Summary
Background
We assessed whether overnight home use of automated closed loop insulin delivery (artificial pancreas) improves glucose control.
Methods
We studied 24 adults with type 1 diabetes in a multicentre crossover study design comparing four weeks of overnight closed loop using a model predictive control algorithm to direct insulin delivery, with four weeks of insulin pump therapy in which participants used real-time display of continuous glucose monitoring independent of their pumps as control. Primary outcome was time when glucose was in the target range of 3·9 and 8·0mmol/l between midnight to 07:00. Analyses were by intention to treat. Trial registration ClinicalTrials.gov NCT01440140.
Findings
Closed loop was utilised over median 8·3 (interquartile range 6·0, 9·6)hours on 555nights (86%). Proportion of time when overnight glucose was in target range was significantly higher during closed loop compared to control by 13·5% (95% CI, 7·3–19·7; p<0·001). Mean overnight glucose (8·2±0·9 vs. 9·0±1·3mmol/l; p=0·005) and time spent above target (44·3%±11·9 vs. 57·1%±15·6; p=0·001) were significantly lower during closed loop. Time spent below target was low and comparable [1·8%(0·6, 3·6) vs. 2·1%(0·7, 3·9); p=0·28]. Lower mean overnight glucose was brought about by increased overnight insulin delivery [6·4 (4·5, 8·1) vs. 4·9 (3·7, 6·3)units; p<0·001) without changing the total daily insulin amount [34·5 (29·3, 48·4) vs. 35·4 (29·7, 45·2)units; p=0·32]. No severe hypoglycaemia episodes occurred during control period and two during closed loop not related to algorithm instructions.
Interpretation
Unsupervised overnight closed loop at home is feasible and may improve glucose control in adults with type 1 diabetes.
Introduction
Intensive insulin therapy has been the standard of care in the management of type 1 diabetes since the Diabetes Control and Complications Trial1. However, tightening glycaemic control increases the risk of hypoglycaemia2,3, alleviated in part by modern insulin analogues4 and educational interventions in adults5 but not youth6. Individuals with type 1 diabetes continue to face daily challenges of complex insulin regimes involving multiple daily insulin boluses, frequent blood glucose monitoring, and unpredictable glucose excursions7. Recent advances in diabetes technology have highlighted their increasing role in clinical care. Continuous glucose monitoring devices measure interstitial glucose every one to five minutes leading to improved glycaemic control8. Randomised control trials demonstrated the benefits of sensor-augmented pump therapy in reducing HbA1c9. The advent of the threshold suspend feature allows insulin delivery to be suspended automatically for up to two hours and has been shown to reduce the duration and frequency of hypoglycaemia10,11.
Closed loop insulin delivery, known as the artificial pancreas, is a novel approach which is more complex and differs from conventional pump therapy and the threshold suspend approach through a control algorithm that autonomously increases and decreases subcutaneous insulin delivery based on real-time sensor glucose levels thereby mimicking physiological insulin delivery12. Clinical research facility studies have demonstrated closed loop insulin delivery to be a feasible and safe improving glycaemic control and reducing the risk of hypoglycaemia13–15.
Follow-up transitional and ‘out-of-hospital’ single night studies have been promising16,17 paving the way towards developing ambulatory closed loop prototypes such as that used in a three-week single centre study in adolescents18. In the present study, we hypothesised that four-week overnight unsupervised closed loop insulin delivery at home in adults may improve glycaemic control without increasing the risk of hypoglycaemia.
Methods
Participants and study design
We identified eligible adults from diabetes clinics attending Addenbrooke’s Hospital, Cambridge, Sheffield Teaching Hospitals, Sheffield, and King’s College Hospital, London. Inclusion criteria were type 1 diabetes (World Health Organisation criteria), C-peptide negative, age 18 years and older, insulin pump therapy for at least 3 months, knowledge of insulin self-adjustment, performing glucose self-monitoring at least four times daily, and HbA1c≤10% (86mmol/mol). Exclusion criteria included established nephropathy, neuropathy or proliferative retinopathy, total daily insulin dose ≥2·0U/kg, regular use of continuous glucose monitoring within one month prior to enrolment, severe visual or hearing impairment, pregnancy or breast feeding. All participants signed informed consent prior to the start of study-related procedures. The study protocol was approved by the East of England Central Cambridge Ethics Committee.
Figure 1 outlines the open label randomised controlled cross-over study design. Following the run-in phase, participants applied insulin pump therapy with real-time continuous glucose monitoring at home on two periods with or without overnight closed loop. Each period lasted four weeks, Identical study insulin pump and real-time continuous glucose monitoring device were used during the two study periods, which were separated by a three to four-week washout during which participants used their own pump and discontinued continuous glucose monitoring.
Figure 1.
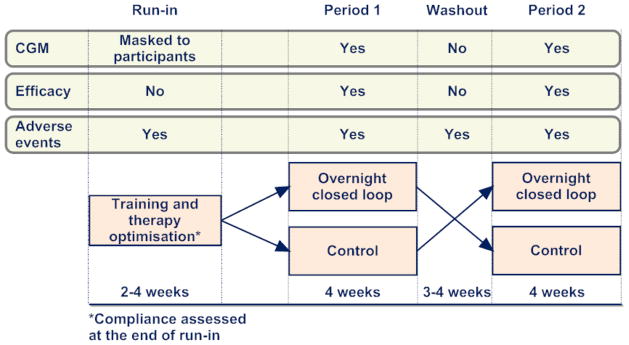
Study design comparing overnight closed loop insulin delivery with control. The outline shows when continuous glucose monitoring data were collected, efficacy assessed, and adverse events monitored.
