Early social isolation results in adult behavioral and cognitive dysfunction that correlates with white matter alterations. However, how social deprivation influences myelination and the significance of these myelin defects in the adult remained undefined. We show that mice isolated for 2 weeks immediately after weaning have alterations in prefrontal cortex function and myelination that do not recover with reintroduction into a social environment. These alterations, which occur only during this critical period, are phenocopied by loss of oligodendrocyte ErbB3 receptors, and social isolation leads to reduced expression of the ErbB3 ligand neuregulin-1. These findings indicate that social experience regulates prefrontal cortex myelination through neuregulin-1/ErbB3 signaling and that this is essential for normal cognitive function, thus providing a cellular and molecular context to understand the consequences of social isolation.
Juvenile social isolation and neglect influence adult cognitive function and social interactions (1–4). Studies of children raised in institutions where neglect was rampant showed that deprivation also correlates with alterations in white matter tracts associated with the medial prefrontal cortex (mPFC), which are not reversed by subsequent foster care placement (5, 6). Similarly, rhesus monkeys isolated as juveniles have white matter disturbances and impaired working memory in adulthood (7), suggesting that juvenile social experience and forebrain white matter development are linked. Studies indicate that experience and neuronal activity influence central nervous system (CNS) myelin (8). However, it remains unclear whether any effect of social experience on oligodendrocytes is important for the establishment of normal adult neuronal circuits and their function, what aspects of oligodendrocyte development are regulated by social experience, and which molecular mechanisms underlie these events. To explore the effects of juvenile social experience on CNS myelination and function, we focused on the murine mPFC.
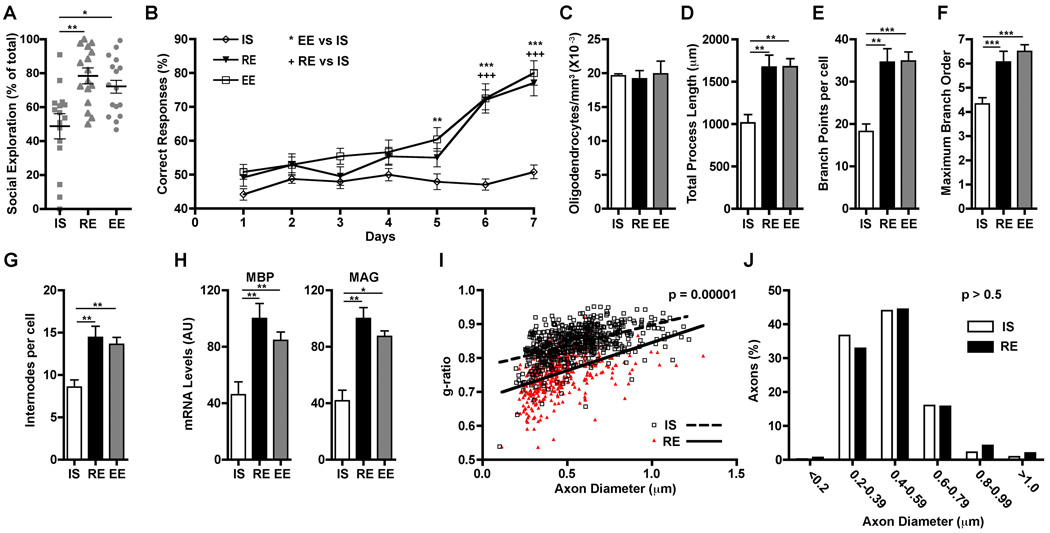
Male mice expressing enhanced green fluorescent protein in oligodendrocytes under the control of the proteolipid protein (PLP) promoter (PLP-eGFP) (9, 10) were housed under one of the following conditions beginning at weaning [postnatal day 21 (P21)]: in isolation (IS, single mouse per standard cage), in a regular environment (RE, four mice per standard cage), or in an enriched environment (EE, large cage with eight mice and novel toys replaced every 48 hours). To determine whether these environments affected mPFC function, 4 weeks later (P50), we tested two mPFC-dependent behaviors, sociability [using a social interaction test; (11)] and working memory [using a non-matching-to-place task; (12)]. Only isolated animals showed alterations, with decreases in both social interaction and acquisition in the non-matching-to-place task (Fig. 1, A and B). General locomotive activity was not altered in isolated mice (fig. S1), which indicated that the change in social behavior was specific. In contrast, as previously described (13), locomotive activity was reduced in animals raised in an enriched environment.
