Summary
Androgen/androgen receptor (AR) signaling plays important roles in normal liver function and in progression of liver diseases. In studies of non-cancerous liver diseases, AR knockout mouse models of liver disease have revealed that androgen/AR signaling suppresses the development of steatosis, virus-related hepatitis, and cirrhosis. In addition, studies have shown that targeting AR in bone marrow-derived mesenchymal stem cells (BM-MSCs) improves their self-renewal and migration potentials, thereby increasing the efficacy of BM-MSC transplantation as a way to control the progression of cirrhosis. Androgen/AR signaling is known to be involved in the initiation of carcinogen- or Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). However, studies have demonstrated that AR, rather than androgen, plays the dominant role in cancer initiation. Therefore, targeting AR might be an appropriate therapy for patients with early-stage HCC. In contrast, androgen/AR signaling has been shown to suppress metastasis of HCC in patients with late-stage disease. In addition, there is evidence that therapy comprising Sorafenib and agents that enhance the functional expression of AR may suppress the progression of late-stage HCC.
Keywords: Androgen receptor (AR), Hepatocellular carcinoma (HCC)
1. Introduction
1.1. Liver function and liver diseases
The liver is the largest visceral organ responsible for systemic homeostasis of blood glucose as well as lipid and protein metabolism. It is also responsible for most xenobiotic clearance even when parts of the liver have been damaged (Rinaldi C. 2011). The liver has extraordinary repair capacity, which partially explains why a diseased/damaged liver is usually asymptomatic. Although the etiologies of liver diseases vary, many share common post-damage healing process pathways. Liver damage can result in the accumulation of matrix proteins, the formation of scars, and the alteration of tissue structure and function. As fibrosis develops, chronic compensatory scar-healing processes in the liver begin to take place. Once irreversible distortion of the hepatic architecture and vascular structure occurs, the cirrhotic liver begins to replace the functional hepatic units (Eugene R. Schiff 2003). Progression of cirrhosis can lead to liver failure or malignant transformation of hepatocytes.
Although cirrhosis is one of the etiological factors contributing to the hepatocarcinogenesis process, a significant number of patients without cirrhosis develop hepatocellular carcinoma (HCC), indicating that the disease process involves oncogenic events and virus-related factors.
Hepatitis B virus (HBV) is a well-known etiological factor contributing to cirrhotic liver progression and early HCC development. Antiviral agents, such as lamivudine and adefovir have been found to improve cirrhotic liver function in some studies (Aspinall, et al. 2011) but have not been shown to have anti-carcenogenic effects in other studies (Kwon and Lok 2011; Peng, et al. 2012). In addition, there is evidence that these anti-viral agents have little effect on survival of patients with advanced-stage disease (Shin, et al. 2012) or on disease recurrence after hepatectomy for HCC (Chan, et al. 2011).
1.2. Gender differences in liver diseases and their linkage to androgen/androgen receptor (AR)
There are four major liver diseases associated with gender (Guy and Yee 2009): steatosis (Wild, et al. 2004; Yang, et al. 2009), hepatitis (Baig 2009; Yang, et al. 2010), cirrhosis (Huang, et al. 2012; Maheshwari and Thuluvath 2011), and liver cancer (Yeh and Chen 2010). A study conducted in the United States by Weston et al in 2005 showed that the prevalence of fatty liver and cirrhosis Non-Alcoholic Fatty Liver Disease (NAFLD) was 3.5 times higher in men than in women (Weston, et al. 2005). In addition, Baig et al showed that the prevalence of HCC was higher among men than among women (range, 2.5:1 to 7:1) (Baig 2009). Several factors may contribute to the gender difference in liver diseases, including age, alcohol consumption, diabetes, hepatic toxins, virus infection, and variation in sex hormones (Yeh and Chen 2010). This review focuses on androgens and their receptors (AR), as they may represent the major factors that contribute to the gender difference in various liver diseases.
Androgens are synthesized mainly in the testes although some androgens form in the adrenal glands (Oshima 1968). Androgens act through the androgen receptor (AR), a transcription factor that belongs to the nuclear receptor superfamily. AR exerts physiological and pathological functions in organisms by translocating to the nucleus upon binding to androgens (Chang, et al. 1988), where it binds to specific DNA sequences known as androgen response elements (AREs) (Claessens, et al. 2001) in conjunction with various AR co-factors (Yeh, et al. 1999). The AR complex can therefore regulate the expression of genes that participate in various physiological and pathological functions (Bluemn and Nelson 2012). In certain conditions, androgens can exert biological functions in some diseases through a non-AR signaling mechanism (Miyamoto, et al. 2007). The effects of androgen and its receptor can be either transitory or long term and can have either local or systemic impacts on organ function. The most well-known androgen/AR-dependent cancer is prostate cancer. Ablation of androgen/AR actions is currently the gold standard for treating patients with prostate cancer; however, not all patients respond to this treatment (Seruga and Tannock 2011). Although androgen/AR plays a role in liver development in the embryonic stage, the maximum dimorphism of androgen/AR effects seems to occur after puberty (Waxman and O'Connor 2006) via influence of the activity of the hypothalamus-pituitary-gonad axis. The differential secretion of growth hormone (GH) between men and women may have different impacts on liver function in the adult stage of life. Studies have shown that in men, GH is released in high-amplitude but low-frequency bursts while in women GH is released in low-amplitude but high-frequency pulses (Lund, et al. 1991; Mode, et al. 1992).
In this review, we focus on the roles androgen/AR play in the processes of liver disease. In the first part of the review, we discuss androgen/AR signaling in non-cancerous liver diseases but include a discussion on the precursors to liver cancer development. In the second part of the review, we discuss the involvement of androgen/AR signaling in liver cancer and potential therapeutic strategies that specifically target AR.
Part I. Androgen/AR signaling in non-cancerous liver diseases
2. Roles of Androgen/AR signaling in the development of steatosis
Steatosis is the abnormal retention of lipids within hepatic cells. Excess lipid accumulation in vesicles displaces the cytoplasm. When the vesicles are large enough to distort the nucleus, the condition is known as macro-vesicular steatosis, otherwise the condition is known as micro-vesicular steatosis (Hashizume, et al. 2007). The most common risk factors associated with steatosis are diabetes mellitus, hypertension, obesity, and alcoholism (Sparks and Sparks 2008). Malnutrition can also cause the over-mobilization of fat from adipocytes to liver where lipid metabolism occurs (Williams 2006). The breakdown of large amounts of ethanol in alcoholic drinks produces large amounts of chemical energy in the form of NADH (reduced form of Nicotinamide adenine dinucleotide), which signals cells to inhibit the breakdown of fatty acids and, simultaneously, to increase the synthesis of fatty acids. This "false sense of energy" may then result in more lipids being created than are needed. Failure of lipid metabolism can also lead to impaired lipid breakdown, resulting in the accumulation of unused lipids in hepatocytes. Finally, certain toxins, such as carbon tetrachloride, aspirin, and diphtheria toxin, can interfere with the cellular machinery involved in lipid metabolism.
2.1. Androgen/AR signaling suppresses the development of steatosis
Many studies have shown that androgen/AR signaling suppresses the development of steatosis. For example, Jacqueson et al reported that androgen [19-nortestosteronephenylpropionate (19-NTPP)] had a protective effect on Amanita phalloides-induced hepatic steatosis (Jacqueson, et al. 1978) and Saint-Aubert et al found that a single injection of testosterone 1 month before subtotal hepatectomy could suppress liver steatosis in rats (Saint-Aubert, et al. 1980).
However, there are inconsistencies in the findings from studies on the roles androgens and their receptors play in the development of non-alcoholic fatty liver disease (NAFLD). Although several human clinical studies (Jones, et al. 2012; Schwingel, et al. 2011a; Schwingel, et al. 2011b; Vassilatou, et al. 2010) and an animal study (Chow, et al. 2011) have shown that androgens might promote NAFLD, other studies have shown that androgens protect against the development of the disease (Haider, et al. 2010)(Magyar, et al. 2011; Zhang, et al. 2013). There is little explanation of such inconsistencies, even the patients with NAFLD in these studies were quite different, with polycyctic ovarian syndrome (PCOS) female patients (Jones et al. 2012) or people using synthetic anabolic androgens (Schwingel et al. 2011a; Schwingel et al. 2011b) in studies that show androgens played positive roles to promote NAFLD vs hypogonadal elderly men (Haider et al. 2010) in other studies showing androgens played negative roles to suppress NAFLD.
In animal models used to study fatty liver, androgens were demonstrated to play a positive role in an aromatase knockout mouse model (Chow et al. 2011); however, androgens were also proven to be suppressors of the disease in rats fed a high fat diet (HFD)(Zhang et al. 2013). Furthermore, C57BL/6 mice receiving the anti-androgen hydroxyflutamide were shown to have a higher incidence of NAFLD (Takahashi, et al. 2012).
In contrast, the effects of AR on steatosis are less controversial. In a HFD-induced NAFLD mouse model, the loss of AR in the whole body (GARKO) was shown to lead to higher insulin insensitivity and the development of diabetes (Lin, et al. 2005). Similar results were confirmed in mice that only lacked hepatic AR (L-ARKO), indicating that hepatic AR might play negative roles in HFD-induced NAFLD (Lin, et al. 2008). Furthermore, aged male mice lacking hepatic AR developed hepatic micro-vesicle steatosis whereas wild-type aged mice did not (Lin et al. 2008). These two in vivo mouse models show that hepatic AR might play a role in the suppression of NAFLD.
Lund et al found that AR might directly regulate carnitine palmitotyltransferase 1 and that AR might exert its function by phosphorylating 3-hydroxy-3-methyl-glutaryl-CoA reductase (Lund J et al., 1991). Furthermore, Lin et al showed that AR might suppress fatty acid de novo synthesis by decreasing the expression of sterol regulatory element binding protein 1c (SREBP1) (Lin et al. 2008). They also found that AR might induce insulin sensitivity by modulating phosphoinositide-3 kinase (PI3K) activity and by suppressing the expression of phosphenolpyruvate carboxykinase (PEPCK) and protein-tyrosine phosphatase 1B (PTP1B) (Lin et al. 2008).
2.2. Potential AR-targeted therapies for steatosis
NAFLD can progress to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and HCC (Dima, et al. 2012). Most treatments for NAFLD focus on life-style modifications without intervention (Dima et al. 2012). Ongoing clinical trials suggest that statins, fibrates, and other lipid-lowering medicines may lead to improvements in liver biochemistry and histology in patients with NAFLD/NASH, although the potential side effects remain unclear (Dima et al. 2012). Metformin, the generally recommended first-line treatment for type 2 diabetes, was also found to be effective in the treatment of NAFLD (Cicero, et al. 2012). However, Weickert, et al. reported that metformin might lead to suppression of serum androgen levels in patients with polycystic ovarian syndrome (PCOS) (Weickert, et al. 2012). Whether this suppressive effect of Metformin on androgen seen in patients with PCOS also occurs in men with NAFLD remains unclear. Importantly, it may be worth testing to see whether therapies that enhance AR signaling, either by increasing AR expression or the expression of its downstream genes can halt the progression of NAFLD.
