Abstract
Upon peripheral nerve injury, specific molecular events, including increases in the expression of selected neurotrophic factors, are initiated to prepare the tissue for regeneration. However, the mechanisms underlying these events and the nature of the cells involved are poorly understood. We used the injury-induced upregulation of glial cell-derived neurotrophic factor (GDNF) expression as a tool to gain insights into these processes. We found that both myelinating and non-myelinating Schwann cells are responsible for the dramatic increase in GDNF expression after injury. We also demonstrate that the GDNF upregulation is mediated by a signaling cascade involving activation of Schwann cell purinergic receptors, followed by protein kinase C signaling which activates protein kinase D (PKD), which leads to increased GDNF transcription. Given the potent effects of GDNF on survival and repair of injured peripheral neurons, we propose that targeting these pathways may yield therapeutic tools to treat peripheral nerve injury and neuropathies.
Keywords: GDNF, purinergic receptor, nerve injury, protein kinase D, apyrase, Schwann cell
INTRODUCTION
Damage to a peripheral nerve can have dramatic consequences on sensory and motor function in the immediate aftermath of an injury, and long-lasting consequences if the process of regeneration is impaired. The cellular and molecular changes that occur within the nerve following an injury are components of a program whose ultimate goal is repair and regeneration (Makwana and Raivich 2005). Numerous studies, utilizing different peripheral nerve injury models, have shown that nerve insult leads quickly to a significant local increase in the expression of several neurotrophic factors and that this induction is important to the process of regeneration (reviewed by (Chen et al. 2007), but little is known concerning the local signals that mediate these events. Identification of the critical physiological modulators and their underlying signal transduction pathways should provide important insights into the intrinsic mechanisms of the injury response and have the potential to reveal new targets for the development of therapies to enhance nerve regeneration.
One of the trophic factors whose expression is induced by peripheral nerve injury is glial cell-derived neurotrophic factor (GDNF). GDNF expression is up-regulated after several types of peripheral nerve injury including sciatic nerve crush, axotomy, and compression (Chao et al. 2008; Hoke et al. 2000; Naveilhan et al. 1997; Trupp et al. 1995). GDNF is a potent survival factor for several types of neurons (Henderson et al. 1994; Lin et al. 1993; Tansey et al. 2000) and application of exogenous GDNF has proven to be beneficial to both peripheral nerve regeneration and functional recovery in multiple experimental nerve injury models, including sciatic nerve transection (Blesch and Tuszynski 2003; Chen et al. 2001; Jubran and Widenfalk 2003; Tannemaat et al. 2008), sciatic nerve crush (Magill et al. 2009), dorsal root avulsion (Ramer et al. 2000), dorsal root compression (Hubbard et al. 2009) and diabetic peripheral neuropathy (Akkina et al. 2001; Anitha et al. 2006). Surprisingly, even though more than 15 years have passed since the first report that peripheral nerve injury induces GDNF expression (Trupp et al. 1995), neither the identity of the cells expressing this factor after injury nor the mechanism responsible for this had been defined. We therefore investigated the temporal, spatial and cellular patterns of injury-induced GDNF expression and the mechanisms that underlie it using adult rat sciatic nerve transection as a model. Using a combination of in vivo, ex vivo and in vitro approaches, we find that injury induces Schwann cells in proximity to the insult to express GDNF and that this depends on the activation of protein kinase C/protein kinase D signaling, which appears to be acting downstream of purinergic receptors.
MATERIALS AND METHODS
Reagents
Drugs were purchased from Calbiochem (La Jolla, CA; Bisindolylmaleimide I hydrochloride (BIM), 12-O-tetradecanoylphorbol-13-acetate (TPA)), Sigma (St. Louis, MO; Forskolin (forsk), 8-bromoadenosine 3′, 5′-cyclic monophosphate (8-Br-cAMP), grade III Apyrase, Adenosine 5′-triphosphate di(Tris) salt dihydrate (ATP)) or Tocris Bioscience (Ellisville, MO; kb NB 142-70).
Rat surgery
8–10 weeks old, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in groups, with a 7 AM-7 PM light-dark cycle. All animal use procedures were in strict accordance with the NIH “Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Care and Use Committee at Children’s Hospital Boston. While under isoflurane anesthesia, the left sciatic nerves were exposed and a full transection just below the sciatic notch was made. Sequential, injury site-adjacent proximal and distal nerve segments (1–1.5 mm length) were removed at various time points and snap frozen in liquid nitrogen. For in vivo drug treatments, Gelfoam® absorbable gelatin compressed sponge (Pfizer, NY, NY) soaked with 200 μl TPA (50 μM in PBS, 3% DMSO final concentration) or vehicle was placed on top of the sciatic nerve around the sciatic notch area. Six hours later, the treated sciatic nerve segment was dissected and then snap frozen.
Sciatic nerve ex vivo culture
Sciatic nerves from adult male rats were dissected, cleaned of overlying connective tissue and cut into 1–1.5 mm segments, which were cultured in low glucose (5.5 mM) Dulbecco’s Modified Eagle Medium (DMEM) with 100 units/ml Penicillin and 100 μg/ml streptomycin at 37° C (Invitrogen, Carlsbad, CA) with the indicated drugs and for the times specified, after which the tissues were snap frozen and stored at −80° C until used to extract RNA or proteins as described below. In each experiment, a group of nerve segments was frozen immediately after cutting and referred to as time 0. Each condition and time point had duplicate samples and at least three independent experiments were performed for each. For samples used in RNA analysis we utilized a pool of 3 to 4, 1–1.5 mm nerve segments for each condition. For samples used in protein extraction and Western blotting, 5 to 6, 1–1.5 mm nerve segments were pooled for each condition tested. We found that the time lag required to clean these segments resulted in a 3 to 6 fold induction in GDNF mRNA compared to nerves that were frozen immediately after euthanasia (data not shown), which explains the apparent lower magnitude of GDNF induction in ex vivo experiments as compared to in vivo injury.
Primary Schwann cell culture
For use in primary cultures, Schwann cells were purified from P1 Long-Evans rats (Charles River, Wilmington, MA) by panning twice on anti-Thy1.1 (AbD Serotec, Raleigh, NC) coated dishes as described previously (Bampton and Taylor 2005). Briefly, sciatic nerves were collected and dissociated with 0.25 % type IV collagenase (Worthington Biochemical Corp., Lakewood, NJ) for one hour, followed by a 15 minute incubation in 0.25% Trypsin (Invitrogen, Carlsbad, CA). The dissociated cell mixture was plated on an anti-Thy1.1-coated dish and incubated at 37° C for one hour. Unattached cells were collected and plated on another anti-Thy1.1 coated dish to further eliminate contaminating fibroblasts. This method results in high purity populations of Schwann cells (> 95%; (Gumy et al. 2008). For GDNF mRNA induction assays, cells were plated at 1.6 × 105 cells/well in laminin (Sigma, St. Louis, MO) coated 24 well plates. Cells were allowed to attach overnight in low glucose DMEM with 0.5% FBS, 1× N2 supplements and penicillin-streptomycin. The next day, cultures were treated with different pharmacological reagents and cells lysed directly in RNA lysis buffer for RNA collection (Qiagen; Valencia, CA). For Western blot assay, Schwann cells were plated at 3.2 × 105 cells per well in laminin (Sigma) coated 12 well plates. The next day, cultures were treated with drugs as indicated and collected using RIPA buffer.
