Abstract
Purpose
Achieving transmural tissue ablation may be necessary for successful treatment of atrial fibrillation. The purpose of this study is to evaluate the reproducibility of transmural left atrial (LA) ablation using a high intensity focused ultrasound (HIFU) energy system in a calf model.
Methods
Nine heparinized bovines underwent a beating-heart LA ablation with a single application of the HIFU device. All animals were acutely sacrificed and the LA was fixed in formalin. Protocolized histological sections (5μm) were obtained throughout each lesion and prepared with Masson's Trichrome and Hematoxylin and Eosin staining. Measurements were performed on a total of 359 slides from the nine lesions. In addition, fresh LA from 18 unused human donor hearts that did not meet criteria for cardiac transplantation were measured at the site where the HIFU device is normally applied.
Results
Calf LA thickness ranged between 2.5 and 20.1 mm, with a mean of 9.10 mm. HIFU ablation consistently produced a 100% transmural lesion in LA thickness up to 6mm. In addition, a transmural lesion was observed in 91% of tissues that were up to 10 mm thick and in 85% up to 15 mm of thickness. Human LA thickness ranged between 1.2 to 6 mm, with a mean of 3.7 mm.
Conclusions
Calf LA thickness in this study was greater than human LA thickness. Human LA thickness is generally less than 6mm, and in this range HIFU ablation achieved 100% transmurality. These histological results may correlate with a high success rate of atrial fibrillation ablation using the HIFU system.
Keywords: Atrial fibrillation, ablation, high intensity focused ultrasound, transmurality, bovine animal model
Introduction
Atrial Fibrillation (AF) is present in approximately 9% of patients older than 70 years of age and has been recognized as an independent risk factor for cardiovascular morbidity and mortality [1]. Reported freedom from AF with medical management and catheter ablation techniques is inferior to the “cut and sew” Cox Maze III procedure, which continues to be the gold standard for the treatment of AF, with 92% to 97% cure rates at 10 years of follow-up [2]. However, this procedure has not been widely adopted because of its technical complexity and because of the required additional cardiopulmonary bypass time. The identification of trigger zones for AF [3], specifically around the pulmonary vein orifices, have lead to the development of simpler, less invasive procedures utilizing different energy sources. Several different energy sources applied endocardially and/or epicardially have been utilized for this purpose with variable results. A common goal when developing new technologies for atrial ablation has been to create transmural lesions similar to what is obtained with the “cut and sew” Cox Maze procedure. Some of the challenges that these new technologies need to overcome include the ability to ensure transmurality without damage of the surrounding structures in the setting of variations in thickness, fat content and the cooling effect of atrial blood flow. High Intensity Focused Ultrasound (HIFU) is a young technology in the armamentarium for AF ablation. Clinical trials performed in Europe [4] and the U.S.[5] using HIFU in patients with AF, undergoing concomitant cardiac surgery, have reported a rate of freedom from AF of 85% at 18 months of follow-up. However, a histological analysis demonstrating the presence of transmural lesions with HIFU had not yet been published. The purpose of this study is to assess the safety and reproducibility of transmural left atrial ablation using the Epicor™ LP HIFU system (St. Jude Medical, St. Paul , MN) in a calf model.
Methods
Device Description
The Epicor Cardiac Ablation System has been previously described [4]. Briefly, the system consists of the Ablation Control System (ACS) generator, a family of disposable ablation devices and a set of accessories. The ACS is a microprocessor-based unit that enables acoustic power to be delivered to the heart from ultrasound transducers. The UltraCinch™ LP device is an array of multiple (7 to 13) ultrasound transducers (cells) that is used to produce a transmural circumferential left atrial lesion, isolating the posterior LA and all four pulmonary vein orifices. An additional tool, the UltraWand™ LP, is a handheld ablation device with only two transducers for the epicardial creation of additional linear lesions such as the line to the mitral annulus. The UltraWand LP was used in this model to assess the effect of HIFU energy applied over coronary vessels.
Animal studies
The study was conducted at Duke University under approval of the Institutional Animal Care and Use Committee (IACUC). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals. Nine calves with a weight range of 63 to 114kg were selected for the study.
System Characteristics
The transducers comprising the UltraCinch LP are positioned on the epicardium but separated from direct contact with the cardiac tissue by a thin perforated membrane. Room temperature normal saline is circulated between the membrane and the transducers during ablation to enhance acoustic coupling and cooling. Each transducer (10 x 15 mm) is designed to deliver HIFU energy up to a distance of 10 mm from its surface. Beyond the focal point, the energy dissipates within the left atrial cavity without exposing the surrounding structures to potential collateral damage. The proprietary UltraCinch LP algorithm generated by the ACS uses a combination of frequency, power, and duration at which the transducers are activated and powered (from 3.8 to 6.4MHz and from 15 to 130W) to generate 3 sequential stages of ablation. The ablation process begins with the deep ablation stage, during which the energy is deposited distal from the transducer in the sub-endocardial zone, followed by the intermediate stage in which energy is deposited in the mid-myocardial layer, followed by the surface stage for epicardial energy deposition. During the first 2 stages, each transducer is activated sequentially, and the energy is pulsed, whereas during the third stage, transducers are staged 3 at a time with continuous wave HIFU to eliminate any residual gap within the epicardium. The lesion is thus built up from the endocardium back to the epicardium and is complete within approximately 10 minutes. The UltraWand LP is activated in a similar fashion but uses a single stage for the creation of linear lesions during a shorter 80-second algorithm that was designed for a hand-held device.
