Abstract
Chaihu, prepared from the dried roots of Bupleurum Chinense DC (also known as bei Chaihu in Chinese) or Bupleurum scorzoneraefolium WILD (also known as nan Chaihu in Chinese), is a herbal medicine for harmonizing and soothing gan (liver) qi stagnation. Substantial pharmacological studies have been conducted on Chaihu and its active components (saikosaponins). One of the active components of Chaihu, saikosaponin-d, exhibited anticancer effects via autophagy induction. This article reviews the pharmacological findings for the roles of autophagy in the pharmacological actions of Chaihu and saikosaponins.
Keywords: Autophagy, Chaihu, saikosaponin, Chinese Medicine, qi
Introduction
Chaihu, prepared from the dried roots of Bupleurum Chinense DC (also known as bei Chaihu in Chinese) or Bupleurum scorzoneraefolium WILD (also known as nan Chaihu in Chinese), is often prescribed as decoctions such as “xiao yao powder”, “da Chaihu decoction”, or “xiao Chaihu decoction” for treating chills and fevers [1-3]. Chaihu facilitates sheng (ascending) and jiang (dispersing) qi to alleviate stagnation of gan (liver) qi[4]. The contemporary clinical indications for Chaihu include common cold, malaria, cholecystitis, globus pharyngitis, gynecological diseases, depression, hepatitis, liver cirrhosis, pancreatitis, and hyperlipidemia [5,6]. Recent research has revealed the pharmacological actions of Chaihu. Specifically, Chaihu and its active components (saikosaponins) exhibited immunomodulatory [7,8], antiviral [9], antipyretic [10,11], hepatoprotective [12,13], anticancer [14], sedative, and analgesic [15] effects. Our recent study further revealed that saikosaponin-a (Ssa) and saikosaponin-d (Ssd), which are related to gan qi regulation [4,13] can induce autophagy [16]. This article reviews the recent findings for the roles of autophagy in the pharmacological actions of Chaihu and saikosaponins (Figure 1).
Figure 1.
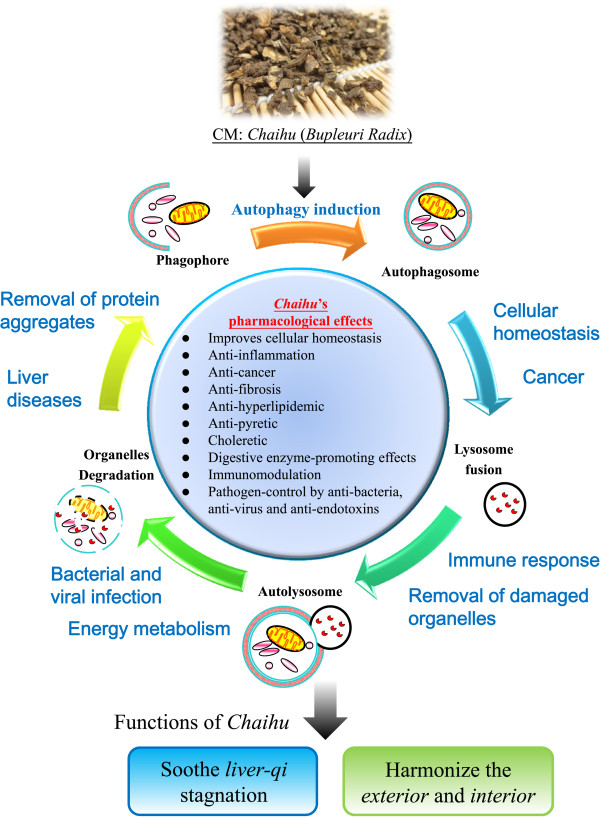
A schematic diagram illustrating the pharmacological effects of Chaihu through autophagy induction. With its major clinical indications in anti-inflammatory, anticancer, antifibrotic, antihyperlipidemic, and antipyretic functions, Chaihu exhibits its pharmacological effects by regulating balanced cellular homeostasis via autophagy induction, leading to harmonization and modulation of qi in the human body.
Chaihu regulates qi stagnation in Chinese Medicine (CM) theory
The CM approach to relieving symptoms (e.g., physical discomfort and emotional instability) is to soothe stagnation of gan qi[17]. Gan qi stagnation can lead to (1) distention and pain in the chest and flank, and menstrual dysregulation, (2) impaired digestive functions such as loss of appetite, dyspepsia, flatulence, and regurgitation, and (3) emotional instabilities such as depression, anxiety, and insomnia [18]. Chaihu is often prescribed to relieve the symptoms of qi stagnation in CM [5].
Modern pharmacological studies on Chaihu and its active components
Chaihu alleviates a wide spectrum of disorders in a multi-target manner through its immunomodulatory [7], antipyretic [10], hepatoprotective [13], choleretic [15], autophagy-inducing [16], sedative and analgesic [15], antihyperlipidemic [15], antiviral [9], and anticancer [14] effects.