Fig. 1.
PFC-dependent behaviors and mPFC oligodendrocytes are altered by juvenile isolation. (A and B) Mice reared in isolation starting at P21 (IS), by P50 show alterations in social interactions (A) and working memory (B) compared with mice in regular (RE) or enriched environment (EE) (n = 16 mice per group). (C to G) Rearing environment does not influence mPFC oligodendrocyte density by P65 (n = 3 mice per group), but social isolation results in mPFC oligodendrocytes with simpler morphology (cells per condition: IS = 12, RE = 16, EE = 16). (H) P21 to P65 isolation reduces mPFC MBP and MAG expression. AU = arbitrary units; (mice per group: IS = 8, RE = 8, EE = 4). (I to J) P21 to P65 isolation leads to reduced mPFC myelin thickness (n = 3 per group) without changes in axon diameters (450 axons per group). *P < 0.05, **P < 0.01, ***P < 0.001. Error bars, SEM.
Then, mPFC myelination was analyzed at P65. The density of mPFC oligodendrocytes was the same in all groups (Fig. 1C), and oligodendrocyte morphology was indistinguishable between mice raised in enriched or regular environments (Fig. 1, D to G). However, the morphology of mPFC oligodendrocytes from isolated mice was markedly simpler, with shorter processes, less branching, and fewer internodes (Fig. 1, D to G). Furthermore, mPFC expression of two myelin genes, myelin basic protein (MBP) and myelin-associated glycoprotein (MAG), was reduced in isolated mice (Fig. 1H). Additionally, electron microscopy showed that isolated mice had reduced mPFC myelin thickness, whereas myelinated axon diameter was unaffected (Fig. 1, I and J). Thus, social isolation from weaning to sexual maturity, which interferes with the elaboration of mPFC-dependent behaviors (14, 15), also alters mPFC myelination and yields oligodendrocytes with simpler morphologies.
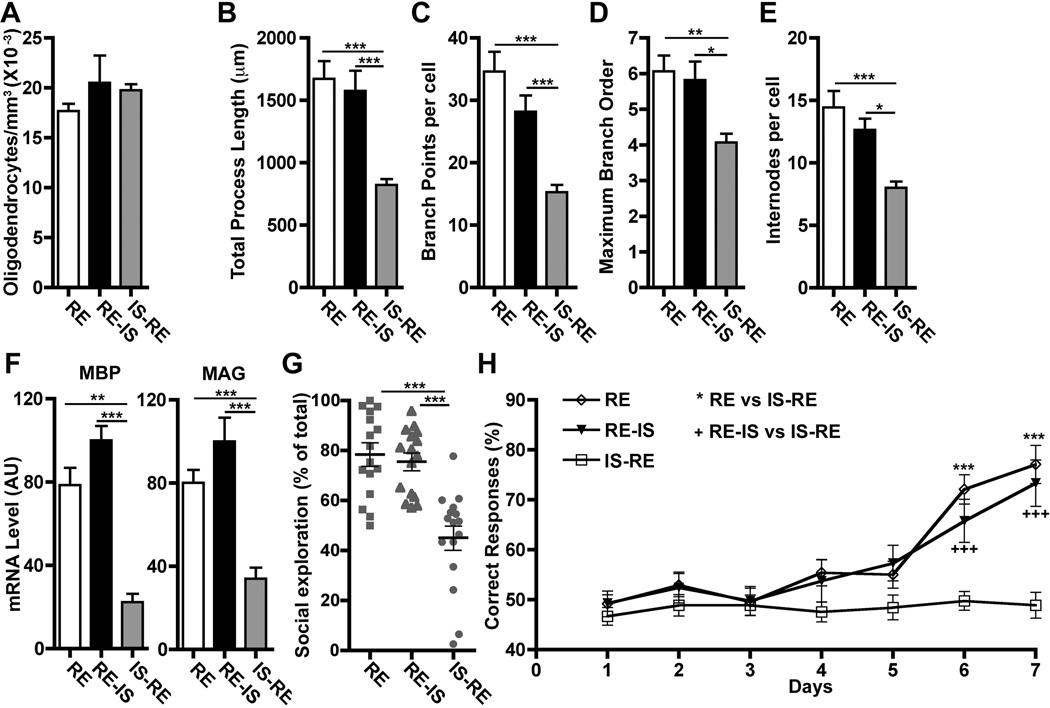
To determine when social experience influences mPFC myelination most, we examined oligodendrocytes in mice housed under regular conditions (RE) and found that P21 to P35 is a period of significant mPFC oligodendrocyte maturation (fig. S2). Further analysis at P35 showed that mice exposed to just 2 weeks of isolation (P21 to P35) exhibited deficits similar to those seen in mice isolated from P21 to P65 (fig. S3). Therefore, we wondered whether P21 to P35 is a critical period for the influence of social isolation on myelination. When isolation was initiated after P35 (RE-IS), oligodendrocyte morphology and myelin gene expression were indistinguishable from those in normally