Surgical Technique
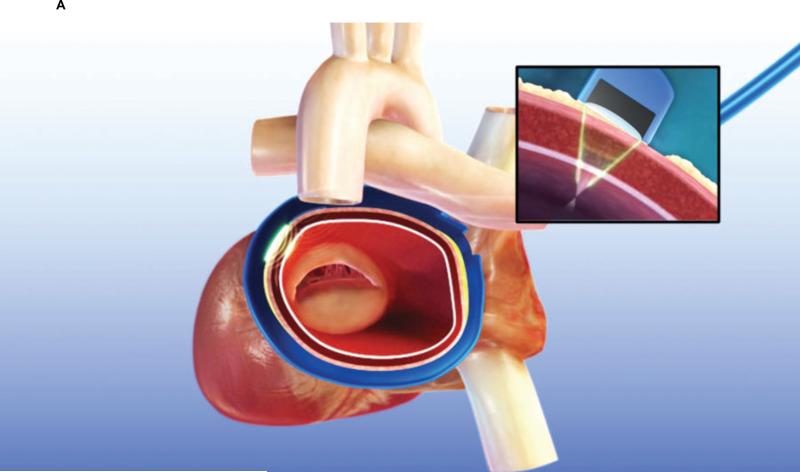
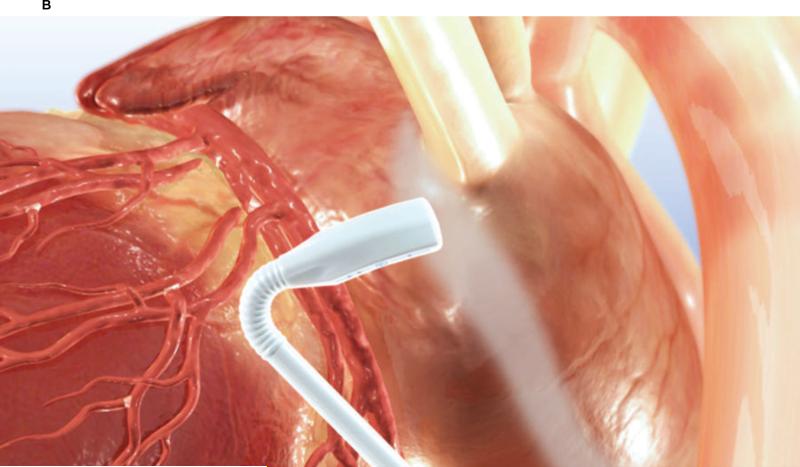
The animals were anesthesized with isoflurane, and a median sternotomy was performed. The surgical ablation was executed as previously described by Ninet et al [4]. The pericardium was opened, and the pericardial reflections around both the superior vena cava (SVC) and inferior vena cava (IVC) were dissected free to gain access to both the transverse and oblique sinuses. A specially designed introducer-sizer was passed behind the superior vena cava into the transverse sinus and guided into the oblique sinus and beneath the inferior vena cava, thereby completely encircling all 4 pulmonary veins. The graduated introducer-sizer was used to measure the circumference of the left atrium to select the proper size of UltraCinch LP device. The introducer was subsequently used to guide the UltraCinch LP around the left atrium. The introducer was then removed, the two ends of the UltraCinch LP device were approximated with tourniquets placed on the appropriate sutures at each end to snug the device securely around the left atrium (Figure 1A). A 7 or 8-cell Epicor UltraCinch LP device was used in all seven cases. A bolus of heparin (100 IU/kg) was administered before the ablation cycle start, and a continuous saline infusion was set by gravity with a bag height of 35 inches, which yields approximately 1.5ml/min per cell of irrigation into the membranes overlying the transducer cells. The ablation cycle was then initiated and progressed automatically until the UltraCinch LP ablation had been completed. Physiologic data was recorded during the ablation run. An additional lesion placed over the coronary sinus was also created epicardially with the UltraWand LP (Figure 1B). A continuous saline infusion was maintained at a rate of 120ml/hr during the UltraWand LP ablation cycle.
Figure 1.