The pharmacological effects of Chaihu are attributed to its active components, Ssa, saikosaponin-c (Ssc), and Ssd [19,20]. Ssa exhibits antiproliferative, anti-inflammatory, anticancer, antioxidative, and hepatoprotective effects [21-26]. Ssc induces umbilical vein endothelial cell proliferation, migration, and capillary vascularization [27], and possesses anti-hepatitis effects [28]. Ssd also exhibits immunomodulatory, antiproliferative, and anticancer effects [29-32]. In particular, Ssd induces autophagy and autophagic cell death in apoptosis-defective cells via direct inhibition of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump (SERCA) and mammalian target of rapamycin (mTOR), with disruption of calcium homeostasis and induction of endoplasmic reticulum (ER) stress [16].
Autophagy in health and diseases
Autophagy has been highlighted for its protective roles in various physiological and pathological conditions including (1) cellular homeostasis and genome stability maintenance, (2) immunomodulation, (3) hepatoprotection and aggregate removal, (4) cancers, and (5) emotional instability conditions [33-35]. Autophagic regulation is mainly responsible for maintenance of normal cellular and hormonal homeostasis, defense against pathogen invasion, and protection against toxic protein aggregate accumulation, and beneficial improvements in all of these at the cellular level are related to improved qi stagnation (Table 1).
Table 1.
Comparisons of CM applications, pharmacological actions, and autophagy effects of Chaihu
| CM applications | Pharmacological effects | Autophagic effects |
|---|---|---|
| Improvement of alternating chills and fever |
Antipyresis Antibacteria, antivirus, and anti-endotoxin Immunomodulation |
Immunomodulation Anti-pathogens Modulation of cytokine secretion Removal of toxic mutant proteins and aggregates |
| Modulation of inflammatory symptoms and diseases |
Immunomodulation Antibacteria and antivirus Modulation of cytokine secretion |
Immunomodulation by pathogen and cytokine control Removal of abnormal protein aggregates Detoxification and degradation of toxins and inflammatory proteins |
| Reduction of distention and pain in the chest and flank Improvement of digestive functions: loss of appetite, dyspepsia, and flatulence |
Hepatoprotection Anti-inflammation Anti-fibrosis Promotion of pancreatic digestive enzyme secretion |
Cellular catabolism for removal of waste materials Immunomodulation Anti-pathogens Removal of toxic mutant proteins and aggregates Regulation of lipid metabolism |
| Improvement of circulation or stasis of blood and body fluid, and accumulation of phlegm |
Promotion of cancer cell death Reduction in cancer cell proliferation Immunomodulation, apoptosis, and anti-angiogenesis |
Maintenance of genomic stability Promotion of autophagic cell death Elimination of damaged proteins and cytotoxic substances |
| Improvement of emotional instability | Reduction in plasma lipid levels Hormonal regulation Glucose metabolism | Regulation of lipid metabolism Removal of toxic mutant proteins and aggregates |
Newborn mice under starvation showed immediate increases of autophagy in various tissues, which returned to the basal levels after nutrient supply restoration [36-38]. Mice deficient in autophagy-related gene (Atg) 5 showed a substantial increase in nutrition deprivation-induced death, suggesting an essential role of autophagy in energy maintenance [39]. Autophagy is a protective mechanism that eliminates abnormal proteins and defective organelles such as mitochondria, peroxisomes, or ER membranes. For example, hepatocytes from Atg7-knockout mice exhibited accumulation of abnormal mitochondria and ER structures [40], and associated cellular degeneration [39]. A recent study further revealed essential roles of autophagy in limiting DNA damage and chromosome instability, and failure of the autophagy process can result in carcinogenesis or cell death [41].
Chaihu-mediated autophagy induction
Maintenance of normal homeostasis by defense against pathogen infections is critical. Fever is an immune response initiated by inflammatory mediators such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, macrophage inflammatory protein 1, and interferon (IFN) for heat production, and depends on antipyretics (IL-10, glucocorticoids, and neuropeptides) for heat dissipation [42,43]. Chaihu is prescribed as the major herbal medicine to resolve alternating chills and fever, headache, distention in the chest and flank, or loss of appetite in CM [18,44]. Chaihu was reported to exert its antipyretic effect through the thermoregulatory center in the hypothalamus [45]. Chaihu inhibited increases in cyclic adenosine monophosphate (c-AMP), an endopyrogen, in the hypothalamus and promoted the release of antipyretic substances [46]. Furthermore, total saikosaponins exerted potent anti-endotoxin effects with a simultaneous reduction in body temperature elevation in vivo[47]. All of these beneficial effects can be attributed to the maintenance of cellular homeostasis, a key process regulated by autophagy.
In liver ischemia-reperfusion injury, autophagy induction attenuated the organ damage, and delayed inflammatory or oxidative damage [48]. Furthermore, autophagy suppression was found to be a response to excessive alcohol intake, which might be a reason for the abnormal protein aggregation observed in liver diseases [40]. In vitro studies further showed a dysfunction of autophagy in cells with hepatitis C virus infection [48,49]. Autophagy was also found to regulate the immunological responses to invading microorganisms [50]. Another study showed that plasmacytoid dendritic cells recognized viruses via Toll-like receptors (TLRs) with a requirement for autophagy [51]. In addition, defective autophagy was involved in inflammatory diseases such as systemic lupus erythematosis and Crohn’s disease [52,53]. Emerging evidence has suggested roles for autophagy in immunological responses including antimicrobial activity, antigen presentation, cytokine production, and regulation of lymphocytes [50,54]. For example, disruption of the virulence factor from the HSV-1 virus, which inhibited the host autophagy proteins, could prevent fatal encephalitis [55]. In addition, autophagy exhibited protective functions in the spleen, bone marrow, or liver through activation of immune responses such as detoxification and degradation of toxins and inflammatory proteins [56-58].