reared mice by P65 (Fig. 2, A to F). In contrast, if mice were isolated from P21 to P35 and then returned to regular housing until P65 (IS-RE), oligodendrocytes displayed abnormal morphology (Fig. 2, A to F). Behavioral tests showed similar results: Mice isolated for 2 weeks immediately after weaning and then returned to regular housing (IS-RE) displayed deficits equivalent to those seen in mice isolated P21 to P65, whereas mice isolated only P35 to P65 (RE-IS) performed like regularly housed mice (RE) (Fig. 2, G and H, and fig. S1B). Thus, social isolation from P21 to P35 alters mPFC oligodendrocyte morphology, myelination, and mPFC-mediated behaviors. These effects persist even when isolated mice are re-exposed to social interactions, which suggests a link between the quality of mPFC myelination established during the juvenile period and adult behaviors.
Fig. 2.
The effects of social isolation on mPFC oligodendrocytes and PFC-dependent behaviors occur only during a critical period and are not reversed by reintroduction to a social environment. (A to E) mPFC oligodendrocytes show morphological alterations in mice isolated P21 to P35 and then returned to regular environment until P65 (IS-RE), but not in mice housed in regular environment until P35 and isolated thereafter (RE-IS). Early social isolation does not affect oligodendrocyte density (n = 3 mice per group), but does alter morphology (cells per group: RE = 16, RE-IS = 11, IS-RE = 14). (F) IS-RE mice showed reductions in mPFC MBP and MAG expression (n = 8 mice per group) and alterations in (G) sociability and (H) working memory (n = 16 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001. Error bars, SEM.
The effects of juvenile social experience likely occur because of changes in signaling pathways important for oligodendrocyte maturation such as neuregulin-1–ErbB (NRG1-ErbB) (16–22). Although there has been some controversy surrounding the role of this signaling pathway in CNS myelination (23, 24), oligodendrocytes express the ErbB2 and ErbB3 receptors (16, 23), and hypomyelination occurs in mice with reduced oligodendrocyte ErbB signaling or NRG1 expression (21, 22). To directly explore the link between ErbB signaling in oligodendrocytes and mPFC myelination in response to isolation, we generated mice with an inducible oligodendrocyte-specific knockout (KO) of ErbB3 using mice carrying an ErbB3 floxed allele (25) and mice expressing the tamoxifen-inducible Cre recombinase (CreERT) under the control of the PLP promoter (PLP/CreERT) (26).
