Abstract
Objective
To assess the effects of a history of alcohol use disorders (AUDs) on risk of severe cognitive and memory impairment in later life.
Methods
We studied the association between history of AUDs and the onset of severe cognitive and memory impairment in 6,542 middle-aged adults born 1931 through 1941 who participated in the Health and Retirement Study, a prospective nationally representative U.S. cohort. Participants were assessed at 1992 baseline and follow-up cognitive assessments were conducted biannually from 1996 through 2010. History of AUDs was identified using the three-item modified CAGE questionnaire. Cognitive outcomes were assessed using the 35-item modified Telephone Interview for Cognitive Status at last follow-up with incident severe cognitive impairment defined as a score ≤8, and incident severe memory impairment defined as a score ≤1 on a 20-item memory subscale.
Results
During up to 19 years of follow-up (mean: 16.7 years, standard deviation: 3.0, range: 3.5–19.1 years), 90 participants experienced severe cognitive impairment and 74 participants experienced severe memory impairment. History of AUDs more than doubled the odds of severe memory impairment (odds ratio [OR] = 2.21, 95% confidence interval [CI] = 1.27–3.85, t = 2.88, df = 52, p = 0.01). The association with severe cognitive impairment was statistically non-significant but in the same direction (OR = 1.80, 95% CI = 0.97–3.33, t = 1.92, df = 52, p = 0.06).
Conclusion
Middle-aged adults with a history of AUDs have increased odds of developing severe memory impairment later in life. These results reinforce the need to consider the relationship between alcohol consumption and cognition from a multifactorial lifespan perspective.
Key Words: Alcohol use disorders, CAGE, memory impairment, cognitive impairment
Dementia presents major clinical and societal challenges and has enormous public health implications. In the United States, dementia affects around 13.9% of people aged over 70 years1 and cost the economy between US$ 159 billion and 215 billion in 2010.2 The considerable human and financial cost of dementia means understanding the causes of dementia and identifying potentially modifiable risk factors are crucial.
Dementia risk is known to be associated with mean levels of current alcohol consumption. Recent meta-analyses suggest a J-shaped relationship between mean alcohol consumption and subsequent risk of Alzheimer dementia and all-cause dementia with lower risk in comparison to moderate drinkers.3, 4 Mean intake captures only one aspect of alcohol consumption, however, and the relationship between dementia and harmful patterns of alcohol consumption such as alcohol use disorders (AUDs), characterized by alcohol dependence or alcohol abuse (recurrent alcohol use associated with a range of problems but not meeting criteria of dependence),5 is largely unknown.
Risks of dementia and short-term mortality were associated with AUDs in the Canadian Study of Health and Aging.6 History of AUDs increased the odds of memory impairment in a 6-year follow up of 3,822 middle-aged men from the U.S. Health and Retirement Study (HRS),7 and was found to be associated with increased prevalence of cognitive and functional impairment and dementia in women but not men in a cross-sectional study of 1,145 older adults aged over 59 years in Brazil.8 AUDs may be related to dementia via a number of mechanisms including brain damage due to toxic effects of alcohol, metabolic changes in the brain, neurotransmitter imbalances,9 and nutritional deficiency.10 No previous study, however, has examined the long-term relationship between history of AUDs and risk of dementia-related outcomes. We used data on middle-aged HRS participants followed for up to 19 years to address the hypothesis that history of AUDs is associated with an increased risk of severe cognitive and memory impairment in later life.
Methods
Study Population
The original HRS study involved a cohort of adults born between 1931 and 1941 and their spouses enrolled in 1992 and re-interviewed biannually thereafter.11 The HRS subsequently expanded, merging with the Asset and Health Dynamic of the Oldest Old survey and adding additional cohorts12 so that in 1998 it became nationally representative of the community-dwelling U.S. population aged 51 years and older.13 The HRS is supported by the U.S. National Institute on Aging and conducted by the Institute for Social Research at the University of Michigan. The HRS was approved by the Health Sciences institutional review board at the University of Michigan. We used anonymous HRS data and a longitudinal data set, the RAND HRS Data file (Version M),14 derived from the HRS data by the RAND Center for the Study of Aging with funding from the U.S. National Institute on Aging and the Social Security Administration. All data can be obtained from the study website (http://hrsonline.isr.umich.edu).