Randomisation and masking
The order of the two study periods was random and was determined following the run-in phase using computer generated permuted block randomisation. During the run-in phase, the continuous glucose monitor receiver was modified and participants were blinded to the recorded sensor glucose levels. Participants had access to sensor glucose readings after the end of the run-in phase.
Procedures
On enrolment, participants were trained on the study insulin pump (Dana R Diabecare, Sooil, Seoul, South Korea) and continuous glucose monitoring device (FreeStyle Navigator, Abbott Diabetes Care, Alameda, CA, USA). Participants calibrated the real-time continuous glucose monitoring device according to manufacturer’s instructions. During the run-in phase, we assessed compliance by assessing the number of days continuous glucose monitoring data were available from sensor glucose downloads. Each participant was required to use the study pump and continuous glucose monitor for at least two weeks. Downloaded sensor glucose readings at the end of the run-in phase were used to optimise insulin pump therapy. Participants used rapid acting insulin analogue normally used in their usual clinical care. The built-in bolus wizard of the study insulin pump was used by participants during both interventions to calculate insulin boluses at mealtimes and also when administering correction boluses.
During the control period, participants used real-time continuous glucose monitor and the study pump. The sensor glucose alarm threshold for hypoglycaemia was initially set at 3·5mmol/l but could be modified by the participants. During the closed loop period, participants were admitted to the local clinical research facility for their first closed loop night and received training on the closed loop system which was used under supervision overnight. Training lasted 60 to 90minutes and covered initiation and discontinuation of the closed loop system as well as problem troubleshooting. Participants were trained to perform calibration checks before evening meal; if sensor glucose was above capillary glucose by more than 3mmol/l, the continuous glucose monitor was re-calibrated and calibration check was repeated before starting closed loop. These instructions reduced the risk of sensor error and the calibration check approach was effective when assessed by computer modelling19. If sensor glucose readings became unavailable or in case of other system failures, participants were alerted by an audible alarm and the system restarted participant’s usual insulin delivery rate within 30 to 60 minutes to mitigate the risk of insulin under- and over-delivery20.
From the following night, participants used the closed loop system unsupervised at home for four weeks. Participants were instructed to initiate the system at home following their evening meal, and to discontinue it before breakfast the next morning. Participants were not restricted in dietary intake or daily activities. A 24-hour telephone support assisted participants in clinical or technical issues that arose during the study. Standard local hypoglycaemia and hyperglycaemia treatment guidelines were followed.
Blood samples for HbA1c, fructosamine, random glucose and C-peptide measurements were taken after enrolment. HbA1c and fructosamine were additionally measured before and after each study period.
Closed loop control algorithm
The Florence automated closed loop system21 comprised a model predictive control (MPC) algorithm residing on a handheld computer linked by cable to the continuous glucose monitoring receiver. Every 12 minutes, the treat-to-target algorithm calculated a new insulin infusion rate, which was automatically set on the study pump using wireless communication. The calculations utilised a compartment model of glucose kinetics22 describing the effect of rapid-acting insulin and the carbohydrate content of meals on glucose levels. The algorithm was initialised using pre-programmed basal insulin delivery. Participant’s weight and total daily insulin dose were entered at setup by the research team on the first night of closed loop. Carbohydrate intake data entered by participants into the insulin pump built-in bolus wizard was automatically downloaded to the handheld computer when closed loop was turned on. Insulin delivery history, including manually instructed insulin boluses, was also automatically downloaded. The algorithm included rules that limited maximum insulin infusion and suspended insulin delivery if glucose was at or below 4·3mmol/l or when glucose was rapidly decreasing. Algorithm version 0·3·24 was used (University of Cambridge).
Assays
We used a chemiluminescence immunoassay (Diasorin Liaison XL, Deutschland GmbH, Dietzenbach, Germany; inter-assay CV 5·6% at 563pmol/l, 4·5% at 2529pmol/l, 5·8% at 5449pmol/l) to measure baseline plasma C-peptide. We determined fructosamine using an enzymatic assay (Randox, Antrim, United Kingdom; interassay CVs 9·5% at 193 μmol/l, 6·4% at 430μmol/l, 5·2% at 669μmol/l). HbA1c was measured centrally using ion exchange high performance liquid chromatography (G8 HPLC Analyzer, Tosoh Bioscience Inc., CA, US; interassay CVs 1·3% at 31·2 mmol/mol, 0·8% at 80·5mmol/mol).
Sample size
The power calculation was based on a previous study14. We anticipated that overnight closed loop insulin delivery would increase the percentage night-time glucose was between 3·9 and 8·0mmol/l by a mean 13% (SD 25%). We calculated that 24 participants would provide 80% power at the 5% level of significance to detect such difference between sensor-augmented pump therapy and overnight closed loop insulin delivery.