3. Androgen/AR signaling in cirrhotic liver with liver regeneration
Cirrhosis is the 12th leading cause of death in the United States (Heron, et al. 2009) and is associated with a 10-year mortality rate ranging from 32% to 66% (Sorensen, et al. 2003), depending on whether the cirrhosis is alcohol-related cirrhosis (ARC), virus-induced hepatic cirrhosis, or non-alcoholic cirrhosis. Since patients with liver cirrhosis are at risk for developing HCC(Aspinall et al. 2011), early treatment of liver cirrhosis with proper therapy will not only improve cirrhotic symptoms but also prevent HCC incidence.
3.1. Androgen/AR signaling is associated with the progression of steatosis to cirrhosis
Cirrhosis generally arises from chronic liver injury. During the injury-healing process, the damaged liver can develop fibrotic lesions that may lead to loss of normal hepatic function, impaired liver regeneration, aberrant polarity for cell proliferation, and obstruction of the portal system.
NAFLD/NASH is the most common cause of chronic liver disease in western countries (Weston et al. 2005), and NAFLD/NASH can lead to permanent liver damage with cirrhosis if liver cells are replaced by scar tissue (Swift, et al. 2001). Scar tissue blocks the flow of blood through the liver and slows the processing of nutrients, hormones, drugs, and naturally produced toxins (Bradbury 2006).
White et al found that high serum testosterone level is associated with advanced steatosis and cirrhosis (White, et al. 2012). Early studies indicated that in males the prevalence of muscular cytochrome P450 enzymes was suppressed during the progression of fatty liver to cirrhosis (K P Littmann 1973; Murray, et al. 1992). Interestingly, it was reported that feminization (e.g. gynecomastia) is an important factor linking the progression of steatosis (Green 1977).
The linkage between male hypogonadism and hypotestosteronemia in patients with alcohol-related cirrhosis (ARC) suggests that androgen/AR signaling might play negative roles in the development of ARC (Green 1977). Cirrhosis in men manifests as hypogonadism with reduced testicular size and clinical features of inadequate testicular function. Between 50% and 75% of cirrhotic men have both macroscopic and histological evidence of testicular atrophy, and as many as 90% of cirrhotic men show some degree of erectile dysfunction (Green 1977). Furthermore, men with cirrhotic liver have a decreased incidence of benign prostatic hypertrophy (BPH) and gynecomastia is found in about 40% of cirrhotic men (Yoshitsugu and Ihori 1997).
3.2. Androgen/AR signaling suppresses the development of cirrhosis
Although transdermal administration of testosterone has been shown to improve symptoms of hypogonadism and gynecomastia (Yurci, et al. 2011), most testosterone replacement therapies have little effect on cirrhosis (Nieschlag, et al. 1977). Similar results were also reported by Gluud et al (Gluud 1988; Gluud, et al. 1987), who showed that oral testosterone treatment yielded little change in liver pathogenesis in men with ARC even though such treatment significantly reduced the prevalence of gynecomastia. Similarly, a large randomized clinical trial also found that administration of androgens had little effect on ARC (Rambaldi and Gluud 2006).
However, Kley HK et al reported that administration of testosterone to male patients with ARC yielded some improvement in ARC symptoms (Kley 1979). Importantly, Thole Z et al (Thole, et al. 2004) reported that administration of steroidal or non-steroidal anti-androgens, such as flutamide or cimentidin, might lead to cirrhosis, suggesting that androgen/AR signaling might protect against the progression of cirrhosis.
3.3. Potential new therapies via targeting AR for treatment of liver cirrhosis via modulation of liver regeneration
Liver regenerative capacity is also associated with the development of cirrhosis (Michalopoulos and DeFrances 1997). Both intrinsic (Cressman, et al. 1996; Cressman, et al. 1994) and extrinsic (El-Ansary, et al. 2012; Takami, et al. 2012) mechanisms of liver regeneration have been reported. Intrinsic factors include the proliferation of hepatocytes and the self-renewal capacity of oval cells (hepatic stem/progenitor cells) (Sherwood, et al. 2005) whereas the extrinsic factors include circulating bone marrow-derived mesenchymal stem cells (BM-MSCs) and infiltrating monocytes/macrophages (Cornell, et al. 1990; Seki, et al. 2000). The intrinsic repair system, however, is not effective in patients with irreversible chronic liver damage. Therefore, potential approaches to treating cirrhosis should focus on the extrinsic repair system.
BM-MSCs are involved in the liver regeneration process. Chen et al revealed that endogenous BM-MSCs could be recruited into fibrotic lesions of mice with injured livers (Chen, et al. 2010). Moreover, Fang et al found that donor transplantation of Flk1 (CD309)-MSCs into mice with CCl4-induced liver fibrosis lead to improvement of liver function (Fang, et al. 2004). Furthermore, autologous transplantation of BM-MSCs into patients with cirrhotic liver resulted in a temporary halt to disease progression (Vanneaux, et al. 2013).
El-Ansary et al conducted a Phase II clinical trial using un-differentiated and differentiated MSCs in patients with HCV-induced cirrhosis and found that both types of MSCs resulted in improvement of liver function (El-Ansary et al. 2012). In a review of trials of autologous MSC infusion in cirrhotic patients, Takami et al found that the therapy resulted in improvement of liver function and in prolonged survival in some patients (Takami et al. 2012).
Using mouse models of CCl4- and thioacetamide (TAA)-induced liver cirrhosis, Huang et al. revealed that androgen/AR exhibited suppressive effects on the renewal capacity of BM-MSCs and adipose-derived MSCs by modulating the EGFR-mediated Erk and Akt pathways (Huang et al. 2012). Targeting AR in BM-MSCs via either AR-siRNA or ASC-J9® [an AR degradation enhancer] (Miyamoto et al. 2007; Yang, et al. 2007) enhanced the self-renewal capacity and migration of BM-MSCs and resulted in reduced inflammation and fibrotic stress. AR-siRNA and ASC-J9® might, therefore, enhance the efficacy of autologous BM-MSC transplantation as treatment for cirrhosis (Hung et al., 2012).
4. Androgen/AR signaling in hepatitis
Hepatitis can be caused by viral infection, chemicals, or drug abuse (Williams 2006). Acute hepatitis is usually defined as inflammation lasting for less than six months and although most patients present with mild symptoms the acute state can present as severe hepatic failure. Chronic hepatitis, however, lasts longer and patients normally are either asymptomatic or mildly symptomatic.
4.1. Androgen/AR signaling in cirrhosis and hepatitis
In addition to toxic/drug and hepatitis virus contributing to hepatitis (Wright and Lau 1993), cirrhosis may also link to hepatitis since many hepatic patients are also found with cirrhotic liver development (Even, et al. 1997).A recent study found that total serum testosterone is associated with increased risk for developing advanced hepatic fibrosis and advanced hepatic inflammatory activity in Hepatitis C virus (HCV)-infected men (White et al. 2012). Theve et al found that the anti-androgen flutamide might prevent the development of cirrhosis and hepatitis in mice (Theve, et al. 2008). Other studies also found a higher incidence of HBV- or HCV-related cirrhosis and HCC among men (Chiu, et al. 2007; DeLoia, et al. 1989; Eugene R. Schiff 2003; Wright and Lau 1993). Importantly, direct linkages have been demonstrated between androgen levels (Tanaka, et al. 2000) and AR gene polymorphisms (Yu, et al. 2000) and the progression of hepatitis and cirrhosis to cancer of the liver.
4.2 Androgen/AR signaling in HBV-induced chronic hepatitis
HBV virus antigens (HBeAg and HBsAg) have been shown to contribute to host immune tolerance to the virus and to be associated with the development of hepatitis. DeLoia et al found that the expression of HBsAg was higher in pubescent male mice and that testosterone injection promoted the expression of HBsAg in female mice (DeLoia et al. 1989). In vitro studies further identified the androgen response element (ARE) located in the HBV virus, suggesting that androgens might be able to go through AR to bind to the ARE in the HBV virus to modulate HBV viral titers (Wang, et al. 2009). Using ARKO mice that lacked AR expression in hepatocytes, Wu et al (Wu, et al. 2010) reported that AR might play key roles in directly regulating HBV replication to influence HBV viral titers, viral particles, and hepatic viral RNA. Results from in vitro cell line studies further confirmed that AR-bound androgen directly binds to ARE in HBV, and that this binding activity is responsible for its transactivation. (Wu et al., 2010)
Wu et al (Wu et al. 2010) revealed that male hormones might promote immune tolerance of HBV in liver. Using HBV transgenic mice, Tian et al found that male hormones promote HBV virus replication, resulting in higher HBV titers in men than in women (Tian, et al. 2012) and concluded that male hormones but not gender could change the HBsAg and HBx antigens.
Current therapies to improve immune tolerance in patients with HBV-related hepatitis involve administration of Interferon to suppress HBV-induced immune activity (Tamori and Kawada 2012) or regimens that boost immune activity to eliminate HBV-infected hepatocytes (Shimizu 2012). Androgen-deprivation therapy with flutamide or cimentidine, however, yielded controversial results. Although Theve et al found that flutamide prevented the development of cirrhosis and hepatitis in mice (Theve et al. 2008), other clinical studies have shown that flutamide can lead to further inflammation of the liver (Manso, et al. 2006; Matsuzaki, et al. 2006; Thole et al. 2004). Wang et al reported that the anti-androgen cimentidine resulted in enhanced immune response when administered in conjunction with HBV DNA vaccine by boosting viral clearance (Wang, et al. 2008). However, Hashimoto et al found that cimentidine also resulted in exaserbation of liver damage (Hashimoto, et al. 1994).
