteric veins and counted. For RNA samples, tissue snips were collected from matched sites of 3 liver lobes and 2–4 sites of the small intestine. Histology specimens were fixed in Bouin’s Hollande, stained with Giemsa, and evaluated by an experienced pathologist. The remaining liver tissue was dissolved in aqueous 4% KOH and eggs were counted using a McMaster slide. Serum IL-13 and IL13Rα2 was assayed as previously described (49).
Ova-Induced Lung Inflammation
On days 0 and 7, mice were sensitized systemically via a 200μl intraperitoneal (i.p.) injection containing either 100 μg Chicken Ova (Sigma) or PBS emulsified in an equal volume mixture with alum (Thermo Scientific). For assessment of pulmonary inflammation, mice were challenged with 100 μg Ova or PBS/30 μl inoculum intratracheally (i.t.) on day 14 and intranasally (i.n.) on day 15. Mice were euthanized 72 hours after the final challenge. Bronchoalveolar lavage (BAL) fluid was obtained by direct cannulation of the lungs with a 20-gauge intravenous catheter and lavage with 500 μl 1% fetal bovine serum (FBS) in PBS (for cytokine analysis) and with 700 μl 1% FBS in PBS (for analysis of cellular infiltration). Lung cell preps were obtained by incubating lung fragments with 100U collagenase for 1h. Cells were stained for surface antigens and intracellular cytokines after stimulation with PMA/ionomycin for 4 h. H&E and PAS stained histological sections were scored by an observer masked to treatment group for peribronchiolar and perivascular cuffing (0–4), goblet cell hyperplasia (0–4) and interstitial infiltrate (0–3).
Induction of papain induced airway inflammation
Mice were anaesthetized with isoflurane and exposed intranasally to 25 μg papain (Calbiochem) in 30 μL PBS on day 0, 2, 4 and 6. 12–16 h after the last challenge bronchoalveolar lavage was performed as described above. Lung isolated cells homogenate were obtained by incubating lung fragments with 100U collagenase for 1h. Cells were stained for surface antigens and intracellular cytokines after stimulation with PMA/ionomycin for 4h. Cells from the BAL were stained using antibodies against CD45, SiglecF, F4/80, Ly6G and CD11b. Eosinophils were identified as CD45+ F4/80− Ly6G− CD11b+ SiglecF+.
Supplementary Material
Acknowledgments
We would like to thank Masaki Terabe and Jay Berzofzky (NCI, NIH) for providing Jα18 and CD1d knockout mouse lines, Juan Rivera for providing W/Wv mice, Anthony Cruz, Sarah Villareal, Odile Gabey, Nathan Bradley, and Allen Cheever for technical assistance, and Yasmine Belkaid, and Aymen Al-Shamkhani for helpful discussions.
References
- 1.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–85. doi: 10.1038/nri2526. Epub 2009/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16(3):479–92. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 3.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178(7):4033–8. doi: 10.4049/jimmunol.178.7.4033. Epub 2007/03/21. [DOI] [PubMed] [Google Scholar]
- 4.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. Epub 2008/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunological Reviews. 2011;244(1):188–96. doi: 10.1111/j.1600-065X.2011.01068.x. Epub 2011/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205(5):1049–62. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull MJ, Williams AS, Mecklenburgh Z, Calder CJ, Twohig JP, Elford C, et al. The Death Receptor 3-TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. Journal of Experimental Medicine. 2008;8 doi: 10.1084/jem.20072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135(2):552–67. doi: 10.1053/j.gastro.2008.04.037. Epub 2008/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchan SL, Taraban VY, Slebioda TJ, James S, Cunningham AF, Al-Shamkhani A. Death receptor 3 is essential for generating optimal protective CD4(+) T-cell immunity against Salmonella. Eur J Immunol. 2012;42(3):580–8. doi: 10.1002/eji.201041950. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 10.Twohig JP, Marsden M, Cuff SM, Ferdinand JR, Gallimore AM, Perks WV, et al. The death receptor 3/TL1A pathway is essential for efficient development of antiviral CD4(+) and CD8(+) T-cell immunity. FASEB J. 2012;26(8):3575–86. doi: 10.1096/fj.11-200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bamias G, Martin C, 3rd, Marini M, Hoang S, Mishina M, Ross WG, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171(9):4868–74. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Wang X, Fahmi H, Wojcik S, Fikes J, Yu Y, et al. Role of TL1A in the pathogenesis of rheumatoid arthritis. J Immunol. 2009;183(8):5350–7. doi: 10.4049/jimmunol.0802645. Epub 2009/09/30. [DOI] [PubMed] [Google Scholar]
- 13.Bamias G, Siakavellas SI, Stamatelopoulos KS, Chryssochoou E, Papamichael C, Sfikakis PP. Circulating levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3 (DcR3) in rheumatoid arthritis. Clin Immunol. 2008;129(2):249–55. doi: 10.1016/j.clim.2008.07.014. Epub 2008/09/02. [DOI] [PubMed] [Google Scholar]