Statistical analysis
The statistical analysis plan was agreed upon by investigators in advance. The analyses were performed on an intention to treat basis. Each night was analysed to the treatment group assigned. The primary efficacy outcome was the time spent in the target glucose range (3·9 to 8·0mmol/l) between midnight and 07:00 as recorded by CGM. Secondary outcomes included mean glucose, time spent below 3·9mmol/l (hypoglycaemia) and above 8·0mmol/l (hyperglycaemia), and insulin delivery. Glucose variability overnight was assessed by the standard deviation and the coefficient of variation of CGM levels. Hypoglycaemia burden was assessed by calculating the glucose sensor area under the curve less than 3·5mmol/l and the number of nights with sensor glucose less than 3·5mmol/l for at least 20minutes. Outcomes were additionally calculated using adjusted sensor glucose assuming a 15% measurement error to correct for bias resulting from simultaneous use of sensor glucose to direct insulin delivery23. Secondary outcomes were calculated from midnight to 07:00 and over the 24 hour period. Differences in HbA1c and plasma fructosamine levels were calculated to determine changes in metabolic control.
Outcomes were calculated using GStat software, Version 2·0 (University of Cambridge, Cambridge, UK) and statistical analyses using SPSS, Version 21 (IBM Software, Hampshire, UK). Normally distributed data were compared using paired t-test while non-normally distributed data using Wilcoxon signed rank test. To assess end-period HbA1c, a regression model adjusted for pre-period HbA1c level was fitted to compare the two treatments. A similar analysis was carried out to assess changes in fructosamine. Values are reported as mean±SD or median (quartile 1, quartile 3), unless stated otherwise. All p-values are two-tailed and values less than 0·05 were considered statistically significant.
Role of the funding and support sources
No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the report. Abbott Diabetes Care read the manuscript before submission. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the publication.
Results
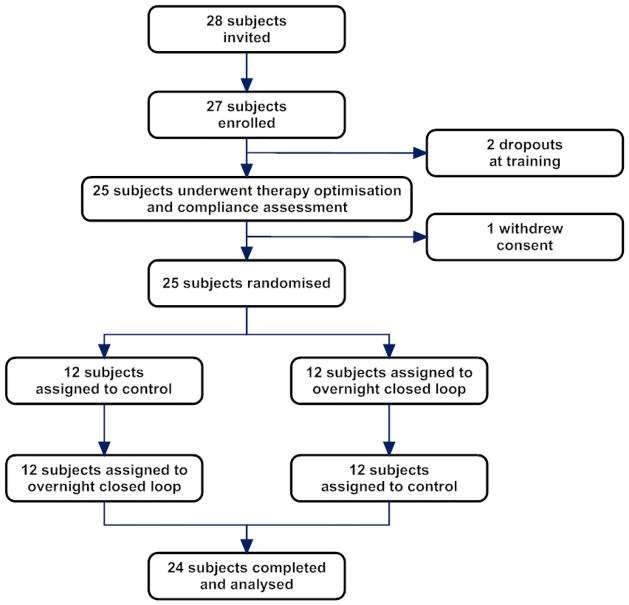
Twenty-seven participants were recruited from December 2012 to September 2013. Figure 2 shows flow of participants through the study. Two participants withdrew during the run-in phase. Twenty-five eligible participants were randomised; nine from Addenbrooke’s Hospital Cambridge, eight from King’s College Hospital London, and eight from Sheffield. One participant withdrew consent following randomisation. Data from 24 participants completing the study were analysed. Table 1 summarises baseline characteristics.
Figure 2.
Flow of participants through the trial.
Table 1.
Baseline characteristics (n = 24).
| Gender (M/F) | 13/11* |
| Age (years) | 43±12 |
| BMI (kg/m2) | 26·0±3·5 |
| HbA1c (%) | 8·1±0·8 |
| HbA1c (mmol/mol) | 65±9 |
| Duration of diabetes (years) | 29±11 |
| Duration on pump (years) | 6·3±4·4 |
| Total daily insulin (U/kg/day) | 0·5±0·1 |
All C-peptide < 33pmol/l
Primary and secondary analyses are shown in Table 2. The time when overnight sensor glucose was in target range, was significantly increased by a mean 13·5% (95% CI, 7·3–19·7, p<0·001) during overnight closed loop insulin delivery. No period (p=0·77) or carry-over effect (p=0·84) on primary endpoint was detected. Sensor glucose profiles are shown in Figure 3. In all but three participants, closed loop improved time spent in target range (Figure 4). In one of the three participants, time spent in hypoglycaemia was reduced by 15·1% and in the other two by 2·4% and 2·7%. Closed loop reduced mean overnight glucose and time above target range without increasing time spent in the hypoglycaemia range. Time spent in hypoglycaemia below 3·9mmol/l was low (median time less than 10 minutes per night) and comparable during the two study periods. There were no significant differences in the burden of hypoglycaemia as measured by the AUC below 3·5 mmol/l (p=0·61), number of nights during the study when sensor glucose was less than 3·5mmol/l for at least 20minutes (p=0·18) and the low blood glucose index24 (p=0·44).
Table 2.