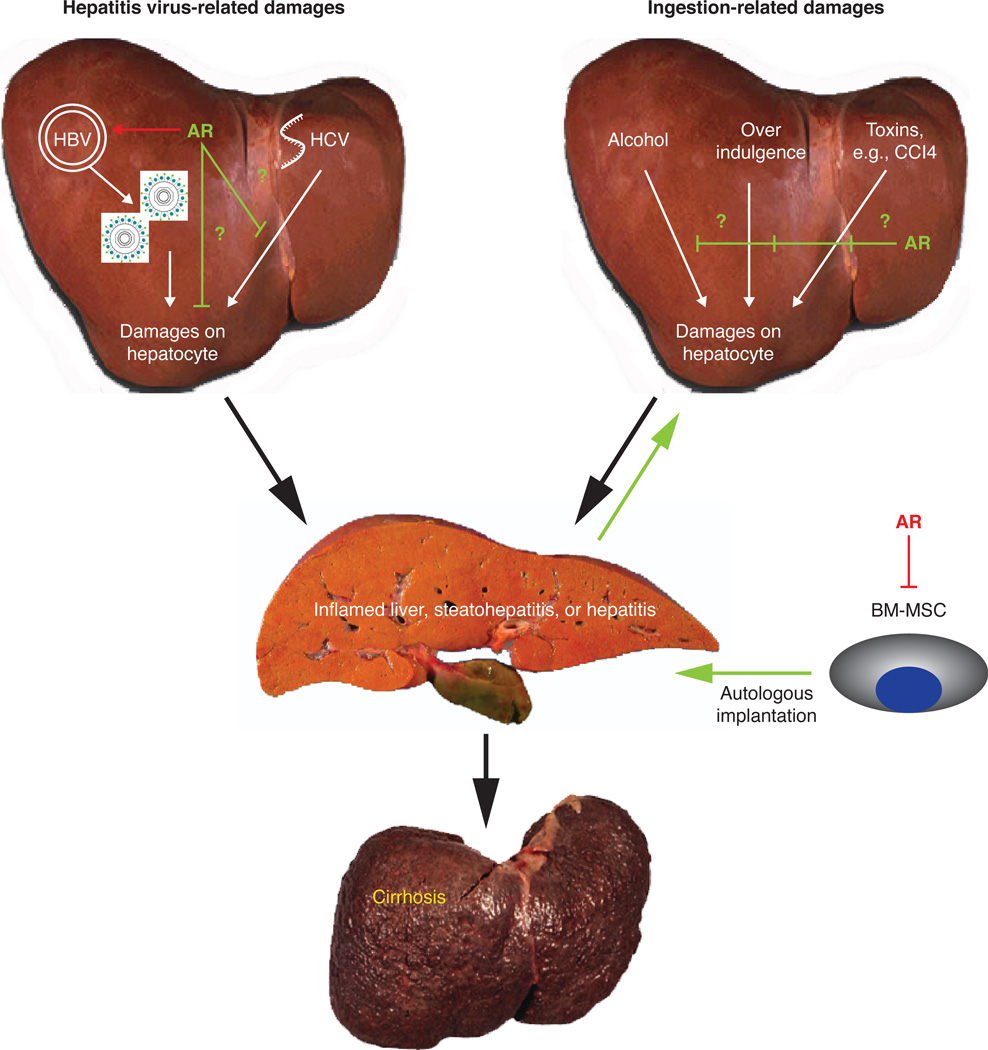
Importantly, in ARKO mouse model studies, Wu et al (Wu et al. 2010) demonstrated that AR, but not androgens, play key roles in the promotion of HBV replication and hepatitis. They found that knockout of AR in hepatocytes resulted in reduced HBV replication. The researchers also found that targeting AR with the newly developed AR degradation enhancer ASC-J9® suppresses HBV-mediated viral replication and the development of HCC in mice (Wu et al. 2010). Figure 1 illustrates the molecular mechanisms governing the effects of androgen/AR on HBV virus replication as well as the positive feedback loop of AR-HBV virus replication.
Figure 1.
Androgen/AR roles in non-cancerous liver disease progression. In hepatitis virus (HBV and HCV)-related hepatic inflammation, androgen/AR promotes HBV virus replication, yet, AR might suppress the hepatitis process. On the other hand, AR also plays a suppressive role in the toxin-related liver inflammation process. Moreover, bone marrow-derived mesenchymal stem cells (BM-MSC) can infiltrate into the damage liver; however, AR suppresses the ability of BM-MSCs to repair liver damage.
4.3. Androgen/AR signaling in HCV-induced chronic hepatitis
HCV-mediated hepatitis is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis. Chronic HCV infection develops in about 85% of infected patients and Lee et al (Lee, et al. 2011) found that female gender was an independent risk factor for HCV infection. Furthermore, Di Matino et al (Di Martino, et al. 2004) reported that hormone replacement therapy was effective at reducing the incidence of HCC development in HCV-infected women. However, other studies have shown that suppression of estrogen signaling reduces the incidence of HCC development in HCV carriers. Using selective estrogen modulators (SERM), researchers have shown that estrogen signaling interferes with the HCV viral cycle (Murakami, et al. 2013) and reduces HCV-mediated Toll-Like receptor 7 (TOLR7) signals (Fawzy, et al. 2012), indicating that SERM are potential adjuvant antiviral treatments (Furusyo, et al. 2012). Interestingly, Huang et al reported that male gender was an independent risk factor for the development of liver disease in patients with HCV-related hepatitis (Huang, et al. 2011). These studies indicate that while women are at greater risk for HCV infection men are at greater risk of developing HCV-related chronic liver disease.
Kanda et al found that HCV viral core antigen could enhance AR transactivation via up-regulation of VEGF and related Stat3 activation (Kanda, et al. 2008). It will be interesting to see in the future whether AR has an effect on HCV virology and related liver diseases by modulating the HCV viral core antigen.
The current therapy for HCV hepatitis is a combination of peginterferon alpha-2a and ribavirin with either boceprevir or telaprevir. However, few studies have linked androgen deprivation therapy or anti-AR therapy to HCV. More studies on how AR influences HCV replication are needed before any potential AR-targeted therapy can be developed.
A summary of the roles that androgen/AR play in non-cancerous liver diseases is illustrated in Figure 1.
Part II. Androgen/AR signaling in liver cancer
The American Cancer Society ranked liver cancer as the 5th highest cause of cancer death, with HCC as the most common form of liver neoplasm (Farazi and DePinho 2006). The etiology of liver cancer includes ingestion of related toxins (e.g., alcohol, aflatoxin B1 or AFB1) and infection with hepatic viruses (HBV, HCV, etc.), which are the major oncogenic factors that contribute to the development of HCC.
Part two of this review will focus on the roles that androgens and their receptors play in the development of HCC and cholangiocarcinoma (CC) and potential therapeutic regimens for patients with HCC that target androgen or AR or both.
5. Androgen/AR signaling in carcinogen-induced HCC
The male predominance in HCC suggests that androgen/AR may promote and that estrogens/ERs may suppress hepatocarcinogenesis (Yeh and Chen 2010). Suppression of carcinogen-induced HCC by estrogen signaling has been shown to regulate MyD88-dependent IL-6 production and to trigger cellular innate immunity (Naugler, et al. 2007). Although the roles female hormones play in HCC development have been established, the roles male hormones play are less clear. A number of studies have shown that carcinogen/AFB1 (aflatoxin B1)-induced HCC predominantly affects men (Li, et al. 2012; Ma, et al. 2008; Tejura, et al. 1989; Yeh and Chen 2010), suggesting that androgen/AR may promote the development of AFB1-induced HCC. Although Yu et al showed that male gender was a risk factor for AFB1-related cancer development(Yu, et al. 2001), Nakatani et al revealed that AFB1 intake was associated with an increased risk of HCC with little association with AFB1-DNA adducts or testosterone levels in men (Nakatani et al., 2001). The reasons for these inconsistent findings remain to be identified.
In men, the testes are the major source of systemic androgen levels with the adrenal gland contributing a small amount. In women, ovarian-derived androgen is an important source of systemic androgen levels. Tejura et al (Tejura et al. 1989) reported an increase in AR expression in ovariectomized rats after hepatic carcinogen injection, suggesting a potential female source of androgen in the development of HCC. Early studies found that the change in endogenous or exogenous androgens in male rodents could lead to altered HCC progression (Nakatani, et al. 2001). In vitro HCC cell line studies also found that androgen/AR-facilitated cell growth were governed by transcriptional regulation of TGFβ1 (Yoon, et al. 2006).
Using an ARKO mouse model, Ma et al found that AR, but not androgens, play major roles in hepatocarcinogenesis (Ma et al. 2008). They also found that the incidence of carcinogen-induced HCC in L-ARKO mice lacking AR in hepatocytes was lower than that in wild-type mice even though the serum testosterone levels in both showed little difference (Ma et al. 2008). Using siRNA to knockdown AR or AR-cDNA to overexpress AR, Ma et al also found that targeting AR could alter HCC cell growth. Based on their findings, they suggested that hepatic AR might influence the degree of cellular oxidative stress by modulating super-oxygen dismutase expression and that it might influence the extent of DNA damage repair by modulating p53 and GADD45α/β expression (Ma et al. 2008).
Feng et al reported that AR might be able to enhance hepatocarcinogenesis by modulating cell cycle-related kinase (CCRK)-β-catenin activation signaling (Feng, et al. 2011). Using system biological approaches to illustrate the interplay between androgen and estrogen signaling in hepatocarcinogenesis, Li et al found that Foxa1 and Foxa2 were the bona fide molecules that governed gender disparity in HCC (Li et al. 2012).
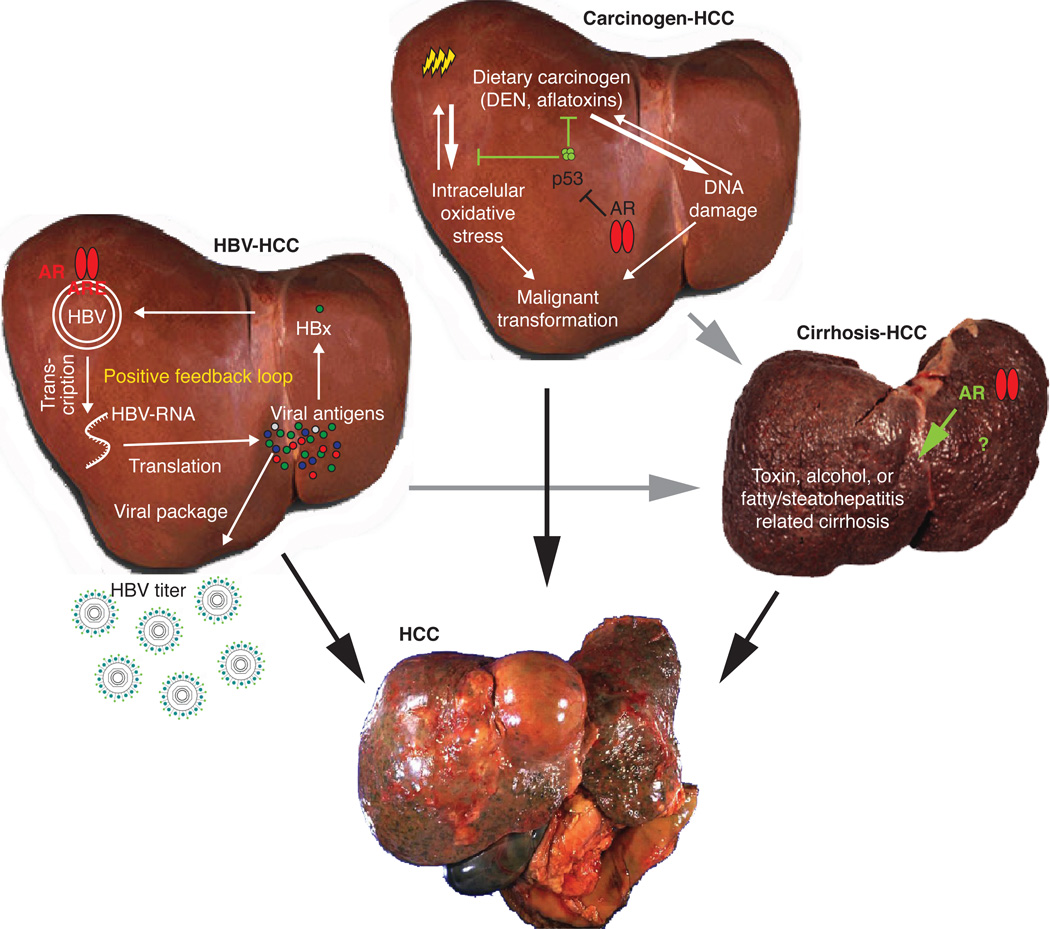
The data suggest, therefore, that androgen/AR signaling promotes hepatocarcinogenesis and HCC development, at least in the early stages. Figure 2 illustrates the androgen/AR roles in carcinogen-induced hepatocarcinogenesis.