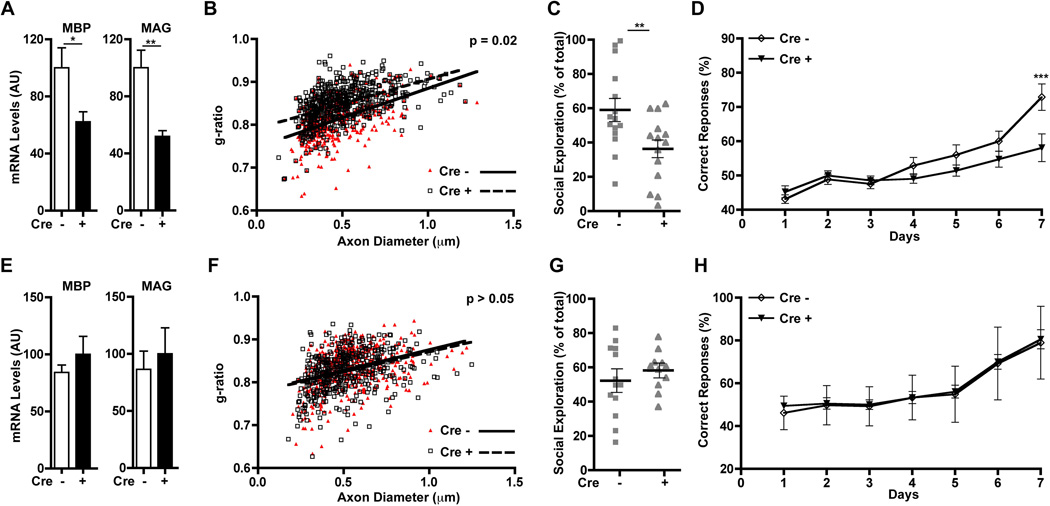
We first tested if ErbB3 could be knocked out in postnatal oligodendrocytes and if this alters myelination in the corpus callosum and the optic nerve. Quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) showed that, when tamoxifen treatment was initiated at P10, by P40 ErbB3 expression in these two regions was reduced by more than 60% in PLP/CreERT::ErbB3flox/flox compared with control (ErbB3flox/flox) mice (fig. S4, A and C). Furthermore, at this age, PLP/CreERT::ErbB3flox/flox mice also had thinner myelin in both structures (fig. S4, B, D, E and F) without changes in axon caliber (fig. S4, G and H), which proved that ErbB3 signaling in oligodendrocytes after P10 is necessary for the generation of myelin of the correct thickness in white matter tracts of the brain. Next, we tested the impact of loss of ErbB3 in oligodendrocytes during the critical period for mPFC myelination and function. Using QRT-PCR, we confirmed that ErbB3 mPFC expression was knocked down whether tamoxifen injections were initiated before or after the critical period, i.e., P19 or P36 (fig. S5). Then, cohorts of PLP/CreERT::ErbB3flox/flox and ErbB3flox/flox mice housed under regular conditions (RE) received tamoxifen injections beginning at either P19 or P36 and were analyzed between P50 and P65. Mice that lost oligodendrocyte ErbB3 expression starting at P19 phenocopied socially isolated wild types; i.e., they exhibited reduced myelin gene expression (Fig. 3A), reduced myelin thickness in mPFC (Fig. 3B), and alterations in sociability and working memory (Fig. 3, C and D, and fig. S1C). In contrast, ablation of oligodendrocyte ErbB3 from P36 onward had no effect on MBP and MAG (Fig. 3E), myelin thickness (Fig. 3F), or mPFC-dependent behaviors (Fig. 3, G and H, and fig. S1C). Thus, ErbB3 signaling in mPFC oligodendrocytes is only required during the critical period to achieve normal myelination, and in its absence, mPFC-dependent behaviors are abnormal.
Fig. 3.
Loss of ErbB3 signaling in oligodendrocytes during the critical period for myelination mimics the effects of social isolation. (A to D) When Cre-mediated ErbB3 knockdown in oligodendrocytes begins at P19 (Cre+), by P65 mice have (A) reduced mPFC MBP and MAG expression (n = 7 per group); (B) reduced myelin thickness (Cre−; n = 3, Cre+; n = 4), (C) altered sociability, and (D) altered working memory (Cre−; n = 15, Cre+; n = 14). (E to H) No effects were observed when tamoxifen was injected starting at P36 (mRNA measurement: Cre−; n = 4, Cre+; n = 5; myelin thickness: n = 3 for, behavioral tests: Cre−; n = 13, Cre+; n = 12). *P < 0.05, **P < 0.01, ***P < 0.001. Error bars, SEM.
To further investigate the involvement of ErbB signaling in mPFC myelination, we used mice expressing a dominant-negative ErbB4 receptor (DN-ErbB4) in oligodendrocytes under the control of the promoter for 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP-DN-ErbB4::PLP-eGFP mice) (21). DN-ErbB4 interferes specifically with ErbB3 and ErbB4 signaling (18, 21, 27). CNP-DN-ErbB4 mice housed in a regular or an enriched environment displayed oligodendrocytic and behavioral phenotypes similar to those of wild types reared in social isolation during the critical period (fig. S6). Thus, interfering with oligodendrocyte ErbB signaling eliminates the positive effects of social experience on mPFC myelination and development of normal behavior.