Chaihu regulated the immune responses against invading pathogens by stimulating the secretion of glucocorticoids and inhibiting inflammation and anaphylaxis [59,60], and was involved in inflammatory processes such as infiltration, capillary permeability, and release of cytokines [46]. Chaihu or its component saikosaponins eliminated exogenous pyrogens through their antibacterial properties [61], and possessed antiviral activities toward hepatitis B [62], human coronavirus 229E [9], influenza virus [11], and respiratory syncytial virus [63]. Ssd reduced the levels of cyclooxygenase and lipoxygenase in vitro, promoted IL-2 and IL-4 production, and inhibited IL-6, TNF-α, and IFN-γ expression in mouse T lymphocytes [64,65]. The prominent anti-inflammatory effects of Chaihu could be mediated through autophagy induction, a key process for pathogen elimination and immunity regulation. Our group was the first to report the autophagic activities of Chaihu and Ssd [16]. We hypothesized that Chaihu harmonizes the exterior and interior of the human body and soothes gan qi stagnation through autophagy induction.
Chaihu-induced autophagy alleviates gan qi stagnation
In CM theory, Chaihu soothes stagnation of gan qi and promotes circulation of qi, and thus alleviates distention and pain in the chest and flank, menstrual dysregulation, impaired digestive functions such as loss of appetite, dyspepsia, flatulence, and regurgitation, and emotional instabilities such as depression, anxiety, and insomnia [18]. Chaihu is used to treat diseases related to the digestive system, e.g., hepatitis, liver cirrhosis, cholecystitis, pancreatitis, gynecological diseases, and hyperlipidemia [5].
Saikosaponins alleviated hepatocytes from oxidative and inflammatory stresses, and inhibited liver fibrosis [66]. Further studies demonstrated the protective effects of saikosaponins in reducing lipid peroxidation in hepatocytes [67], regulating intracellular calcium levels to prevent hepatocyte injury [68], suppressing activation of hepatic stellate cells as the major matrix-producing cells in liver fibrosis [69,70], and reducing collagen I deposition in the rat liver [71]. Saikosaponins exhibited regulatory effects on cytokines such as ILs, TNF, and IFN [64,65], inhibitory effects on infiltration of macrophages and T lymphocytes [72], and bidirectional modulation of splenic T lymphocyte proliferation [64]. These findings suggest that the hepatoprotective effects of Chaihu and saikosaponins are related to improvement of gan qi stagnation. In addition to liver diseases, Chaihu is commonly used for chronic pancreatitis [73]. Saikosaponins exhibited potent stimulatory effects on pancreatic enzyme secretion in rats [74]. Chai-hu-shu-gan powder inhibited the expression of nuclear factor-κB (NF-κB) and TNF-α mRNA in the pancreas to achieve anti-inflammatory and antifibrotic effects [75]. Moreover, the same prescription reduced the abnormally high plasma level of cholecystokinin in chronic pancreatitis, improved the gastric movement, and avoided nausea and flatulence [76,77].
In liver ischemia-reperfusion injury, autophagy induction attenuated the ischemic and reperfusion damage to the organ, probably because a decrease in autophagy would lead to accumulation of dysfunctional mitochondria, resulting in cellular damage and failure in energy production, and eventually cell death [48]. In liver disease, suppression of autophagy caused abnormal protein aggregation [40]. In liver fibrosis, autophagy activation might be beneficial to the recovery of the liver function [78]. All of these findings indicate that Chaihu-induced autophagy might relieve liver disease-related symptoms through anti-inflammatory, organ-protective, and aggregate removal functions, which are related to alleviation of gan qi stagnation.
Chaihu-mediated autophagy intervenes in carcinogenesis
In CM theory, tumor formation is the result of stasis of xue (blood), retention of jin ye (fluid), and accumulation of tan (phlegm) [79]. A recent study demonstrated the anticancer effects of Ssa and Ssd via autophagy induction and autophagic cell death [16]. In addition, Chaihu is a commonly prescribed herb in contemporary formulations (Table 2) with preventive or therapeutic effects on cancer [80]. Patients treated with “xiao Chaihu” decoction exhibited a significantly lower incidence of hepatocellular carcinoma [81], reductions in cancer pain and tumor size [82,83], and prevention of liver cancer relapses [84]. The decoction had multiple functions in immunomodulation, apoptosis, and anti-angiogenesis [85-87].
Table 2.