RNA extraction, cDNA synthesis and quantitative real time PCR
Nerve samples were homogenized in Qiazol (Qiagen) using a Polytron homogenizer. Primary Schwann cells were lysed directly in RNeasy lysis buffer provided in the RNA micro collection kit (Qiagen). RNA was extracted according to the manufacturer’s instructions and genomic DNA was eliminated with an on-column DNase treatment (Qiagen). RNA quantity was determined by absorbance at 260 nm. For first-strand cDNA synthesis, 250 ng RNA was used as template for reverse transcription with an iScript kit (Bio-Rad Laboratories, Hercules, CA). Real time PCR was performed using SYBR Green in reactions run on an iCycler (Bio-Rad Laboratories). The amplification conditions were as follows: step 1, 3 mins at 95° C; step 2, 30 sec at 95° C for denaturation; 30 sec at 60° C for annealing and extension for 40 cycles. The following PCR primers were used. Rat GDNF (NCBI Reference Sequence: NM_019139.1): forward, 5′-ACGAAACCAAGGAGGAACTGA-3′; reverse, 5′-TTTGTCGTACATTGTCTCGGC-3′ with an expected 74 bp fragment; rat Neurturin (NCBI Reference Sequence: NM_053399.1): forward, 5′-CGTGCGTGTGATGCTACCT-3′; reverse, 5′-TGTGAATTCAGTTCTCCTGAAAGT-3′ with an expected 73 bp fragment; rat 18S rRNA (GenBank: X01117.1), 5′-CGGCTACCACATCCAAGGAA-3′; reverse, 5′-GCTGGAATTACCGCGGCT-3′ with an expected 187 bp fragment. 18S rRNA was used as the internal control. The primer efficiency was measured by standard curves and amplicon specificity was determined by melting curve analysis and DNA sequencing. All samples were run in triplicate and the relative gene expression was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001; Vandesompele et al. 2002) with the iQ5 optical system software (Bio-Rad). Since GDNF mRNA levels were nearly undetectable at the initial time points in our experiments, we normalized expression to later time points in order to get more accurate quantitation.
Western Blot
Samples from dissected sciatic nerve, nerve segments or Schwann cells were prepared in RIPA buffer containing 1 μg/ml leupeptin, 2 μg/ml antipain, 10 μg/ml benzamidine, 2 μg/ml aprotinin, 1 mM NaF, 2 mM Na3VO4, 1 mM Na pyrophosphate, 0.5 mM PMSF, 50 mM Tris-HCl (pH=7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton, 1% Sodium deoxycholate and 0.2% SDS. The protein concentrations of the lysates were determined using the Pierce BCA Protein Assay (Thermo Fisher Scientific, Rockford, IL). For experiments examining kinase activation, 30 microgram protein samples were separated on 4–15% SDS-PAGE and transferred to PVDF membrane. For examination of GDNF protein levels following nerve segment explantation, samples were prepared as above and 40 micrograms of each extract were run on 4 – 12% Novex Bis-Tris gels in MES running buffer (Invitrogen). The membranes were incubated with primary antibodies against phospho-PKD (pS916) (1:1000, Epitomics), phospho-PKD (pS744/748) (1:1000, Cell Signaling), phospho-PKD (pS744) (1:1000, Cell Signaling), PKC μ C-20 (total PKD, 1:1000, Santa Cruz), GDNF (1:250, Santa Cruz), actin (1:5000, Calbiochem) or α-tubulin (1:1000, Abgen). After overnight incubation, membranes were washed and incubated with appropriate secondary antibodies (1:2000 HRP-conjugated goat anti-rabbit or anti-mouse antibodies, GE Healthcare Bio-Sciences Corp.) and the bands detected using enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp.).
In situ hybridization
Both sense and antisense probes were generated against the rat GDNF sequence which was produced with the following primer set: forward: 5′-GACTCCAATATGCCCGAAGA-3′, reverse: 5′-CTGGAGCCAGGGTCAGATAC-3′. Schwann cells were identified by hybridization with a probe encoding S100β, a known marker of both myelinating and non-myelinating Schwann cells in humans (Gonzalez-Martinez et al. 2003) and rodents (Sugimura et al. 1989). Sense and antisense probes for rat S100β were generated with the following primers: forward: 5′-ATAGCACCTCCGTTGGACAG-3′, reverse: 5′-CATGACACCCAGCAGCTAAA-3′. For PCR-generated cDNAs, the consensus sequence for the T7 promoter was added to the forward primer and the consensus sequence for the T3 promoter was added to the reverse primer. Probes were synthesized using the DIG RNA Labeling Kit following the manufacturer’s instructions (Roche, Indianapolis, IN). OCT or paraffin embedded sciatic nerve samples were sectioned at 15 μm and processed for in situ hybridization. In brief, sections were fixed with 4% paraformaldehyde-PBS and treated with proteinase K (45 U/ml). After acetylation with 0.25% acetic anhydride in triethanolamine buffer, slides were prehybridized at room temperature for one hour and then incubated with 1 μg/ml antisense or sense probes at 65° C for 15 hrs. Unbound probes were washed off and signals were revealed with NBT/BCIP (Roche). Sections incubated with sense probe did not show any signals.
Immunolabeling of sciatic nerves
Freshly dissected (control) and ex vivo (9 hours in culture media) nerve segments were fixed in 4% paraformaldehyde in 0.1M phosphate buffer (PB) for 2 hours, equilibrated in 30% sucrose in PB, embedded in OCT and sectioned (transverse) at 15μm. Sections were blocked in PB containing 10% normal goat serum and 0.3% Triton-X 100 followed by labeling with rabbit anti-GDNF (1:400, Santa Cruz) and either mouse anti-MBP (1:1000, Sternberger Monoclonals) or chicken anti-GFAP (1:1000; Millipore) overnight. Secondary detection was performed using anti-rabbit Alexa 594 and either anti-mouse Alexa 488 or anti-chicken Alexa 488 (Invitrogen). DAPI counterstaining was used to visualize nuclei.
Histology on plastic embedded sciatic nerves
Rats were terminally anesthetized with pentobarbital 100 mg/kg, and underwent transcardial perfusion with 30 mL of cold phosphate buffered saline, followed by 100 mL of cold fixative, containing 1.25% PFA, 2.5% GA, 0.03% Picric Acid in 0.1 M Cacodylate buffer. Sciatic nerves were dissected, immersed in the fixative for 48 hours, washed 2X in 0.1 M cacodylate buffer, and post fixed with 1% OsO4 in 0.1 M cacodylate buffer for 48 hours. After fixation, nerves were washed 2X for 1 hour in 0.1 M cacodylate buffer, dehydrated in graded ethanol solutions: 50, 75, 95, 100% (2X), and suspended in Propylene Oxide for 1 hour 2X. Before embedding the nerve fragments in plastic resin, they were suspended in a solution of Propylene Oxide:araldite-ddsa 1:1 overnight, and serial sections of 1 μm thickness were cut with an ultra-microtome (Reichert Ultracuts, Leica AG 702501, Austria).