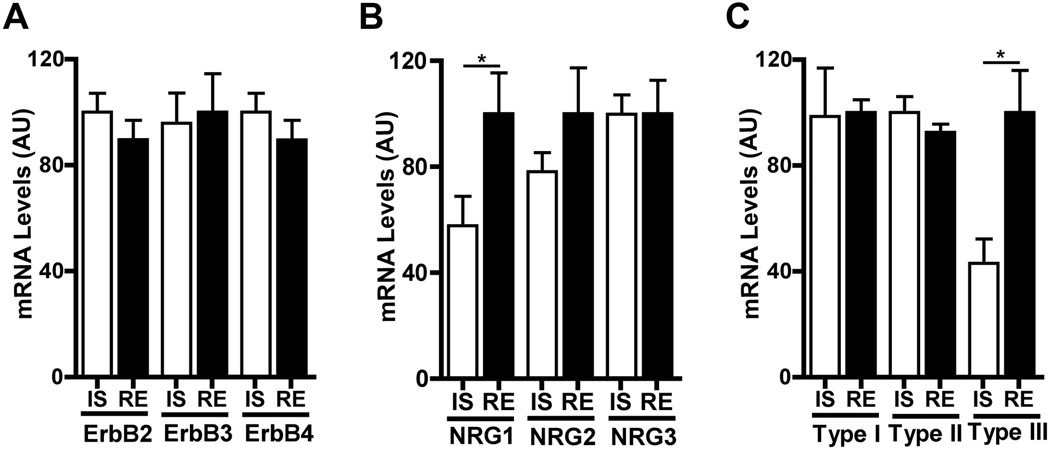
To test if mPFC NRG1-ErbB signaling is regulated by juvenile social experience, we compared the levels of expression of components of this pathway between mice isolated P21 to P35 and those in regular housing. Isolation did not change expression of ErbB2, ErbB3, ErbB4, NRG2, or NRG3 (Fig. 4, A and B). However, isolated mice showed a significant decrease in expression of the epidermal growth factor–like domain of NRG1, common to all NRG1 isoforms (28) (Fig. 4B). The Nrg1 gene produces numerous isoforms through alternative splicing and the activity of several promoters (28). The decrease in NRG1 expression was limited to type III NRG1 (Fig. 4C), the isoform most highly expressed in the mPFC (fig. S7). mPFC type III NRG1 mRNA levels in isolated mice were lower at P35 than at P21 (fig. S8A), and isolation resulted in reduced mPFC NRG1 expression in CNP-DN-ErbB4 mice (fig. S8B), showing that isolation reduces mPFC type III NRG1 expression independently of oligodendrocyte ErbB signaling.
Fig. 4.
Social isolation alters mPFC type III NRG1 expression. (A and B) Isolation from P21 to P35 (IS) did not change mPFC expression levels of ErbB2, ErbB3, and ErbB4 but reduced NRG1 mRNA levels. (C) Isoform specific QRT-PCR showed that the effect of isolation was limited to type III NRG1 (n = 8 per group). *P < 0.05. Error bars, SEM.
Our results demonstrate that juvenile social experience influences oligodendrocyte maturation and myelination in the murine mPFC during a temporally restricted “critical period” between P21 and P35. This effect depends on oligodendrocyte ErbB3 signaling. Note that mice in which oligodendrocyte maturation and/or myelination is abnormal because of loss of ErbB3 signaling phenocopy the behaviors elicited by social isolation during the critical period, which indicates that experience-dependent oligodendrocyte maturation is necessary for normal social behavior and working memory in the adult. At the same time, the motor cortex, a region adjacent to mPFC, showed none of the social experience–dependent changes in gene expression observed in the mPFC (fig. S9), which highlights the specificity of the effects of social experience.