Sites of HIFU ablation
A. Position of Epicor UltraCinch LP
B. Position of Epicor UltraWand LP
Tissue processing
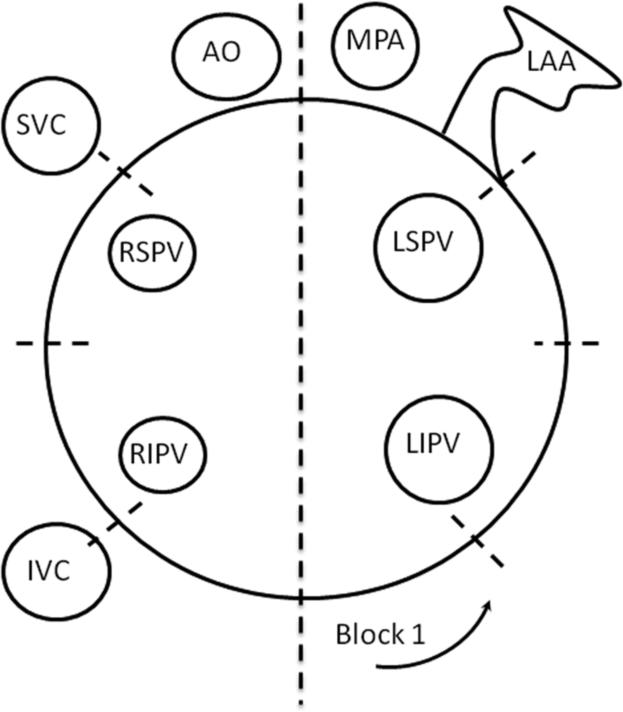
All animals were sacrificed at the end of the procedure and the heart was immediately explanted for macroscopic examination. The left atrium (LA) and additional cardiac tissue surrounding the UltraCinch LP and UltraWand LP lesions was widely resected en-block. Endocardial and epicardial surfaces were examined for color, appearance, evidence of perforation or rupture, presence of intramural hemorrhage, presence of endocardial thrombus, integrity of the pulmonary vein wall and the mitral valve apparatus. The resected tissue was then fixed in 10% formalin until sectioning. The fixed LA was radially sectioned into 8 blocks, approximately equivalent to the locations of the individual device cells (Figure 5 on-line). The wand lesion was serially sectioned perpendicular to the coronary sinus. Five standard 5μm sections were prepared from each block, alternating Masson trichrome and hematoxylin and eosin staining, and additional sections were prepared in the same manner until each block was completely sectioned. All sections were examined, and measurements were taken from two separate sections of each block using an ocular micrometer.
Figure 5.
(on-line) Representation of how the fixed LA was divided into 8 sections (blocks) along HIFU lesion to obtain histology slides
Measurements
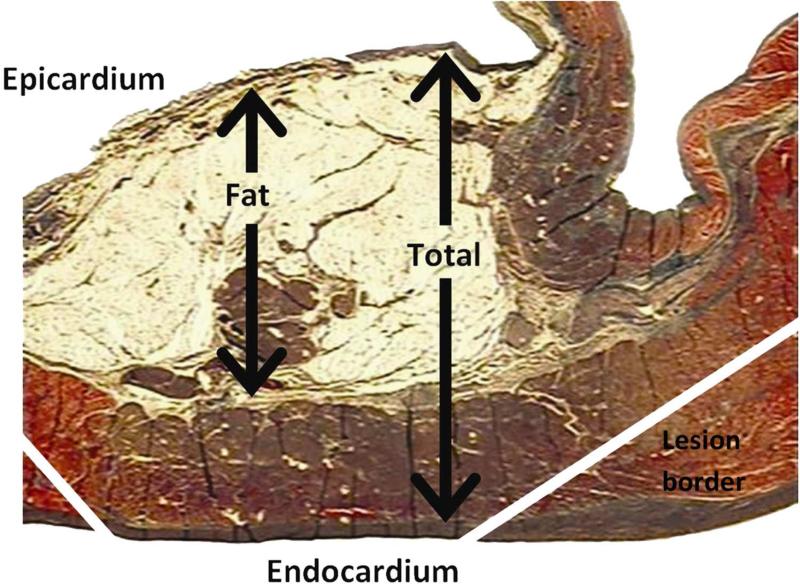
Measurements were obtained from a total of 359 slides. Tissue thickness was measured at the site of the lesion. Lesion thickness was measured, in addition the fat and myocardial thickness which composed the lesion was recorded; lastly, any gap of uninjured tissue was measured. Lesion transmurality was calculated by comparing lesion depth and total tissue thickness as shown in figure 2.
Figure 2.
Histology of HIFU lesion with total versus fat thickness measurement
Human studies
Human hearts that did not meet criteria for cardiac transplantation were procured after research consent was obtained from our local organ procurement organization. Hearts were procured in standard fashion from donors in which the lungs were also not used for transplantation to allow for dissection and isolation of 2-3 cm of each pulmonary vein. The LA was separated from the left ventricle at the AV groove immediately above the mitral valve similar to the procurement of the bovine LAs. Measurements were made with a micrometer adjacent to but 2mm central to each of the four pulmonary veins in the perceived location of the UltraCinch LP device. Three measurements were taken at each location and averaged. Data are presented as mean±SEM.
Results
Animal studies
Macroscopic examination
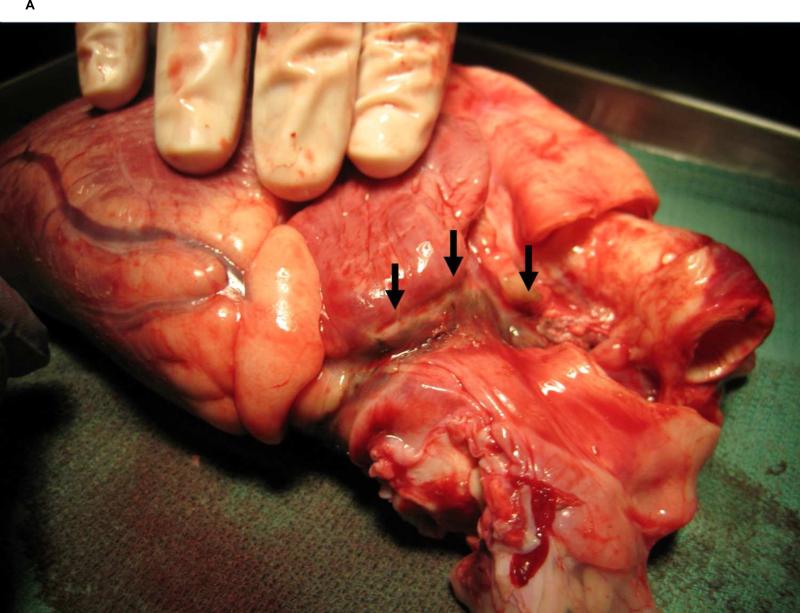
There was no evidence of perforation or rupture of the cardiac tissues subjected to the ablation in any of the 9 animals. Gross exam of the UltraCinch LP application site revealed a wide, distinct, circumferential lesion on the epicardium with corresponding linear tracts on the endocardial surface upon inversion of the specimen, indicating transmural penetration of the lesion (Figure 3A). Importantly, there was no evidence of left atrial endocardial injury distant to the lesion. No intramural hemorrhage or endocardial thrombus was identified in any of the 9 animals. The pulmonary vein wall and mitral valve apparatus demonstrated freedom from any injury in all 9 animals. Gross exam of the UltraWand LP application site did not show evidence of acute cavitation, perforation, thrombosis of epicardial coronary vessels or other unintended trauma to the target tissue.
