Abstract
Objective
To report a case of seizures and supraventricular tachycardia (SVT) following confirmed synthetic cannabinoid ingestion.
Background
Despite widespread use of legal synthetic cannabinoids, reports of serious toxicity following confirmed use of synthetic cannabinoids are rare. We report severe toxicity including seizures following intentional ingestion of the synthetic cannabinoid JWH-018 and detail confirmation by laboratory analysis.
Case Report
A healthy 48 year old man had a generalized seizure within thirty minutes of ingesting an ethanol mixture containing a white powder he purchased from the Internet in an attempt to get high. Seizures recurred and abated with lorazepam. Initial vital signs were: pulse, 106/min; BP, 140/88 mmHg; respirations, 22/min; temperature, 37.7 °C. A noncontrast computed tomography of the brain and EEG were negative, and serum chemistry values were normal. The blood ethanol concentration was 3.8 mg/dL and the CPK 2,649 U/L. Urine drug screening by EMIT was negative for common drugs of abuse, including tetrahydrocannabinol. On hospital day 1, he developed medically refractory SVT. The patient had no further complications and was discharged in his normal state of health 10 days after admission. The original powder was confirmed by gas chromatography mass spectrometry to be JWH-018, and a primary JWH-018 metabolite was detected in the patient’s urine (200 nM) using liquid chromatography tandem mass spectrometry.
Discussion
Synthetic cannabinoids are legal in many parts of the world and easily obtained over the Internet. Data on human toxicity are limited and real-time confirmatory testing is unavailable to clinicians. The potential for toxicity exists for users mistakenly associating the dose and side effect profiles of synthetic cannabinoids to those of marijuana.
Conclusion
Ingestion of JWH-018 can produce seizures and tachyarrhythmias. Clinicians, lawmakers, and the general public need to be aware of the potential for toxicity associated with synthetic cannabinoid use.
Keywords: Synthetic Cannabinoids, CNS/Psychological/Heart, JWH-018
Introduction
Synthetic cannabinoids marketed under brand names like “K2” and “SPICE” herbal preparations have been previously reported in Western Europe and Asia and are gaining popularity in the United States. These products are widely available through local “head shops” and Internet resources and are typically labeled as “incense” and “not for human consumption,” or “for aromatherapy only”. Despite package labeling, these herbal mixtures are frequently laced with a variety of synthetic cannabinoids, which when smoked can deliver a “legal” high and avoid detection in common drug screens. 1–4
In 2008, The United States (US) Customs and Border Protection service analyzed “SPICE” products and found them to contain pharmacologically active compounds, some of which were originally manufactured as investigative drugs but not further developed because of their undesirable psychoactive properties. 5 At the time of identification, these products were not subject to regulatory authority in the US due to their unique chemical classification and the packaging designating them as “not for human consumption.” With increased prevalence of human use came the recognition of significant public health threats, and several states and municipalities adopted ordinances to control the sale and distribution of synthetic cannabinoids. In response to increasing reports through the National Forensic Laboratory Information System and the American Association of Poison Control Centers, the US Department of Justice initiated the process of formally scheduling several synthetic cannabinoids in November 20106 resulting in temporary Schedule I status for JWH-018, JWH-073, JWH-200, CP 47,497 and a C8 homologue of CP 47,497 effective from March 2011.
The clinical effects of the synthetic cannabinoids laced in herbal products are mediated through agonism at the CB1 receptor, the same receptor through which Δ9-THC exerts its psychoactive effects. 7,8 However, the lack of quality control for these products raises concern regarding the potential for toxicity following human consumption. In addition, emerging data suggest that there may be stark differences in relative potency between synthetic cannabinoids and Δ9-THC. Unlike the low efficacy partial agonist effects of Δ9-THC, JWH-018, one of the more popular synthetic cannabinoids found in “K2” products, has been characterized as a full agonist with significantly increased potency relative to Δ9-THC.7,9–15 This increased potency is likely to be associated with an increased likelihood of adverse effects.
Case reports of human exposure and toxicity are limited. The few cases published in the medical literature report similar adverse clinical effects to those of marijuana including anxiety, tachycardia and hallucinations. 16–19 Like marijuana, withdrawal from synthetic cannabinoid use is also reported after abrupt cessation in tolerant individuals. 20 Additionally, two deaths have been reported in the US with “K2” product use. The first case involved an adolescent who committed suicide after experiencing extreme anxiety following use, and the second case involved an adolescent who died following a coronary ischemic event. 21,22 In previously reported cases, “K2” or “SPICE” was smoked in the same manner as traditional cannabinoids and to our knowledge no account of a large ingestion of pure JWH-018 has been reported.