How do myelin defects produce these behavioral and/or cognitive deficiencies? One scenario is that thinner myelin changes conduction velocity of myelinated mPFC axons, which leads to abnormal information processing, which in turn contributes to the aberrant social behaviors and working memory. Also, loss of oligodendrocyte ErbB signaling by DN-ErbB4 produces changes in dopamine neurotransmission similar to those generated by juvenile isolation (21, 29). Thus, a second possibility is that myelin defects caused by isolation change dopaminergic functionand contribute to the deficits in working memory and social interactions (30, 31). Although the changes in NRG1 expression induced by isolation might also modify ErbB4 signaling in interneurons (32), because elimination of ErbB3 signaling in oligodendrocytes phenocopies isolation, it is evident that oligodendrocyte ErbB3 signaling is essential in this context.
There is consensus that NRG1-ErbB signaling plays a central role in peripheral nervous system myelination (18–20, 24), but its roles in the CNS were controversial. It is now apparent that ErbB signaling is not required for the initiation of CNS myelination, but that ErbB3 is necessary for late events in oligodendrocyte maturation and myelination; i.e., oligodendrocyte ErbB3 and ErbB4 loss does not produce myelin defects by P11 (24), but loss of oligodendrocyte ErbB3 starting at P10 causes hypomyelination in the corpus callosum and optic nerve. Furthermore, in the mPFC, hypomyelination occurs even when oligodendrocyte ErbB3 is lost at later stages (after P19). Moreover, mPFC myelination in CNP-DN-ErbB4 mice is not different from wild type at P21 but is clearly altered by P35 (fig. S10). ErbB4 KO mice do not have alterations in CNS myelin even upon reaching adulthood (24), and loss of ErbB3 produces the same phenotype as expression of DN-ErbB4, which blocks both ErbB3 and ErbB4 function; this indicates that ErbB3 is the critical ErbB receptor for CNS myelination. Our data suggest that normal type III NRG1 expression is necessary for mature mPFC myelination, in agreement with the observation that reduced type III NRG1 expression leads to hypomyelination in several brain regions (22), whereas NRG1 overexpression results in thicker CNS myelin (24).
The effects of social experience on mPFC myelination depend, at least in part, on the social experience–dependent regulation of NRG1-ErbB3 signaling. We propose that lack of social interactions during the juvenile period leads to reduced type III NRG1 expression by mPFC neurons, resulting in reduced oligodendrocyte ErbB3 signaling and thus incomplete oligodendrocyte maturation and myelination. Previous studies showed that neuronal activity influences expression of type I but not type III NRG1 mRNA in the intact brain and cultures of cortical neurons (33), a finding we replicated in primary cultures of frontal cortex neurons (fig. S11A). Furthermore, expression of type III NRG1 was unaffected in the mPFC of mice with oligodendrocyte ErbB3 KO or those expressing DN-ErbB4 in oligodendrocytes (fig. S11B). These results suggest that social experience affects mPFC myelination by influencing the levels of type III NRG1 in mPFC neurons independent of neuronal depolarization and ErbB receptor signaling in oligodendrocytes. Nevertheless, the possibility that other molecular events also contribute to the effects of social experience on myelination should not be dismissed. For example, in vitro studies by Wake et al. (34) showed that release of glutamate along axons induces myelination in part by increasing synthesis of myelin proteins in the associated oligodendrocytes. Thus, electrical activity in mPFC might influence the translation of the myelin transcripts regulated by type III NRG1 in an experience-dependent fashion and so contribute to normal myelination.
Our findings indicate that the effects of childhood isolation and neglect on adult mental health might be caused, at least in part, by alterations in oligodendrocytes and myelin development. Furthermore, we provide a cellular and/or molecular context and genetic models in which to begin to understand the effects of juvenile social experience on brain development in general and myelin maturation in particular. Our results may be also relevant to neuropsychiatric disorders such as schizophrenia and mood disorders, which usually manifest after the juvenile period and have been linked to alterations in white matter and myelination (35, 36). In this respect, it is noteworthy that the NRG1-ErbB signaling pathway has been genetically linked to neuropsychiatric disorders (36, 37), which suggests that genetic liabilities in this pathway and life experience may interact in the pathogenesis of these diseases.
Supplementary Material
Acknowledgments
We thank T. Schwarz, C. J. Woolf, R. A. Corfas, M. Rao, F. Sher and M. Lozada for critical reading of the manuscript; C. Hart for help with mouse colony; G. Gorski for help with electron microscopy; C. Arteaga for the generous gift of the ErbB3 floxed mouse line; J. C. Dodart for advice on behavioral tests; M. Fagiolini for allowing us to use behavioral equipment. This research was supported in part by the National Institute of Neurological Disorders and Stroke, NIH, grant R01 NS35884 (to G.C.), by Lori and Joel Freedman (to G.C.), an NIH Intellectual and Developmental Disabilities Research Center grant P30-HD 18655, and Nara Medical University (M.M.).