Comparison of overnight glucose control from midnight to 07:00 during closed loop and control period using unadjusted (raw) sensor glucose over 28 days in the home setting.
| Closed loop (n=24) | Control (n=24) | Paired difference* (n=24) | p value | |
|---|---|---|---|---|
| Mean glucose (mmol/l) | 8·2±0·9 | 9·0±1·3 | −0·8±1·3 | 0·005 |
| SD of glucose (mmol/l) | 2·0±0·3 | 1·9±0·3 | 0·1±0·4 | 0·18 |
| Within night CV of glucose (%) | 24±3 | 21±4 | 3±6 | 0·01 |
| Between nights CV of glucose (%) | 26±6 | 29±7 | −3±9 | 0·11 |
| Time spent at glucose level (%) | ||||
| 3·9 to 8·0mmol/l†, | 52·6±10·6 | 39·1±12·8 | 13·5±14·7 | <0·001 |
| 3·9 to 10·0mmol/l | 73·2±9·0 | 61·2±13·7 | 12·0±14·2 | <0·001 |
| > 8·0mmol/l | 44·3±11·9 | 57·1±15·6 | −12·8±16·5 | 0·001 |
| > 16·7mmol/l** | 1·1 (0·0, 2·8) | 1·5 (0·1, 3·4) | −0·0(−1·6, 0·5) | 0·54 |
| < 3·9mmol/l** | 1·8 (0·6, 3·6) | 2·1 (0·7, 3·9) | −0·3 (−2·4, 1·0) | 0·28 |
| < 3·5 mmol/l** | 0·7 (0·3, 1·4) | 0·7 (0·3, 2·0) | 0·3 (−17·4, 3·4) | 0·3 |
| < 2·8mmol/l** | 0·2 (0·0, 0·7) | 0·2 (0·0, 1·3) | 0·0 (−0·9, 0·2) | 0·63 |
| AUCDay below 3·5mmol/l** (mmol/l × minutes) | 4·0 (0·8, 15·1) | 5·3 (0·4, 25·6) | 0·3 (−17·4, 3·8) | 0·61 |
| Number of nights when glucose <3·5mmol/l‡ | 36 (5·4%) | 58 (8·6%) | - | 0·18 |
| LBGI** | 0·67 (0·27, 0·96) | 0·62 (0·25, 1·21) | −0·02 (−0·69, 0·32) | 0·44 |
| Glucose at 21:00 (mmol/l) | 8·6±0·9 | 9·3±1·3 | −0·6±1·3 | 0·02 |
| Glucose at midnight (mmol/l) | 9·2±1·3 | 9·2±1·7 | 0·01±1·2 | 0·9 |
| Glucose at 07:00 (mmol/l) | 7·2±0·9 | 8·8±1·2 | −1·6±1·5 | <0·001 |
Closed loop minus control. Positive value indicates measurement was higher on night of closed loop delivery compared with night of control
Data are presented as mean ± SD, or **median (interquartile range)
Primary endpoint
Number of nights over four weeks when sensor glucose was < 3·5mmol/l for at least 20 minutes.
LBGI = low blood glucose index; CGM = continuous glucose monitoring
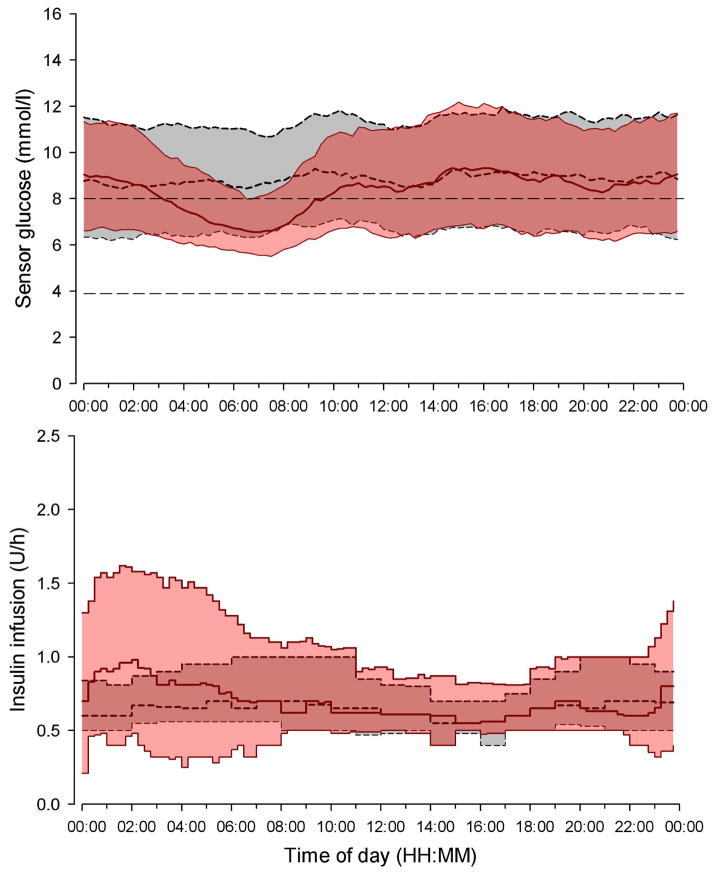
Figure 3.
Median (interquartile range) of sensor glucose (top panel) and insulin delivery (bottom panel) during closed loop (solid red line and red shaded area) and control (dashed black line and gray shaded area) period for the 24-hour duration. The glucose range 3·9 to 8·0 mmol/l is denoted in the top panel by horizontal dashed lines.
Figure 4.
Individual values of time when glucose was in target glucose range from 3·9 to 8·0 mmol/l (left panel) and mean overnight glucose (right panel) (n=24).