We report serious toxicity in a 48 year-old man who ingested the synthetic cannabinoid JWH-018 obtained from a bulk supply. We confirm exposure to JWH-018 through forensic evaluations of the product and through the use of a quantitative assay for a primary metabolite of JWH-018 excreted urine.
Case Report
A previously healthy 48 year-old man was witnessed to ingest a mixture of ethanol and an unknown a powder he purchased over the Internet. Within thirty minutes after ingestion the patient exhibited agitation and had a generalized seizure witnessed by his wife. EMS providers observed additional seizure activity while on route to the Emergency Department (ED). On arrival, he was awake with the following vital signs: pulse, 106/min; BP, 140/88 mmHg; respirations, 22/min; temperature, 37.7 °C. Initial electrocardiogram showed sinus tachycardia with regular intervals. Seizure activity recurred in the ED and abated following the administration of intravenous lorazepam. The patient was subsequently intubated for airway protection. Initial laboratory values were: sodium 134 mEq/L, potassium 4.6 mEq/L, chloride 98 mEq/L, bicarbonate 24 mEq/L, blood urea nitrogen 23 mg/dL, creatinine 1.0 mg/dL, glucose 145 mg/dL, ethanol 3.8 mg/dL, and creatinine phosphokinase 2649 U/L. Acetaminophen and salicylates were not detected. A non-contrast computed tomography (CT) scan of the brain and EEG were unremarkable. Urine toxicology screening by the hospital clinical laboratory was negative for drugs of abuse, including THC. The patient’s family corroborated that he had no recent use of marijuana. On hospital day 1 the patient developed refractory supraventricular tachycardia that required electrical cardioversion. No elevations in cardiac enzymes were detected and the patient had no further dysrhythmias. On the third day of hospitalization his wife produced a small bag of the powder he had ingested as well as a receipt for its purchase via the Internet. The rest of his clinical course was unremarkable and the patient was subsequently discharged home in his baseline state of good health.
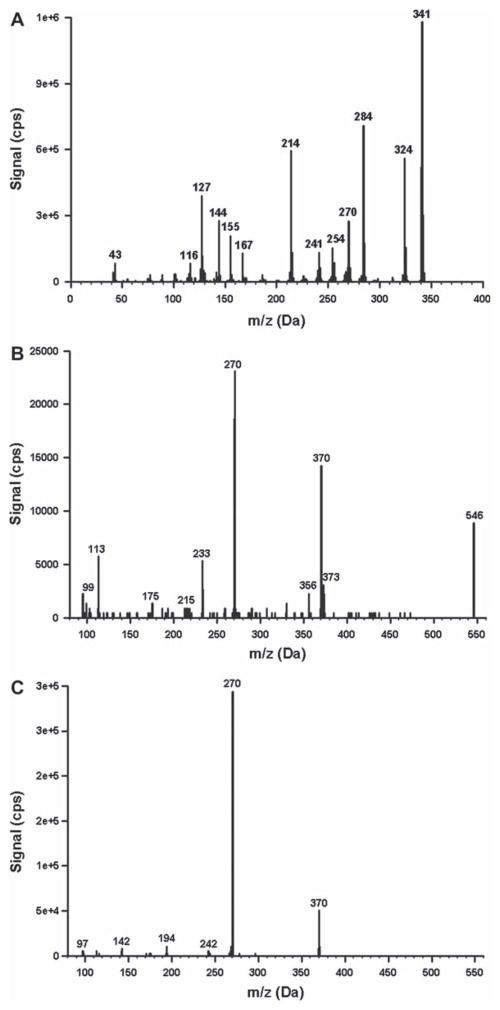
The product suspected of being self-administered was characterized as an off-white powder containing JWH-018. The product was analyzed by gas chromatography mass spectrometry (GC-MS) to assess cannabinoid content and to determine if other drugs were present. GC-MS procedures were validated forensic procedures commonly used by the Arkansas State Crime Laboratory. GC-MS characteristics of the powder were consistent with a JWH-018 analytical standard (Cayman Chemical) (Fig. 1). No other drugs were detected using analytical procedures capable of detecting trace levels of many Aminoalkylindoles (AAIs) and other synthetic cannabinoids and cathinones commonly present in a variety of “K2” products.
Fig. 1.