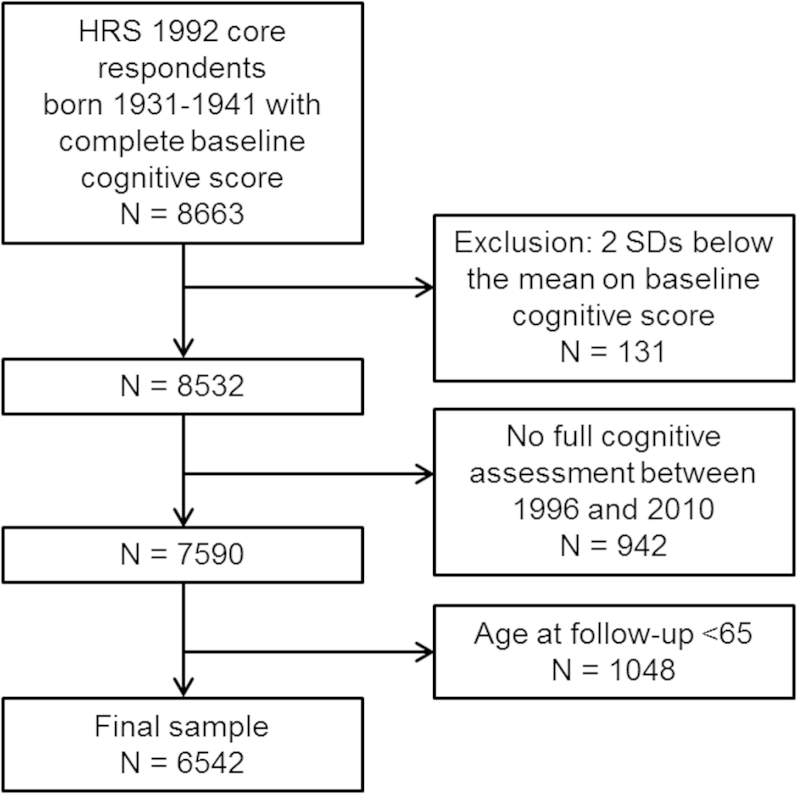
Our analyses were based on data from 8,663 self-respondents born 1931 through 1941 with complete 1992 baseline cognitive score (see Fig. 1). Excluding those who were two standard deviations below the mean on baseline cognitive score reduced the analysis group to 8,532. Between 1996 and 2010, a total of 6,542 participants aged 65 years or over at follow-up completed at least one follow-up cognitive assessment (mean length of follow-up [SD]: 16.7 [3.0], range: 3.5–19.1 years).
Figure 1.
Diagram of the study population. SD: Standard deviation.
Compared with the analyzed group, those not included in our analyses were younger (t = −8.20, df = 8661, p <0.001), less likely to be women (χ² = 55.38, df = 1, p <0.001), less educated (t = −10.15, df = 8661, p <0.001), and had a lower baseline memory score (t = −15.21, df = 8661, p <0.001).