Statistical analyses
Unpaired t-test was used for two group comparisons and one-way analysis of variance (ANOVA) followed by Tukey test was used for multiple group comparisons. Data is presented as mean ± S.E.M. and significance is indicated with asterisks: *** p<0.001, ** p<0.01, * p< 0.05 (GraphPad Prism 5 software).
RESULTS
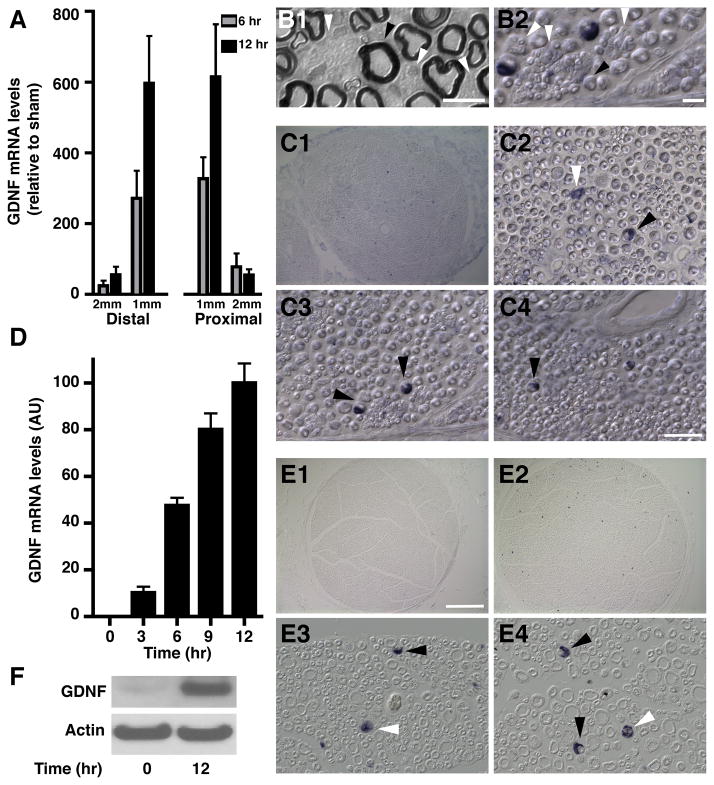
To characterize the temporal and spatial pattern of GDNF mRNA expression after peripheral nerve injury, sciatic nerves of adult rats were transected and RNA was collected from the first and second millimeter segments proximal and distal to the injury site at either 6 or 12 hours following injury. Axotomy caused a dramatic induction in the levels of GDNF mRNA on both sides of the injury site in a time dependent fashion (Fig. 1A), with the greatest increase found in the 1 mm of nerve immediately adjacent to the injury site, suggesting a local response. Interestingly, expression of another member of the GDNF family of trophic factors, Neurturin (Kotzbauer et al. 1996), was not altered by the injury (not shown), indicating that the increase in GDNF expression is specific.
Fig. 1. Nerve injury induces GDNF mRNA in Schwann cells.
A) GDNF mRNA levels in adjacent 1 mm segments both proximal and distal to the injury site in sciatic nerve 6 and 12 hours post-axotomy were measured using real time q-RT-PCR. Values are expressed relative to non-axotomized sham using 18S rRNA as normalizer.
B) Images of sections of plastic embedded (B1) and frozen (B2) sciatic nerves illustrating the distinct morphology of myelinating (black arrowheads) and non-myelinating (white arrowheads) Schwann cells. The scale bars represent 20 μm for B1 and 150μm for B2.
C) In situ hybridization on frozen sections of adult rat sciatic nerve immediately adjacent to the site of injury, 12 hours post axotomy, show GDNF mRNA is restricted to Schwann cells. B1 shows a low magnification image, B2–4 show representative fields at high magnification to illustrate the morphology of GDNF-expressing cells. Black arrowheads in B2–4 indicate myelinating Schwann cells, white arrowheads indicate non-myelinating Schwann cells. The scale bar represents 200 μm for C1 and 50 μm for C2–4.
D) GDNF mRNA levels measured in ex vivo nerve segments at 0, 3, 6, 9, and 12 hours post-explant. Values were normalized to the maximum expression at 12 hours. ANOVA showed that most time points are significantly different from the others with p < 0.001, except for 0 hr vs. 3 hr and 9 hr vs. 12 hr (p > 0.05).
E) In situ hybridization for GDNF performed on sections prepared from paraffin embedded ex vivo sciatic nerve explants. D1: low magnification image of ex vivo nerve segment at time 0. D2: low magnification image of GDNF expression in an ex vivo nerve segment at 12 hours post-explant. D3–4: high magnification view of representative fields of ex vivo nerve sections 12 hours post-explant. Black arrowheads indicate myelinating Schwann cells, white arrowheads indicate non-myelinating Schwann cells. The scale bar represents 200 μm for D1–2 and 50 μm for D3–4.
F) Western blot analysis of ex vivo nerve segments at 0 and 12 hours post-explant shows that the levels of GDNF protein are increased after explantation. Actin levels were measured to control for loading.
It had been proposed that Schwann cells are the source of the GDNF mRNA in injured nerves (Hammarberg et al. 1996), but this was not formally proven because the methods used (radioactive in situ hybridization) did not provide the necessary resolution. Other studies (Bar et al. 1998; Hoke et al. 2002) showed that injury leads to increased GDNF immunostaining in Schwann cells, but this approach could not discriminate between these glial cells being the source of this factor or a site of its accumulation. Therefore, to explore the identity of the GDNF-expressing cells, we used non-radioactive in situ hybridization.
Within peripheral nerves there are several cell types, including Schwann cells, endothelial cells and fibroblasts. Two types of Schwann cells can be observed in the nerve, myelinating and non-myelinating. Each myelinating Schwann cell wraps around one large-diameter axon, creating the myelin sheath while each non-myelinating cell associates with several small diameter axons forming a Remak bundle. Both of these types of cells can be readily identified in histological preparations, including electron microscopy (Corfas et al. 2004) as well as in conventional microscopy using plastic (Fig 1B1), frozen (Fig. 1B2) and paraffin sections (Fig 1E3 and E4).
Non-radioactive in situ hybridization failed to detect GDNF mRNA in the un-injured nerve (Fig. 1E1) but clear signal was observed after injury (Fig. 1C1C2–4 and ). These results (going from no signal to robust signal) are consistent with the dramatic increase we measured using real-time RT-PCR. The cellular resolution of the non-radioactive in situ hybridization identified Schwann cells as the source of increased GDNF mRNA, and the morphology of the labeled cells indicated that GDNF expression was induced in both myelinating and non-myelinating cells (white arrow in Fig. 1C2). No GDNF mRNA was observed in endothelial cells, which did show increased expression of NGF (data not shown).