Figure 2.
Androgen/AR signaling in hepatocarcinogenesis. There are three major factors that contribute to the development of liver tumors, e.g., HBV, carcinogens, and cirrhosis. In HBV-related HCC, AR promotes HBV virus replication to increase viral titers, as well as viral antigens to positively feedback HBV replication. In carcinogen- and DEN-related HCC, AR suppresses p53 and related cellular oxidative stress and DNA damage repair, thereby promoting hepatocyte transformation. However, it is still unknown how AR is involved in cirrhosis-related HCC.
6. Androgen/AR signaling in HBV-induced HCC
In a nested case-control study, Yuan et al concluded that high androgen level is a risk factor for developing HCC in men with chronic HBV infection (Yuan, et al. 1995). Other studies found that higher serum testosterone, increased number of steroid 5α-reductase type II (SRD5A2) V89L polymorphisms, and fewer AR gene CAG repeats in exon 1 (<23 repeats) in HBV patients were correlated with high risk of HCC (Yu et al. 2000; Yu et al. 2001), suggesting that more active androgen/AR signaling might lead to higher risk of HBV-induced HCC.
Using HBV transgene mice lacking hepatic AR who were fed sub-minimal doses of the carcinogen DEN, Wu et al found that the incidence of HBV-induced HCC was lower among mice with loss of hepatic AR than in wild-type mice (Wu et al. 2010). They revealed that AR enhanced HBV virus replication by binding directly to the HBV core promoter region, which up-regulated the expression of the HBV X antigen (HBx). AR-induced HBx expression subsequently promotes AR transactivation, thereby amplifying its role in hepatic cell transformation. Importantly, AR was also shown to enhance HBV-mediated cell proliferation and to suppress apoptosis (Wu et al. 2010).
Other studies have reported that HBV antigen interacts with AR to promote HCC initiation and that HBx enhances hepatic cell transformation by interacting with AR signaling (Chiu et al. 2007; Zheng, et al. 2007a). The mechanism of action governing the effects of HBx on enhanced AR transactivation might involve an increase in phosphorylation of AR rather than binding to ARE sites (Chiu et al. 2007). In contrast, androgen/AR signaling might promote HCC development by enhancing HBx function.
Although some clinical and animal studies have provided evidence that androgen/AR signaling promotes HBV-induced HCC development, other studies have shown that inhibition of androgens has little effect on preventing the progression of hepatitis. Using the LHRH analog triptorelin to suppress testosterone production in male patients with chronic HBV infection, Jilma et al (Jilma, et al. 1998) found no significant effect on serum HBsAg and HBV-DNA concentrations. Using murine Helicobacter hepaticus infection to study the effects androgen has on chronic hepatitis and HCC progression in mice, Arlin et al (Rogers, et al. 2007) also found little effect of castration on hepatitis and HCC incidence when compared with control mice. Supplementation with dihydrotestosterone also failed to change the outcome. They concluded that an imprinting effect of endogenous androgens might be responsible for chronic hepatitis and HCC in men.
7. Androgen/AR signaling in HCV-induced HCC
Although the prevalence of HCV-induced HCC is highest among men, the gender difference in prevalence is not as obvious for HBV-induced HCC. Huang et al conducted a prospective cohort study of newly developed HCC and studied the cumulative lifetime incidence rates of HCC in men and women who were positive for both HBV surface antigen (HBsAg) and antibodies against HCV (anti-HCV) (Huang et al. 2011). They found that there was a significant male predominance in incidence of HCC for chronic HBV carriers but not for chronic carriers of HCV (Lee et al. 2011). Epidemiological studies have shown polymorphisms of three enzymes involved in androgen and estrogen biosynthesis among anti-HCV positive patients, namely SRD5A2, cytochrome P450c17α (CYP17), and catechol-O-methyltransferase (COMT) (Rossi, et al. 2003). The researchers found that the frequency of the CYP17 C/C polymorphism in female patients with hepatitis or HCC was higher than that in asymptomatic carriers (Rossi et al. 2003). Kanda et al reported that HCV core protein, but not NS5A oncogene, up-regulated AR target genes in Huh7 human HCC cells by augmenting androgen/AR activity (Kanda et al. 2008). In addition, they found that the HCV-induced increase in AR activity also up-regulated VEGF expression and tube formation in human coronary microvascular endothelial cells (Kanda et al. 2008). Interestingly, Vizoso et al examined AR expression in HCV-related HCC lesions and found little evidence for a linkage between AR or ER expression and HCC progression (Vizoso, et al. 2007). Similar negative results also occurred in a study by Wang et al, who showed that AR expression was not linked to HCV-related HCC (Wang, et al. 2006). These controversial results indicate that more studies, especially those on HCV mice lacking hepatic AR, are needed to better understand the roles AR plays in HCV-induced HCC.
8. Androgen/AR signaling in cirrhosis-induced HCC
Regardless of the etiological factors, cirrhosis and HCC progress at unequal rates in the two sexes, with more frequent disease in men than in women (Giannitrapani, et al. 2006). In addition, Tanaka et al found that elevated serum testosterone, together with decreased serum estrogens, may promote the development of HCC in patients with cirrhosis (Tanaka et al. 2000).
Although an increase in HCC incidence was reported in patients with NAFLD and cirrhosis (Donna L. White 2012), the evidence of direct linkage of androgen/AR signaling in NAFLD with cirrhosis-induced HCC remains unclear.
A direct positive linkage between androgen/AR signaling and the progression of HBV-cirrhosis to HCC is still lacking (Iloeje, et al. 2012). Interestingly, Gong et al found that loss of an X chromosome might result in altered transformation of hepatocytes in patients with HBV-induced cirrhosis (Gong, et al. 2010), suggesting the potential linkage between AR loss and the initiation of HCC. Furthermore, Rossi et al reported that higher CYP17 activity might increase serum androgen levels and that these increased levels might be associated with risk for HCV-related cirrhosis (Rossi et al. 2003).
The androgen/AR roles in HBV-, carcinogen-, and cirrhosis-related hepatocarcinogenesis are summarized and illustrated in Figure. 2.
9. Androgen/AR signaling in HCC progression and invasion
Although there is sound evidence that androgen/AR promote hepatocarcinogenesis and HCC development in the early stages, there is less evidence showing a direct link between androgen/AR signaling and HCC progression in advanced stages. Yuan et al reported that testosterone levels were higher in men than in women with advanced HCC, indicating that androgen might play positive roles in advanced HCC (Yuan et al. 1995). In addition, Kew et al found that testosterone levels were positively correlated with survival in patients with HCC (Kew, et al. 1977).
However, the correlation between AR expression and progression of HCC remains controversial (Kalra, et al. 2008). Nagasue et al reported up-regulation of AR protein in peripheral tumor lesions (Nagasue, et al. 1995), while Tavian et al reported down-regulation of AR mRNA in poorly differentiated HCC lesions (Tavian, et al. 2002). The reason for the inconsistencies in results is unknown. It could be due to small sampling size and different measurement methods. However, the reason is most likely misclassification of samples. Most of the studies failed to differentiate HCC samples by stage, tumor size, and malignant cellularity. Ma et al found that AR was upregulated only in tumors smaller than 3 cm (Ma, et al. 2012b). They also found little AR expression in severe HCC lesions, which was consistent with results from Zhu et al, which showed that AR was expressed only in the tumor margins and not in the tumor centers (Zhu, et al. 2011).
In a study using L-ARKO mice lacking hepatic AR with carcinogen-induced HCC, Ma et al demonstrated that mice with loss of hepatic AR developed more malignant tumors, had poorer survival, and had a higher incidence of metastatic lung tumors (Ma et al. 2012b). They further used in vitro HCC cell lines to investigate whether knock down of hepatic AR or overexpression of AR altered HCC cell migration and invasion and found that AR alters HCC invasion by indirectly suppressing NFκB. These results indicate that AR might suppress HCC metastasis in the advanced stage of HCC.
HCC cell survival in the detached environment (circulation) and the ability to metastasize to distant organs or micrometastasize to neighboring liver tissues is critical for malignant progression of HCC in the advanced stage. A recent report revealed that AR enhanced HCC cell anoikis by suppressing p38 phosphorylation (Ma et al. 2012b), raising the possibility that detecting AR expression in circulating HCC-tumor cells could be a diagnostic marker for HCC recurrence/invasion after curative surgery.
Taken together, the ability of AR to enhance HCC cell anoikis and suppress HCC cell invasion suggests that AR might function as a suppressor of metastasis in late-stage HCC.
10. Cholangiocarcinoma
Cholangiocarcinoma (CC), a liver tumor arising from the epithelial cells (cholangiocytes) lining the biliary tree, is characterized by poor prognosis and poor response to current therapies (Marsh Rde, et al. 2012a, b; Valero, et al. 2012). The incidence of and mortality associated with CC are increasing worldwide and an etiological study found that the daily ingestion of germs, including Helicobacter spp., and parasites might contribute to the development of CC (Samaras, et al. 2010). Liossi et al found 17β-estradiol (E2), but not testosterone, immunoactivity in CC lesions (Liossi, et al. 1988), suggesting potential protective/redundant roles of male hormones in CC progression (Liossi et al. 1988). Other studies found that estradiol levels were higher among patients with CC than among patients with HCC or cirrhosis (Kuper, et al. 2001a). Further studies found that estrogenic signaling might promote CC progression via the estrogen receptor alpha (ERα)-AKT pathway (Alvaro, et al. 2006) and up-regulation of VEGF (Mancino, et al. 2009), and that E2 and ERα levels were increased in male CC patients.
11. Potential AR-targeted therapies for HCC
11.1 Dual and opposite roles of AR in HCC progression
Hepatic AR play positive roles in hepatocarcinogenesis and in the early development of HCC (Nagasue et al. 1995; Tavian et al. 2002) but have been shown to play negative roles in the advanced stage of HCC (Nagasue et al. 1995; Tavian et al. 2002). A clear understanding of the mechanisms governing these opposite and dual functions of AR in HCC progression might lead to the development of different therapeutic approaches for HCC at different stages (see sections 11.3 and 11.4).