Assessment of AUDs
We identified history of AUDs based on responses to the CAGE instrument, a validated screening questionnaire.15, 16 The CAGE consists of four questions and its key elements build the acronym: Have you ever felt you should Cut down on your drinking? Have people ever Annoyed you by criticizing your drinking? Have you ever felt bad or Guilty about drinking? Have you ever taken a drink first thing in the morning (Eye-opener) to steady your nerves or get rid of a hangover? We used a modified three-item version of the CAGE which omits the “cut-down” question because a wish to reduce alcohol consumption, possibly due to health concerns, is common in those aged 50 years and over and reduces this item's discriminatory value.17 The three-item version of the CAGE with a cutoff point of 1 has a sensitivity of 84% and a specificity of 69% for alcohol abuse or dependence (i.e., alcohol use disorders).17
Assessment of Cognitive Function
At baseline in 1992, memory was assessed using immediate and delayed word recall tasks of 20 nouns (with a total score ranging from 0–40) as previously described.18 Abstract reasoning was assessed at baseline using a modified similarities test18 from the Wechsler Adult Intelligence Scale-Revised.19 Participants were asked to describe similarities between seven pairs of words—for example, orange and banana (score ranging from 0–14).18 We z-standardized both scores (mean of 0 and SD of 1), summed them and z-standardized them again to derive a baseline global cognitive function score which gave equal weighting to memory and abstract reasoning. Full cognitive assessment was neither available in 1992 nor in 1994.18
At follow-up from 1996 through 2010 global cognitive function was measured using a modified version of the Telephone Interview for Cognitive Status (mTICS),20 an adaptation of the widely used Mini-Mental State Examination (MMSE).21 The mTICS includes orientation items from the MMSE, immediate and delayed recall of 10 words, the Serial 7s subtraction test, backwards counting from 20, object naming, and naming the current U.S. President and Vice President.18 Scores on the mTICS range from 0 to 35 points and higher scores represent better cognitive function. Based upon previous research,22 we used a cutoff score of 8 (two standard deviations below the mean) to define severe global cognitive impairment indicative of dementia. For each respondent we used the last available mTICS score between 1996 and 2010, thus ensuring all participants had at least four years of follow-up and reducing loss of information due to subsequent mortality. Missing values for partially completed mTICS assessments were imputed by HRS investigators as previously described.23
Memory at follow-up was measured using the immediate and delayed recall of 10 words with higher scores (ranging from 0–20) representing better memory.18 We derived a cut-point to define severe memory impairment based upon the distribution of scores in HRS participants aged 65 years and older in 1998 when the study became population representative of community-dwelling U.S. elders13 (participant characteristics in Supplemental Table 1; available online). Applying the same approach used previously to define severe global cognitive impairment (two standard deviations below the mean) resulted in a cutoff score of 1 to define severe memory impairment that we applied to the last available wave of follow-up.
Statistical Analyses
We calculated baseline characteristics for our sample and compared those with and without history of AUDs using a Pearson's χ2 test for categorical variables and a Student's t-test for continuous variables. To investigate the association between history of AUDs and odds of severe cognitive and memory impairment we used multivariate logistic regression models, weighted to ensure sample representativeness and to account for clustering and stratification. We adjusted our models for variables that have previously been identified as potential confounders in studies of alcohol consumption and dementia risk.3, 4 In basic adjusted models, we controlled for age, sex, years of formal education, and years of follow-up. In fully adjusted models, we also included race (categorized as White or Caucasian versus Other; the Other category includes Black or African American, American Indian, Alaskan Native, Asian, and Pacific Islander), socioeconomic status (high versus low resulting from a median split of the total household income in U.S. dollars), smoking status (current smoker versus nonsmoker), obesity (body mass index ≥30, calculated as kg/m2), physical inactivity (defined as engaging in light or vigorous physical activity or heavy housework less than once weekly), and depressive symptoms (cutoff of 924 on the 11-item Center for Epidemiologic Studies Depression Scale,25 range: 0–33). After estimating the fully adjusted models we calculated the average predicted probability for those with and without history of AUDs.
In a series of secondary analyses we investigated the influence of potential mediators (hypertension, cardiovascular disease, and head injury) as suggested by the existing literature.26, 27, 28, 29 Self-reported doctor-diagnosed history of hypertension and cardiovascular disease (heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems as indicative of cardiovascular disease), and history of unconsciousness due to head injury were added to fully adjusted models to investigate whether they accounted for any observed association.
We also carried out a number of sensitivity analyses. To investigate whether our results were sensitive to the operationalization of dementia-related outcomes at follow-up we repeated our main analyses substituting our dichotomous outcome variables (severe cognitive and memory impairment) with z-standardized continuous outcomes using linear rather than logistic regression models. To determine whether any observed association was driven by current heavy drinking we excluded participants consuming three or more drinks per day. At baseline participants who consumed alcohol were asked if they had less than one drink a day, one to two drinks a day, three or four drinks a day, or five or more drinks a day. We also repeated our analyses using the four-item CAGE with the standard 2 cut-point to examine the effect of using the standard four-item rather than the modified three-item CAGE.