Chaihu -containing formulated decoctions prescribed for modulation of cancers in CM[80]
| Cancer | Chaihu -containing prescriptions |
|---|---|
| Hepatocellular cancer |
Xiao Chaihu Decoction |
| Supplemented Da Chaihu Decoction | |
|
Si ni Powder combined with Liu jun zi Decoction | |
| Supplemented Xiao yao Powder | |
| No. 1 anticancer formula | |
|
Chaihu zhe chong Decoction | |
| Experienced prescription | |
| Pancreatic cancer |
Xiao Chaihu Decoction |
| Experienced prescription | |
| Gall bladder cancer |
Shu gan li dan Decoction |
| Breast cancer |
Yi qi shu gan Decoction |
|
Xiao ru Decoction | |
| Supplemented Xiao yao powder combined with Si jun zi Decoction | |
| Experienced prescription | |
| Cervical cancer |
Jia wei xiao yao Powder |
|
Chaihu gui zhi Decoction | |
| Thyroid carcinoma |
Jia xian ping Decoction |
| Esophageal carcinoma |
Jin fo yin |
|
Er chen xuan fu Decoction | |
| Gastric cancer | Chaihu shu gan Decoction combined with Xi shu jian |
The signaling pathway of autophagy is associated with the key regulatory proteins of carcinogenesis, such as tumor suppressor gene p53, phosphatase and tensin homolog (PTEN), death-associated protein kinase, and proto-oncoprotein B-cell CLL/lymphoma 2 (Bcl-2) [39,88]. Autophagy was responsible for massive cancer cell death in vitro and in vivo[89-91]. Autophagic inducers also promoted autophagic cell death in tumors or augmented the efficacy of chemotherapeutic agents when used in combination during cancer therapy [92,93]. By eliminating genomic mutations, damaged proteins, and cytotoxic substances, autophagy protected cells against cancers [94]. However, the roles of autophagy in cancers remain controversial, because autophagy might promote tumor growth by providing energy to poorly-vascularized tumor cells [95].
Despite its adaptive and pro-survival roles, autophagy can lead to type II programmed cell death [96]. Autophagy promoted autophagic cell death in cells [97], and killed apoptosis-resistant cancer cells under chemotherapy [98]. Moreover, autophagy was associated with massive cancer cell death in cancerous tissues derived from different organs [99,100]. Ssd was able to induce autophagic cell death in a panel of apoptosis-resistant cells via direct inhibition of SERCA [16]. The anticancer effects of Chaihu can be attributed to its autophagy-inducing ability.
Chaihu-mediated autophagy modulates stress hormone-regulated metabolism
Chaihu could mediate its protective effects on gan qi stagnation-induced emotional instability through lipid metabolism and hormonal regulation [101]. In fact, analyses of plasma metabolites in a rat model of gan qi stagnation stimulated by chronic immobilization stress revealed elevated levels of lactic acid, saturated fatty acid, and blood sugar, and reduced levels of unsaturated fatty acid and high density lipoprotein [102]. Another study applied stress to a macaque model with premenstrual syndrome, and demonstrated increased plasma levels of serotonin (5-HT), noradrenalin, and prolactin [103].
As a regulator of lipid and glucose metabolism [104], loss of autophagy caused abnormal accumulation of lipids in mouse hepatocytes and a significant increase in plasma triglycerides, with reductions in fatty acid beta-oxidation [105] and pancreatic β-cell mass [106]. Coincidently, saikosaponins increased hepatic uptake of cholesterol and decreased plasma levels of cholesterol and triglycerides [107]. Furthermore, a study on depressive patients revealed correlations between the plasma levels of cholesterol, triglycerides, and serum neurotransmitters, and depression [108]. As saikosaponins were able to reduce the plasma levels of cholesterol, triglycerides, and phospholipids [107], Chaihu might attenuate depressive symptoms by regulating metabolite, hormone, and neurotransmitter levels via autophagy-mediated lipid metabolism in the human body.
Conclusions
The function of Chaihu in harmonizing the exterior and interior of the body is related to its pathogen control and immunomodulation properties. Furthermore, Chaihu’s function in resolving gan qi stagnation might arise through its supportive roles in protecting organs, preventing damage to cells and organs, and restoring visceral and cellular metabolic conditions. All of these protective pharmacological effects of Chaihu might be attributed to its autophagy induction.
Abbreviations
Ssa: Saikosaponin-a; Ssc: Saikosaponin-c; Ssd: Saikosaponin-d; ER: Endoplasmic reticulum; PTEN: Phosphatase and tensin homolog; TLRs: Toll-like receptors; Bcl-2: B-cell CLL/lymphoma 2; IL: Interleukin; TNF: Tumor necrosis factor; IFN: Interferon; c-AMP: Cyclic adenosine monophosphate; SERCA: Sarcoplasmic/endoplasmic reticulum calcium ATPase pump; NF-κB: Nuclear factor-κB; CM: Chinese medicine; Atg: Autophagy-related gene; mTOR: Mammalian target of rapamycin; 5-HT: 5-hydroxytryptamine.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VKWW conceived and planned the review. BYKL and JFO carried out the review plan and wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Betty Yuen-Kwan Law, Email: yklaw@must.edu.mo.
Jing-Fang Mo, Email: djmaybee@hotmail.com.
Vincent Kam-Wai Wong, Email: kawwong@must.edu.mo.
Acknowledgment
This work was supported by grants from the Science and Technology Development Fund (FDCT) of Macao (Project codes: 013/2012/A1 and 076/2011/A3).