References and Notes
- 1.Egeland B, Sroufe LA, Erickson M. The developmental consequence of different patterns of maltreatment. Child Abuse Negl. 1983;7:459. doi: 10.1016/0145-2134(83)90053-4. [DOI] [PubMed] [Google Scholar]
- 2.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch. Gen. Psychiatry. 2007;64:49. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Bos K, et al. Psychiatric outcomes in young children with a history of institutionalization. Harv. Rev. Psychiatry. 2011;19:15. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollak SD, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 2010;81:224. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chugani HT, et al. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 6.Eluvathingal TJ, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 8.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 2002;22:876. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murtie JC, Macklin WB, Corfas G. Morphometric analysis of oligodendrocytes in the adult mouse frontal cortex. J. Neurosci. Res. 2007;85:2080. doi: 10.1002/jnr.21339. [DOI] [PubMed] [Google Scholar]
- 11.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur. J. Neurosci. 2000;12:4457. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 13.Van de Weerd HA, et al. Effects of environmental enrichment for mice: Variation in experimental results. J. Appl. Anim. Welf. Sci. 2002;5:87. doi: 10.1207/S15327604JAWS0502_01. [DOI] [PubMed] [Google Scholar]
- 14.Winterfeld KT, Teuchert-Noodt G, Dawirs RR. Social environment alters both ontogeny of dopamine innervation of the medial prefrontal cortex and maturation of working memory in gerbils (Meriones unguiculatus) J. Neurosci. Res. 1998;52:201. doi: 10.1002/(SICI)1097-4547(19980415)52:2<201::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav. Brain Res. 1999;100:91. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 16.Park SK, Miller R, Krane I, Vartanian T. The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes. J. Cell Biol. 2001;154:1245. doi: 10.1083/jcb.200104025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Sun Q, Oglesbee M, Yoon SO. The role of ErbB2 signaling in the onset of terminal differentiation of oligodendrocytes in vivo. J. Neurosci. 2003;23:5561. doi: 10.1523/JNEUROSCI.23-13-05561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J. Neurosci. 2006;26:3079. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 20.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy K, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8131. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taveggia C, et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 23.Schmucker J, et al. erbB3 is dispensable for oligodendrocyte development in vitro and in vivo. Glia. 2003;44:67. doi: 10.1002/glia.10275. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu S, et al. Gene targeting of ErbB3 using a Cre-mediated unidirectional DNA inversion strategy. Genesis. 2006;44:477. doi: 10.1002/dvg.20243. [DOI] [PubMed] [Google Scholar]
- 26.Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- 27.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J. Neurosci. 2003;23:230. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp. Cell Res. 2003;284:14. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 29.Gariépy JL, Gendreau PL, Mailman RB, Tancer M, Lewis MH. Rearing conditions alter social reactivity and D1 dopamine receptors in high- and low-aggressive mice. Pharmacol. Biochem. Behav. 1995;51:767. doi: 10.1016/0091-3057(95)00028-u. [DOI] [PubMed] [Google Scholar]
- 30.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 31.Li CR, Huang GB, Sui ZY, Han EH, Chung YC. Effects of 6-hydroxydopamine lesioning of the medial prefrontal cortex on social interactions in adolescent and adult rats. Brain Res. 2010;1346:183. doi: 10.1016/j.brainres.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 32.Rico B, Marín O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr. Opin. Genet. Dev. 2011;21:262. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. Specific regulation of NRG1 isoform expression by neuronal activity. J. Neurosci. 2011;31:8491. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 37.Buonanno A. The neuregulin signaling pathway and schizophrenia: From genes to synapses and neural circuits. Brain Res. Bull. 2010;83:122. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stankovic KM, Corfas G. Real-time quantitative RT-PCR for low-abundance transcripts in the inner ear: Analysis of neurotrophic factor expression. Hear. Res. 2003;185:97. doi: 10.1016/s0378-5955(03)00298-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.