11.2 Controversial results of using anti-androgens to battle HCC
The dual and opposite roles of AR during HCC progression might also explain the controversial results of past clinical studies involving the targeting of androgens (but not AR) to battle HCC. Results from several studies on the use of anti-androgens to manage HCC remain controversial (Di Maio, et al. 2008; Hépatocellulaire. 2004). For example, in a large cohort nested case-control study, Yuan et al found that serum testosterone levels in HCC patients were significantly higher than those in non-HCC controls (Yuan et al. 1995). Ex vivo studies using human HCC primary cells also demonstrated a positive correlation between androgen/AR signaling and HCC progression (Yu, et al. 1997). Recent studies also suggest that HBx protein might function as a coactivator to promote AR-mediated anchorage-independent cell growth via AR-HBx protein interaction (Chiu et al. 2007). Importantly, clinical studies using the anti-androgen cyproterone acetate (300 mg daily) showed some positive improvement in HCC cell growth (Forbes, et al. 1987). Similarly, the results of an ex vivo study using the anti-androgen flutamide showed that flutamide suppressed androgen-induced HCC cell growth. In contrast, other studies found that serum testosterone levels were lower in HCC patients than in non-HCC control patients (Kuper, et al. 2001b; Lampropoulou-Karatzas, et al. 1993). A small-scale phase II clinical trial of flutamide failed to show improvement in HCC patient survival (Chao, et al. 1996). A similar result was found in a large-scale population study using leuprorelin and flutamide (Hépatocellulaire. 2004).
There are several possible explanations for the controversial findings among clinical trials and basic studies. One of the explanations is that most of the anti-androgen trials included patients with advanced stage, unresectable HCC. Anti-androgens only suppress a small portion of fast growing cancer cells, but not poorly differentiated lesions. Another explanation is that AR expression is low in advanced HCC; therefore, anti-androgens no longer exert tumor suppressive effects. The controversial results of the effects of androgens or anti-androgens on HCC progression suggest that targeting androgens to suppress HCC progression might have limited value. Therefore, targeting AR may represent a better therapeutic approach to battle HCC progression.
11.3 ASC-J9®, a new AR-targeted therapy for early stage HCC
Using testicular feminized (Tfm) mice that lack AR in the whole body with little serum testosterone, Kemp et al found that AR expression in liver tumors was lower than that in the surrounding normal liver tissue (Kemp and Drinkwater 1989). They concluded that testosterone might function through AR to promote HCC progression, and suggested that either testosterone or AR might be able to promote HCC progression. Using GARKO mice that lack AR in the whole body with little serum testosterone, Ma et al also showed a reduced incidence of HCC development, cancer growth, and apoptosis (Ma et al. 2008).
However, since it was impossible to distinguish between the effects of androgen and the effects of AR in the two mouse models mentioned above, Ma et al developed a new mouse model that only lacks hepatic AR (L-ARKO) with little change in serum testosterone. They found that the suppressive effects of AR on hepatocarcinogenesis in mice without hepatic AR were similar to those observed in GARKO mice, suggesting that AR, and not androgens, might play key roles in promoting hepatotumorigenesis and the development of HCC (Ma et al. 2008).
These results provide a new therapeutic approach for treating patients with early-stage HCC. Using a newly developed AR degradation enhancer, ASC-J9®, which has been shown to degrade AR in selected cells with few side effects (Lai, et al. 2013; Lai, et al. 2012; Miyamoto et al. 2007; Yang et al. 2007), Ma et al found that targeting AR could suppress HCC progression in a carcinogen-induced HCC mouse model (Ma et al. 2008) and in an HBV-lowDEN-induced HCC mouse model (Wu et al. 2010). Those findings suggest that ASC-J9® might be an effective therapy for stage I or grade 1 HCC. Considering the fact that the rate of recurrence of HCC is as high as 60% in patients with HCC stage I~II or grade 1~2 disease who are treated with preventive chemotherapy or transcatheter arterial chemoembolization (Jung, et al. 2013)(Lim, et al. 2012), targeting the remaining AR-induced HCC cells via ASC-J9® at the early stage of HCC may prevent or delay the recurrence of tumors after treatment. The impact of using ASC-J9® to suppress HBV-lowDEN-induced HCC may be huge, especially in countries with a high rate of HBV infection as there have been around 522,400 new liver cancer patients yearly in the US (Jemal, et al. 2011) and Jemal et al also reported that 60% of over 1 million newly identified liver cancer patients yearly were from HBV infections in the Eastern and South-Eastern Asia (Jemal et al. 2011), especially in China, considering the HBV infected population in China is around 8% of the over 1.3 billion people. The value of any successful therapy to prevent or delay the development of HCC from HBV hepatitis in these HBV-hepatitis patients can be also huge.
11.4 AR-targeted therapy in combination with other therapeutic agents as treatment for late-stage HCC
In the advanced stages of HCC, chemotherapy with Sorafenib has been shown to have beneficial effects in selected patients (Llovet, et al. 2008; Yau, et al. 2009). Increasing the effectiveness of therapies so that they benefit more HCC patients is challenging and needed. Ma et al found that AR could suppress HCC metastasis by modulating p38. They also found that the addition of functional AR in SKhep1 and HepG2 HCC cells to decrease p38 enhanced the effectiveness of Sorafenib against HCC cells in later stages of development (Ma et al. 2012b). Importantly, results from tail-vein injection cancer metastasis mouse model showed using a much lower Sorafenib dosage (30 mg/kg/mouse) with combined therapy to increase AR expression could lead to similar therapeutic effects as compared to the higher dosage used in the Bayer preclinical trial (40~60 mg/kg/mouse) (Hoshino-Yoshino, et al. 2011). This suggests that pre-screening for higher expression of hepatic AR in patients with advanced HCC may help to increase the efficacy of treatment with Sorafenib. Alternatively, a new combinational therapy with Sorafenib and some compounds to enhance the functional AR expression may be developed to battle HCC at later advanced stages in the future.
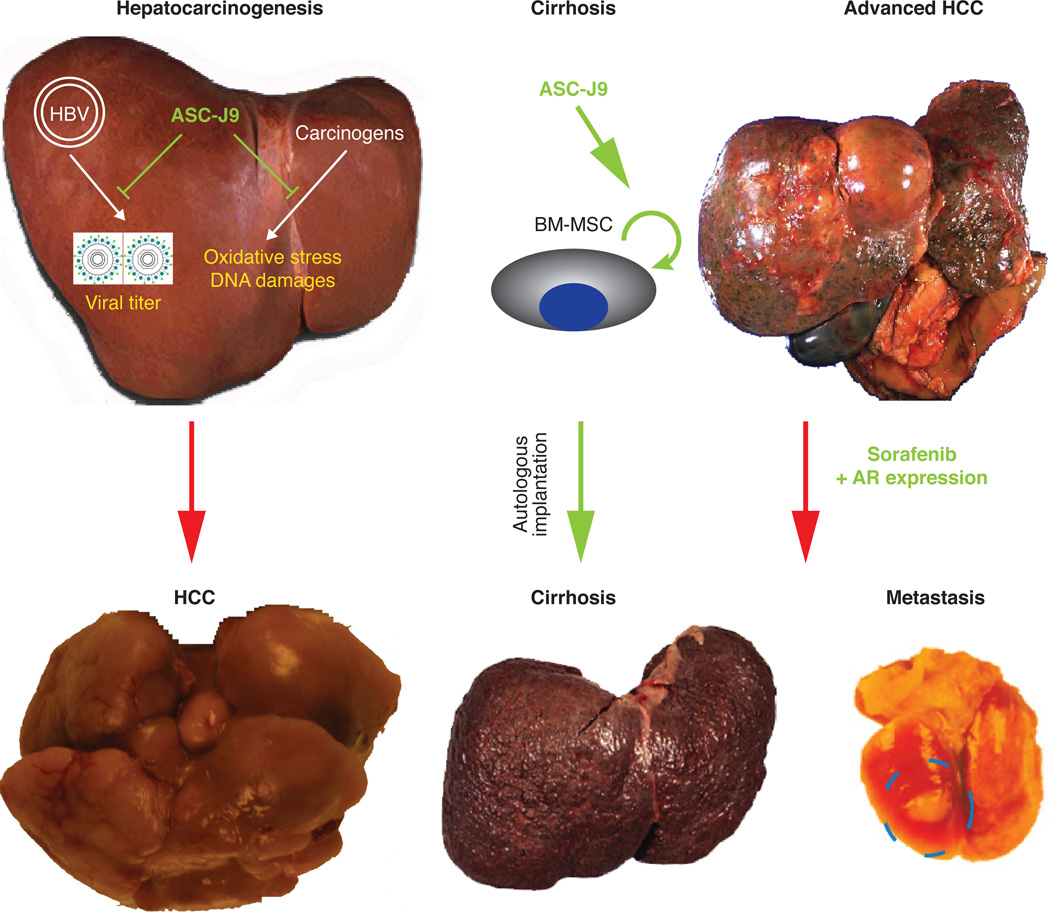
Together, the findings of differential androgen/AR roles in early HCC development vs advanced HCC progression provided insights for targeting AR in these two stages of cancers, which is illustrated in Figure 3.
Figure 3.
Novel therapeutic strategy targeting AR to battle liver diseases. In the early stage of HCC development, targeting AR using ASC-J9 might suppress early cancer progression. And in the cirrhotic liver, ASC-J9 applied to BM-MSC for autologus implantation might be effective against cirrhosis. At last, treatment of patients with advanced-stage HCC with Sorafenib and expressing AR in the liver might improve the therapeutic efficiency of Sorafenib at reducing the incidence of metastasis or recurrence.
12. Summary and future prospects
Androgen/AR signaling plays different roles in various liver diseases. Androgen/AR signaling suppresses the development of steatosis and may also suppress the development of cirrhosis. In HBV-induced hepatitis, androgen/AR signaling enhances HBV replication and promotes HBV- and carcinogen-induced hepatotumorigenesis and HCC development. However, in the advanced stages of HCC progression, androgen/AR signaling suppresses HCC metastasis. We have summarized the overall androgen/AR signaling effects in liver disease in Table 1.
Table 1.