All analyses were performed using STATA SE 13 (StataCorp, College Station, TX). Two-sided p values of less than 0.05 were considered to be statistically significant.
Results
Baseline characteristics of the study population are presented in Table 1. Compared with those with no history of AUDs, those with history of AUDs were younger, less educated, less likely to be female, more likely to have lower socioeconomic status, to smoke, to consume more than one alcoholic drink per day, to have a history of cardiovascular disease or of having been unconsciousness due to head injury, and to have depressive symptoms, lower baseline cognitive function, and shorter follow-up. No group differences were found regarding race, obesity, physical inactivity, or history of hypertension.
Table 1.
Baseline Sample Characteristics of 6,542 HRS Participants
| Variable | History of AUDs M (SD) or N (%) |
No History of AUDs M (SD) or N (%) |
t/χ² | df | p |
|---|---|---|---|---|---|
| Total | 1065 (16.3) | 5477 (83.7) | |||
| Age | 55.4 (3.2) | 55.7 (3.2) | 2.60 | 6540 | 0.01a |
| Sex | |||||
| Female | 333 (31.3) | 3400 (62.1) | 345.48 | 1 | <0.001b |
| Race | |||||
| White/Caucasian | 862 (80.9) | 4559 (83.2) | 3.32 | 1 | 0.07b |
| Other | 203 (19.1) | 918 (16.8) | |||
| Education (years in school) | 12.1 (3.2) | 12.5 (3.0) | 3.88 | 6540 | <0.001a |
| High socioeconomic status | 529 (49.7) | 2936 (53.6) | 5.54 | 1 | 0.02b |
| Current smoker | 366 (34.4) | 1232 (22.5) | 68.08 | 1 | <0.001b |
| Current alcohol consumption | |||||
| Non-drinker | 358 (33.6) | 2106 (38.5) | |||
| <1 drink a day | 355 (33.3) | 2751 (50.2) | |||
| 1–2 drinks a day | 195 (18.3) | 476 (8.7) | 436.96 | 4 | <0.001b |
| 3–4 drinks a day | 109 (10.2) | 119 (2.2) | |||
| ≥5 drinks a day | 48 (4.5) | 25 (0.5) | |||
| Depressive symptoms (CES-D)c | 262 (24.6) | 759 (13.9) | 78.13 | 1 | <0.001b |
| Obesity | 238 (22.4) | 1326 (24.2) | 1.70 | 1 | 0.19b |
| Physical inactivity | 181 (17.0) | 948 (17.3) | 0.06 | 1 | 0.80b |
| Cognitive score | −0.04 (0.97) | 0.12 (0.97) | 4.81 | 6540 | <0.001a |
| History of hypertension | 356 (33.4) | 1713 (31.3) | 1.91 | 1 | 0.17b |
| History of cardiovascular disease | 116 (10.9) | 450 (8.2) | 8.08 | 1 | <0.01b |
| History of unconsciousness due to head injury | 196 (18.4) | 575 (10.5) | 53.60 | 1 | <0.001b |
| Length of follow-up (years) | 16.5 (3.1) | 16.7 (3.0) | 2.45 | 6540 | 0.01a |
Notes: All values are shown unweighted. SD: standard deviation; df: degrees of freedom.
p value for a Student's t-test.
p value for a Pearson's χ² test.
CES-D: Center for Epidemiologic Studies Depression Scale (11 items; range: 0–33; cutoff of 9 indicating depressive symptoms).