(A) Mass spectra generated from the white powder (A), the glucuronic acid conjugate excreted in urine (B), and the JWH-018 carboxy metaboloite excreted in urine (C). The white powder was analyzed using GC-MS while the urinary metabolites were analyzed using LC-MS/MS. Mass spectra were compared with purchased standards of JWH-018 and its carboxy metabolite (Cayman Chemical). An analytical standard of the glucuronic acid conjugate was not available.
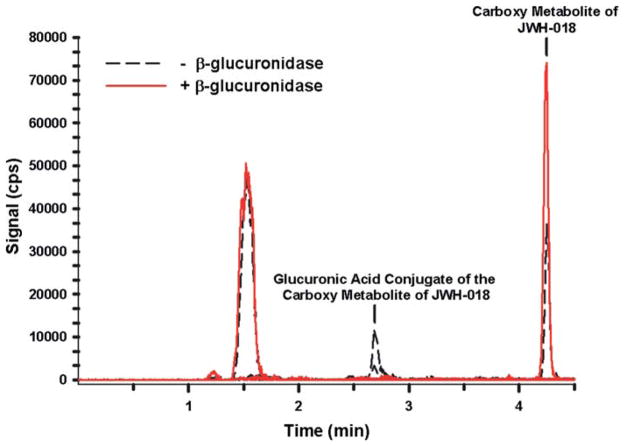
As a way to confirm product use, the patient’s urine sample was analyzed by negative ion mode, liquid chromatography tandem mass spectrometry methods established to detect major JWH-018 metabolites, 34–36 as approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. The method used a purchased standard (Cayman Chemical, Ann Arbor, Michigan) to confirm the presence of a major omega carboxyl metabolite previously shown to be excreted after JWH-018 use. 23,34–36 The urinary concentration of this metabolite was approximately 200 nM (Figs. 1, 2). In addition, analysis of resulting mass spectra and reactions with β-glucuronidase showed that approximately 50% of the carboxyl metabolite was excreted as the glucuronic acid conjugate (Figs. 2, 3). This is consistent with previous reports showing that JWH-018 metabolites are primarily excreted as conjugates. 23,34–36
Fig. 2.
Extracted ion chromatographs from LC-MS/MS experiments performed with the urine specimen before (black dash tracing) and after (red tracing) β-glucuronidase incubation (see colour version of this figure online).
Discussion
JWH-018 is a relatively potent agonist of G-protein coupled cannabinoid receptors and is commonly used to lace “K2” and “SPICE” products.24 It has nonselective agonist effects at both centrally and peripherally located CB1 and CB2 receptors. 7 Studies in animals have shown that CB1 receptors are located presynaptically on both glutamanergic and GABAergic synapses, suggesting a role for these receptors in modulation of neurotransmitter signaling mechanisms. 25
Although traditional and nontraditional cannabinoids lower the seizure threshold in animal models, seizures following THC use in humans are rarely reported. 26,27 However, the selective CB1 antagonist, rimonabant induced seizures in animal models. 28 It is possible that the patient experienced seizures through a dose-response mechanism. One postulation is that high doses of JWH-018 and the resulting CB1 agonism result in a presynaptic release of excitatory neurotransmitters or a decrease in inhibitory neurotransmitters.
Tachycardia and tachyarrhythmias have been reported following use of traditional 29–31 as well as synthetic cannabinoids.16–19 The patient’s medically refractory supraventricular tachycardia may have resulted from increased circulating catecholamines or oxidative demands on the myocardium, both of which have been previously reported with CB1 receptor agonists. 32
Public health risks of synthetic cannabinoids are of great concern due to the known variability in potency in “K2” and “SPICE” products.1–4,7 As marijuana is the most frequently abused illicit drug in the US, 33 the continued availability of a legal marijuana substitute to both users and retailers may result in consequential exposures to a novel drug that is likely more dangerous than the illicit one it supplants. Further development of analytical methods for the analysis of these emerging drugs of abuse will help delineate the clinical pharmacology and toxicology of these compounds in man. This case study indicates that JWH-018 is orally bioavailable in humans, and with the quick onset of seizure, appears to be rapidly distributed.
Conclusion
We describe a patient with repetitive seizures and supraventricular tachycardia following confirmed ingestion of the synthetic cannabinoid JWH-018. Clinicians should consider synthetic cannabinoid exposure in the differential diagnosis of patients who present with agitated delirium and seizures following recreational drug abuse.
Footnotes
Declaration of interest
This work was supported by a Centers for Disease Control (Contract No. 200-2007-21729) (J.H.M.) and by a Pilot Research Award (L.P.J.) from the University of Arkansas for Medical Sciences Center for Clinical and Translational Research, supported by a grant from the National Center For Research Resources (No. 1UL1RR029884).