References
- Huang Z. The overview of xiao yao powder in clinical practice. J Guangxi Tradit Chin Med Univ. 2009;12:76–78. [Google Scholar]
- Wei DY, Wang GY. The clinical application of da chaihu decoction. Mod J Integr Tradit Chin Wes Med. 2013;22:1476–1478. [Google Scholar]
- Liu CL. Treated chronic cholecystitis alternating chills and fevers syndrome with xiao chai hu decoction in 59 cases. Guangming J Chin Med. 2012;27:63. [Google Scholar]
- Xuan X, Rong ZB, Liu C, Li YH, Duan LH, Li LJ, Wu ZZ. Effects of CHSGS on behavior and hippocampal monoamine neurotransmitter in Alzheimer's disease rats with liver-qi. Shenzhen J Integ Tradit Chin Wes Med. 2013;23:129–134. [Google Scholar]
- Zhao ZZ, Xiao PG. Encyclopedia of Medicinal Plants. Shanghai: Shanghai World Publishing Corporation; 2009. [Google Scholar]
- Li TL, Du XW. The comparison of the pharmacological effect of nan Chaihu and bei Chaihu: the pilot study of anti-pyretic and hepatoprotective effect. Acta Chin Med Pharmacol. 1992;3:34–37. [Google Scholar]
- Benito PB, Martinez MJA, Sen AMS, Gomez AS, Matellano LF, Contreras SS, Lanza AMD. In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci. 1998;63:1147–1156. doi: 10.1016/S0024-3205(98)00376-2. [DOI] [PubMed] [Google Scholar]
- Yen MH, Lin CC, Yen CM. The immunomodulatory effect of saikosaponin derivatives and the root extract of Bupleurum kaoi in mice. Phytother Res. 1995;9:351–358. doi: 10.1002/ptr.2650090509. [DOI] [Google Scholar]
- Cheng PW, Ng LT, Chiang LC, Lin CC. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin Exp Pharmacol Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman MSI, Africa LJ, Akuodor GC, Ugwu TC, Osunkwo UA. Antinociceptive and antipyretic properties of the pharmaceutical herbal preparation, Radix bupleuri in rats. J Medic Plants Res. 2010;4:659–663. [Google Scholar]
- Wang SC, Zhao HP. Effects of chaihu on antipyretic and antivirus. Lishizhen medicine mat Med Res. 1998;9:418–419. [Google Scholar]
- Shimaoka A, Seo S, Minato H. Saponins isolated from Bupleurum falcatum L.; components of saikosaponin b. J Chem Soc Perkin 1. 1975;20:2043–2048. doi: 10.1039/p19750002043. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhao Y, Sun R. Research development on hepatoprotective effect and hepatotoxicity based on bupleurum saikosaponin components. Chin J Pharmacovigilance. 2011;8:38–40. [Google Scholar]
- Kang SJ, Lee YJ, Kim BM, Kim YJ, Woo HD, Jeon HK, Chung HW. Effect of Bupleuri Radix extracts on the toxicity of 5-fluorouracil in HepG2 hepatoma cells and normal human lymphocytes. Basic Clin Pharmacol Toxicol. 2008;103:305–313. doi: 10.1111/j.1742-7843.2008.00280.x. [DOI] [PubMed] [Google Scholar]
- Wang YS. Pharmacology and Applications of Chinese Materia Medica. Beijing: People's Health Publisher; 1983. [Google Scholar]
- Wong VK, Li T, Law BY, Ma ED, Yip NC, Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL, Liu L. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013;4:e720. doi: 10.1038/cddis.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HW, Zhou ZM. The clinical application and modern research progress of CHSGS. Lishizhen medicine and materia medica Res. 2007;18:1234–1236. [Google Scholar]
- Liu ZW, Liu L. Essentials of Chinese Medicine. London: Foundations of Chinses Medicine. Springer; 2009. [Google Scholar]
- Wang YL, He SX, Luo JY. Progress in research on antitumor activity of saikosaponin and its mechanism. Zhong Xi Yi Jie He Xue Bao. 2006;4:98–100. doi: 10.3736/jcim20060129. [DOI] [PubMed] [Google Scholar]
- Yang YY, Tang YZ, Fan CL, Luo HT, Guo PR, Chen JX. Identification and determination of the saikosaponins in Radix bupleuri by accelerated solvent extraction combined with rapid-resolution LC-MS. J Sep Sci. 2010;33:1933–1945. doi: 10.1002/jssc.201000100. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Shimokawa S, Tanaka H, Shoyama Y. Development of an assay system for saikosaponin a using anti-saikosaponin a monoclonal antibodies. Biol Pharm Bull. 2004;27:66–71. doi: 10.1248/bpb.27.66. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chang NW, Chung JG, Chen KC. Saikosaponin-a induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 2003;31:363–377. doi: 10.1142/S0192415X03001065. [DOI] [PubMed] [Google Scholar]