During follow-up 90 participants experienced severe cognitive impairment and 74 participants experienced severe memory impairment. In both basic and fully adjusted logistic regression models there was an increase in the odds of severe memory impairment for participants with a history of AUDs (Table 2). History of AUDs more than doubled the odds of memory impairment in a fully adjusted model (odds ratio [OR]: 2.21, 95% confidence interval [CI]: 1.27–3.85, t = 2.88, df = 52, p = 0.01). Those with a history of AUDs had a 1.84% (95% CI: 1.02%–2.66%) chance of developing severe memory impairment later in life compared with a 0.85% (95% CI: 0.58%–1.12%) chance in those without a history of AUDs. In a basic adjusted model, history of AUDs almost doubled the odds of severe cognitive impairment (OR: 1.96, 95% CI: 1.04–3.70, t = 2.13, df = 52, p = 0.04). The association was non-significant in a fully adjusted model but in the same direction (OR: 1.80, 95% CI: 0.97–3.33, t = 1.92, df = 52, p = 0.06). Those with a history of AUDs had a 1.86% (95% CI: 0.93%–2.79%) chance of developing severe cognitive impairment later in life whereas those without a history of AUDs had a 1.06% (95% CI: 0.73%–1.39%) chance. Fully adjusted sensitivity analyses incorporating continuous cognitive outcomes confirmed that history of AUDs was statistically significantly associated with lower memory (β = −0.08, 95% CI: −0.15 to −0.02, t = −2.65, df = 52, p = 0.01) and lower cognitive function (β = −0.07, 95% CI: −0.13 to −0.01, t = −2.26, df = 52, p = 0.03) at follow-up.
Table 2.
Multivariate Logistic Regression Models of Severe Cognitive and Memory Impairment by History of AUDs
| Model | Total No. of Participants | No. of Cases | History of AUDs OR (95% CI) |
No History of AUDs | Wald test | df | p |
|---|---|---|---|---|---|---|---|
| Severe cognitive impairment | |||||||
| Basic adjusted modela | 6,542 | 90 | 1.96 (1.04–3.70) | 1 [Reference] | 2.13 | 52 | 0.04 |
| Fully adjusted modelb | 6,542 | 90 | 1.80 (0.97–3.33) | 1 [Reference] | 1.92 | 52 | 0.06 |
| Severe memory impairment | |||||||
| Basic adjusted modela | 6,542 | 74 | 2.27 (1.31–3.91) | 1 [Reference] | 3.01 | 52 | <0.01 |
| Fully adjusted modelb | 6,542 | 74 | 2.21 (1.27–3.85) | 1 [Reference] | 2.88 | 52 | 0.01 |
Notes: Based on weighted data. Ns are shown unweighted. OR: odds ratio; CI: confidence interval; df: degrees of freedom.
Adjusted for age, gender, education, length of follow-up.
Adjusted for age, sex, race, education, socioeconomic status, length of follow-up, smoking, depressive symptoms, physical inactivity, and obesity.
Additional adjustment for cardiovascular disease and hypertension in midlife did not substantially attenuate the results in secondary analyses (Table 3), suggesting they do not mediate the relationship between history of AUDs and severe cognitive and memory impairment later in life. Our findings also remained mostly unchanged after adjusting for history of unconsciousness due to head injury (Table 3), suggesting history of head injury does not mediate the observed associations either.
Table 3.
Multivariate Logistic Regression Models of Severe Cognitive and Memory Impairment by History of AUDs with Additional Adjustment for Potential Mediatorsa
| Mediation Analysis | Total No. of Participants | No. of Cases | History of AUDs OR (95% CI) |
No History of AUDs | Wald test | df | p |
|---|---|---|---|---|---|---|---|
| Severe cognitive impairment | |||||||
| History of hypertension | 6,542 | 90 | 1.80 (0.97–3.32) | 1 [Reference] | 1.92 | 52 | 0.06 |
| History of cardiovascular disease | 6,542 | 90 | 1.82 (0.98–3.38) | 1 [Reference] | 1.94 | 52 | 0.06 |
| History of unconsciousness due to head injury | 6,542 | 90 | 1.83 (1.00–3.33) | 1 [Reference] | 2.02 | 52 | 0.05 |
| Severe memory impairment | |||||||
| History of hypertension | 6,542 | 74 | 2.22 (1.27–3.85) | 1 [Reference] | 2.89 | 52 | 0.01 |
| History of cardiovascular disease | 6,542 | 74 | 2.21 (1.27–3.83) | 1 [Reference] | 2.89 | 52 | 0.01 |
| History of unconsciousness due to head injury | 6,542 | 74 | 2.22 (1.28–3.83) | 1 [Reference] | 2.93 | 52 | 0.01 |
Notes: Based on weighted data. Ns are shown unweighted. OR: odds ratio; CI: confidence interval; df: degrees of freedom.