- 14.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–62. doi: 10.1038/NG.175. Epub 2008/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taraban VY, Slebioda TJ, Willoughby JE, Buchan SL, James S, Sheth B, et al. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4(2):186–96. doi: 10.1038/mi.2010.70. Epub 2010/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meylan F, Song YJ, Fuss I, Villarreal S, Kahle E, Malm IJ, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4(2):172–85. doi: 10.1038/mi.2010.67. Epub 2010/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih DQ, Barrett R, Zhang X, Yeager N, Koon HW, Phaosawasdi P, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS ONE. 2011;6(1):e16090. doi: 10.1371/journal.pone.0016090. Epub 2011/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twohig JP, Roberts MI, Gavalda N, Rees-Taylor EL, Giralt A, Adams D, et al. Age-dependent maintenance of motor control and corticostriatal innervation by death receptor 3. J Neurosci. 2010;30(10):3782–92. doi: 10.1523/JNEUROSCI.1928-09.2010. Epub 2010/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren JE, Calder CJ, McSharry BP, Sexton K, Salter RC, Singh NN, et al. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010;184(10):5827–34. doi: 10.4049/jimmunol.0903782. Epub 2010/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. Epub 2013/01/08. [DOI] [PubMed] [Google Scholar]
- 21.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–9. doi: 10.1038/nri3365. Epub 2013/01/26. [DOI] [PubMed] [Google Scholar]
- 22.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330(6004):665–9. doi: 10.1126/science.1194597. Epub 2010/10/12. [DOI] [PubMed] [Google Scholar]
- 23.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13(3):229–36. doi: 10.1038/ni.2208. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38(4):694–704. doi: 10.1016/j.immuni.2012.12.003. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–4. doi: 10.1038/nature08636. Epub 2009/12/22. [DOI] [PubMed] [Google Scholar]
- 26.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–U9. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187(11):5505–9. doi: 10.4049/jimmunol.1102039. Epub 2011/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–41. doi: 10.1038/nature11551. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–48. doi: 10.1016/j.immuni.2012.06.020. Epub 2012/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107(25):11489–94. doi: 10.1073/pnas.1003988107. Epub 2010/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–7. doi: 10.1038/ni.2133. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129(1):191–8. e1–4. doi: 10.1016/j.jaci.2011.09.041. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 33.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):564–73. doi: 10.1038/ni.2584. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110(1):282–7. doi: 10.1073/pnas.1206587110. Epub 2012/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–16. doi: 10.1084/jem.20051615. Epub 2006/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104(6):777–85. doi: 10.1172/JCI7325. Epub 1999/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162(2):920–30. Epub 1999/01/23. [PubMed] [Google Scholar]
- 38.Sokol CL, Chu N-Q, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10(7):713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11(7):608–17. doi: 10.1038/ni.1883. Epub 2010/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, et al. IL-33-Mediated Innate Response and Adaptive Immune Cells Contribute to Maximum Responses of Protease Allergen-Induced Allergic Airway Inflammation. J Immunol. 2013;190(9):4489–99. doi: 10.4049/jimmunol.1201212. Epub 2013/04/03. [DOI] [PubMed] [Google Scholar]
- 41.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–95. doi: 10.4049/jimmunol.1101977. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 42.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–63. doi: 10.1016/j.immuni.2011.12.020. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 43.Zhu WEP, Jinfang How are TH2-type immune responses initiated and amplified? Nature Reviews Immunology. 2010;10(4):225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–54. doi: 10.1031/ni.2131. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62. doi: 10.1038/ni.2104. Epub 2011/09/13. [DOI] [PubMed] [Google Scholar]
- 46.Wang ECY, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ. DR3 Regulates Negative Selection during Thymocyte Development. Molecular and Cellular Biology. 2001;21(10):3451. doi: 10.1128/MCB.21.10.3451-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camberis M, Le Gros G, Urban J., Jr Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol. 2003;Chapter 19(Unit 19):2. doi: 10.1002/0471142735.im1912s55. Epub 2008/04/25. [DOI] [PubMed] [Google Scholar]
- 48.Lewis F. Schistosomiasis. Curr Protoc Immunol. 2001;Chapter 19(Unit 19):1. doi: 10.1002/0471142735.im1901s28. Epub 2008/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khodoun M, Lewis CC, Yang JQ, Orekov T, Potter C, Wynn T, et al. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J Immunol. 2007;179(10):6429–38. doi: 10.4049/jimmunol.179.10.6429. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.