- Kim BM, Hong SH. Sequential caspase-2 and caspase-8 activation is essential for saikosaponin a-induced apoptosis of human colon carcinoma cell lines. Apoptosis. 2011;16:184–197. doi: 10.1007/s10495-010-0557-x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cai TT, Zhou XB, Xu Q. Saikosaponin a inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int Immunopharmacol. 2009;9:978–983. doi: 10.1016/j.intimp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Wu WS, Hsu HY. Involvement of p-15(INK4b) and p-16(INK4a) gene expression in saikosaponin a and TPA-induced growth inhibition of HepG2 cells. Biochem Biophys Res Commun. 2001;285:183–187. doi: 10.1006/bbrc.2001.5152. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Lin YH, Chu CC, Tsai YH, Chao JC. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J Med Food. 2008;11:224–229. doi: 10.1089/jmf.2007.555. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Tsai SC, Wang BW, Liu YC, Lee CC. Saikosaponin C induces endothelial cells growth, migration and capillary tube formation. Life Sci. 2004;76:813–826. doi: 10.1016/j.lfs.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69:705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- Wong VK, Zhang MM, Zhou H, Lam KY, Chan PL, Law CK, Yue PY, Liu L. Saikosaponin-d enhances the anticancer potency of TNF-alpha via overcoming its undesirable response of activating NF-Kappa B signalling in cancer cells. Evid Based Complement Alternat Med. 2013;2013:745295. doi: 10.1155/2013/745295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Orita M, Konishi H, Arichi S, Odashima S. Effects of saikosaponin-d on enhanced CCl4-hepatotoxicity by phenobarbitone. J Pharm Pharmacol. 1985;37:555–559. doi: 10.1111/j.2042-7158.1985.tb03066.x. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Kuo PL, Lin CC. The proliferative inhibition and apoptotic mechanism of saikosaponin d in human non-small cell lung cancer A549 cells. Life Sci. 2004;75:1231–1242. doi: 10.1016/j.lfs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Hsu YL, Kuo PL, Chiang LC, Lin CC. Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in induction of apoptosis and cell cycle arrest by saikosaponin d in human hepatoma cell lines. Cancer Lett. 2004;213:213–221. doi: 10.1016/j.canlet.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan S, Waghray A, Larner SF, Dunn WA Jr, Hayes RL, Wang KK. Amino acid starvation induced autophagic cell death in PC-12 cells: evidence for activation of caspase-3 but not calpain-1. Apoptosis. 2006;11:1573–1582. doi: 10.1007/s10495-006-7690-6. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S, Zhukovsky DS. Pathophysiology and management of fever. J Support Oncol. 2006;4:9–16. [PubMed] [Google Scholar]
- Kleef R, Hager ED. In: Hyperthermia in Cancer Treatment: A Primer. Volume 3. Baronzio GF, Hager ED, editor. London: Springer; 2006. Fever, Pyrogens and Cancer; pp. 276–337. [Google Scholar]
- Han DW, Ma XH, Zhou LM, Zhao YC. The therapeutic effect of experimental liver injury by xiao yao san. Shan Xi Yi Yao Za Zhi. 1976;2:71–75. [Google Scholar]
- Jin GT, Li B, Wang SR. Experimental study on material basis, efficacy and mechanism of antipyretic effect of Bupleuri Radix. J Chengdu Univ TCM. 2013;36:28–30. [Google Scholar]
- Zhang YB, Liang Y, Xia AJ. The progress of pharmacological study on the anti-pyretic effect of Chaihu. China Pharmaceuticals. 2011;20:79–80. [Google Scholar]
- Liu YH, Chen YS, Xi W, Bai J. Studies on antiendotoxin action of total saponins from radix Bupleuri. Zhong Yao Cai. 2003;26:423–425. [PubMed] [Google Scholar]
- Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48:1054–1061. doi: 10.1002/hep.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Grossmayer GE, Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M. Removal of dying cells and systemic lupus erythematosus. Mod Rheumatol. 2005;15:383–390. doi: 10.3109/s10165-005-0430-x. [DOI] [PubMed] [Google Scholar]
- Massey DC, Parkes M. Genome-wide association scanning highlights two autophagy genes, ATG16L1 and IRGM, as being significantly associated with Crohn's disease. Autophagy. 2007;3:649–651. doi: 10.4161/auto.5075. [DOI] [PubMed] [Google Scholar]
- Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Miller BC, Zhao Z, Stephenson LM, Cadwell K, Pua HH, Lee HK, Mizushima NN, Iwasaki A, He YW, Swat W, Virgin HWT. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy. 2008;4:309–314. doi: 10.4161/auto.5474. [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Kourtzelis I, Papadopoulos VP, Mimidis K, Speletas M, Ritis K. In vivo induction of the autophagic machinery in human bone marrow cells during Leishmania donovani complex infection. Parasitol Int. 2009;58:475–477. doi: 10.1016/j.parint.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QL, Zhang ZQ, Nagasawa T, Hiai S. The structure activity relationship of saikosaponins and glycyrrhizin derivatives for Na+, K(+)-ATPase inhibiting action. Yao Xue Xue Bao. 1996;31:496–501. [PubMed] [Google Scholar]