Also adjusted for age, sex, race, education, socioeconomic status, length of follow-up, smoking, depressive symptoms, physical inactivity, and obesity.
In a series of further sensitivity analyses excluding those currently consuming three or more drinks per day did not change the pattern of observed associations (Table 4). Similarly, using the four-item version of the CAGE questionnaire with a cut-point of two or more positive answers indicating history of AUDs also gave a similar pattern of results (Table 4).
Table 4.
Sensitivity Analyses: Multivariate Logistic Regression Models by History of AUDsa
| Sensitivity Analysis | Total No. of Participants | No. of Cases | History of AUDs OR (95% CI) |
No History of AUDs | Wald test | df | p |
|---|---|---|---|---|---|---|---|
| Severe cognitive impairment | |||||||
| Exclusion: current baseline consumption of 3+ drinks | 6,241 | 82 | 1.75 (0.86–3.55) | 1 [Reference] | 1.59 | 52 | 0.12 |
| 4-item CAGE | 6,542 | 90 | 1.72 (0.85–3.50) | 1 [Reference] | 1.54 | 52 | 0.13 |
| Severe memory impairment | |||||||
| Exclusion: current baseline consumption of 3+ drinks | 6,241 | 64 | 2.40 (1.34–4.31) | 1 [Reference] | 3.01 | 52 | <0.01 |
| 4-item CAGE | 6,542 | 74 | 2.08 (1.11–3.88) | 1 [Reference] | 2.35 | 52 | 0.02 |
Notes: Based on weighted data. Ns are shown unweighted. OR: odds ratio; CI: confidence interval; df: degrees of freedom.
Adjusted for age, sex, race, education, socioeconomic status, length of follow-up, smoking, depressive symptoms, physical inactivity, and obesity.
Discussion
Our results suggest odds of late-life severe memory impairment are substantially increased in middle-aged adults with a history of AUDs. There was little evidence that this relationship is mediated by history of hypertension, cardiovascular disease, or head injury. The observed pattern of associations remained unchanged when continuous cognitive and memory outcomes were used, current heavy drinkers were excluded, and an alternative version of the CAGE questionnaire was used.
Our findings are consistent with a previous HRS study showing an increased odds of memory impairment in 3,822 middle-aged men who were followed for 6 years (OR: 1.71, 95% CI: 1.14–2.56).7 Our study substantially improves on this previous work by incorporating a much longer follow-up period, using a larger cohort representative of both the male and female U.S. population, and examining both cognitive and memory impairment. This increases our understanding of the role of personal drinking history before late life and suggests that the CAGE questionnaire is an appropriate instrument to identify individuals at higher risk of future adverse cognitive outcomes associated with history of AUDs. Our research confirms that in order to understand the relationship between alcohol consumption and dementia more fully we need to take into account more than just mean consumption. Excluding current heavy drinkers did not markedly change the pattern of associations, suggesting that the association between history of AUDs and severe memory impairment does not simply reflect current heavy mean consumption.
A number of mechanisms have been identified that help to explain why history of AUDs may be linked to cognitive impairment and dementia risk. Alcohol dependence is associated with volume reduction in white and gray matter, particularly in the frontal lobes, parts of the limbic system, and the cerebellum.9 It is linked to decreased glucose metabolism in cortical and subcortical structures and imbalance of neurotransmitters including GABAergic, serotonergic, dopaminergic, and opioidergic systems.9 Alcohol dependence is also related to head injuries, liver cirrhosis, and nutritional deficiency.10, 30 Severe thiamine deficiency may lead to Wernicke encephalopathy (confusion, ataxia, nystagmus, and ophthalmoplegia) followed by Korsakoff syndrome (severe memory disorder), commonly referred to as Wernicke-Korsakoff syndrome.10, 31 In contrast to moderate alcohol consumption, alcoholism can be also detrimental to the cardiovascular system resulting in cardiomyopathy, cardiac arrhythmias, hypertension, and stroke.32 The potential role of hypertension, cardiovascular disease and head injury is less likely given the results of our mediation analyses indicating that these conditions did not substantially mediate the relationship between history of AUDs and odds of severe cognitive and memory impairment.