Figure 3.
Characteristics of Epicor UltraCinch LP ablation
A. Macroscopic examination
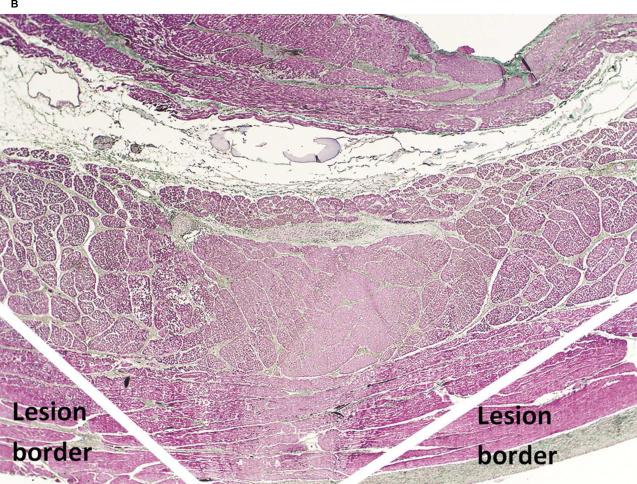
B. Microscopic examination
Microscopic examination
Microscopically, the type of injury caused by the Epicor UltraCinch LP device was characterized by a wedge-shaped lesion with a wide base at the epicardial surface that progressively narrowed to the endocardial surface. The histologic features include edema, nuclear hyperchromasia, and contraction bands, typical of irreversible myocardial injury (Figure 3B).
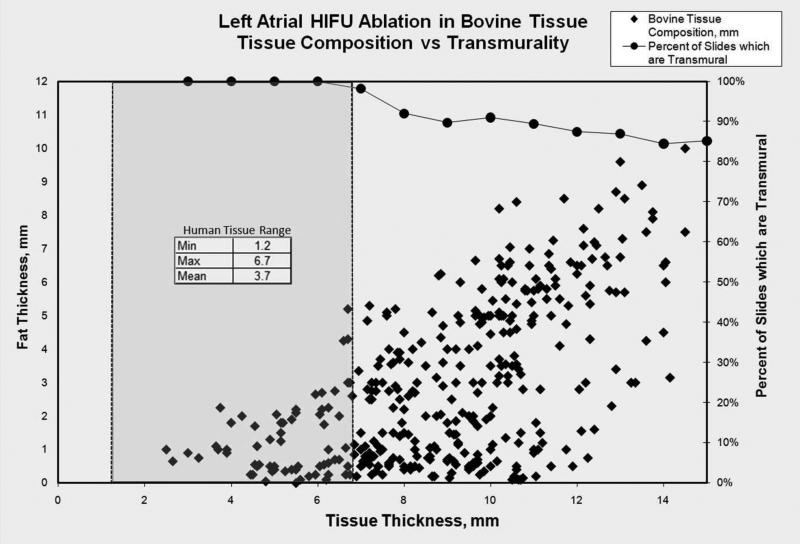
Calf total LA thickness for all 359 slides ranged between 2.5 and 20.1 mm, with a mean of 9.10mm. Adipose tissue thickness at the lesion site ranged between 0 and 16 mm, with a mean of 3.10 mm. The distribution of total thickness and percentage of adipose tissue for the entire cohort is represented in figure 4. Seventy-one percent of the measured slides contained LA tissue that was 10mm or less in total thickness, which is the greatest depth at which the HIFU beam is focused.
Figure 4.
Percentage of transmural lesions in relation to distribution of total thickness and percentage of adipose tissue using the Epicor UltraCinch LP.