References
- 1.EMCDDA. European Monitoring Centre for Drugs and Drug Addiction EMCDDA 2009 Thematic paper ed. Office for Official Publications of the European Communities; 2009. Understanding the ‘Spice’ phenomenon; pp. 1–25. [Google Scholar]
- 2.Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45:1186–94. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- 3.Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull. 2009;57:439–441. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- 4.Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan Pl, Wood DM. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol chem. 2010;34:252–260. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- 5.Iversen LL. The Science of Marijuana. 2. Vol. 2. New York: Oxford University Press, Inc; 2008. [Google Scholar]
- 6.21 Code of Federal Regulations: Part 1308, Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I. 2010;75:71635–71638. [Google Scholar]
- 7.Huffman JW. Cannabimimetic indoles, pyrroles, and indenes: structure-activity relationships and receptor interactions. In: Reggio PH, editor. The Cannabinoid Receptors, The Receptors. Vol. 1. Humana Press; New York, NY 10013: 2009. pp. 49–94. [Google Scholar]
- 8.Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR. 1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: new high affinity ligands for the cannabinoid CB1 and CB2 receptors. Bioorg Med Chem Lett. 2006;16:5432–5435. doi: 10.1016/j.bmcl.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 9.Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- 10.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:89–999. [PubMed] [Google Scholar]
- 11.Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- 12.Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- 13.Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- 14.Bell MR, D’Ambra TE, Kumar V, Eissenstat JL, Jr, WEtzel JR, Rosi D, Phillon RE, Daum SJ, Hlasta DJ. Antinociceptive (aminoalkyl)indoles. J Med Chem. 1991;34:1099–1110. doi: 10.1021/jm00107a034. [DOI] [PubMed] [Google Scholar]
- 15.Eissenstat MA, Bell MR, D’Ambra TE, Alexander EJ, Daum SJ, Ackerman JH, Gruett MD, Kumar V, Estep KG, Olefirwicz EM. Aminoalkylindoles: structure-activity relationships of novel cannabinoid mimetics. J Med Chem. 1995;38:3094–3105. doi: 10.1021/jm00016a013. [DOI] [PubMed] [Google Scholar]
- 16.Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. 2010;26:462–465. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- 17.Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118:309–310. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105:1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- 19.Schneir AB, Cullen J, Ly BT. “Spice” Girls: Synthetic Cannabinoid Intoxication. J Emerg Med. 2011;40:296–9. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay M. The New York Times. Jul 10, 2010. Synthetic Marijuana Spurs State Bans. [Google Scholar]
- 22.Fisher WG. Musings in the life of an internist, cardioloist and cardiac electrophysiologist. Evanston, IL: 2010. Aug 15, Inhaled incense “K2” may cause heart damage. http://drwes.blogspot.com/2010/08/inhaled-incense-k2-may-cause-heart.html? [Google Scholar]
- 23.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Tilelli JA, Spack LD. Marijuana intoxication presenting as seizure—comment. Pediatr Emerg Care. 2006;22:141. doi: 10.1097/01.pec.0000204831.96139.52. [DOI] [PubMed] [Google Scholar]
- 27.Bonkowsky JL, Sarco D, Pomeroy SL. Ataxia and shaking in a 2-year-old girl: acute marijuana intoxication presenting as seizure. Pediatr Emerg Care. 2005;21:527–528. doi: 10.1097/01.pec.0000173349.38024.33. [DOI] [PubMed] [Google Scholar]
- 28.Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- 29.Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(11 Suppl):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 30.Kiplinger GF, Manno JE. Dose-response relationships to cannabis in human subjects. Pharmacol Rev. 1971;23:339–347. [PubMed] [Google Scholar]
- 31.Petronis KR, Anthony JC. An epidemiologic investigation of marijuana- and cocaine-related palpitations. Drug Alcohol Depend. 1989;23:219–226. doi: 10.1016/0376-8716(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 32.Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int J Cardiol. 2007;118:141–144. doi: 10.1016/j.ijcard.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- 34.Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, et al. Conjugation of Synthetic Cannabinoids, JWH-018 [Naphthalen-1-yl-(1-pentylindol-3-yl)methanone] and JWH-073 [naphthalen-1-yl-(1-butylindol-3-yl)methanone], Metabolites by Human UDP-glucuronosyltransferases. Drug Metab Dispos. 2011 Jul 11; doi: 10.1124/dmd.111.040709. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal Chem. 2011;1;83:4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, et al. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. 2011;83:6381–8. doi: 10.1021/ac201377m. Epub 2010 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]