The strengths of the present study include its large nationally representative population-based sample, the prospective design with long follow-up, the use of validated measures of history of AUDs and cognitive function, and the adjustment for a wide range of potential confounders. The lifetime AUDs prevalence for those aged 45 to 64 years is 31% according to the U.S. National Epidemiologic Survey on Alcohol and Related Conditions,33 whereas in our study 16% reported a history of AUDs. A limitation of this study, one it holds in common with all population-based studies of alcohol consumption, is its reliance on self-reported AUDs. Study participants tend to underreport their alcohol consumption34 and those engaging in risk behaviors are less likely to participate in surveys.35 The CAGE questionnaire has been shown to discriminate well between subjects with and without history of AUDs, however, and is a validated and widely used screening instrument.36 A further limitation relates to the timing and duration of AUDs. We were not able to take into account when the AUDs occurred or their magnitude because they are not assessed by the CAGE questionnaire.37 The covariates were also based on self-report. Clinical dementia diagnoses were not available and as a result validated screening instruments were used to identify participants with severe cognitive impairment indicative of dementia. Given that the association with severe memory impairment was stronger than that with severe global cognitive impairment, future research incorporating dementia subtypes is warranted to establish whether the link between AUDs and Alzheimer disease is stronger than that with other dementia subtypes. As with all observational studies the possibility of unmeasured confounding—for example, illicit drug use related to AUDs—remains, and our results in themselves do not demonstrate causality.
In conclusion, our findings suggest middle-aged adults with a history of AUDs are at increased risk of developing severe memory impairment later in life. These results reinforce the need to consider the relationship between alcohol consumption and cognition from a multifactorial lifespan perspective. Further research is therefore warranted to investigate patterns of alcohol consumption including the timing and duration of AUDs. Gaining greater insight into the role that comorbid conditions, such as AUDs, play in the natural history of dementia may lead to new opportunities for prevention. The CAGE questionnaire may offer clinicians a practical way to identify individuals at risk of adverse dementia-related outcomes who may benefit from interventions targeting AUDs.
Acknowledgments
Elżbieta Kuźma, David J. Llewellyn, and Iain A. Lang acknowledge support from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. Iain A. Lang is funded by a National Institute for Health Research Knowledge Mobilisation Research Fellowship. This article presents independent research partly funded by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health in England. Elżbieta Kuźma and David J. Llewellyn also acknowledge funding from the Mary Kinross Charitable Trust. Additionally, David J. Llewellyn receives funding from the Alzheimer Association (NIRG-11-200737), the Norman Family Charitable Trust, the James Tudor Foundation, and the Halpin Trust. The National Institute on Aging (NIA) provided funding for the Health and Retirement Study (U01AG09740), data from which were used for this analysis. The Health and Retirement Study is performed at the Survey Research Center, Institute for Social Research, University of Michigan.
The authors have no disclosures to report.
Footnotes
Supplemental digital content is available for this article in the HTML and PDF versions of this article on the journal’s Web site (www.ajgponline.org).
Supplemental digital content.