Increased time spent in target range and reduced mean overnight glucose (Table 2 and Figure 4) was brought about by closed loop delivering 30% more insulin overnight (Table 3 and Figure 3, bottom panel) but total daily insulin delivery did not differ between the two study interventions (Table 3). Overnight closed loop was utilised on 555 nights (86%); turned on at 22:52 (22:05, 23:44) and turned off at 07:23 (06:41, 08:29) operating over 8·3 (6·0, 9·6) hours (Table 4). Closed loop was unintentionally interrupted on average every 41 hours (once every 5 nights). The commonest cause (over 60%) of interruptions was the loss of wireless connectivity between handheld computer and insulin pump (Table 4). Other causes included inability to initiate closed loop cycle within 30 minutes, glucose sensor unavailability, and change of temporary infusion by user. Participants contacted the 24 hour support line approximately four times during the closed loop period.
Table 3.
Insulin delivery overnight (00:00 – 07:00) and over 24-hour period.
| Closed loop (n=24) | Control (n=24) | p value | |
|---|---|---|---|
| Overnight insulin delivery (U)* | 6·4 (4·5, 8·1) | 4·9 (3·7, 6·3) | <0·001 |
| Total daily insulin delivery (U)* | 34·5 (29·3, 48·4) | 35·4 (29·7, 45·2) | 0·32 |
| SD of overnight insulin delivery (U) | 0·6±0·2 | 0·1±0·1 | <0·001 |
Data shown are mean ± SD, or *median (interquartile range)
Table 4.
Utility and failure analysis of closed loop operation.
| Number of nights when closed loop turned on | 555 (86%) |
| Time of day when closed loop turned on* | 22:52 (22:05, 23:44) |
| Time of day when closed loop turned off* | 07:23 (06:41, 08:29) |
| Duration of closed loop operation (hours)* | 8·3 (6·0, 9·6) |
| Total duration of closed loop operation (h) | 4613 |
| Number of events when closed loop interrupted (% of total interruptions) | |
| lack of pump connectivity | 68 (61) |
| unable to start closed loop cycle within 30 mins | 21 (20) |
| sensor data unavailability | 7 (6) |
| Temporary infusion changed by user | 7 (6) |
| extended bolus changed by user | 4 (4) |
| handheld computer operating system failure | 4 (4) |
| handheld computer software system error | 1 (1) |
Median (interquartile range) from all study nights when closed loop turned on
Overnight glucose variability measured as the standard deviation was comparable during the two interventions. The coefficient of variation of overnight glucose within each night was increased during closed loop. Conversely, a trend towards a reduced between-nights coefficient of variation was observed during closed loop, accompanied by consistently lower morning glucose (Table 2). This was not associated with increased time spent below 3·9mmol/l or AUC below 3·5mmol/l (Supplementary material, Table S1). Outcomes based on adjusted sensor glucose values were in concordance with outcomes based on unadjusted sensor glucose; the proportion of time when adjusted overnight glucose was in target increased during closed loop compared to sensor-augmented therapy by a mean of 13·4% (p<0·001). Time above target was reduced by a mean of 11·9% (p=0·001) and time below target was comparable [2·2 (0·7, 3·9) vs. 2·5 (1·0, 4·5) %, p=0·21].
Endpoints calculated from midnight to midnight are shown in Table 5. Overnight closed loop significantly reduced 24-hour glucose by 0·5mmol/l (p<0·001) and increased proportion of time spent within wider target range (p=0·002). Similar to the overnight period analyses, time when glucose was above 10·0mmol/l was significantly reduced. Participants performed on average eight capillary glucose measurements per day. Overall sensor accuracy in relation to capillary glucose was good with the median absolute deviation 0·8 (0·3, 1·5)mmol/l and the median absolute relative deviation of 10·4% (4·7, 19·3). Seventy-eight percent of values were in Clarke Error Grid zone A. Median absolute relative deviation of sensor glucose during closed loop and control interventions was 10·1% and 10·7%, respectively.
Table 5.
Comparison of 24-hour glucose control during closed loop and control using unadjusted (raw) sensor glucose over 28 days in the home setting.
| Closed loop (n=24) | Control (n=24) | Paired difference* (n=24) | p value | |
|---|---|---|---|---|
| Mean glucose (mmol/l) | 8·7±0·8 | 9·3±1·1 | −0·5±0·7 | 0·001 |
| SD of glucose (mmol/l) | 2·9±0·4 | 2·9±0·4 | −0·0±0·3 | 0·79 |
| Within day CV of glucose (%)** | 34·1 (31·1, 35·8) | 32·6 (30·0, 34·1) | 1·9 (−0·6, 3·4) | 0·02 |
| Between day CV of glucose (%)** | 14·9 (12·4, 16·6) | 15·3 (13·6, 21·3) | -- | 0·11 |
| Time spent at glucose level (%) | ||||
| 3·9 to 10·0mmol/l | 66·0±7·7 | 59·7±10·8 | 6·4±8·7 | 0·002 |
| >10·0mmol/l | 30·8±9·3 | 37·3±12·3 | −6·5±8·7 | 0·001 |
| > 16·7mmol/l** | 1·9 (1·0, 2·9) | 2·2 (1·0, 3·0) | −0·6 (−1·2, 0·5) | 0·33 |
| < 3·9mmol/l** | 1·7 (0·9, 3·1) | 1·7 (1·1, 3·5) | −0·2 (−1·8, 0·5) | 0·27 |
| < 3·5mmol/l** | 0·8 (0·4, 1·4) | 0·7 (0·5, 1·6) | −0·2 (−0·8, 0·3) | 0·11 |
| < 2·8mmol/l** | 0·2 (0·0, 0·6) | 0·2 (0·1, 0·6) | 0·0 (−0·3, 0·2) | 0·84 |
| AUCDay below 3·5mmol/l (mmol/l × minutes)** | 4·7 (1·3, 11·9) | 4·5 (1·8, 17·2) | −0·2 (−7·2, 1·9) | 0·42 |
| LBGI** | 0·57 (0·36, 0·84) | 0·54 (0·34, 0·96) | 0·0 (−0·5, 0·2) | 0·57 |
Closed loop minus control. Positive value indicates measurement was higher during closed loop delivery intervention compared with control
Data shown are mean ± SD, or **median (IQR)
Closed loop reduced HbA1c whilst no change was observed during control (7·9%±0·8 vs. 7·7%±0·8, pre- vs. post-closed loop; 7·9%±0·7 vs. 7·9%±0·8, pre- vs. post-control; p=0·033). Fructosamine was unchanged (460±76 vs. 454±77; 458±98 vs. 464±84μmol; p=0·754).