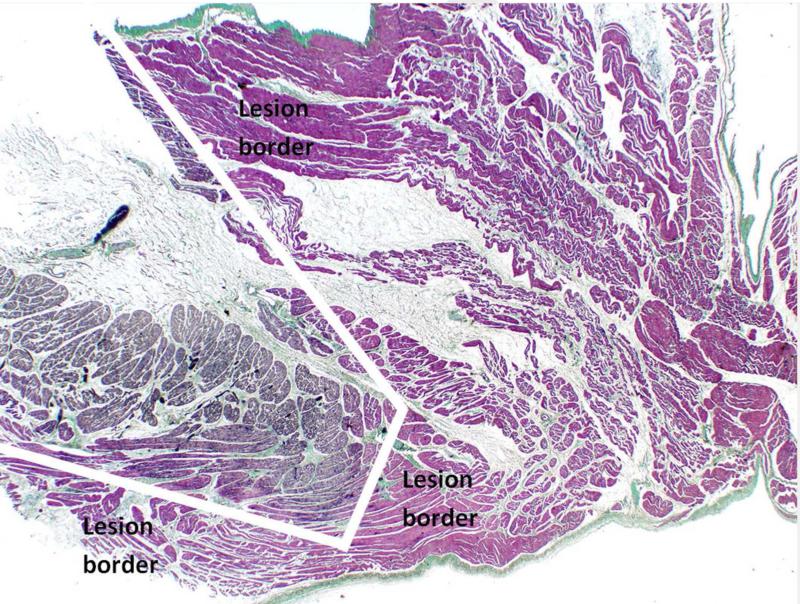
The Epicor UltraCinch LP consistently produced a 100% transmural lesion in LA thickness up to 6mm, which includes adipose tissue of up to 2.7mm. In addition, a transmural lesion was observed in 91% of tissues that were up to 10mm thick and in 85% of tissues that were up to 15mm of thickness (Figure 4). The greatest of total thickness that underwent a 100% transmural lesion was 14.5mm. The greatest of adipose tissue that underwent a 100% transmural lesion was 9.6mm. Non-transmural lesions were not located at a defined anatomical landmark in every animal nor was there a correlation between non-transmural lesions and the cell number within the Epicor UltraCinch LP. The amount of adipose tissue present on the target LA did not appear to correlate with lesion transmularity. In contrast, the shape of the target tissue and the orientation of the HIFU lesion (the direction of the wedge-shaped lesion) seemed to play an important role in obtaining transmurality. (Figure 6 on-line)
Figure 6.
(on-line) Oblique lesion presumably related to the shape of the target tissue and the orientation of the HIFU lesion
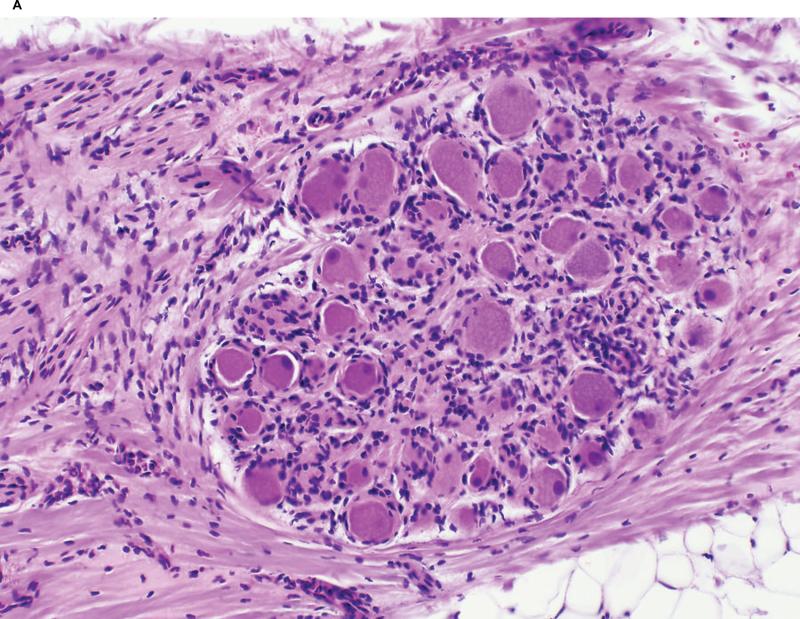
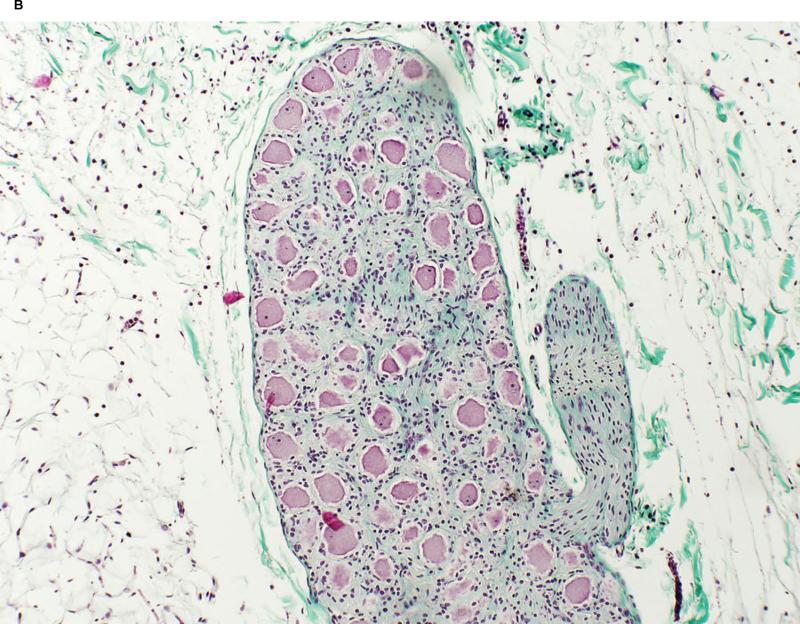
All nerve bundles identified within the HIFU beam appeared irreversibly injured independent of size or tissue depth (Figure 7 on-line).