References
- 1.Plassman B.L., Langa K.M., Fisher G.G. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurd M.D., Martorell P., Delavande A. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstey K.J., Mack H.A., Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- 4.Peters R., Peters J., Warner J. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 5.Zucker R.A. In: Developmental Psychopathology: Risk, Disorder, and Adaptation: 3. Cohen D.J., Cicchetti D., editors. John Wiley & Sons; Hoboken, NJ: 2006. Alcohol use and the alcohol use disorders: a developmental–biopsychosocial systems formulation covering the life course; pp. 620–657. [Google Scholar]
- 6.Thomas V.S., Rockwood K.J. Alcohol abuse, cognitive impairment, and mortality among older people. J Am Geriatr Soc. 2001;49:415–420. doi: 10.1046/j.1532-5415.2001.49085.x. [DOI] [PubMed] [Google Scholar]
- 7.Perreira K.M., Sloan F.A. Excess alcohol consumption and health outcomes: A 6-year follow-up of men over age 50 from the Health and Retirement Study. Addiction. 2002;97:301–310. doi: 10.1046/j.1360-0443.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 8.Lopes M.A., Furtado E.F., Ferrioli E. Prevalence of alcohol-related problems in an elderly population and their association with cognitive impairment and dementia. Alcohol Clin Exp Res. 2010;34:726–733. doi: 10.1111/j.1530-0277.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 9.Buhler M., Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 10.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 11.Juster F.T., Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7–S56. [Google Scholar]
- 12.Hauser R.M., Weir D. Recent developments in longitudinal studies of aging in the United States. Demography. 2010;47(suppl):S111–130. doi: 10.1353/dem.2010.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ofstedal MB, Weir D, Chen K-T, et al: Updates to HRS sample weights. June 2011. Available at: http://hrsonline.isr.umich.edu/index.php. Accessed November 4, 2013.
- 14.RAND: HRS Data, Version M. Produced by the the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA. September 2013. Available at: http://hrsonline.isr.umich.edu/index.php. Accessed November 7, 2013.
- 15.Buchsbaum D.G., Buchanan R.G., Centor R.M. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115:774–777. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- 16.Mayfield D., McLeod G., Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 17.Hinkin C.H., Castellon S.A., Dickson-Fuhrman E. Screening for drug and alcohol abuse among older adults using a modified version of the CAGE. Am J Addict. 2001;10:319–326. [PubMed] [Google Scholar]
- 18.Ofstedal MB, Fisher GG, Herzog AR: Documentation of cognitive functioning measures in the Health and Retirement Study. March 2005. Available at: http://hrsonline.isr.umich.edu/index.php. Accessed November 20, 2012.
- 19.Wechsler D. Psychological Corporation; New York: 1981. Manual for the Wechsler Adult Intelligence Scale - Revised. [Google Scholar]
- 20.Brandt J., Spencer M., Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 21.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Langa K.M., Chernew M.E., Kabeto M.U. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med. 2001;16:770–778. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher GG, Hassan H, Rodgers WL, et al: Health and Retirement Study. Imputation of cognitive functioning measures: 1992–2010 (final release version). September 15, 2013. Available at: http://hrsonline.isr.umich.edu/index.php. Accessed November 1, 2013.
- 24.Steffick DE: Documentation of affective functioning measures in the Health and Retirement Study. 2000. Available at: http://hrsonline.isr.umich.edu/index.php. Accessed February 25, 2013.
- 25.Radloff L.S. The CES-D Scale: a self-report depression scale for research in the general population. App Psych Meas. 1977;1:385–401. [Google Scholar]
- 26.Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135–143. [PMC free article] [PubMed] [Google Scholar]
- 27.Whitmer R.A., Sidney S., Selby J. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 28.Newman A.B., Fitzpatrick A.L., Lopez O. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan J.D. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76:302–309. doi: 10.1016/s0003-9993(95)80654-7. [DOI] [PubMed] [Google Scholar]
- 30.Harper C.G., Kril J.J. Neuropathology of alcoholism. Alcohol Alcohol. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- 31.Kopelman M.D., Thomson A.D., Guerrini I. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- 32.Zakhari S. Alcohol and the cardiovascular system: molecular mechanisms for beneficial and harmful action. Alcohol Health Res World. 1997;21:21–29. [PMC free article] [PubMed] [Google Scholar]
- 33.Hasin D.S., Stinson F.S., Ogburn E. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 34.Feunekes G.I., van 't Veer P., van Staveren W.A. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150:105–112. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 35.Galea S., Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Conigliaro J., Kraemer K., McNeil M. Screening and identification of older adults with alcohol problems in primary care. J Geriatr Psychiatry Neurol. 2000;13:106–114. doi: 10.1177/089198870001300303. [DOI] [PubMed] [Google Scholar]
- 37.Boyle A.R., Davis H. Early screening and assessment of alcohol and substance abuse in the elderly: clinical implications. J Addict Nurs. 2006;17:95–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.