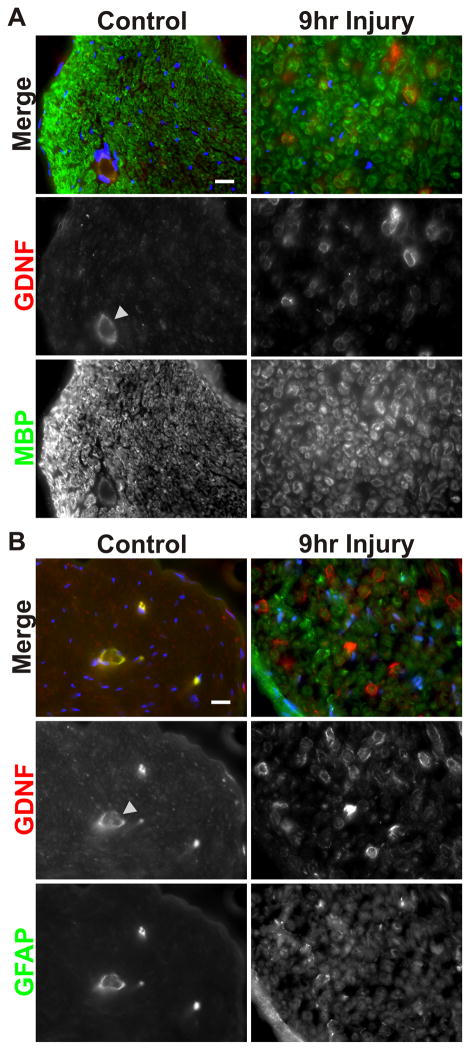
To investigate the mechanisms underlying this injury-induced increase in Schwann cell GDNF expression, we adopted an ex vivo sciatic nerve segment culture system (Banner and Patterson 1994; Kinameri and Matsuoka 2003). Samples consisting of several, 1–1.5 mm long segments of acutely isolated sciatic nerve were cultured for 0, 3, 6, 9, and 12 hours post-dissection, after which RNA was isolated and used to measure GDNF expression. Quantitative RT-PCR revealed a dramatic time-dependent increase in levels of GDNF mRNA in a pattern similar to that seen in vivo (Fig. 1D). As we found with the in vivo axotomy experiments described above, Neurturin expression was not induced by the incubation (data not shown), showing that this system retains its specificity. Furthermore, in situ hybridization of explanted sciatic nerve segments mirrored those obtained in vivo, i.e. Schwann cells are the source of GDNF mRNA after injury. These histological analyses also showed that nerve morphology was well maintained over the course of the experiment (Fig. 1E). Finally, to determine if the change seen in GDNF mRNA was reflected by an increase in GDNF protein, we performed both Western blot and immunofluorescence analyses. Immunoblot analysis showed that extracts of nerve explants that had been in culture for 12 hours contained much greater amount of GDNF protein than freshly isolated segments (Fig. 1F). Immunostaining demonstrated that GDNF protein is clearly upregulated in myelinating (MBP+) Schwann cells in nerve explants after 9 hours in culture compared to freshly dissected ones (Fig. 2A). Changes in GDNF expression in non-myelinating Schwann cells could not be assessed due to the overall increase in the expression of their marker GFAP (Fig. 2B). Some of the secondary antibodies showed non-specific binding to endothelial cells (arrowheads in Figs. 2A and B).
Fig. 2. Nerve injury increases GDNF protein expression in myelinating Schwann cells.
A) Immunofluorescence analysis shows that ex vivo nerve segments 9 hours post-explantation have higher GDNF protein levels associated with myelinating (MBP+) Schwann cells compared to freshly dissected nerve segments. The arrowhead shows blood vessels, which display non-specific staining (see also Panel B). Scale bar = 20μm.
B) Immunofluorescence analysis shows that staining for GFAP, a marker for non-myelinating Schwann cells, is widely increased in ex vivo nerve segments at 9 hours post-explantation compared to freshly dissected nerve segments. As a result, changes in GDNF protein levels in non-myelinating Schwann cells could not be validated. Scale bar = 20μm.
Since the sciatic nerve contains both sensory and motor axons, in situ hybridization of nerve sections did not provide insights into any potential differences in the injury response between Schwann cells associated with sensory versus motor axons. To address this, we performed in situ hybridization experiments using explants of L4 dorsal and ventral roots, which contain predominantly sensory or motor fibers respectively. Hybridization with the GDNF probe showed that injury induces GDNF expression in both roots, peaking at 12 hr, but the density of GDNF-expressing cells was higher in the dorsal root (Table 1). In contrast, hybridization with a probe for S100β, a molecule expressed by both myelinating and non-myelinating cells in rodents (Li et al. 1997; Sugimura et al. 1989), showed no difference in Schwann cell density between dorsal and ventral root sections. These results indicate that the percent of Schwann cells expressing GDNF after injury is significantly higher in the dorsal than the ventral root, suggesting a greater response by Schwann cells associated with sensory axons.
Table 1. Effects of injury on Schwann cell GDNF expression in the dorsal and ventral roots.
The density of GDNF expressing Schwann cells was measured in sections of dorsal and ventral roots 12 hr after explantation. The density of S100β expressing Schwann cells was measured of sections of uninjured roots.
| L4 dorsal | L4 ventral | p value | |
|---|---|---|---|
| Density of GDNF expressing cells (cells/mm2) | 66.7 ± 13.7 | 13.9 ± 1.7 | p < 0.02 |
| Density of S100β expressing cells (cells/mm2) | 165.3 ± 15.4 | 170.7 ± 24.1 | p = 0.86 |
| GDNF expressing Schwann cells (% of S100β+ cells) | 42.1 ± 16.6 | 8.4 ± 1.8 | p < 0.05 |
Some studies have suggested that protein kinase C (PKC) is instrumental in the regulation of GDNF expression in Schwann cells (Kinameri and Matsuoka 2003), while data from studies using glioma and other cell lines have suggested that cAMP-dependent protein kinase (PKA) may also be involved (Verity et al. 1999), including the identification of a cAMP response element in the promoter of the gene for GDNF (Grimm et al. 1998). Therefore, we used the ex vivo system to test the effects of modulators of both pathways. Treatment with TPA, an activator of PKC, increased GDNF mRNA levels significantly beyond that found with “injury” alone (Fig. 3A) while the PKC inhibitor bis-indolyl-maleimide I (BIM) nearly completely inhibited the expression of GDNF mRNA in explants (Fig. 3B), indicating that PKC activity is required for the injury-induced elevation in GDNF mRNA. Conversely, treatment with forskolin, an activator of adenylate cyclase and therefore of PKA, significantly decreased the levels of GDNF mRNA in explanted nerve segments (Fig. 3C), indicating that PKA is not involved in the injury-induced GDNF upregulation, but that rather it acts as an inhibitor. Based on these results we considered the possibility that injury-associated signals activate PKC in Schwann cells, which leads to an induction in GDNF expression. To test this hypothesis we subjected primary cultures of rat sciatic nerve Schwann cells to treatment with TPA and/or BIM. As was observed with the explanted nerve segments, TPA treatment of Schwann cells led to a significant induction of GDNF mRNA, an effect that was blocked by BIM (Fig. 3D). Consistent with what we found in explants, PKA activation in Schwann cells by either forskolin or 8-bromo-cAMP led to a reduction in GDNF mRNA production (Fig. 3E).