Two participants with history of hypoglycaemia unawareness each had an episode of severe hypoglycaemia during the closed loop period (Supplementary material, Figure S1 and S2). Both events occurred at a time when closed loop was not operational and one subject was receiving standard while the other 50% of standard insulin pump therapy insulin rate. A post-hoc analysis identified that closed loop was interrupted about an hour prior to these events due to lack of wireless connectivity with insulin pump and at the time when insulin delivery was suspended due to predicted low glucose levels. The events were not attributable to control algorithm insulin advice and, whilst it is not possible to know the cause of the episodes definitely, they were likely compounded by increased physical activity during the day in one participant, and a user-error resulting in over-delivery of insulin whilst changing pump infusion set at night in the second participant. Both participants recovered fully without any clinical sequelae.
No other episodes of severe adverse events and no episodes of hyperglycaemia with ketosis were reported. Four participants had mild to moderate respiratory tract infections during the closed loop period and one during control period. Two participants had viral gastroenteritis episodes during the closed loop period. One participant underwent an elective inguinal hernia repair during the washout period and continued the study after recovery.
Discussion
We demonstrated the feasibility of four-week home use of overnight closed loop insulin delivery in adults. Glucose control improved including increased time spent in the target range and reduced mean glucose by delivering 30% more insulin overnight. During closed loop intervention, glucose levels remained lower compared to control throughout the day-time after closed loop was stopped (Supplementary material, Table S2), allowing participants to give less insulin bolus during breakfast and dinner periods (Supplementary material, Table S3). As a result, total daily insulin amount during both interventions were comparable. Time spent in hypoglycaemia was low with few nights with glucose below 3·5mmol/l during both interventions.
Achieving glycaemic level within the euglycaemic range, as safely as possible, presents a major challenge in type 1 diabetes. The risk of hypoglycaemia is increased when glycaemic control is tightened25. Threshold suspend pump therapy, which allows insulin delivery to be automatically suspended for up to two hours when sensor glucose falls below a preset threshold, represents the first step towards glucose responsive insulin delivery. Studies in children and adults report reductions in the frequency and duration of nocturnal hypoglycaemia in those at greatest risk26,27. However, the threshold suspend approach is not designed to step up insulin delivery and does not address the problem of overnight hyperglycaemia. Following the use of masked continuous glucose monitoring during the run-in period, participants utilised real-time sensor glucose during the control intervention to reduce time spent in hypoglycaemia demonstrating that the primary driver for these subjects was hypoglycaemia avoidance (Supplementary material, Tables S4 and S5). During control intervention, glucose outcomes were similar between Week 1 and Week 4 indicating rapid settling of glucose levels once real-time continuous glucose monitoring was initiated (Supplementary material, Table S6). Corrected for baseline HbA1c, continuous glucose monitoring data collected during control intervention were comparable to the JDRF continuous glucose monitoring trial8; the latter recruited adults with baseline HbA1c of 7·6% achieving mean glucose levels of 68% in the target range between 3·9 and 10·0mmol/l over 24 hours and 4·2% below 3·9mmol/l. The present study recruited adults with a slightly higher baseline HbA1c of 8·1% reflected by a lower time in the target range of 60% and a lower time spent in hypoglycaemia of 1·7%.
The advantage of a closed loop system such as ours is the finely tuned modulation of insulin delivery below and above the preset pump regimen. Day-to-day variations in insulin sensitivity are present in individuals with type 1 diabetes28. Using information from participant’s total daily insulin dose, basal insulin requirements and sensor glucose values, our control algorithm was able to adapt and safely cope with variations in overnight insulin requirements trading variability in insulin delivery for glucose consistency (Figure 3).
Early overnight closed loop studies with our model predictive control algorithm in the research facility setting showed increased time spent in the target range and reduced time spent in hypoglycaemia14,29. A single centre three-week overnight closed loop study in the home setting demonstrated improved glucose control and fewer nights with sensor glucose below 3·5mmol/l (10% vs. 17%) in adolescents18. Prior to the present study, no other study had been conducted assessing the safety and efficacy of unsupervised closed loop at home in adults for a period longer than one week. A four week study intervention period was considered sufficient to provide useful experience with unsupervised overnight closed loop home use by adults, and to allow progression to longer studies. Although nights with glucose level below 3·5mmol/l were not significantly different, we observed lower baseline hypoglycaemia compared to previous studies16 with median time of less than 10 minutes spent below 3·9mmol/l per night. Demonstrating reductions in hypoglycaemia will be difficult to achieve without studying a larger or more hypoglycaemia prone population.