Figure 7.
(on-line) A. Irreversibly injured nerve bundle identified within the HIFU beam caused by the Epicor UltraCinch LP. Hematoxylin and Eosin stain.
B. Ganglionic plexi ablated by the Epicor UltraWand LP. Masson's Trichrome stain.
The Epicor UltraWand LP lesion was microscopically similar to the UltraCinch LP lesion. No thrombosis was seen inside the coronary sinus or circumflex artery over which the Epicor UltraWand LP was applied. However, there is some evidence of insult to smaller vessels that cannot be fully characterized. The endothelial cell nuclei are rounded, enlarged, and protrude into the lumen; these are characteristic features of injured or reactive endothelial cells. There are no specific histologic features that allow differentiation between reversible versus irreversible endothelial cell injury. It should be noted that no injury to the circumflex artery or other nearby vessels has been observed in chronic human studies extending as far out as 18 months post-procedure [5, 6].
Human studies
To determine the relationship of the calf model to adult human LA thickness, we also measured LA thickness in 18 adult human unused donor hearts. The average age of hearts examined was 51.8±4.6 years and 28% had history of cardiovascular disease. The mean human LA thickness was 3.7mm with a range of 1.2 to a maximum thickness of 6mm.
Discussion
AF is a significant cause of cardiovascular morbidity and mortality [1]. The success rate of medical therapy and catheter ablation remains suboptimal. The “cut and sew” Cox Maze III surgical procedure has proven to be very effective but still carries a significant risk for complications and is technically challenging [2]. The development of new technologies to reproduce the outcomes obtained with the Cox Maxe III but to minimize risks is an area of active investigation. Alternative energy sources used to treat AF include hypothermic sources (cryoablation) and hyperthermic sources (radiofrequency (RF), microwave, laser, ultrasound).
Since Haissaguerre et al. identified the pulmonary vein orifices as the trigger zones for AF [3], a great interest has emerged to design ablation devices capable of isolating these trigger zones. The Epicor LP HIFU system has been designed to electrically isolate these areas in a safe and reproducible manner.
Although the necessity to achieve conduction block acutely is still a debatable topic, mature transmural lesions will always result in chronic electrical block. Accordingly, the only guarantee of long-term effectiveness is a fully transmural lesion. For instance, Melby et al have shown that AF can conduct through gaps ≥1mm in ablation lines [6]. Furthermore, gaps along the lines of prior ablation have been identified in patients with unsuccessful surgical ablation [7]. One of the endpoints of this study was to determine the presence of transmurality in the ablations created with the Epicor LP HIFU system. A single epicardial, beating-heart application of the Epicor UltraCinch LP device was 100% effective in producing a transmural lesion in calf atrial tissue up to 6mm thick; which was the maximal thickness of measured adult human left atrium.
In comparison, animal studies using different energy sources have reported a wide range of lesion thickness in beating–heart models (Table 1). Cryoablation has produced transmural lesions in 25-100% of samples in beating-heart models with mean LA thickness of 2.0 ± 0.3mm [8]. Transmularity with microwave ablations has only been achieved in 20%-46% of samples in beating-heart animal models with mean LA thickness of 2.62 ± 1.31mm [9-11]. Only one study has reported transmularity using a laser energy source for surgical AF in beating hearts, which showed all lesions to be transmural in dogs with an average tissue thickness of 3.62 ± 1.50mm (range, 0.95mm to 7.06mm) [12]. Bipolar RF clamp devices have been shown to create transmural lesions in 92-100% of samples in beating-heart animal models [13, 14]. However, the average tissue thickness was 4.2 ± 0.1mm in the swine model [13] and 1.9 ± 1.1mm in the sheep model [14]. In this study, we tested the HIFU product in an animal model with much greater LA thickness relative to other beating-heart models and with only a single application, yet the experimental lesions in this study were deeper than those observed in the other models.
Table 1.
Transmurality in beating-heart models
| Study | Energy source | Animal model | Mean LA thickness | % Transmurality |
|---|---|---|---|---|
| Doll et al. 2003 [8] | Cryoablation | Dog | 2.0 ± 0.3mm | 96% |
| Van Brakel et al. 2004[9] | Epicardial microwave | Dog | NS | 33% |
| Melby et al. 2006 [10] | Epicardial microwave | Pig | 2.62 ± 1.31mm | 46% |
| Gaynor et al. 2006 [11] | Epicardial microwave | Pig | 2.88 ± 0.4mm | 20% |
| Williams et al. 2006 [12] | Laser | Dog | 3.62 ± 1.5mm | 100% |
| Melby et al. 2006 [13] | Bipolar RF | Pig | 4.2 ± 0.1mm | 100% |
| Bugge et al. 2005 [14] | Bipolar RF | Sheep | 1.9 ± 1.1 mm | 92% |
| Villamizar et al. 2010 | HIFU | Calf | 9.10 ± 2.6mm | 84% |
NS: non-specified
In our analysis of human LA thickness, we found that the average human LA measures 3.7mm (1.2 – 6mm). Our results are similar to those of other anatomical studies aimed to define the human LA anatomy to provide a firm background for the development of AF ablation techniques. Cabrera et al [15] reported a mean LA thickness of 2.8 ± 1.1mm (1.5–4.2mm) between the orifices of the left pulmonary veins and the os of the left atrial appendage in a total of 22 human hearts. Ho et al [16] found a mean LA thickness of 3.7 ± 0.6mm (2.9-4.5mm) at the venoatrial junction of the left superior pulmonary vein in a total of 26 human hearts. Sanchez et al [17] showed a mean LA thickness of 6.5 ± 2.5mm (2.8 to 12mm) at the middle of the posterior atrial wall in a total of 15 cadavers. These results suggest that the LA thickness of the dog, swine and sheep models in previous reports falls short of maximal adult human LA thickness. Thus, these models may not be ideal for testing transmurality. In contrast, the calf model provides a tissue thickness much greater than human LA as demonstrated by our findings.