Fig. 3. PKC up-regulates and PKA downregulates GDNF expression in sciatic nerve segments and cultured Schwann cells.
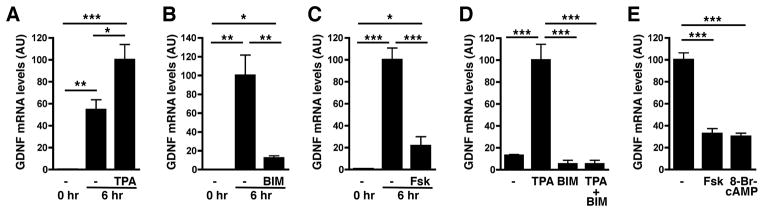
A) The PKC activator TPA enhances the upregulation of GDNF expression in ex vivo sciatic nerve segments after 6hr of culture.
B) PKC inhibition by Bisindolylmaleimide I hydrochloride (BIM) blocks the injury-induced increase of GDNF expression in ex vivo nerve segments.
C) Forskolin reduces the injury-induced increase of GDNF expression in ex vivo nerve segments.
D) TPA treatment induces GDNF expression in Schwann cell cultures, an effect that is blocked by BIM.
E) Forskolin and 8-Br-cAMP treatments reduce GDNF expression by Schwann cells.
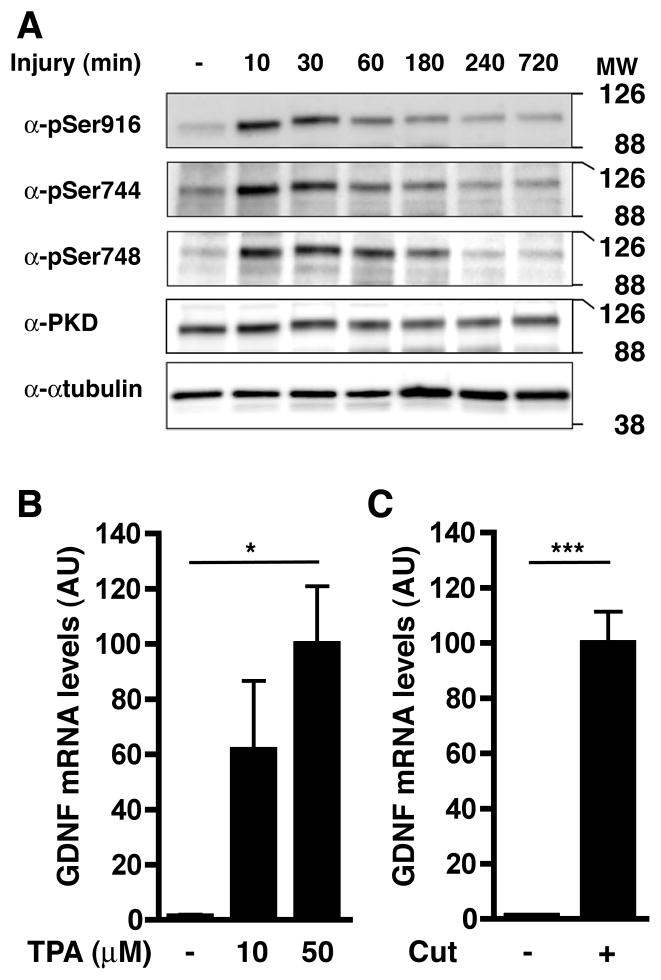
Together, the results obtained with both explants and dissociated Schwann cells suggested that injury causes the activation of PKC in Schwann cells close to the injury site, leading to increased GDNF expression. To monitor endogenous PKC activation after injury, we measured the phosphorylation state of a PKC substrate, protein kinase D (PKD; (Valverde et al. 1994), in nerve explants. Western blots using phospho-residue-specific antibodies showed that PKD became activated and phosphorylated on known PKC-dependent sites, i.e. Ser744 and Ser748 (Jacamo et al. 2008), within minutes of being explanted, with phosphorylation reaching maximum levels rapidly and then decreasing gradually over several hours (Fig. 4A). Importantly, this occurs in the absence of changes in the total amount of PKD. To test if PKC activation is sufficient to upregulate GDNF expression in peripheral nerves, rat sciatic nerves were surgically exposed and then overlaid with GelFoam® saturated with either TPA or vehicle (DMSO) without injuring them. Then, the incision was sutured and the treatment continued for 6 hours, at which time the animals were sacrificed and the nerves were harvested. Exposure of the intact sciatic nerve to the membrane permeable PKC activator TPA caused a dramatic induction of GDNF mRNA (Fig. 4B) of approximately the same order of magnitude as that induced by injury (Fig. 1A). Importantly, neither the procedure nor the presence of the DMSO soaked GelFoam® itself had any impact on the typical response to transection over the same time course (Fig. 4C).
Fig. 4. PKC becomes activated in the injured nerve and PKC activation is sufficient to induce GDNF expression in intact peripheral nerve.
A) Western blot depicting the rapid site-specific phosphorylation of protein kinase D, a PKC substrate, at Ser916, Ser744, and Ser748 in ex vivo nerve segments at the indicated times following culture. Levels of total PKD are unchanged by nerve injury. α-tubulin was used as loading control.
B) Exposure of uninjured nerve to GelFoam® containing the PKC activator TPA for 6 hr induces GDNF expression.
C) Exposure of sciatic nerve to GelFoam® soaked with vehicle (DMSO) does not interfere with GDNF induction in the 2 mm segment adjacent to the injury site.
The observation that PKD becomes phosphorylated in response to injury raised the possibility that its activity could be involved in the injury-induced GDNF expression. Therefore, we tested the effects of CRT0066101, a PKD inhibitor (Thrower et al. 2011), using nerve explants. Western blot analysis showed that CRT0066101 blocked PKD catalytic activity specifically, as revealed by the inhibition of PKD Ser 916 autophosphorylation (Fig. 5A). Importantly, this drug did not interfere with the phosphorylation of MARCKS, another PKC substrate, indicating that PKC activity was not compromised (Fig. 5A). Quantitative RT-PCR showed that PKD inhibition blocks the induction of GDNF by injury even when PKC is activated by TPA (Fig. 5B), indicating a key role for PKD in GDNF expression and that it acts downstream of PKC. Furthermore, PKD inhibition using two different inhibitors, CRT0066101 and kb NB 142-70 (Lavalle et al. 2010) also blocks induction of GDNF expression by TPA in Schwann cells (Fig. 5C and D). Thus, PKC-driven PKD activity is a positive regulator of Schwann cell GDNF expression and is involved in the injury-induced GDNF expression in sciatic nerve.
Fig. 5. PKD activation, downstream of PKC, is necessary for induction of GDNF expression by nerve injury.