The strength of our study is its multicentre design, allowing the assessment of a novel technology over a wider patient demographic demonstrating generalizability. No restrictions were placed on participants’ dietary intake or physical activity assessing system’s performance during free living conditions. Previous trials demonstrated improved glucose control with continuous glucose monitoring alone particularly in regular users8. Our study was limited by a duration of the control period which was too short to demonstrate a fall in HbA1c observed in previous trials of continuous glucose monitoring over three months or longer. Nevertheless, compliance with continuous glucose monitoring was high with most participants using it regularly during this period. Regular use of hyperglycaemia threshold alarms and further increase in nocturnal insulin supplementation during the control period might have diminished the difference in mean glucose between the two interventions. However, this might have resulted in more hypoglycaemia during the control intervention, or reduced sensor glucose use compliance due to alarm fatigue. Longer duration studies may provide additional information. We adopted cross-over design which had the benefit of each subject serving as its own control and the possible confounding period or carry-over effects were not detected. The study was limited by disruptions of wireless connectivity and other reasons causing closed loop to be interrupted on average every five nights. Improved connectivity and reliability of follow up prototypes may further increase usage above the present in excess of 85%.
In conclusion, unsupervised overnight closed loop at home is feasible and may improve glucose control in adults with type 1 diabetes. Longer term assessments are needed to strengthen the evidence of overnight closed loop benefits using systems with improved reliability.
Supplementary Material
Panel. Research in context.
Systematic review
We searched PubMed for articles published up to 24 January 2014 with the search terms (“closed loop” OR “artificial pancreas”) AND “type 1 diabetes”, and identified four randomised controlled trials which assessed closed loop use outside hospital settings. No multicentre randomised control trial in adults at home has been performed to date with or without remote monitoring, or of similar duration to the present study. A single night study at a diabetes youth camp involving remote monitoring showed reduction in the number of hypoglycaemic episodes with high baseline levels of hypoglycaemia; however no significant improvement in the median glucose values overnight was observed16. A 48-hour home study using a portable bi-hormonal closed loop system, combining the delivery of insulin with subcutaneous glucagon did not show any improvement in time spent within target range. Reduction of median glucose on the second day of closed loop period was shown, but at the expense of greater time spent in the hypoglycaemic range30. An interim analysis of overnight closed loop over four nights at home demonstrated improvements in hypoglycaemia endpoints, without improvement in the percentage of nights with normal mean glucose levels31. The only unsupervised single centre study published was one of three week duration in adolescence18. The reported benefits of overnight closed loop included increased time when glucose is in target, reduced mean glucose, and fewer nights with hypoglycaemia.
Interpretation
The use of overnight closed loop at home for an extended period without remote monitoring or continuous supervision is feasible in adults with type 1 diabetes. We showed improved time spent in target range and reduced glucose. These improvements were achieved by increasing insulin delivery overnight but without changing the total daily insulin delivery or the time spent in hypoglycaemia.
Acknowledgments
Funding/support: Supported by Diabetes UK (BDA07/0003549), Juvenile Diabetes Research Foundation (#22-2006-1113, #22-2007-1801, #22-2009-801, #22-2009-802), and National Institute for Health Research Cambridge Biomedical Research Centre. Abbott Diabetes Care supplied continuous glucose delivery devices and sensors and modified devices to facilitate real-time connectivity.
Footnotes
Author contributions: RH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. RH coordinated the study. RH, MLE, SRH, SAA, DBD, KK, HT, EW, MEW and KDB co-designed the studies. HT, ALS, MS, LL, EW, AP, AI, and CN were responsible for screening and enrolment of participants and arranged informed consent from the participants. HT, ALS, MS, LL, EW, AP, JMA, PC, AI, and CN provided patient care and/or took samples. MEW managed randomisation. HT, MN, LL, and MEW carried out or supported data analysis, including the statistical analyses. RH designed and implemented the glucose controller. HT, RH, MLE, SRH, SAA, ALS, MS, LL, EW, AI, PC, KK, MEW and DBD contributed to the interpretation of the results. All authors critically reviewed the report. No writing assistance was provided.
Conflict of interest disclosures: RH reports having received speaker honoraria from Minimed Medtronic, Lifescan, Eli Lilly, BBraun, and Novo Nordisk, serving on advisory panel for Animas, Minimed Medtronic, and Eli Lilly, receiving license fees from BBraun and Beckton Dickinson; and having served as a consultant to Beckton Dickinson, BBraun, Sanofi-Aventis, and Profil. SRH has undertaken consultancy for Novo Nordisk, Eli Lilly for which his institution has received payment. He has spoken at meetings for which he has received payment from NovoNordisk, Eli Lilly, BD. Medtronic has provided research support for some of his work. MLE has received speaker honorariums from Eli Lilly, Animas and Abbott Diabetes Care and served on advisory panels for Medtronic, Roche, Sanofi-Aventis and Cellnovo. PC declares speaker honoraria and travel support from Medtronic, Roche and Lifescan, and has undertaken consultancy for Novo Nordisk, Eli Lilly for which his institution has received payment. He has spoken at meetings for which he has received payment from NovoNordisk, Eli Lilly, and Beckton Dickinson. Medtronic has provided research support for some of his work. KK has spoken at meetings for which she received personal fees from Eli Lilly, Merck Sharpe & Dohme and Sanofi. MEW reports receiving licensing fees from Beckton Dickinson. RH, DBD and MEW report patent applications. HT, ALS, MS, LL, EW, AP, JMA, AI, MN, CN, KDB and SAA declare no competing financial interests exist.