Summary of the androgen/AR roles in liver diseases
| Liver diseases | Etiological factor(s) | Androgen/AR roles |
|---|---|---|
| Inflammaed liver (hepatitis) | 1. HBV/HCV | Promoter (Jacquesonet al, 1978; Theve et al, 2008; Kanda et al, 2008; White et al, 2012) |
| 2. Hepatic toxins (chemical/alcohol etc.) | Protective effect (Jacqueson et al, 1978) | |
| 3. Diet (metabolic) | Promoter (Vassilatou et al, 2010; Schwingel et al, 2011a, b; Jones et al, 2012) | |
| Fatty liver/NASH/Steatosis | 1. HBV/HCV | Suppressor (Lin et al, 2005, 2008) |
| 2. Hepatic toxins (chemical/alcohol etc.) | Promoter (Chow et al, 2011)/ Suppressor (Zhang et al 2013) | |
| Cirrhosis | 1. HBV/HCV | Promoter/suppressor (DeLoia et al, 1989; Wright and Lau, 1993; Tanaka et la, 2000; Yu et al, 2000; Eugene R Schiff 2003; Chiu et al, 2007; Wang et al, 2009; Wu et al, 2010) |
| 2. Hepatic toxins (chemical/alcohol etc.) | Inconslusive (Rambaldi and Gluud, 2006; Thole et al, 2004) | |
| 3. Diet (metabolic) | Unknown | |
| Hepatocarcinogenesis | 1. HBV/HCV | Promoter/suppressor (Yu et al, 2000, 2001; Zhang et al, 2007a; Chiu et al, 2007; Wang et al, 2008; Kanda et al, 2008; Wu et al, 2010; Huang et al, 2011) |
| 2. Hepatic toxins (chemical/alcohol etc.) | Promoter (Tejura et al, 1989; Rossi et al, 2003; Ma et al, 2008; Li et al, 2012) | |
| 3. Diet (metabolic) | Unknown | |
| HCC progression/ cancer metastasis | 1. HBV/HCV | Unknown |
| 2. Hepatic toxins (chemical/alcohol etc.) | Unknown | |
| 3. Diet (metabolic) | Suppressor (Tavian et al, 2002; Zhu et al, 2011; Ma et al, 2012) |
There is mounting evidence that the effects of androgen are not equal to those of AR on HCC progression and that targeting AR instead of targeting androgens may lead to better therapies for patients with HCC. Additional studies are needed to uncover how AR influences individual infiltrating cells and their interaction within the HCC microenvironment(Ma, et al. 2012a).
Although hepatocytes comprise more than 85% of all cells in normal livers, the cancerous liver presents with an increased number of infiltrating immune cells (Doumba, et al. 2013; Guo, et al. 2013), including dendrocytes (Butterfield 2004), monocytes/macrophages (Shirabe, et al. 2012), CD4/8+ T cells (Guo et al. 2013), neutrophils (Motomura, et al. 2013), B cells (Chen, et al. 2012) and mast cells (Ju, et al. 2009). Other cells in the liver, such as endothelial cells (Sato and Mori 2011) or BM-MSCs (Garcia, et al. 2011) may also be altered due to chronic inflammation or tumorigenesis. Ma et al found that infiltration of monocytes/macrophages might alter the growth and invasion patterns of HCC and that targeting AR in HCC might reduce the infiltration of monocytes/macrophages, thereby suppressing monocyte/macrophage-mediated cancer growth (Wen-Lung Ma, Yin- Yi Chen, Chawnshang Chang, 2013). Huang et al (Huang, et al. 2013) also revealed that infiltrating BM-MSCs in the cirrhotic liver might result in enhanced liver repair and subsequent improvement in liver function. It will be interesting to see whether targeting AR in BM-MSCs suppresses or prevents the progression of cirrhosis to HCC.
Many studies have focused on sex hormone signaling to try to explain the gender difference associated with HCC. In a recent review article, Ruiggieri et al (Ruggieri, et al. 2010) provided an abundance of evidence that estrogens inhibit IL6 production by suppressing NFκB/MyD88 and that they also reduce cellular oxidative stress. However, they also found that androgen signaling promotes the VEGF/Stat3 pathway and suppresses p53-mediated antitumor effects. Therefore, there appears to be a crossover of estrogen and androgen signals in the regulation of cellular oxidative stresses.
In summary, the dual yet opposite roles of AR in early HCC initiation vs. later advanced stages, and findings developing new and differential therapeutic approaches via targeting AR to battle HCC at different stages may help us to better battle the HCC.
Acknowledgement
Financial Support: This work was supported by NIH Grant CA127300, George Whipple Professorship Endowment at University of Rochester to C Chang, and Taiwan National Science Council grant (NSC101-2314-B-039-027-MY3), and National Health Research Institution grant (NHRI-EX102-10214BC) to WL Ma.
We appreciate Professor Charles Sparks (University of Rochester) for critical review, discussion, and suggestions. Thanks also to Karen Wolf and Jeffery Conrad for English editing on the manuscript.
Footnotes
Conflict of interest: ASC-J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and C.C. own royalties and equity in AndroScience.
References
- Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–888. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–540. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- Baig S. Gender disparity in infections of Hepatitis B virus. J Coll Physicians Surg Pak. 2009;19:598–600. [PubMed] [Google Scholar]
- Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–257. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW. Lipid metabolism and liver inflammationIHepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232–S241. doi: 10.1053/j.gastro.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Chan AC, Chok KS, Yuen WK, Chan SC, Poon RT, Lo CM, Fan ST. Impact of antiviral therapy on the survival of patients after major hepatectomy for hepatitis B virus-related hepatocellular carcinoma. Arch Surg. 2011;146:675–681. doi: 10.1001/archsurg.2011.125. [DOI] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Chao Y, Chan WK, Huang YS, Teng HC, Wang SS, Lui WY, Whang-Peng J, Lee SD. Phase II study of flutamide in the treatment of hepatocellular carcinoma. Cancer. 1996;77:635–639. [PubMed] [Google Scholar]
- Chen T, Song D, Min Z, Wang X, Gu Y, Wei B, Yao J, Chen K, Jiang Z, Xie H, et al. Perioperative dynamic alterations in peripheral regulatory T and B cells in patients with hepatocellular carcinoma. J Transl Med. 2012;10:14. doi: 10.1186/1479-5876-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, Zhang GR. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14:1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Chen PJ, Yang WJ, Chen DS. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007;104:2571–2578. doi: 10.1073/pnas.0609498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JD, Jones ME, Prelle K, Simpson ER, Boon WC. A selective estrogen receptor alpha agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J Endocrinol. 2011;210:323–334. doi: 10.1530/JOE-10-0462. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Tartagni E, Ertek S. Metformin and its clinical use: new insights for an old drug in clinical practice. Arch Med Sci. 2012;8:907–917. doi: 10.5114/aoms.2012.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens F, Verrijdt G, Schoenmakers E, Haelens A, Peeters B, Verhoeven G, Rombauts W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol. 2001;76:23–30. doi: 10.1016/s0960-0760(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916–922. doi: 10.1002/hep.1840110603. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429–30435. [PubMed] [Google Scholar]
- DeLoia JA, Burk RD, Gearhart JD. Developmental regulation of hepatitis B surface antigen expression in two lines of hepatitis B virus transgenic mice. J Virol. 1989;63:4069–4073. doi: 10.1128/jvi.63.9.4069-4073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio M, Daniele B, Pignata S, Gallo C, De Maio E, Morabito A, Piccirillo MC, Perrone F. Is human hepatocellular carcinoma a hormone-responsive tumor? World J Gastroenterol. 2008;14:1682–1689. doi: 10.3748/wjg.14.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- Dima A, Marinescu AG, Dima AC. Non-alcoholic fatty liver disease and the statins treatment. Rom J Intern Med. 2012;50:19–25. [PubMed] [Google Scholar]
- Donna L, White FK, Hashem B, El-Serag Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clinical Gastroenterology and Hepatology. 2012;10:1342–1359. doi: 10.1016/j.cgh.2012.10.001. .e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumba PP, Nikolopoulou M, Gomatos IP, Konstadoulakis MM, Koskinas J. Co-culture of primary human tumor hepatocytes from patients with hepatocellular carcinoma with autologous peripheral blood mononuclear cells: study of their in vitro immunological interactions. BMC Gastroenterol. 2013;13:17. doi: 10.1186/1471-230X-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- Eugene R, Schiff MFS, Willis C, Maddrey . Schiff's disease of the liver. Lippincott William & Wilkins; 2003. [Google Scholar]
- Even C, Launoy G, Collet T, Duval O, Piquet MA, Rougereau A, Verwaerde JC, Dao T. [Epidemiology of hepatocellular carcinoma in the department of Calvados] Gastroenterol Clin Biol. 1997;21:450–458. [PubMed] [Google Scholar]
- Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. The genetic and environmental basis of hepatocellular carcinoma. Discovery Medicine. 2006;6:182–186. [PubMed] [Google Scholar]
- Fawzy IO, Negm M, Ahmed R, Esmat G, Hamdi N, Abdelaziz AI. Tamoxifen alleviates hepatitis C virus-induced inhibition of both toll-like receptor 7 and JAK-STAT signalling pathways in PBMCs of infected Egyptian females. J Viral Hepat. 2012;19:854–861. doi: 10.1111/j.1365-2893.2012.01612.x. [DOI] [PubMed] [Google Scholar]
- Feng H, Cheng AS, Tsang DP, Li MS, Go MY, Cheung YS, Zhao GJ, Ng SS, Lin MC, Yu J, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2011;121:3159–3175. doi: 10.1172/JCI45967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Wilkinson ML, Iqbal MJ, Johnson PJ, Williams R. Response to cyproterone acetate treatment in primary hepatocellular carcinoma is related to fall in free 5 alpha-dihydrotestosterone. Eur J Cancer Clin Oncol. 1987;23:1659–1664. doi: 10.1016/0277-5379(87)90446-9. [DOI] [PubMed] [Google Scholar]
- Furusyo N, Ogawa E, Sudoh M, Murata M, Ihara T, Hayashi T, Ikezaki H, Hiramine S, Mukae H, Toyoda K, et al. Raloxifene hydrochloride is an adjuvant antiviral treatment of postmenopausal women with chronic hepatitis C: a randomized trial. J Hepatol. 2012;57:1186–1192. doi: 10.1016/j.jhep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, Rodriguez A, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8:1538–1548. doi: 10.1021/mp200137c. [DOI] [PubMed] [Google Scholar]
- Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006;1089:228–236. doi: 10.1196/annals.1386.044. [DOI] [PubMed] [Google Scholar]
- Gluud C. Testosterone and alcoholic cirrhosis. Epidemiologic, pathophysiologic and therapeutic studies in men. Dan Med Bull. 1988;35:564–575. [PubMed] [Google Scholar]
- Gluud C, Christoffersen P, Eriksen J, Wantzin P, Knudsen BB. No effect of long-term oral testosterone treatment on liver morphology in men with alcoholic cirrhosis. Am J Gastroenterol. 1987;82:660–664. [PubMed] [Google Scholar]