- Chen RJ, Chung TY, Li FY, Yang WH, Jinn TR, Tzen JT. Steroid-like compounds in Chinese medicines promote blood circulation via inhibition of Na+/K+ -ATPase. Acta Pharmacol Sin. 2010;31:696–702. doi: 10.1038/aps.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LL, Ye YK, Pan SL, Wu JM. Activity of monomers of pentaeyclic triterpennoids of saikosaponines against methicillin-resistant staphylococcus aureus. J Tongji UnivMed Sci. 2008;29:15–46. [Google Scholar]
- Chang JS, Wang KC, Liu HW, Chen MC, Chiang LC, Lin CC. Sho-saiko-to (xiao-chai-hu-tang) and crude saikosaponins inhibit hepatitis B virus in a stable HBV-producing cell line. Am J Chin Med. 2007;35:341–351. doi: 10.1142/S0192415X07004862. [DOI] [PubMed] [Google Scholar]
- Liao DS, Yu DW. Suppresive effect on respiratory syncytial virus through combination of Ribavirin and Chaihu injection. Chin J of mis diagnostics. 2003;3:230–231. [Google Scholar]
- Kato M, Pu MY, Isobe K, Iwamoto T, Nagase F, Lwin T, Zhang YH, Hattori T, Yanagita N, Nakashima I. Characterization of the immunoregulatory action of saikosaponin-d. Cell Immunol. 1994;159:15–25. doi: 10.1006/cimm.1994.1291. [DOI] [PubMed] [Google Scholar]
- Wong VK, Zhou H, Cheung SS, Li T, Liu L. Mechanistic study of saikosaponin-d (Ssd) on suppression of murine T lymphocyte activation. J Cell Biochem. 2009;107:303–315. doi: 10.1002/jcb.22126. [DOI] [PubMed] [Google Scholar]
- Liao B, Chen N. The study of the protective mechanism of hepatocyte in obstructive jaundice by saikosaponin. Guide of China Med. 2011;9:52–53. [Google Scholar]
- He Y, Hu ZF, Li P, Xiao C, Chen YW, Li KM, Guo JZ, Pan L, Xiong JP. Experimental study of saikosaponin-d (SSd) on lipid peroxidation of hepatic fibrosis on rat. Zhongguo Zhong Yao Za Zhi. 2008;33:915–919. [PubMed] [Google Scholar]
- Han XH, Gai XD, Xue YJ, Chen M. Effects of the extracts from Bupleurum Chinese DC on intracelluar free calcium concentration and vincristine accumulation in human hepatoma BEL-7402 cells. Tumor. 2006;26:314–317. [Google Scholar]
- Chen SA, Ben CG, Yang MJ. A clinical study on the saikosaponin on FSC activation and extracellular matrix synthesis. J Beijing University of TCM. 1999;22:31–34. [Google Scholar]
- Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- Fan J, Li X, Li P, Li N, Wang T, Shen H, Siow Y, Choy P, Gong Y. Saikosaponin-d attenuates the development of liver fibrosis by preventing hepatocyte injury. Biochem Cell Biol. 2007;85:189–195. doi: 10.1139/O07-010. [DOI] [PubMed] [Google Scholar]
- Li P, Gong YW, Zhao SP, Ming LK, Chen YW, Fu GX, Zhen GJ, Li X, Zhang Y. Experimental investigation of suppressive effect of saikosaponin-d on the progression of glomerulosclerosis. Sci Technol Rev. 2006;24:37–41. [Google Scholar]
- Huang HQ, Feng LY. Cognition of traditional Chinese medicine about chronic pancreatitis and its treating progression. Mod J Integrated Traditional Chin West Med. 2007;16:3762–3763. [Google Scholar]
- Yang WX, Yu Y, Wang H, Zhao YY, Liang H, Wu XZ. Kinetics of enzymes secretion and mechanism stimulated by effective component of bupleurum in rat pancreatic acini. Modernization of Traditional Chinese Medicine and Materia Medica–World. Sci Technolx. 2001;3:21–24. [Google Scholar]
- Chen Y, Zhou XL, Xue CR. Therapeutic effects and mechanisms of chaihushugan decoction in experimental chronic pancreatitis rats. Chi J Sur Integrated Traditional Wes Med. 2010;16:330–333. [Google Scholar]
- Liu J, Zhao Z, Xue CR. Effect of chaihu shugan pulvis on dysfunction of pancreatic exocrine secretion in patients with chronic pancreatitis. Chinese J Sur Integrated Traditional and We Med. 2010;16:275–277. [Google Scholar]
- Miyasaka K, Ohta M, Kanai S, Yoshida Y, Sato N, Nagata A, Matsui T, Noda T, Jimi A, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Enhanced gastric emptying of a liquid gastric load in mice lacking cholecystokinin-B receptor: a study of CCK-A, B, and AB receptor gene knockout mice. J Gastroenterol. 2004;39:319–323. doi: 10.1007/s00535-003-1297-2. [DOI] [PubMed] [Google Scholar]
- Mao YL, Chen RR, Yang HY, Zhang JC, Zhang YD, Ma JH, Sang XT, Lu X, Zhong SX, Huang JF. Autophagy in fibrotic and postoperative remnant liver in rat. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008;30:421–425. [PubMed] [Google Scholar]
- Fang XH, Wu Q, Han XM, Ren XL, Bai X, Zhu B, Li JY, Yang YH. A clinical study of chaihu shugan powder in the treatment of malignant tumor patients with depression. J Chin Oncol. 2013;19:726–729. [Google Scholar]
- Yang JK. Xian Dai Zhong Yi Zhong Liu Xue (Modern oncology study-Chinese medicine) Shanghai: Shanghai University of Traditional Chinese Medicine Press; 2004. [Google Scholar]
- Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, Monna T, Kobayashi K, Tango T. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9) Cancer Lett. 1995;76:743–749. doi: 10.1002/1097-0142(19950901)76:5<743::AID-CNCR2820760506>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Chang MY. Treatment of 15 cases of primary liver cancer by using supplemented xiao-chai-hu decoction. J Practical Trad Chin Med. 1995;1:14. [Google Scholar]
- Zhao HY. Clinical observation of the treatment of primary liver cancer by using xiao-chai-hu decoction. J Tradit Chin Med. 1995;18:32. [Google Scholar]
- Li SJ, Li L, Yi C. Clinical observation of the preventive effect of xiao-chai-hu-pian after surgery of liver cancer, attached with 40 case reports. J Chengdu Unv Tarditional Chin Med. 2001;25:16–18. [Google Scholar]
- Inada Y, Watanabe K, Kamiyama M, Kanemitsu T, Clark WS, Lange M. In vitro immunomodulatory effects of traditional Kampo medicine (sho-saiko-to: SST) on peripheral mononuclear cells in patients with AIDS. Biomed Pharmacotherapy. 1990;44:17–19. doi: 10.1016/0753-3322(90)90064-G. [DOI] [PubMed] [Google Scholar]
- Motoo Y, Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994;86:91–95. doi: 10.1016/0304-3835(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Liang JJ, Yin DF, Zhou LJ. Experimental study on the expression of VEGF of the minor Bupleuri Decoction in mice with Lewis lung cancer cells. Acta Chin Med Pharmacol. 2008;36:15–17. [Google Scholar]
- Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M, Walker R, Hermann RS. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- Onodera R, Motoyama K, Tanaka N, Ohyama A, Okamatsu A, Higashi T, Kariya R, Okada S, Arima H. Involvement of autophagy in antitumor activity of folate-appended methyl-beta-cyclodextrin. Sci Rep. 2014;4:4417. doi: 10.1038/srep04417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liersch R, Detmar M. The miR-290-295 cluster suppresses autophagic cell death of melanoma cells. Sci Rep. 2012;2:808. doi: 10.1038/srep00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law BY, Wang M, Ma DL, Al-Mousa F, Michelangeli F, Cheng SH, Ng MH, To KF, Mok AY, Ko RY, Lam SK, Chen F, Che CM, Chiu P, Ko BC. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther. 2010;9:718–730. doi: 10.1158/1535-7163.MCT-09-0700. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- DiPaola RS, Dvorzhinski D, Thalasila A, Garikapaty V, Doram D, May M, Bray K, Mathew R, Beaudoin B, Karp C, Stein M, Foran DJ, White E. Therapeutic starvation and autophagy in prostate cancer: a new paradigm for targeting metabolism in cancer therapy. Prostate. 2008;68:1743–1752. doi: 10.1002/pros.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippert MM, O'Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yoshida T, Tsujioka M, Arakawa S. Autophagic cell death and cancer. Int J Mol Sci. 2014;15:3145–3153. doi: 10.3390/ijms15023145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- Mujumdar N, Saluja AK. Autophagy in pancreatic cancer: an emerging mechanism of cell death. Autophagy. 2010;6:997–998. doi: 10.4161/auto.6.7.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opipari AW Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.CAN-03-2404. [DOI] [PubMed] [Google Scholar]
- Li D, Jiang T, Fan HQ, Liang WN, Xiong KC, Tang CP. Influence of chaihushugan powder on lipid metabolism and liver function in nonalcoholic fatty liver rats. Zhong Yao Yao Li Yu Lin Chuang. 2013;29:8–12. [Google Scholar]
- Luo HG. PhD thesis. Beijing: Beijing University of Traditional Chinese Medicine, Chinese Medicine Department; 2007. Studies of prescriptions corresponding to syndromes of xiaoyaosan decoction based on metabonomics. [Google Scholar]
- Shi ZF. Master thesis. Shan Dong: Shan Dong University of Traditional Chinese Medicine, Traditional Chinese Medicine Department; 2002. The new evidence of the hypothesis of liver governs normal flow of qi relates to monoamine neurotransmitter and sex hormone and their regulative hormone. [Google Scholar]
- Kotoulas OB, Kalamidas SA, Kondomerkos DJ. Glycogen autophagy in glucose homeostasis. Pathol Res Pract. 2006;202:631–638. doi: 10.1016/j.prp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kumagai A, Yamamura Y. Structure and action of saikosaponins isolated from Bupleurum falcatum L. II. Metabolic actions of saikosaponins, especially a plasma cholesterol-lowering action. Arzneimittelforschung. 1975;25:1240–1243. [PubMed] [Google Scholar]
- Yuan YG, Zhang XB, Wu AQ, Zhang SN, Chen YQ. The relationship between plasma monoamine neurotransmitters and serum lipid concentrations in depressive patients. J Clin Psychological Med. 2003;13:67–68. [Google Scholar]