Another advantage of the Epicor LP HIFU system is that epicardial fat dissection is not necessary to achieve transmural ablation. The average amount of adipose tissue in the 9 animals included in this study was 3.10 mm (range 0-16mm). As an example, a lesion of 20.2mm total thickness with 16mm of adipose tissue had a gap of only 0.55mm of non-ablated tissue after a single application of the Epicor UltraCinch LP device, which demonstrates the efficacy of HIFU to penetrate through fat. In contrast, Turek et al reported that microwave energy has diminished penetration through adipose tissue [18]. Assessment of adipose tissue penetration by other energy sources (cryoablation, RF, laser) has not been reported.
Some of the factors that may influence the depth of the ablation achieved by alternative energy sources are anatomy, tissue composition, device sizing and position, and saline flow rates. It did not appear that the amount of adipose tissue present on the target LA significantly contributed to the depth of the ablation in this model. However, the shape of the target tissue and the orientation of the HIFU beam seem to play an important role in obtaining transmurality. All animals underwent ablation with either a 7 or 8-cell Epicor UltraCinch LP device. As each cell measures 1.0cm x 1.5cm, it is possible to create a significant gap interface between the cell surface and the epicardium when over-sizing the circumference around the pulmonary veins orifices. On the other hand, under-sizing may inihibit saline flow to cool off the Epicor UltraCinch LP cells and maintain their integrity throughout the ablation cycle. Thus, appropriate sizing is important when using this epicardial technology.
Another endpoint of this study was to determine the presence of vascular injury and thrombogenic complications in areas where the Epicor LP HIFU system is applied. No thrombogenic complications were appreciated with the use of the Epicor LP HIFU system in this study. In comparison, unipolar RF has been associated with thromboembolic complications [19], which appear to be absent with the use of bipolar RF devices [20]. There have also been reports of coronary artery stenosis using microwave technology [21]. Whereas experience has shown that cryothermal energy appears to have no permanent effects on valvular tissue or the coronary sinus, experimental studies have shown late minimal hyperplasia of coronary arteries [22-24]. There are no reports about the impact of laser ablation on the coronary vessels. The impact of HIFU ablation on the small vessels found within the HIFU beams appeared to be less destructive to what has been observed using other energy sources, such as microwave ablation [18]. The ability of HIFU to ablate tissue surrounding coronary arteries without damaging the arteries themselves has been attributed to the protective cooling effect provided by the high blood flow through the coronary arteries and the absence of acoustic heating of the blood [25].
This study has some limitations that deserve discussion. First, it employs a calf model to investigate the ablative characteristics of a device designed to ablate human cardiac tissue. It is unclear how healthy calf atrial tissue compares to diseased human atria in AF patients. Second, this study represents an acute examination of the injured tissue; some cells which appear dead may resurrect, and cells which appear viable may die later. Anatomy or dimensions of the scar at 1-3 months may appear different, and therefore, we are planning more chronic studies. Third, conduction block was not evaluated. The calf LA thickness was considerably greater than human LA thickness, and therefore gaps were anticipated. Also, calves have a left SVC which traverses the site of the HIFU lesion resulting in greater total tissue thickness and preventing transmurality at this site. Finally, acute conduction block may not correlate with late conduction block.
Despite these limitations, some assumptions could be made from the observed results. The average calf left atrial thickness in this study was 9.10 mm and the range was between 2.5 and 20.1 mm. In contrast, the average LA in 18 human hearts ranged between 1.2 to 6mm, with a mean of 3.7mm. HIFU ablation consistently produced a 100% transmural lesion in LA tissue thickness up to 6mm. Human LA thickness is generally less than 6mm, and in this range HIFU ablation should achieve transmurality.
Acknowledgments
This work was supported by an unrestricted grant from St. Jude Medical and NIH grant # P01-HL075443 to Dr. Carmelo A. Milano. The authors report no conflicts of interest other than previously stated. The authors wish to thank Tom Eby and Jennifer Teng for their technical assistance and contributions throughout the course of this protocol.