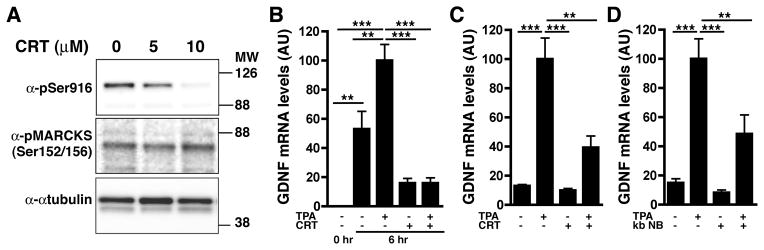
A) Inhibition of PKD using CRT0066101 blocks PKD catalytic activity as revealed by the inhibition of its autophosphorylation (pSer916) but has no impact on the phosphorylation of MARCKS, another target of PKC. α-tubulin was used as a control for loading.
B) TPA treatment of ex vivo nerve segments increases GDNF expression to levels above those induced by injury alone. Both the injury and TPA-induced effects are blocked by CRT0066101.
C) The TPA-induced increase in Schwann cell GDNF expression is significantly reduced by PKD blockade using CRT0066101 (CRT).
D) Blocking PKD activity using the inhibitor kb NB 142-70 (kb NB) greatly diminishes the TPA-induced increase in GDNF expression in cultured Schwann cells.
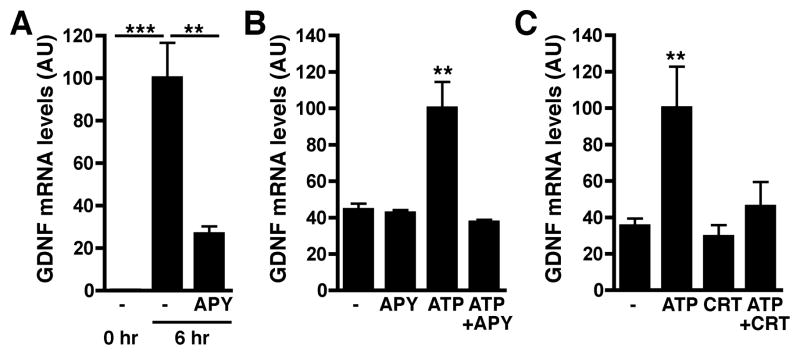
To obtain insights into the potential ligand/receptor systems that might be responsible for the injury-induced activation of the PKC/PKD signaling pathway in Schwann cells, we searched for receptors known to be expressed by Schwann cells and capable of activating the above mentioned kinases. We focused on P2 type purinergic receptors because they are expressed by these glial cells (Braun et al. 2004; Grafe et al. 1999; Stevens 2006), are known to activate PKC and PKD (Bradford and Soltoff 2002; Franke and Illes 2006) and previous studies have shown that their ligand ATP is released as a result of injury in the nervous system (Neary and Kang 2005; Sawynok and Liu 2003). To test the involvement of this pathway in the activation of GDNF in sciatic nerve, we exposed nerve explants to apyrase, an ectonucleotidase that degrades nucleoside di- and triphosphates to their monophosphate form and can thus modulate the activity of P2 type purinergic receptors (Knowles 2011; Zimmermann 1996). Inclusion of apyrase in the culture medium markedly decreased the levels of GDNF expression in explants (Fig. 6A). Furthermore, we found that the addition of exogenous ATP induces GDNF expression in Schwann cells and this effect is blocked by apyrase (Fig. 6B). Finally, inhibiting PKD activation through the addition of CRT0066101 was able to block the ATP-induced increase in GDNF expression in Schwann cells, recapitulating the results that were seen in nerve explants (Fig. 6C).
Fig. 6. Purinergic signaling induces GDNF expression in explants and Schwann cells through PKD activation.
A) Induction of GDNF expression by injury in ex vivo nerve segments is reduced by the presence of apyrase (APY), an ectonucleotidase that degrades ATP and ADP.
B) ATP stimulates GDNF expression by Schwann cells, an effect that is blocked by apyrase (APY).
C) Inhibition of PKD by CRT0066101 (CRT) blocks the ATP-mediated increases in GDNF expression by Schwann cells.
DISCUSSION
There is strong experimental evidence that molecules expressed by Schwann cells play important roles in peripheral nerve regeneration (e.g. see Morisaki et al. 2010; Morton et al. 2012; Tomita et al. 2007; Webber et al. 2011). Schwann cell expression of GDNF in the mature peripheral nerve is quite low, but there is evidence that it is necessary for the maintenance of sensory neurons and their axons (Chen et al. 2003). It has been proposed that induction of GDNF expression by injury is important for neuronal survival and nerve regeneration (Hammarberg et al. 1996; Hoke et al. 2000). Here we delineate a cascade of events that underlie the induction of GDNF expression in response to peripheral nerve injury, i.e. trauma causes release of ATP/ADP at the site of injury, which, in turn, activates Schwann cell purinergic receptors leading to the stimulation of PKC/PKD signaling, which induces transcription of the GDNF gene, a process that might involve Schwann cell c-Jun (Fontana et al. 2012). The source of the ATP/ADP and the mechanisms responsible for their release remain undefined, but the similarity in the magnitude of the GDNF induction on both sides of the lesion supports the hypothesis that purinergic ligands are released by axons in response to electrical activity caused by the injury (Fields and Ni 2010; Mandolesi et al. 2004).
Most previous studies on the effects of nerve injury on GDNF expression focused on time points days to weeks after injury, and showed that 48hr after injury GDNF expression increases 500–1000 fold (Bar et al. 1998; Hammarberg et al. 1996; Hoke et al. 2000; Trupp et al. 1995). We found that this level of induction is already evident within 12 hr after injury, making this 12hr period key for understanding the mechanisms of the injury response. Furthermore, we observed that both myelinating and non-myelinating Schwann cells adjacent to the injury site are responsible for the GDNF expression. We were somewhat surprised to find that a greater proportion of Schwann cells in the dorsal root showed increased GDNF expression following injury compared to the ventral root. This is consistent with the fact that GDNF responsive cells represent a larger portion of the neurons located within the sensory component of the postnatal spinal nerve as compared to the motor (Baudet et al. 2000; Linnarsson et al. 2001; Mikaels et al. 2000). The differences in responses in the roots are not simply due to differences between myelinating and non-myelinating cells, and therefore indicate a significant diversity in the behavior of the Schwann cells dependent upon the type of axons they contact. It is important to note that, along with the injury-induced increase in GDNF, the expression of components of the Ret/GFRα receptor complex is also strongly induced in multiple locations in the nerve, the spinal cord and the dorsal root ganglion (DRG) following peripheral nerve injury (Fundin et al. 1999; Keast et al. 2010; Naveilhan et al. 1997). Furthermore, Keast et al (2010) have proposed that an upregulation of GDNF receptors may increase the number of GDNF responsive cells beyond that seen in the healthy organism. Thus, the increased GDNF expression by Schwann cells at the injury site might influence the behavior of cells that would not normally be sensitive to this growth factor.