- Gong L, Li YH, Su Q, Chu X, Zhang W. Clonality of nodular lesions in liver cirrhosis and chromosomal abnormalities in monoclonal nodules of altered hepatocytes. Histopathology. 2010;56:589–599. doi: 10.1111/j.1365-2559.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- Green GR. Mechanism of hypogonadism in cirrhotic males. Gut. 1977;18:843–853. doi: 10.1136/gut.18.10.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CL, Yang HC, Yang XH, Cheng W, Dong TX, Zhu WJ, Xu Z, Zhao L. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;13:5913–5917. doi: 10.7314/apjcp.2012.13.11.5909. [DOI] [PubMed] [Google Scholar]
- Guy J, Yee HF., Jr Health disparities in liver disease: Time to take notice and take action. Hepatology. 2009;50:309–313. doi: 10.1002/hep.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Gooren LJ, Padungtod P, Saad F. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes. 2010;118:167–171. doi: 10.1055/s-0029-1202774. [DOI] [PubMed] [Google Scholar]
- Hashimoto F, Davis RL, Egli D. Hepatitis following treatments with famotidine and then cimetidine. Ann Pharmacother. 1994;28:37–39. doi: 10.1177/106002809402800106. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Sato K, Takagi H, Hirokawa T, Kojima A, Sohara N, Kakizaki S, Mochida Y, Shimura T, Sunose Y, et al. Primary liver cancers with nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2007;19:827–834. doi: 10.1097/MEG.0b013e3282748ef2. [DOI] [PubMed] [Google Scholar]
- Hépatocellulaire. GdEedTdC. Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology. 2004;40:1361–1369. doi: 10.1002/hep.20474. [DOI] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- Hoshino-Yoshino A, Kato M, Nakano K, Ishigai M, Kudo T, Ito K. Bridging from preclinical to clinical studies for tyrosine kinase inhibitors based on pharmacokinetics/pharmacodynamics and toxicokinetics/toxicodynamics. Drug Metab Pharmacokinet. 2011;26:612–620. doi: 10.2133/dmpk.DMPK-11-RG-043. [DOI] [PubMed] [Google Scholar]
- Huang CK, Lee SO, Lai KP, Ma WL, Lin TH, Tsai MY, Luo J, Chang C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology. 2012 doi: 10.1002/hep.26135. [DOI] [PubMed] [Google Scholar]
- Huang CK, Lee SO, Lai KP, Ma WL, Lin TH, Tsai MY, Luo J, Chang C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology. 2013;57:1550–1563. doi: 10.1002/hep.26135. [DOI] [PubMed] [Google Scholar]
- Huang YT, Jen CL, Yang HI, Lee MH, Su J, Lu SN, Iloeje UH, Chen CJ. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol. 2011;29:3643–3650. doi: 10.1200/JCO.2011.36.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL revealed? Liver Int. 2012;32:1333–1341. doi: 10.1111/j.1478-3231.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- Jacqueson A, Thevenin M, Warnet JM, Claude JR, Truhaut R. Comparative study of the protective effect of an anabolic steroid. The 19-nortestosterone-phenylpropionate (19 NTPP), on liver steatosis induced by Amanita phalloides and white phosphorus in rats. Arch Toxicol Suppl. 1978:193–196. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jilma B, Eichler HG, Koppl C, Weber B, Pidlich JP, Ferenci P, Muller C. Effects of testosterone suppression on serum levels of hepatitis B surface antigen and HBV-DNA in men. Liver. 1998;18:162–165. doi: 10.1111/j.1600-0676.1998.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- Ju MJ, Qiu SJ, Gao Q, Fan J, Cai MY, Li YW, Tang ZY. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci. 2009;100:1267–1274. doi: 10.1111/j.1349-7006.2009.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ES, Kim JH, Yoon EL, Lee HJ, Lee SJ, Suh SJ, Lee BJ, Seo YS, Yim HJ, Seo TS, et al. Comparison of the Methods for Tumor Response Assessment in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.01.039. [DOI] [PubMed] [Google Scholar]
- K P Littmann GW, Gerdes H. Aktivitätsänderungen testosteroninaktivierender Enzyme in menschlichem Lebergewebe bei Cholostase und Lebercirrhose. Verhandlungen der Deutschen Gesellschaft für Innere Medizin (Verh Dtsch Ges Inn Med) 1973;79:1258–1261. [PubMed] [Google Scholar]
- Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14:5945–5961. doi: 10.3748/wjg.14.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Steele R, Ray R, Ray RB. Hepatitis C virus core protein augments androgen receptor-mediated signaling. J Virol. 2008;82:11066–11072. doi: 10.1128/JVI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp CJ, Drinkwater NR. Genetic variation in liver tumor susceptibility, plasma testosterone levels, and androgen receptor binding in six inbred strains of mice. Cancer Res. 1989;49:5044–5047. [PubMed] [Google Scholar]
- Kew MC, Kirschner MA, Abrahams GE, Katz M. Mechanism of feminization in primary liver cancer. N Engl J Med. 1977;296:1084–1088. doi: 10.1056/NEJM197705122961903. [DOI] [PubMed] [Google Scholar]
- Kley HK. Plasma-estrogens and liver cirrhosis. Z Gastroenterol. 1979;17:406–412. [PubMed] [Google Scholar]
- Kuper H, Lagiou P, Mucci LA, Tamimi R, Benetou V, Trichopoulos D. Risk factors for cholangiocarcinoma in a low risk Caucasian population. Soz Praventivmed. 2001a;46:182–185. doi: 10.1007/BF01324254. [DOI] [PubMed] [Google Scholar]
- Kuper H, Mantzoros C, Lagiou P, Tzonou A, Tamimi R, Mucci L, Benetou V, Spanos E, Stuver SO, Trichopoulos D. Estrogens, testosterone and sex hormone binding globulin in relation to liver cancer in men. Oncology. 2001b;60:355–360. doi: 10.1159/000058532. [DOI] [PubMed] [Google Scholar]
- Kwon H, Lok AS. Does antiviral therapy prevent hepatocellular carcinoma? Antivir Ther. 2011;16:787–795. doi: 10.3851/IMP1895. [DOI] [PubMed] [Google Scholar]
- Lai KP, Huang CK, Chang YJ, Chung CY, Yamashita S, Li L, Lee SO, Yeh S, Chang C. New Therapeutic Approach to Suppress Castration-Resistant Prostate Cancer Using ASC-J9 via Targeting Androgen Receptor in Selective Prostate Cells. Am J Pathol. 2013;182:460–473. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KP, Yamashita S, Huang CK, Yeh S, Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med. 2012;4:791–807. doi: 10.1002/emmm.201101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou-Karatzas C, Goritsas P, Makri MG. Low serum testosterone: a special feature of hepatocellular carcinoma. Eur J Med. 1993;2:23–27. [PubMed] [Google Scholar]
- Lee MH, Yang HI, Jen CL, Lu SN, Yeh SH, Liu CJ, You SL, Sun CA, Wang LY, Chen WJ, et al. Community and personal risk factors for hepatitis C virus infection: a survey of 23,820 residents in Taiwan in 1991-2. Gut. 2011;60:688–694. doi: 10.1136/gut.2010.220889. [DOI] [PubMed] [Google Scholar]
- Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008 doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- Liossi AK, Aroni KG, Kyrkou KA, Kittas C, Markaki SP. Immunohistochemical study of sex steroid hormones in primary liver cancer. Cancer Detect Prev. 1988;13:195–201. [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Lund J, Zaphiropoulos PG, Mode A, Warner M, Gustafsson JA. Hormonal regulation of cytochrome P-450 gene expression. Adv Pharmacol. 1991;22:325–354. doi: 10.1016/s1054-3589(08)60040-x. [DOI] [PubMed] [Google Scholar]
- Ma W, Jeng L, Yeh C, Chang C. Androgen and Androgen Receptor Signals Jamming Monocyte/Macrophage Functions in Premalignant Phase of Livers. BioMedicine. 2012a;2:155–159. [Google Scholar]
- Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, Jou YS, Chen CW, Yeh S, Chang C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–955. 955, e941–e945. doi: 10.1053/j.gastro.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hsu CL, Yeh CC, Wu MH, Huang CK, Jeng LB, Hung YC, Lin TY, Yeh S, Chang C. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012b;56:176–185. doi: 10.1002/hep.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Bekesi G, Racz K, Feher J, Schaff Z, Lengyel G, Blazovics A, Illyes G, Szombath D, Hrabak A, et al. Increased total scavenger capacity and decreased liver fat content in rats fed dehydroepiandrosterone and its sulphate on a high-fat diet. Gerontology. 2011;57:343–349. doi: 10.1159/000321385. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Thuluvath PJ. Endocrine diseases and the liver. Clin Liver Dis. 2011;15:55–67. doi: 10.1016/j.cld.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156–163. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso G, Thole Z, Salgueiro E, Revuelta P, Hidalgo A. Spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmacovigilance system. Pharmacoepidemiol Drug Saf. 2006;15:253–259. doi: 10.1002/pds.1168. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W, Alonzo M, Bajaj S, Baker M, Elton E, Farrell TA, Gore RM, Hall C, Nowak J, Roy H, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part I: diagnosis-clinical staging and pathology. J Surg Oncol. 2012a;106:332–338. doi: 10.1002/jso.23028. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W, Alonzo M, Bajaj S, Baker M, Elton E, Farrell TA, Gore RM, Hall C, Nowak J, Roy H, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part II: multidisciplinary management. J Surg Oncol. 2012b;106:339–345. doi: 10.1002/jso.23027. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Nagai D, Ichimura E, Goda R, Tomura A, Doi M, Nishikawa K. Metabolism and hepatic toxicity of flutamide in cytochrome P450 1A2 knockout SV129 mice. J Gastroenterol. 2006;41:231–239. doi: 10.1007/s00535-005-1749-y. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- Mode A, Tollet P, Strom A, Legraverend C, Liddle C, Gustafsson JA. Growth hormone regulation of hepatic cytochrome P450 expression in the rat. Adv Enzyme Regul. 1992;32:255–263. doi: 10.1016/0065-2571(92)90021-q. [DOI] [PubMed] [Google Scholar]
- Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Fukasawa M, Kaneko Y, Suzuki T, Wakita T, Fukazawa H. Selective estrogen receptor modulators inhibit hepatitis C virus infection at multiple steps of the virus life cycle. Microbes Infect. 2013;15:45–55. doi: 10.1016/j.micinf.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Murray M, Cantrill E, Mehta I, Farrell GC. Impaired expression of microsomal cytochrome P450 2C11 in choline-deficient rat liver during the development of cirrhosis. J Pharmacol Exp Ther. 1992;261:373–380. [PubMed] [Google Scholar]
- Nagasue N, Yu L, Yukaya H, Kohno H, Nakamura T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resection. Br J Surg. 1995;82:542–547. doi: 10.1002/bjs.1800820435. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res. 2001;92:249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Cuppers HJ, Wickings EJ. Influence of sex, testicular development and liver function on the bioavailability of oral testosterone. Eur J Clin Invest. 1977;7:145–147. doi: 10.1111/j.1365-2362.1977.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Oshima H. [Androgens] Horumon To Rinsho. 1968;16:863–872. [PubMed] [Google Scholar]
- Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442–450. doi: 10.1016/j.jhep.2012.02.033. [DOI] [PubMed] [Google Scholar]