Funding of project: This work was supported in part by an unrestricted grant from St. Jude Medical to Dr. Carmelo A. Milano and an NIH grant # P01-HL075443 to Dr. Carmelo A. Milano.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr Mark Groh is a consultant to the Atrial Fibrillation Division of SJM
Bibliography
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 2.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, III, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Ninet J, Roques X, Seitelberger R, Deville C, Pomar JL, Robin J, et al. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. J Thorac Cardiovasc Surg. 2005;130:803–9. doi: 10.1016/j.jtcvs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Groh MA, Binns OA, Burton HG, Ely SW, Johnson AM. Ultrasonic cardiac ablation for atrial fibrillation during concomitant cardiac surgery: long-term clinical outcomes. Ann Thorac Surg. 2007;84:1978–83. doi: 10.1016/j.athoracsur.2007.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Melby SJ, Lee AM, Schuessler RB, Damiano RJ. The effect of residual gaps in ablation lines for the treatment of atrial fibrillation. Heart Rhythm. 2005;2(5):S15. doi: 10.1016/j.hrthm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 19:16–24. doi: 10.1053/j.semtcvs.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Doll N, Kornherr P, Aupperle H, Fabricius AM, Kiaii B, Ullmann C, et al. Epicardial treatment of atrial fibrillation using cryoablation in an acute off-pump sheep model. Thorac Cardiovasc Surg. 2003;51(5):267–273. doi: 10.1055/s-2003-43086. [DOI] [PubMed] [Google Scholar]
- 9.van Brakel TJ, Bolotin G, Salleng KJ, Nifong LW, Allessie MA, Chitwood WR, Jr, et al. Evaluation of epicardial microwave ablation lesions: histology versus electrophysiology. Annals Thorac Surg. 2004;78(4):1397–1402. doi: 10.1016/j.athoracsur.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Melby SJ, Zierer A, Kaiser SP, Schuessler RB, Damiano RJ., Jr Epicardial microwave ablation on the beating heart for atrial fibrillation: the dependency of lesion depth on cardiac output. JThorac Cardiovasc Surg. 2006;132:355–360. doi: 10.1016/j.jtcvs.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor SL, Byrd GD, Diodato MD, Ishii Y, Lee AM, Prasad SM, et al. Microwave ablation for atrial fibrillation: dose-response curves in the cardioplegia-arrested and beating heart. Annals Thorac Surg. 2006;81(1):72. doi: 10.1016/j.athoracsur.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 12.Williams MR, Casher JM, Russo MJ, Hong KN, Argenziano M, Oz MC. Laser energy source in surgical atrial fibrillation ablation: preclinical experience. Ann Thorac Surg. 2006;82:2260–2264. doi: 10.1016/j.athoracsur.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Melby SJ, Gaynor SL, Lubahn JG. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J Thorac Cardiovasc Surg. 2006;132(4):853–860. doi: 10.1016/j.jtcvs.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Bugge E, Nicholson IA, Thomas SP. Comparison of bipolar and unipolar radiofrequency ablation in an in vivo experimental model. European J Cardio-thorac Surg. 2005;28(1):76–80. doi: 10.1016/j.ejcts.2005.02.028. discussion 80-82. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera JA, Ho SY, Climent V, Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008 Feb;29(3):356–62. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 16.Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999 Nov;10(11):1525–33. doi: 10.1111/j.1540-8167.1999.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, Mendonça MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005 Sep 6;112(10):1400–5. doi: 10.1161/CIRCULATIONAHA.105.551291. Epub 2005 Aug 29. [DOI] [PubMed] [Google Scholar]
- 18.Turek JW, Dibernardo LR, Lodge AJ, Lin SS, Davis RD, Milano CA, Simsir SA. Histopathology and transmurality of acute microwave lesions on the beating human atrium. Interact Cardiovasc Thorac Surg. 2006 Jun;5(3):202–6. doi: 10.1510/icvts.2005.126649. [DOI] [PubMed] [Google Scholar]
- 19.Epstein MR, Knapp LD, Martindill M, Lulu JA, Triedman JK, Calkins H, et al. Embolic complications associated with radiofrequency ablation. Am J Cardiol. 1996;77:655–658. doi: 10.1016/s0002-9149(97)89327-7. [DOI] [PubMed] [Google Scholar]
- 20.Lall SC, Damiano RJ. Surgical ablation devices for atrial fibrillation. J Interv Card Electrophysiol. 2007;20:73–82. doi: 10.1007/s10840-007-9186-x. [DOI] [PubMed] [Google Scholar]
- 21.Manasse E, Medici D, Ghiselli S, Ornaghi D, Gallotti R. Left main coronary arterial lesion after microwave epicardial ablation. Annals Thorac Surg. 2003;76(1):276–277. doi: 10.1016/s0003-4975(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 22.Manasse E, Colombo P, Roncalli M, Galloti R. Myocardial acute and chronic histological modifications induced by cryoablation. European J Cardio-thorac Surg. 2000;17(3):339–340. doi: 10.1016/s1010-7940(99)00361-9. [DOI] [PubMed] [Google Scholar]
- 23.Mikat EM, Hackel DB, Harrison L, Gallagher JJ, Wallace AG. Reaction of the myocardium and coronary arteries to cryosurgery. Laboratory Investigation. 1977;37(6):632–641. [PubMed] [Google Scholar]
- 24.Holman WL, Ikeshita M, Ungerleider RM, Smith PK, Ideker RE, Cox JL. Cryosurgery for cardiac arrhythmias: acute and chronic effects on coronary arteries. Amer JCardiol. 1983;51(1):149–155. doi: 10.1016/s0002-9149(83)80026-5. [DOI] [PubMed] [Google Scholar]
- 25.Curra F, Mourad P, Crum LA. High intensity focused ultrasound and tissue heating: the effect of nonlinear sound propagation and vessel presence. Proc IEEE Ultrasonics Symposium. 1998;2:1419–22. [Google Scholar]