Additional contributions: We are grateful to study volunteers for their participation. We acknowledge support by the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility, Sheffield’s Centre for Biomedical Research Clinical Research Facility, and the NIHR-Welcome Trust King’s Clinical Research Facility. Jasdip Mangat and John Lum (Jaeb Centre) supported development and validation of the closed loop system. Josephine Hayes (University of Cambridge) provided administrative support. Arti Gulati (University of Cambridge) provided data management support. Karen Whitehead (University of Cambridge) provided laboratory support. Biochemical assays were performed by the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory (Keith Burling). Gopal Kotecha (University of Cambridge) supported utility analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med. 1991;90(4):450–9. [PubMed] [Google Scholar]
- 3.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19(5):792–9. doi: 10.4158/EP12382.OR. [DOI] [PubMed] [Google Scholar]
- 4.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11(4):372–8. doi: 10.1111/j.1463-1326.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- 5.Rankin D, Cooke DD, Clark M, et al. How and why do patients with Type 1 diabetes sustain their use of flexible intensive insulin therapy? A qualitative longitudinal investigation of patients’ self-management practices following attendance at a Dose Adjustment for Normal Eating (DAFNE) course. Diabet Med. 2011;28(5):532–8. doi: 10.1111/j.1464-5491.2011.03243.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy HR, Wadham C, Hassler-Hurst J, Rayman G, Skinner TC Group FaACaTSF. Randomized trial of a diabetes self-management education and family teamwork intervention in adolescents with Type 1 diabetes. Diabet Med. 2012;29(8):e249–54. doi: 10.1111/j.1464-5491.2012.03683.x. [DOI] [PubMed] [Google Scholar]
- 7.Aschner P, Horton E, Leiter LA, Munro N, Skyler JS Management GPfED. Practical steps to improving the management of type 1 diabetes: recommendations from the Global Partnership for Effective Diabetes Management. Int J Clin Pract. 2010;64(3):305–15. doi: 10.1111/j.1742-1241.2009.02296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group JDRFCGMS. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–62. doi: 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32. doi: 10.1056/NEJMoa1303576. [DOI] [PubMed] [Google Scholar]
- 11.Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240–7. doi: 10.1001/jama.2013.277818. [DOI] [PubMed] [Google Scholar]
- 12.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385–95. doi: 10.1038/nrendo.2011.32. [DOI] [PubMed] [Google Scholar]
- 13.Sherr JL, Cengiz E, Palerm CC, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36(10):2909–14. doi: 10.2337/dc13-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:d1855. doi: 10.1136/bmj.d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luijf YM, DeVries JH, Zwinderman K, et al. Day and night closed-loop control in adults with type 1 diabetes: a comparison of two closed-loop algorithms driving continuous subcutaneous insulin infusion versus patient self-management. Diabetes Care. 2013;36(12):3882–7. doi: 10.2337/dc12-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–33. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 17.Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013;36(7):1851–8. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204–11. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabetes Sci Technol. 2010;4(1):132–44. doi: 10.1177/193229681000400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilinska ME, Budiman ES, Taub MB, et al. Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Technol. 2009;3(5):1109–20. doi: 10.1177/193229680900300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elleri D, Allen JM, Biagioni M, et al. Evaluation of a portable ambulatory prototype for automated overnight closed-loop insulin delivery in young people with type 1 diabetes. Pediatr Diabetes. 2012;13(6):449–53. doi: 10.1111/j.1399-5448.2012.00903.x. [DOI] [PubMed] [Google Scholar]
- 22.Hovorka R, Shojaee-Moradie F, Carroll PV, et al. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab. 2002;282(5):E992–1007. doi: 10.1152/ajpendo.00304.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hovorka R, Nodale M, Haidar A, Wilinska ME. Assessing performance of closed-loop insulin delivery systems by continuous glucose monitoring: drawbacks and way forward. Diabetes Technol Ther. 2013;15(1):4–12. doi: 10.1089/dia.2012.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–5. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 25.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–76. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ly TT, Nicholas JA, Retterath A, Davis EA, Jones TW. Analysis of glucose responses to automated insulin suspension with sensor-augmented pump therapy. Diabetes Care. 2012;35(7):1462–5. doi: 10.2337/dc12-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhary P, Shin J, Wang Y, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34 (9):2023–5. doi: 10.2337/dc10-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams RM, Dunger DB. Insulin treatment in children and adolescents. Acta paediatrica. 2004;93(4):440–6. doi: 10.1080/08035250410024934. [DOI] [PubMed] [Google Scholar]
- 29.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–51. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 30.van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, Devries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2013 doi: 10.1089/dia.2013.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nimri R, Muller I, Atlas E, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes. 2014;15(2):91–9. doi: 10.1111/pedi.12071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.