Our finding that the increase in GDNF expression depends on purinergic receptors in Schwann cells provides evidence that extracellular ATP/ADP acts as an injury signal in peripheral nerves, something that had been observed in the CNS (Neary et al. 2005; Volonte et al. 2003; Wang et al. 2004). For example, a recent study reported that astroglia utilize signaling through P2X7 receptors as a cellular sensor for the pathological release of ATP (Oliveira et al. 2011), and purinergic signaling activated via ATP release has been implicated in bidirectional communication between neurons and satellite cells in the trigeminal ganglion, where it modulates calcium signaling in both cell types (Suadicani et al. 2010). Also, of particular relevance to our results, mechanical injury to spinal nerve roots has been shown to lead to a rapid and significant release of ATP to the extracellular space (Grafe et al. 2006), and GDNF itself has been shown to regulate the expression of purinergic receptors following injury (Bradbury et al. 1998). Schwann cells have been shown to express a number of P2 type receptors (Fields and Burnstock 2006) and thus are capable of responding to injury-released ATP/ADP, but the specific set of receptors involved in the regulation of GDNF expression remain to be defined. It is important to note that purinergic signaling in the context of axon-glia interactions also has been implicated in the process of myelination, but the specifics in this respect remain poorly defined as they have been reported to promote myelination by oligodendrocytes (Ishibashi et al. 2006) but inhibit it in Schwann cells (Stevens and Fields 2000).
Downstream of purinergic receptor activation in Schwann cells, we have identified PKD signaling as a critical component of the pathway leading to GDNF production following axotomy. Our study provides the first evidence that PKD is critical in Schwann cells and establishes this pathway as an important intracellular signaling mediator downstream of axon-glia communication. PKD, originally characterized as a PKC isoform (PKCμ) (Valverde et al. 1994), plays important roles in numerous cell types outside of the nervous system, regulating proliferation, migration and differentiation, immune function and angiogenesis (Rozengurt 2011). In contrast, there has been little information concerning the involvement of PKD signaling in nervous system development and function. PKD has been implicated in the establishment of neuronal polarity (Bisbal et al. 2008; Yin et al. 2008) and the maintenance of Golgi apparatus integrity and dendritic arborization in hippocampal neurons (Czondor et al. 2009). PKD activation is also part of the early response by dopaminergic neurons to oxidative stress (Asaithambi et al. 2011). In sensory neurons, PKD activation has been linked to the phosphorylation of transient receptor potential (TRP) channels leading to pathological changes in nociception (Amadesi et al. 2009). From the point of view of glia biology, it has been reported that cultured rat primary cerebellar astrocytes express PKD and that it is activated downstream of signaling by different purinergic receptors (Carrasquero et al. 2010).
Our data also identify an important new role for PKD, i.e. a critical component of the injury-induced increase in GDNF expression in the peripheral nerve. The compelling evidence that exogenous GDNF improves/facilitates recovery after peripheral nerve damage (Akkina et al. 2001; Blesch and Tuszynski 2003; Chen et al. 2001), coupled with data indicating that a reduction in GDNF expression by non-myelinating Schwann cells causes small-fiber neuropathy (Chen et al. 2003), suggest that the signaling cascade we have uncovered for the regulation of GDNF expression by Schwann cells could be an interesting potential target for pharmacological approaches for the treatment of peripheral neuropathies. Future studies could also address whether PKD activation is important in Schwann cells during normal development and in processes such as myelination.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS35884 (to G.C.) and National Institutes of Health (NIH) Intellectual and Developmental Disabilities Research Center grant P30-HD 18655 (to G.C.).
BIBLIOGRAPHY
- Akkina SK, Patterson CL, Wright DE. GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp Neurol. 2001;167(1):173–82. doi: 10.1006/exnr.2000.7547. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Grant AD, Cottrell GS, Vaksman N, Poole DP, Rozengurt E, Bunnett NW. Protein kinase D isoforms are expressed in rat and mouse primary sensory neurons and are activated by agonists of protease-activated receptor 2. J Comp Neurol. 2009;516(2):141–56. doi: 10.1002/cne.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaithambi A, Kanthasamy A, Saminathan H, Anantharam V, Kanthasamy AG. Protein kinase D1 (PKD1) activation mediates a compensatory protective response during early stages of oxidative stress-induced neuronal degeneration. Mol Neurodegener. 2011;6:43. doi: 10.1186/1750-1326-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bampton ET, Taylor JS. Effects of Schwann cell secreted factors on PC12 cell neuritogenesis and survival. J Neurobiol. 2005;63(1):29–48. doi: 10.1002/neu.20119. [DOI] [PubMed] [Google Scholar]
- Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91(15):7109–13. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Saldanha GJ, Kennedy AJ, Facer P, Birch R, Carlstedt T, Anand P. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. Neuroreport. 1998;9(1):43–7. doi: 10.1097/00001756-199801050-00009. [DOI] [PubMed] [Google Scholar]
- Baudet C, Mikaels A, Westphal H, Johansen J, Johansen TE, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development. 2000;127(20):4335–44. doi: 10.1242/dev.127.20.4335. [DOI] [PubMed] [Google Scholar]
- Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, Quiroga S, Diaz Anel A, Malhotra V, Marzolo MP, Caceres A. Protein kinase d regulates trafficking of dendritic membrane proteins in developing neurons. J Neurosci. 2008;28(37):9297–308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467(3):403–17. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12(4–5):256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Bradford MD, Soltoff SP. P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J. 2002;366(Pt 3):745–55. doi: 10.1042/BJ20020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45(2):124–32. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- Carrasquero LM, Delicado EG, Sanchez-Ruiloba L, Iglesias T, Miras-Portugal MT. Mechanisms of protein kinase D activation in response to P2Y(2) and P2X7 receptors in primary astrocytes. Glia. 2010;58(8):984–95. doi: 10.1002/glia.20980. [DOI] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506(2):180–93. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6(11):1186–93. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–33. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Chai YF, Cao L, Lu CL, He C. Glial cell line-derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res. 2001;902(2):272–6. doi: 10.1016/s0006-8993(01)02395-2. [DOI] [PubMed] [Google Scholar]
- Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(42):9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czondor K, Ellwanger K, Fuchs YF, Lutz S, Gulyas M, Mansuy IM, Hausser A, Pfizenmaier K, Schlett K. Protein kinase D controls the integrity of Golgi apparatus and the maintenance of dendritic arborization in hippocampal neurons. Mol Biol Cell. 2009;20(7):2108–20. doi: 10.1091/mbc.E08-09-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7(6):423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Ni Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci Signal. 2010;3(142):ra73. doi: 10.1126/scisignal.2001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. The Journal of cell biology. 2012;198(1):127–41. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109(3):297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Mikaels A, Westphal H, Ernfors P. A rapid and dynamic regulation of GDNF-family ligands and receptors correlate with the developmental dependency of cutaneous sensory innervation. Development. 1999;126(12):2597–610. doi: 10.1242/dev.126.12.2597. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Perez-Pinera P, Diaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60(6):633–8. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- Grafe P, Mayer C, Takigawa T, Kamleiter M, Sanchez-Brandelik R. Confocal calcium imaging reveals an ionotropic P2 nucleotide receptor in the paranodal membrane of rat Schwann cells. J Physiol. 1999;515 ( Pt 2):377–83. doi: 10.1111/j.1469-7793.1999.377ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Schaffer V, Rucker F. Kinetics of ATP release following compression injury of a peripheral nerve trunk. Purinergic Signal. 2006;2(3):527–36. doi: 10.1007/s11302-006-9018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm L, Holinski-Feder E, Teodoridis J, Scheffer B, Schindelhauer D, Meitinger T, Ueffing M. Analysis of the human GDNF gene reveals an inducible promoter, three exons, a triplet repeat within the 3′-UTR and alternative splice products. Hum Mol Genet. 1998;7(12):1873–86. doi: 10.1093/hmg/7.12.1873. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37(2):298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7(4):857–60. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–4. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hoke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport. 2000;11(8):1651–4. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Experimental neurology. 2002;173(1):77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Martinez JJ, Burdick JA, Winkelstein BA. Controlled release of GDNF reduces nerve root-mediated behavioral hypersensitivity. J Orthop Res. 2009;27(1):120–7. doi: 10.1002/jor.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(748) phosphorylation. J Biol Chem. 2008;283(19):12877–87. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubran M, Widenfalk J. Repair of peripheral nerve transections with fibrin sealant containing neurotrophic factors. Exp Neurol. 2003;181(2):204–12. doi: 10.1016/s0014-4886(03)00041-4. [DOI] [PubMed] [Google Scholar]
- Keast JR, Forrest SL, Osborne PB. Sciatic nerve injury in adult rats causes distinct changes in the central projections of sensory neurons expressing different glial cell line-derived neurotrophic factor family receptors. J Comp Neurol. 2010;518(15):3024–45. doi: 10.1002/cne.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinameri E, Matsuoka I. Autocrine action of BMP2 regulates expression of GDNF-mRNA in sciatic Schwann cells. Brain Res Mol Brain Res. 2003;117(2):221–7. doi: 10.1016/s0169-328x(03)00326-7. [DOI] [PubMed] [Google Scholar]
- Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011;7(1):21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384(6608):467–70. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- Lavalle CR, Bravo-Altamirano K, Giridhar KV, Chen J, Sharlow E, Lazo JS, Wipf P, Wang QJ. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol. 2010;10:5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Terenghi G, Hall SM. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997;20(4):333–47. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Mikaels A, Baudet C, Ernfors P. Activation by GDNF of a transcriptional program repressing neurite growth in dorsal root ganglia. Proc Natl Acad Sci U S A. 2001;98(25):14681–6. doi: 10.1073/pnas.251548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magill CK, Tuffaha SH, Yee A, Luciano JP, Hunter DA, Mackinnon SE, Borschel GH. The short- and long-term effects of Seprafilm on peripheral nerves: a histological and functional study. J Reconstr Microsurg. 2009;25(6):345–54. doi: 10.1055/s-0029-1215526. [DOI] [PubMed] [Google Scholar]
- Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272(11):2628–38. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004;18(15):1934–6. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- Mikaels A, Livet J, Westphal H, De Lapeyriere O, Ernfors P. A dynamic regulation of GDNF-family receptors correlates with a specific trophic dependency of cranial motor neuron subpopulations during development. Eur J Neurosci. 2000;12(2):446–56. doi: 10.1046/j.1460-9568.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Morisaki S, Nishi M, Fujiwara H, Oda R, Kawata M, Kubo T. Endogenous glucocorticoids improve myelination via Schwann cells after peripheral nerve injury: An in vivo study using a crush injury model. Glia. 2010;58(8):954–63. doi: 10.1002/glia.20977. [DOI] [PubMed] [Google Scholar]
- Morton PD, Johnstone JT, Ramos AY, Liebl DJ, Bunge MB, Bethea JR. Nuclear factor-kappaB activation in Schwann cells regulates regeneration and remyelination. Glia. 2012;60(4):639–50. doi: 10.1002/glia.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9(7):1450–60. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y. Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol Neurobiol. 2005;31(1–3):95–103. doi: 10.1385/MN:31:1-3:095. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Tran M, Feld J. Traumatic injury activates protein kinase B/Akt in cultured astrocytes: role of extracellular ATP and P2 purinergic receptors. J Neurotrauma. 2005;22(4):491–500. doi: 10.1089/neu.2005.22.491. [DOI] [PubMed] [Google Scholar]
- Oliveira JF, Riedel T, Leichsenring A, Heine C, Franke H, Krugel U, Norenberg W, Illes P. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex. 2011;21(4):806–20. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–6. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011;26(1):23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69(5):313–40. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Stevens B. Cross-talk between growth factor and purinergic signalling regulates Schwann cell proliferation. Novartis Found Symp. 2006;276:162–75. discussion 175–80, 233–7, 275–81. [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287(5461):2267–71. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010;6(1):43–51. doi: 10.1017/S1740925X09990408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Haimoto H, Nagura H, Kato K, Takahashi A. Immunohistochemical differential distribution of S-100 alpha and S-100 beta in the peripheral nervous system of the rat. Muscle Nerve. 1989;12(11):929–35. doi: 10.1002/mus.880121109. [DOI] [PubMed] [Google Scholar]
- Tannemaat MR, Eggers R, Hendriks WT, de Ruiter GC, van Heerikhuize JJ, Pool CW, Malessy MJ, Boer GJ, Verhaagen J. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci. 2008;28(8):1467–79. doi: 10.1111/j.1460-9568.2008.06452.x. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Baloh RH, Milbrandt J, Johnson EM., Jr GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25(3):611–23. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Thrower EC, Yuan J, Usmani A, Liu Y, Jones C, Minervini SN, Alexandre M, Pandol SJ, Guha S. A novel protein kinase D inhibitor attenuates early events of experimental pancreatitis in isolated rat acini. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G120–9. doi: 10.1152/ajpgi.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Kubo T, Matsuda K, Fujiwara T, Yano K, Winograd JM, Tohyama M, Hosokawa K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia. 2007;55(11):1199–208. doi: 10.1002/glia.20533. [DOI] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130(1):137–48. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci U S A. 1994;91(18):8572–6. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity AN, Wyatt TL, Lee W, Hajos B, Baecker PA, Eglen RM, Johnson RM. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J Neurosci Res. 1999;55(2):187–97. doi: 10.1002/(SICI)1097-4547(19990115)55:2<187::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Volonte C, Amadio S, Cavaliere F, D’Ambrosi N, Vacca F, Bernardi G. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2(6):403–12. doi: 10.2174/1568007033482643. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Webber CA, Christie KJ, Cheng C, Martinez JA, Singh B, Singh V, Thomas D, Zochodne DW. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5H2. Glia. 2011;59(10):1503–17. doi: 10.1002/glia.21194. [DOI] [PubMed] [Google Scholar]
- Yin DM, Huang YH, Zhu YB, Wang Y. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J Neurosci. 2008;28(35):8832–43. doi: 10.1523/JNEUROSCI.1291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996;49(6):589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]