- Rambaldi A, Gluud C. Anabolic-androgenic steroids for alcoholic liver disease. Cochrane Database Syst Rev. 2006:CD003045. doi: 10.1002/14651858.CD003045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AB, Theve EJ, Feng Y, Fry RC, Taghizadeh K, Clapp KM, Boussahmain C, Cormier KS, Fox JG. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–11546. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- Rossi L, Leveri M, Gritti C, De Silvestri A, Zavaglia C, Sonzogni L, Silvestri L, Civardi E, Mondelli MU, Silini EM. Genetic polymorphisms of steroid hormone metabolizing enzymes and risk of liver cancer in hepatitis C-infected patients. J Hepatol. 2003;39:564–570. doi: 10.1016/s0168-8278(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Ruggieri A, Barbati C, Malorni W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int J Cancer. 2010;127:499–504. doi: 10.1002/ijc.25298. [DOI] [PubMed] [Google Scholar]
- Saint-Aubert B, Vic P, Brissac C, Bories P, Humeau C, Joyeux H, Solassol C. [Hepatic regeneration in the rat after subtotal (90%) hepatectomy treated with testosterone] C R Seances Acad Sci D. 1980;291:653–655. [PubMed] [Google Scholar]
- Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME. Chronic bacterial and parasitic infections and cancer: a review. J Infect Dev Ctries. 2010;4:267–281. doi: 10.3855/jidc.819. [DOI] [PubMed] [Google Scholar]
- Sato K, Mori M. Evolving molecular mechanism-based strategies for control of hepatocellular carcinoma. Curr Med Chem. 2011;18:4375–4388. doi: 10.2174/092986711797200462. [DOI] [PubMed] [Google Scholar]
- Schwingel PA, Cotrim HP, Salles BR, Almeida CE, dos Santos CR, Jr, Nachef B, Andrade AR, Zoppi CC. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int. 2011a;31:348–353. doi: 10.1111/j.1478-3231.2010.02346.x. [DOI] [PubMed] [Google Scholar]
- Schwingel PA, Zoppi CC, Cotrim HP. Increased liver steatosis in anabolic-androgenic steroid users: more evidence towards toxicant-associated fatty liver disease development. Liver Int. 2011b;31:1240–1241. doi: 10.1111/j.1478-3231.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011;29:3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. T cell immunopathogenesis and immunotherapeutic strategies for chronic hepatitis B virus infection. World J Gastroenterol. 2012;18:2443–2451. doi: 10.3748/wjg.v18.i20.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, Baatarkhuu O, Ahn SH. Antiviral efficacy of lamivudine versus entecavir in patients with hepatitis B virus-related advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1528–1534. doi: 10.1111/j.1440-1746.2012.07145.x. [DOI] [PubMed] [Google Scholar]
- Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- Sorensen HT, Thulstrup AM, Mellemkjar L, Jepsen P, Christensen E, Olsen JH, Vilstrup H. Long-term survival and cause-specific mortality in patients with cirrhosis of the liver: a nationwide cohort study in Denmark. J Clin Epidemiol. 2003;56:88–93. doi: 10.1016/s0895-4356(02)00531-0. [DOI] [PubMed] [Google Scholar]
- Sparks JD, Sparks CE. Overindulgence and metabolic syndrome: is FoxO1 a missing link? J Clin Invest. 2008;118:2012–2015. doi: 10.1172/JCI35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift LL, Farkas MH, Major AS, Valyi-Nagy K, Linton MF, Fazio S. A recycling pathway for resecretion of internalized apolipoprotein E in liver cells. J Biol Chem. 2001;276:22965–22970. doi: 10.1074/jbc.M100172200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300–2308. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Terai S, Sakaida I. Advanced therapies using autologous bone marrow cells for chronic liver disease. Discov Med. 2012;14:7–12. [PubMed] [Google Scholar]
- Tamori A, Kawada N. [Interferon] Nihon Rinsho. 2012;70:620–624. [PubMed] [Google Scholar]
- Tanaka K, Sakai H, Hashizume M, Hirohata T. Serum testosterone:estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res. 2000;60:5106–5110. [PubMed] [Google Scholar]
- Tavian D, De Petro G, Pitozzi A, Portolani N, Giulini SM, Barlati S. Androgen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinoma. Histol Histopathol. 2002;17:1113–1119. doi: 10.14670/HH-17.1113. [DOI] [PubMed] [Google Scholar]
- Tejura S, Rodgers GR, Dunion MH, Parsons MA, Underwood JC, Ingleton PM. Sex-steroid receptors in the diethylnitrosamine model of hepatocarcinogenesis: modifications by gonadal ablation and steroid replacement therapy. J Mol Endocrinol. 1989;3:229–237. doi: 10.1677/jme.0.0030229. [DOI] [PubMed] [Google Scholar]
- Theve EJ, Feng Y, Taghizadeh K, Cormier KS, Bell DR, Fox JG, Rogers AB. Sex hormone influence on hepatitis in young male A/JCr mice infected with Helicobacter hepaticus. Infect Immun. 2008;76:4071–4078. doi: 10.1128/IAI.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A. Hepatotoxicity induced by antiandrogens: a review of the literature. Urol Int. 2004;73:289–295. doi: 10.1159/000081585. [DOI] [PubMed] [Google Scholar]
- Rinaldi C, Lesmana A, Laurentus A. the role of Liver Biopsy in the non-invasive methods ERa and liver stiffness measurement using transient elastography. In: Takahashi Hirokazu., Dr, editor. Liver Biopsy. 2011. ISB:978-953-307-644-7. [Google Scholar]
- Tian Y, Kuo CF, Chen WL, Ou JH. Enhancement of hepatitis B virus replication by androgen and its receptor in mice. J Virol. 2012;86:1904–1910. doi: 10.1128/JVI.06707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero V, 3rd, Cosgrove D, Herman JM, Pawlik TM. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol. 2012;6:481–495. doi: 10.1586/egh.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneaux V, Farge-Bancel D, Lecourt S, Baraut J, Cras A, Jean-Louis F, Brun C, Verrecchia F, Larghero J, Michel L. Expression of transforming growth factor beta receptor II in mesenchymal stem cells from systemic sclerosis patients. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatou E, Lafoyianni S, Vryonidou A, Ioannidis D, Kosma L, Katsoulis K, Papavassiliou E, Tzavara I. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod. 2010;25:212–220. doi: 10.1093/humrep/dep380. [DOI] [PubMed] [Google Scholar]
- Vizoso FJ, Rodriguez M, Altadill A, Gonzalez-Dieguez ML, Linares A, Gonzalez LO, Junquera S, Fresno-Forcelledo F, Corte MD, Rodrigo L. Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3221–3227. doi: 10.3748/wjg.v13.i23.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AG, Lee KY, Kim SY, Choi JY, Lee KH, Kim WH, Wang HJ, Kim JM, Park MG, Yeom YI, et al. The expression of estrogen receptors in hepatocellular carcinoma in Korean patients. Yonsei Med J. 2006;47:811–816. doi: 10.3349/ymj.2006.47.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Su B, Ding Z, Du X, Wang B. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T cells. Biochem Biophys Res Commun. 2008;372:491–496. doi: 10.1016/j.bbrc.2008.04.191. [DOI] [PubMed] [Google Scholar]
- Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50:1392–1402. doi: 10.1002/hep.23163. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- Weickert MO, Hodges P, Tan BK, Randeva HS. Neuroendocrine and endocrine dysfunction in the hyperinsulinemic PCOS patient: the role of metformin. Minerva Endocrinol. 2012;37:25–40. [PubMed] [Google Scholar]
- Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- White DL, Tavakoli-Tabasi S, Kuzniarek J, Pascua R, Ramsey DJ, El-Serag HB. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55:759–768. doi: 10.1002/hep.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- Wright TL, Lau JY. Clinical aspects of hepatitis B virus infection. Lancet. 1993;342:1340–1344. doi: 10.1016/0140-6736(93)92250-w. [DOI] [PubMed] [Google Scholar]
- Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, Hung YC, Yeh S, Chang C. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001143. 32ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Yin Y, Wang F, Zhang L, Wang Y, Sun S. An altered pattern of liver apolipoprotein A-I isoforms is implicated in male chronic hepatitis B progression. J Proteome Res. 2010;9:134–143. doi: 10.1021/pr900593r. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang YK, Esterly N, Klaassen CD, Wan YJ. Gender disparity of hepatic lipid homoeostasis regulated by the circadian clock. J Biochem. 2009;145:609–623. doi: 10.1093/jb/mvp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- Yau T, Chan P, Epstein R, Poon RT. Management of advanced hepatocellular carcinoma in the era of targeted therapy. Liver Int. 2009;29:10–17. doi: 10.1111/j.1478-3231.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang HC, Miyamoto H, Takatera H, Rahman M, Kang HY, Thin TH, Lin HK, Chang C. Differential induction of the androgen receptor transcriptional activity by selective androgen receptor coactivators. Keio J Med. 1999;48:87–92. doi: 10.2302/kjm.48.87. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl 1):172–179. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
- Yoon G, Kim JY, Choi YK, Won YS, Lim IK. Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem. 2006;97:393–411. doi: 10.1002/jcb.20638. [DOI] [PubMed] [Google Scholar]
- Yoshitsugu M, Ihori M. [Endocrine disturbances in liver cirrhosis--focused on sex hormones] Nippon Rinsho. 1997;55:3002–3006. [PubMed] [Google Scholar]
- Yu L, Kubota H, Imai K, Yamaguchi M, Nagasue N. Heterogeneity in androgen receptor levels and growth response to dihydrotestosterone in sublines derived from human hepatocellular carcinoma line (KYN-1) Liver. 1997;17:35–40. doi: 10.1111/j.1600-0676.1997.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, Lin SM, Lee SD, Chen PJ, et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023–2028. doi: 10.1093/jnci/92.24.2023. [DOI] [PubMed] [Google Scholar]
- Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, Chen CJ. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–1651. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Ross RK, Stanczyk FZ, Govindarajan S, Gao YT, Henderson BE, Yu MC. A cohort study of serum testosterone and hepatocellular carcinoma in Shanghai, China. Int J Cancer. 1995;63:491–493. doi: 10.1002/ijc.2910630405. [DOI] [PubMed] [Google Scholar]
- Yurci A, Yucesoy M, Unluhizarci K, Torun E, Gursoy S, Baskol M, Guven K, Ozbakir O. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:845–854. doi: 10.1016/j.clinre.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu Y, Wang L, Li Z, Wu J, Rahman N, Guo Y, Li D, Li N, Huhtaniemi I, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345–357. doi: 10.1194/jlr.M028969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chen WL, Ma WL, Chang C, Ou JH. Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein. Virology. 2007a;363:454–461. doi: 10.1016/j.virol.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Zhang JS, Zhu YZ, Fan J, Mao Y, Chen Q, Zhu HG. HBx-induced androgen receptor expression in HBV-associated hepatocarcinoma is independent of the methylation status of its promoter. Histol Histopathol. 2011;26:23–35. doi: 10.14670/HH-26.23. [DOI] [PubMed] [Google Scholar]